Introduction
Chronic and refractory wounds do not heal normally,
and the integrity of the skin of the affected patients is not
achieved in 1 month, leading to an increased inflammatory response
and tissue damage (1). Chronic
refractory wounds pose a great psychological burden to patients and
seriously affect their quality of life and are therefore are
considered one of the most urgent problems to be resolved in the
field of surgery (2).
Chronic and refractory wounds are usually caused by
microbial colonization and are treated as serious infections. One
of the main factors that influences the wound healing process is
bacterial infection (3). The
formation of bacterial biofilms is an important factor in the
development of bacterial resistance and avoidance of the body's
immune defense mechanisms. The bacterial density sensing system
serves an important role in the formation of biofilms, which
directly affects the formation and dissociation of biofilms
(2).
The self-induction of the molecule autoinducer
(AI)-2, which is the only molecule currently known to be capable of
conducting signal transduction during intra- and interspecific
interactions of bacteria, serves a key role in the formation and
stability of the bacterial density induction system (2,4). AI-2
regulates the expression pattern of genes that promote biofilm
development. An increased concentration of AI-2 leads to a high
level of bioluminescence in Vibrio harveyi. AI-2 has been
reported to be involved in numerous important processes in a number
of Gram-positive and Gram-negative bacteria, including biofilm
formation, excretion of poison, production of antimicrobial agents,
migration and genetic competence (5,6). It is
well known that hypoxia-inducible factors (HIFs) serve a
transcriptional role under conditions of hypoxic stress and
activate a number of target genes, including phosphoglycerate
kinase and vascular endothelial growth factor (VEGF) in cells
(7–9). These processes contribute to
endothelial proliferation, glycolysis and angiogenesis, which
facilitates the generation of new cells (8). Therefore, there is close association
between HIFs, VEGF and wounds healing.
Clinical observations of patients with chronic
refractory wounds have revealed that these patients can
significantly control and eliminate bacterial infection of the
wound upon oral treatment with the QBM. The authors' previous
results revealed that the traditional Chinese medicine QBM served
an antibacterial role by regulating the immune function in mice
with Staphylococcus aureus infection (10). Based on the clinical efficacy of the
QBM, the present study focused on the effect of the self-inducer
molecule AI-2 on chronic and refractory wounds in rats with
Staphylococcus aureus and Pseudomonas aeruginosa
infection.
Materials and methods
Animals
Male Sprague-Dawley rats (n=30; age, 6–8 weeks)
weighing 200–250 g were obtained from Shanghai SLAC Laboratory
Animal Co., Ltd., (Shanghai, China). Rats were housed under
conventional conditions at an appropriate temperature (22.0±0.5°C)
and humidity (50–60%), and under a 12-h light/dark cycle (lights
were switched on from 8:00 a.m. to 8:00 p.m.). the rats were
allowed free access to food and water. All experiments and
procedures were carried out according to the Regulations of
Experimental Animal Administration issued by the State Committee of
Science and Technology of China [animal production license number:
SCXK (Shanghai) 2012-0002].
Drugs and reagents
The QBM (cat. no. 160120) was purchased from the
LongHua Hospital Shanghai University of Traditional Chinese
Medicine (Shanghai, China). Its main ingredients are Chinese
medicines, including Angelica sinensis, Viola mandshurica,
honeysuckle, Red Peony root, Salvia miltiorrhiza, Forsythia
suspensa, Astragali radix, Gleditsia sinensis and
liquorice. The WXD (cat. no. 160413) was purchased from the LongHua
Hospital Shanghai University of Traditional Chinese Medicine. The
main ingredients are Chinese medicines including honeysuckle, wild
chrysanthemum, Viola mandshurica, Gynura bicolor and
dandelion. Pseudomonas aeruginosa was purchased from the
American Type Culture Collection (cat. no. 27853; Manassas, VA,
USA).
The bacteria culture medium contained 1.5% tryptone
(g/100 ml), 0.5% soy peptone (g/100 ml) and 0.5% NaCl dissolved in
distilled water (pH 7.2±0.2).
Establishment of a refractory wound
model
Rats were anesthetized with an intraperitoneal (IP)
injection of sodium pentobarbital (50 mg/kg) and their lumbar spine
was then sheared. The wound area was marked with a plastic bottle
cap of 2 cm in diameter. A 2 cm-deep skin defect was produced by a
surgical method under aseptic conditions for intramuscular
injection of cortisone sodium succinate (8 mg/100 g). A
Pseudomonas aeruginosa suspension wrapped in alginate, which
was used as a type of biofilm, was prepared with a microball maker
(quantitative quality control strain; Hangzhou Microball Science
& Technology Co., Ltd., Hangzhou, China). The bacterial
concentration was 109 colony-forming units/ml. Next, 0.5
ml artificial Pseudomonas aeruginosa was injected into the
wound surface of the animals to create a refractory wound model
with bacterial biofilm infection.
The rats were randomly divided into five groups,
namely a control group, model group, Cef control group, WXD control
group and the QBM group, and treated for 20 days (n=6). The control
group received water every day. The model and other groups were
injected with 0.5 ml artificial Pseudomonas aeruginosa via
the wound surface. Then, drug administration commenced at day 1
subsequent to the establishment of the model. The stomach and the
wound surface were treated with saline every day. The wound surface
was cleaned with 1:5,000 nitrofurazone solution prior to switching
drugs. The rats in all groups had saline gauze topically applied
according to the size of the wound. Then, two layers of aseptic and
disinfected dry gauze were put on the wound, which was fixed with
medical tape once daily. Concomitantly, each group was orally
administrated with the corresponding drug (40 mg/kg) or
physiological saline (10 ml/kg, once per day). The rats were
anesthetized with an IP injection of sodium pentobarbital (50
mg/kg) and euthanized by decapitation following collection of the
plasma at days 1, 3, 8, 15 and 20. One rat from each group was
euthanized at each indicated time point. An area of 3×3
cm2 containing wound tissue and surrounding skin was
cut. Part of the tissue was fixed with 4% formaldehyde at room
temperature for 24 h and part was frozen at −80°C.
Measurement of AI-2 production in
bacteria by Vibrio harveyi BB170 bioluminescence
Luminescence-based broth assays for AI-2 production
were performed using Vibrio v BB170 as a biosensor strain.
Briefly, Vibrio harveyi was cultured for 36 h on marine agar
plates (cat. no. BD2216) at 26°C. Single colonies were cultured
overnight on a rotary shaker (150 rpm) in 5 ml Marine Broth medium
(Shanghai Canspec Scientific Instruments Co., Ltd., Shanghai,
China) until the reaching stationary phase at 26°C. Then, the
colonies were diluted 1:500 and cultured overnight. Pseudomonas
aeruginosa, DH5α and Vibrio harveyi BB170 were cultured
overnight. The indicated bacteria, while growing at stationary
phase, were centrifuged at 12,000 × g for 10 min at 26°C and then
the supernatant was purified with 0.22-µm filtration membranes. A
total of 180 µl diluted BB170 bacterial solution was mixed with the
target bacterial supernatant at 26°C for 2, 4 or 6 h.
Immunohistochemistry (IHC)
IHC was performed on 5 µm sections of
paraffin-embedded tissue with the indicated enzyme-labeled antibody
at room temperature. Briefly, IHC using anti-HIFα monoclonal
(1:200; cat. no. 36169; Cell Signaling Technology, Inc., Danvers,
MA, USA) and anti-VEGF polyclonal (1:400; cat. no. AF-293-NA;
R&D Systems, Inc., Minneapolis, MN, USA) antibodies was
performed according to the manufacturer's protocol. For detection,
the paraffin embedded sections (5 µm) were heated at 60°C for 2 h
and then deparaffinized with toluene and rehydrated in a graded
alcohol series. To retrieve antigens, the sections were boiled for
15 min at 120°C in 10 mM citrate buffer (pH 6.0). The sections were
then washed 3 times with PBS at room temperature and pre-incubated
with goat immunoglobulin G (IgG; cat. no. ab150077; 1:5,000, Abcam,
Cambridge, UK) dissolved in PBS containing 1% bovine serum albumin
(BSA; Thermo Fisher Scientific, Inc., Waltham, MA, USA; pH 7.4) for
1 h. Upon incubation with the primary antibodies overnight, the
sections were washed with PBS and then incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG (cat. no. ab6721;
1:5,000; Abcam) in PBS containing 1% BSA for 50 min. Upon washing
with PBS 3 times, the reaction was visualized with
3,3′-diaminobenzidine and H2O2 in the
presence of nickel and cobalt ions. Nonspecific rabbit IgG (cat.
no. ab188776; 1:5,000; Abcam) was used as the negative control and
the sections were imaged by a fluorescent microscope (Olympus CX23;
Olympus Corporaion, Tokyo, Japan).
ELISA
ELISA kits were used to determine the concentration
of HIF-1α (cat. no. DYC1935-2; R&D Systems, Inc.), HIF-2α (cat.
no. DYC1997-2; R&D Systems, Inc.), HIF-3α (Spbio, Wuhan, China)
and VEGF (cat. no. MMV00; R&D Systems, Inc.) or interleukin-27
(cat. no. DY2274; R&D Systems, Inc.) in the bacterial
supernatants. The indicated sample solutions were added to ELISA
well plates and the specific-kit cytokine was bound via the
immobilized antibody on the plate. Upon washing off the unbound
substances, an enzyme-linked polyclonal antibody that was specific
to each cytokine was added to the wells. Upon washing the unbound
antibody-enzyme molecules, a substrate solution was added to the
wells and the intensity of the color in the reaction developed
proportionally to the quantity of specific cytokine bound in the
initial step, as indicated in the manufacturer's protocol. The
reaction was terminated and the color intensity was determined
using a microplate reader at 450 nm.
Hematoxylin and eosin staining
The sections were heated at 60°C for 2 h,
deparaffinized with toluene and rehydrated in graded alcohol prior
to being immersed in hematoxylin for 5 min at room temperature. The
sections were then washed with acidic alcohol (0.5% HCl in 70%
ethanol), immersed in PBS for 5 min and stained by several
immersions in eosin for 3 min at room temperature. Upon removing
the excess eosin, the slides were dehydrated with ethanol
(75–100%), cleared with xylene and mounted with a xylene-based
mounting medium. The stained sections were observed under a light
microscope and images were captured.
Immunofluorescence detection of
biofilm formation
For detection of biofilm formation, the sections
were heated at 60°C for 2 h, deparaffinized with toluene and
rehydrated in graded alcohol. To retrieve antigens, the sections
were boiled for 15 min at 120°C in 10 mM citrate buffer (pH 6.0).
The sections were washed 3 times with PBS at room temperature and
then pre-incubated with goat IgG dissolved in PBS containing 1% BSA
(pH 7.4) for 1 h. Subsequently, the sections were incubated with
concanavalin A-fluorescein isothiocyanate (0.3 mg/ml) at 4°C for 30
min. Upon washing with PBS, the sections were incubated for 15 min
with propidium iodide (50 µg/ml). The sections were washed with PBS
and mounted on slides (75×25×1 mm) with a drop of mounting medium.
After sealing the edges with nail polish, the slides were stored in
the dark at 4°C. Antibody localization and cell structures were
visualized with a confocal microscope.
Immunofluorescence detection of
macrophages
For detection of macrophages, the sections were
heated at 60°C for 2 h, deparaffinized with toluene and rehydrated
in graded alcohol. To retrieve antigens, the sections were boiled
for 15 min at 120°C in 10 mM citrate buffer (pH 6.0). The sections
were washed 3 times with PBS at room temperature and then
pre-incubated with goat IgG at room temperature dissolved in PBS
containing 1% BSA (pH 7.4) for 1 h. Upon overnight incubation with
primary antibodies against cluster of differentiation (CD) 68 (cat.
no. ab125212; 1:1,000, Abcam), the sections were washed with PBS
and incubated with HRP-conjugated goat anti-rabbit IgG in PBS
containing 1% BSA for 50 min at room temperature. Upon washing with
PBS, the sections were incubated for 7 min with DAPI at room
temperature. Then, the sections were washed with PBS and mounted on
slides (75×25×1 mm) with a drop of mounting medium. Upon sealing
the edges with nail polish, the slides were stored in the dark at
4°C. Antibody localization and cell structures were visualized with
a confocal microscope.
Statistical analysis
Each experiment was performed at least three times,
independently. A one-way analysis of variance followed by Dunnett's
test analysis was used to compare the values in multiple-groups.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were conducted with the SPSS
19.0 software (IBM Corp., Armonk, NY, USA).
Results
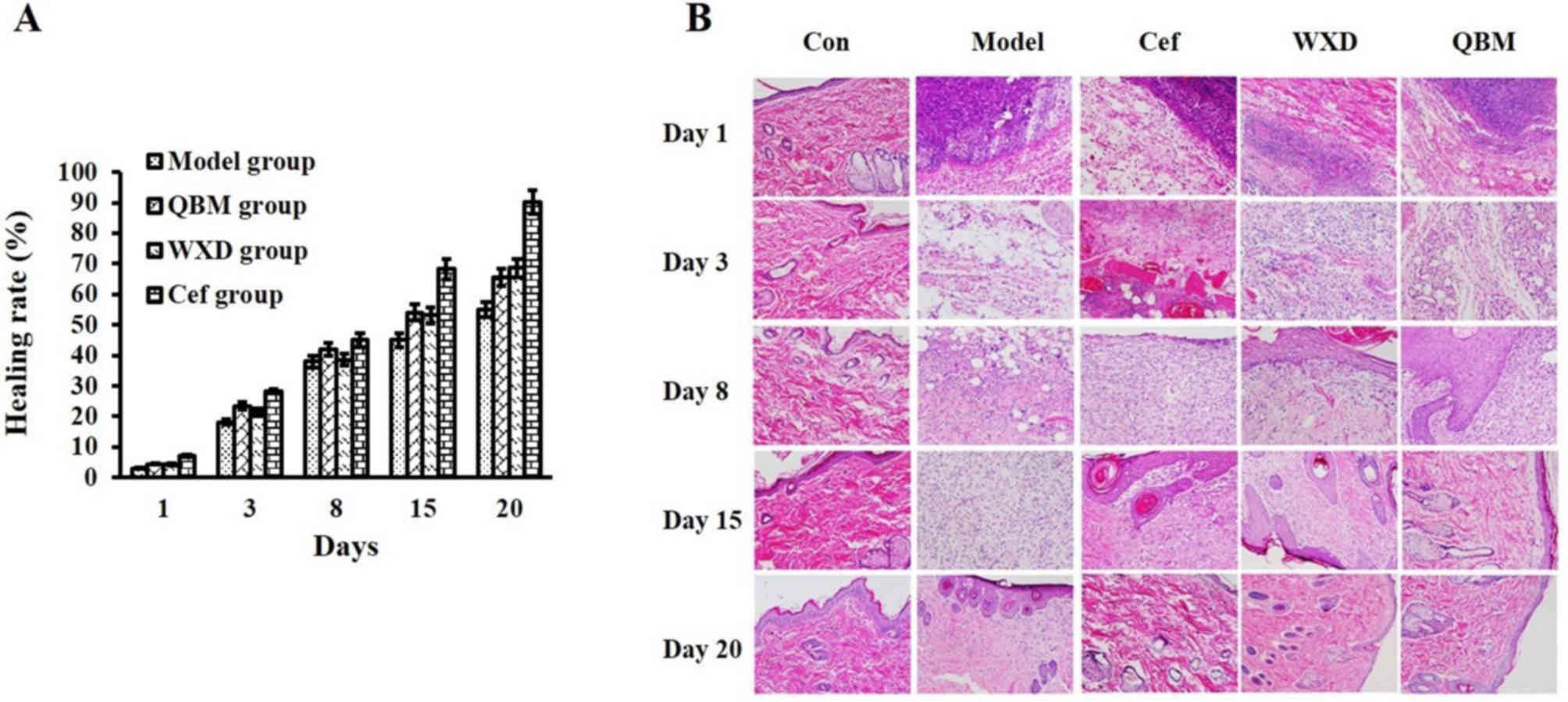
QBM increases the healing rate of
refractory wounds
The effect of the QBM on the healing rate of
refractory wounds in rats was first evaluated. The results revealed
that the QBM-treated group exhibited an increased healing rate of
refractory wounds. However, there was no obvious difference between
the Cef group and the WXD group at day 8 (Fig. 1A and Table
I). No abnormalities were observed in the morphology of the
skin of the sham-operated group during the whole experimental
period, while the animals in the other groups displayed varying
degrees of damage (Fig. 1B).
Specifically, on day 1, the inflammatory cells were markedly
concentrated in the wound tissue of these groups, while on day 3,
the animals in these groups exhibited diffuse distribution of
inflammatory cells. There was an increased concentration of
fibroblasts in the Cef, WXD and QBM groups. Telangiectasia and
granulated tissue were observed in the QBM group. On day 8, the
granulated tissues and newborn epithelia were increased in the
other 3 groups. On day 15, there was an increased number of
capillary vessels in the granulated tissue of the model group. The
epithelium increased in the Cef and WXD, while the fibers in the
heat-clearing group increased. The number of collagen cells
increased prior to returning to normal levels. The tissue expressed
more fibroblasts and collagen, and it almost returned to a normal
state, in the QBM group. On day 20, the epithelium expressed in the
model group, while the tissue expressed more fibers and collagen
prior to almost returning to a normal state in the other groups. In
summary, the healing speed of each group was faster than the
healing speed of the model group. The QBM group exhibited the best
curative effect, since it promoted granulation growth and improved
healing speed (Fig. 1B).
 | Table I.Healing rate of refractory wounds
regulated by Cef, WXD or QBM. |
Table I.
Healing rate of refractory wounds
regulated by Cef, WXD or QBM.
|
| Day (%) |
|---|
|
|
|
|---|
| Group | 1 | 3 | 8 | 15 | 20 |
|---|
| Model | 3.10 | 18.10 | 38.00 | 45.00 | 55.00 |
| QBM | 4.50 | 23.50 | 42.00 | 54.10 | 65.50 |
| WXD | 4.40 | 21.40 | 38.60 | 53.20 | 68.60 |
| Cef | 7.10 | 28.40 | 45.00 | 68.20 | 90.20 |
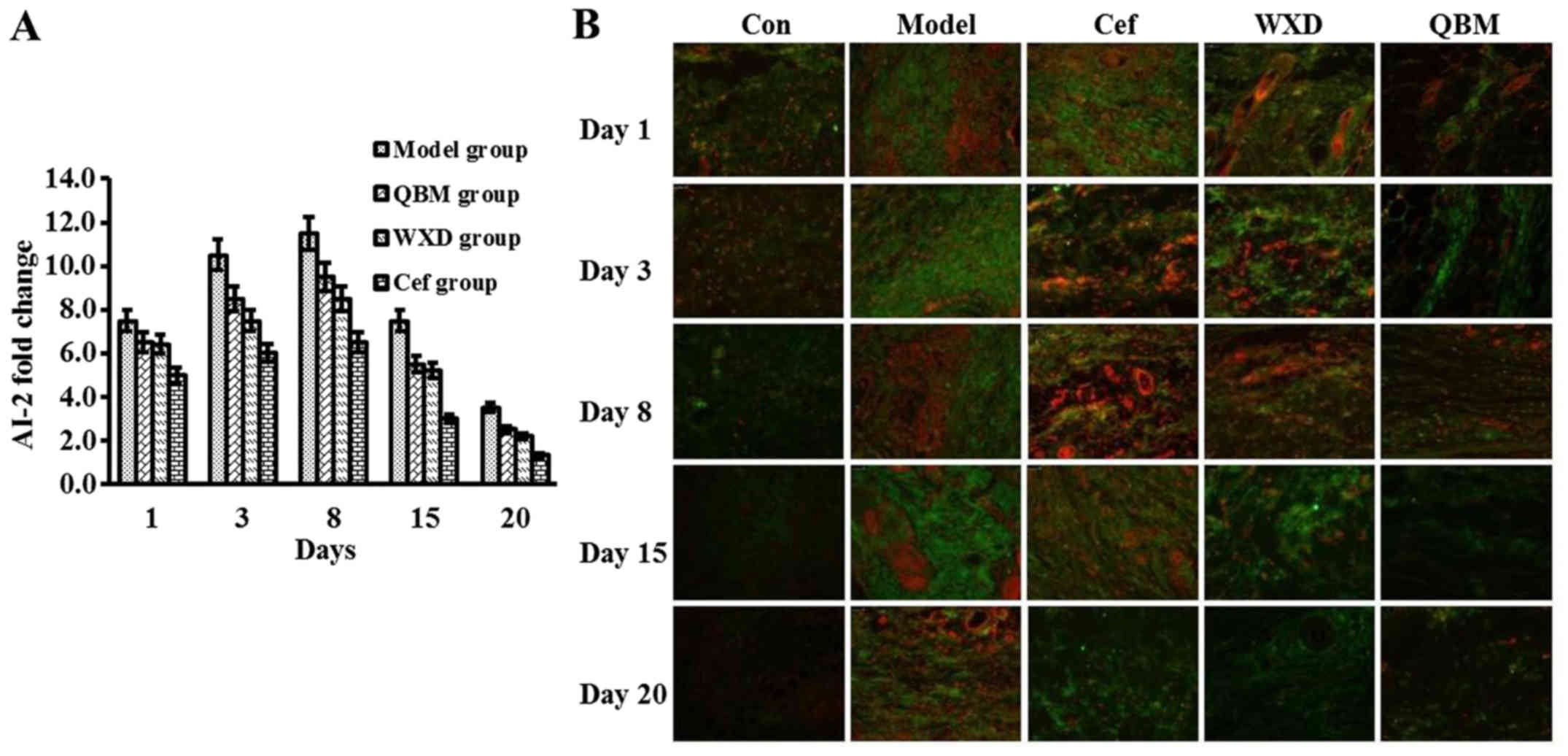
QBM downregulates AI-2 and inhibits
the formation of bacterial biofilms in chronic and refractory
wounds
Group differences in terms of the expression of AI-2
are presented in Fig. 2. Following
wound infection with Pseudomonas aeruginosa, the expression
of AI-2 peaked at day 8 in the control group. Compared with the
model treatment, the QBM markedly downregulated the expression of
AI-2. The level of AI-2 in the Cef and WXD groups was lower
compared with in the control group, but it remained higher than
that in the QBM group (Fig. 2A). To
detect bacterial biofilm formation, immunofluorescence was
performed. The results indicated that the biofilm formation rate
was downregulated at day 20 in the QBM-treated group compared with
the model group. This result indicated that QBM has a strong
inhibitory effect on the formation of bacterial biofilms (Fig. 2B).
HIF-1α, HIF-2α and VEGF expression
levels are upregulated by the QBM in chronic and refractory
wounds
The present results revealed that the expression of
HIF-1α, HIF-2α and VEGF in the serum of animals in the refractory
wound model with bacterial biofilm infection was markedly
upregulated by the QBM. The WXD also increased the level of HIF-1α,
HIF-2α and VEGF, although not as markedly as the QBM. The effect of
Cef on the expression of HIF-1α, HIF-2α and VEGF was decreased
compared with the model group (Fig.
3A). Unexpectedly, the expression of HIF-3α in the serum of
animals in the refractory wound model with bacterial biofilm
infection was markedly upregulated by the WXD. The QBM also
increased the level of HIF-1α, HIF-2α and VEGF, although not as
markedly as the WXD. The effect of Cef on the expression of HIF-1α,
HIF-2α and VEGF in the Cef group was decreased compared with in the
model group (Fig. 3A). The same
results were obtained by IHC for the HIF-1α and VEGF groups
(Fig. 3B).
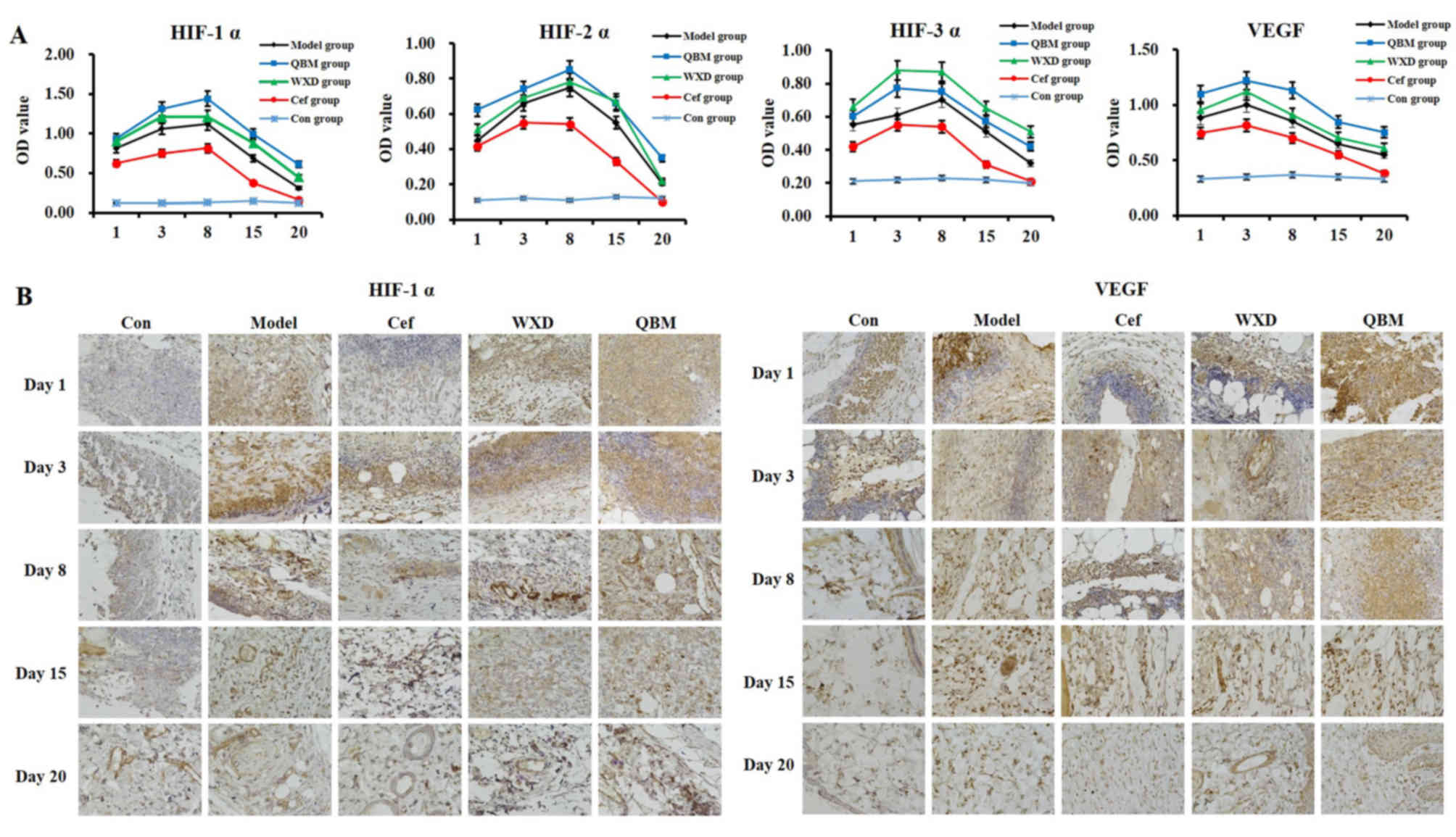 | Figure 3.The expression level of HIF-1α,
HIF-2α and VEGF in the serum of animals in the refractory wound
model group with bacterial biofilm infection is increased by the
QBM. (A) The protein content of HIF-1α, HIF-2α, HIF-3α and VEGF in
the serum of animals in the refractory wound model was measured by
ELISA. (B) Immunohistochemical staining of HIF-1α and VEGF was
performed in the refractory wound animal model (magnification,
×400). Data are presented as the mean ± standard deviation; n=3;
HIF, hypoxia-inducible factor; VEGF, vascular endothelial growth
factor; QBM, Qingre Baidu mixture; WXD, Wu Wei Xiao Du drink; Cef,
cefoperazone; Con, control. |
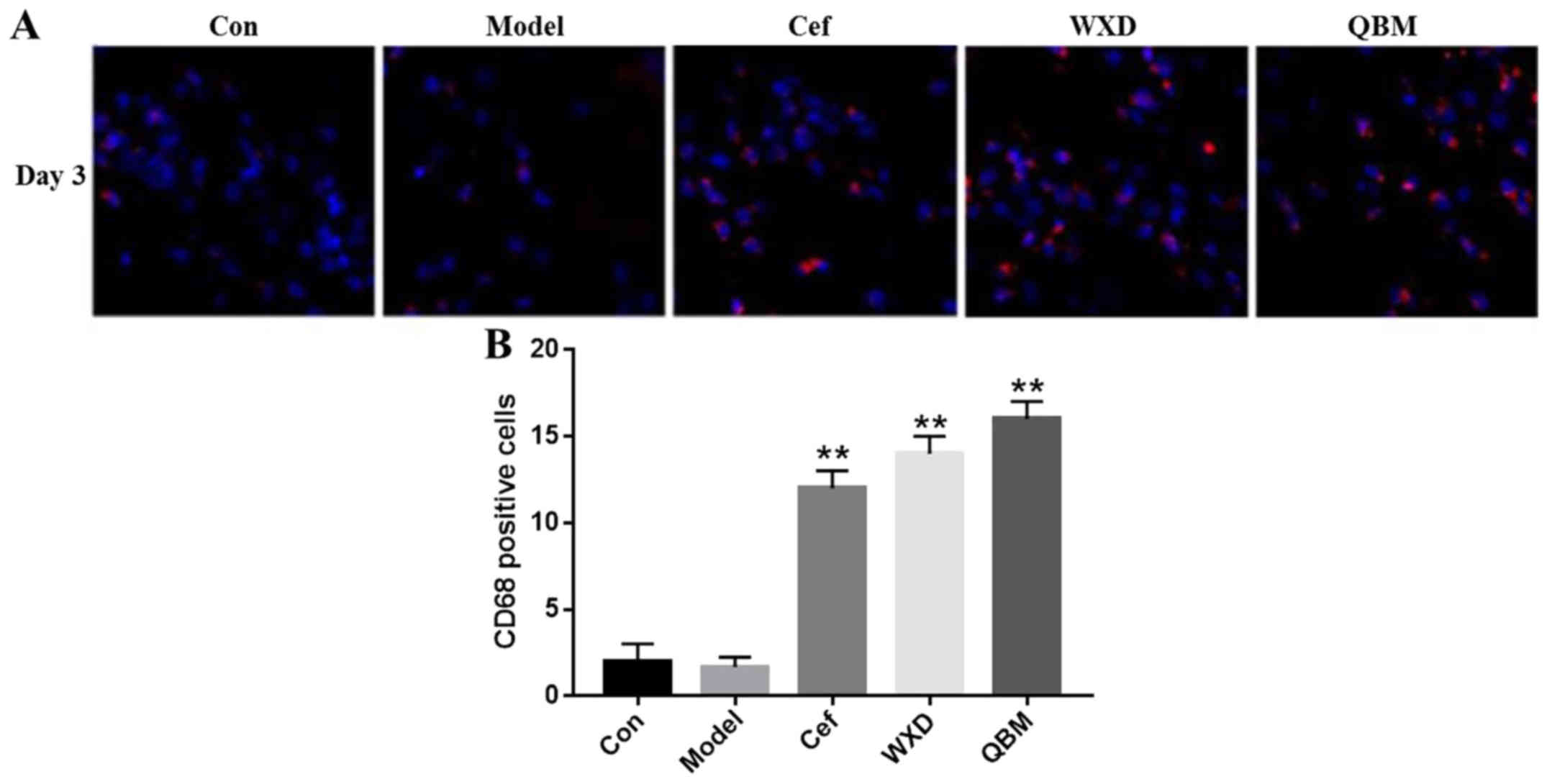
QBM recruits macrophages to chronic
and refractory wounds
CD68 is a marker of macrophages. Macrophages rapidly
engulf bacteria to restrict autoimmune disease or inflammation. The
present study examined the content of macrophages in wounds. The
results indicated that the QBM and WXD significantly recruited
numerous macrophages to the wound compared with the model group
(P<0.01). However, in QBM, WXD and Cef groups, the content of
macrophages in the Cef-treated group was the lowest compared with
the QBM and WXD groups (Fig. 4).
Discussion
Chronically refractory wounds require considerable
medical resources every year. The key factor responsible for the
failure of healing of such wounds is the presence of multiple
species of bacterium, including Staphylococcus aureus and
Pseudomonas aeruginosa with highly organized biofilms
(11). Biofilm protection prevents
the body's immune response from reaching the bacteria, which is an
important factor in the failure of therapeutic treatment (12). The present study has demonstrated for
the first time that the expression of AI-2 induced by the QBM on
the biofilm of Staphylococcus aureus and Pseudomonas
aeruginosa promoted the healing of chronic and refractory
wounds (13).
The QBM is mainly composed of 10 herbs, including
Radix Astragali, the root of red-rooted salvia and honeysuckle,
which are traditionally used in the treatment of furunculosis and
erysipelas. In the chronic refractory wound, the main types of
bacterium detected are Staphylococcus aureus and
Pseudomonas aeruginosa. Therefore, the present study
compared the effect of the QBM with another traditional Chinese
medicine or Cef on the bacterial biofilm formation of
Pseudomonas aeruginosa and Staphylococcus aureus. The
QBM-treated group displayed a significantly increased healing rate
in refractory wounds. However, there was no obvious difference
between the Cef group and the WXD group. Furthermore, the tissue
expressed increased levels of fiber and collagen, and almost
returned to a normal state, in the QBM group.
Quorum sensing (QS) is the cell-to-cell signaling
system that reflects the ability of bacteria to respond to small
signaling molecules secreted by various microbial species (14,15). The
QS system is activated by extracellular receptors and is important
in the formation of biofilms (16).
Therefore, the QS system may be a key target for antimicrobial
treatment. Numerous anti-infectious approaches against
Staphylococcus aureus and Pseudomonas aeruginosa
biofilms have been studied during the past decade, including
antibiotic combinations. However, several problems remain to be
resolved (17), including the
extended use of antibiotics, which results in a high prevalence of
bacterial resistance. Currently, traditional Chinese medical
compounds that inhibit QS systems are being investigated. AI-2 is
one of the QS molecules that mediates intra- and interspecies
communication. AI-2 is formed from spontaneous rearrangement of
4,5-dihydroxy-2,3-pentanedione, which is produced by the enzyme
LuxS and is the primary QS molecule produced by Gram-positive and
Gram-negative bacteria (18). AI-2
has been demonstrated to serve a pivotal role in biofilm formation,
including in the initial bacterial aggregation and the production
of virulence factors. AI-2 inhibits biofilm formation in
Bacillus cereus (19),
Candida albicans (20) and
Eikenella corrodens (21),
and promotes biofilm formation in Escherichia coli (22) and Streptococcus mutans
(23). The present study
demonstrated that the QBM downregulates AI-2 in biofilms of
Staphylococcus aureus and Pseudomonas aeruginosa, and
inhibits the formation of bacterial biofilms in chronic and
refractory wounds (24).
HIF-α and VEGF serve important roles in cancer
progression in various cancer cell lines (25). Previously, HIF-α and VEGF have been
demonstrated to exhibit a high level of expression when a bacterial
infection occurs (26). The results
of the present study revealed that the levels of HIF-1α, HIF-2α and
VEGF were upregulated by the QBM. The WXD also increased the levels
of HIF-1α, HIF-2α and VEGF, although not to the same extent as the
QBM. However, the effect of Cef on the expression of HIF-1α, HIF-2α
and VEGF was decreased compared with the model group.
Macrophages have been reported to participate in the
induction of inflammation (27).
Infections with human pathogens that require macrophages in the
infected wounds for control and recognition include infections with
Staphylococcus aureus and Pseudomonas aeruginosa
(28). In addition to recognition of
bacteria by the host immune system, macrophages are recruited to
the wound site and phagocytose the invading organisms. In
particular, deficiencies in macrophage phagocytosis, as well as
bactericidal potential, have been associated with reduced bacterial
clearance, as well as chronic and refractory wounds (29). The present study used CD68, a marker
of macrophages, to detect the content of macrophages, and observed
that the QBM recruited macrophages to chronic and refractory
wounds. This result demonstrated that the QBM serves a prominent
effect in anti-bacterial defense.
A previous study reported that bacterial biofilms
served a key role in the emergence of resistance to antibacterial
agents (30). However, it is widely
known that a mature biofilm matrix may provide the conditions for
bacteria to elude the host immune response while forming a barrier
against the majority of conventional antimicrobial treatments
(31). Furthermore, numerous
biofilm-associated mechanisms of drug resistance should also be
considered. Increased mutation frequencies have been described in
biofilm cultures of Staphylococcus aureus and Pseudomonas
aeruginosa, suggesting that the biofilm matrix provides an
important environment to promote mutational resistance to
antibiotics (32). In the present
study, the effect of Cef on inflammation and wound healing in
chronic and refractory wounds was decreased compared with the
QBM.
The present study examined the effect of the
QBM-regulated biofilm formation of Staphylococcus aureus and
Pseudomonas aeruginosa in chronic and refractory wounds. The
QBM upregulated the expression of AI-2, HIF-1α, HIF-2α and HIF-3α
and increased the levels of VEGF. These results provide novel
insight into the complex interrelationships between chronic and
refractory wounds and bacterial biofilms, and open the possibility
of treating chronic and refractory wounds with traditional Chinese
medicines.
Acknowledgements
Not applicable.
Funding
The current study was funded by grants obtained from
the following institutions: The foundation of national natural
science in China (grant no. 81503582); The Chinese medicine
research fund of Shanghai health and family planning commission
(grant no. 2014JP032A); The National TCM clinical research base,
longhua hospital affiliated to Shanghai university of Chinese
medicine (Seedling program; grant no. LYTD-37); The third batch of
young doctors in Shanghai (personal funding); and China's 6th group
of medicinal experts (personal funding).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS, YW, ZZ, JXi and JXu participated in experiment
design, tissue collection and experiment execution. WX, YS and SG
analyzed and interpreted the patient data, and were major
contributors to the development of the first draft of the present
manuscript. HQ conceived and designed the current study. WS and HQ
reviewed and approved the final draft of the manuscript prior to
submission.
Ethics approval and consent to
participate
Ethics approval for the present study was provided
by the Longhua Hospital Affiliated to Shanghai University of
Traditional Chinese Medicine Animal Experimental Ethics Committee.
The National Institutes of Health guide for the care and use of
laboratory animals was strictly followed by the authors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Merckoll P, Jonassen TØ, Vad ME, Jeansson
SL and Melby KK: Bacteria, biofilm and honey: A study of the
effects of honey on ‘planktonic’ and biofilm-embedded chronic wound
bacteria. Scand J Infect Dis. 41:341–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clinton A and Carter T: Chronic wound
biofilms: Pathogenesis and potential therapies. Lab Med.
46:277–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kathju S, Nistico L, Hall-Stoodley L, Post
JC, Ehrlich GD and Stoodley P: Chronic surgical site infection due
to suture-associated polymicrobial biofilm. Surg Infect (Larchmt).
10:457–461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun J, Daniel R, Wagner-Döbler I and Zeng
AP: Is autoinducer-2 a universal signal for interspecies
communication: A comparative genomic and phylogenetic analysis of
the synthesis and signal transduction pathways. BMC Evol Biol.
4:362004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li M, Villaruz AE, Vadyvaloo V, Sturdevant
DE and Otto M: AI-2-dependent gene regulation in Staphylococcus
epidermidis. BMC Microbiol. 8:42008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taga ME, Semmelhack JL and Bassler BL: The
LuxS-dependent autoinducer AI-2 controls the expression of an ABC
transporter that functions in AI-2 uptake in Salmonella
typhimurium. Mol Microbiol. 42:777–793. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wiesener MS, Turley H, Allen WE, Willam C,
Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, et
al: Induction of endothelial PAS domain protein-1 by hypoxia:
Characterization and comparison with hypoxia-inducible
factor-1alpha. Blood. 92:2260–2268. 1998.PubMed/NCBI
|
|
8
|
Ikeda E, Achen MG, Breier G and Risau W:
Hypoxia-induced transcriptional activation and increased mRNA
stability of vascular endothelial growth factor in C6 glioma cells.
J Biol Chem. 270:19761–19766. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agarwala V, Choudhary N and Gupta S: A
risk-benefit assessment approach to selection of adjuvant
chemotherapy in elderly patients with early breast cancer: A mini
review. Indian J Med Paediatr Oncol. 38:526–534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shan W and Que HF: The effect of qingre
baidu mixture on the biofilm of Staphylococcus aureus and
Pseudomonas aeruginosa in chronic and refractory wounds.
Guiding J Trad Chin Med Pharm. 23:12–16. 2017.
|
|
11
|
Driver VR and Blume PA: Evaluation of
wound care and health-care use costs in patients with diabetic foot
ulcers treated with negative pressure wound therapy versus advanced
moist wound therapy. J Am Podiatr Med Assoc. 104:147–153. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Price LB, Liu CM, Melendez JH, Frankel YM,
Engelthaler D, Aziz M, Bowers J, Rattray R, Ravel J, Kingsley C, et
al: Community analysis of chronic wound bacteria using 16S rRNA
gene-based pyrosequencing: Impact of diabetes and antibiotics on
chronic wound microbiota. PLoS One. 4:e64622009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rondas AA, Schols JM, Stobberingh EE and
Halfens RJ: Prevalence of chronic wounds and structural quality
indicators of chronic wound care in Dutch nursing homes. Int Wound
J. 12:630–635. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Camilli A and Bassler BL: Bacterial
small-molecule signaling pathways. Science. 311:1113–1116. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miller MB and Bassler BL: Quorum sensing
in bacteria. Annu Rev Microbiol. 55:165–199. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hentzer M, Wu H, Andersen JB, Riedel K,
Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen
P, et al: Attenuation of Pseudomonas aeruginosa virulence by
quorum sensing inhibitors. EMBO J. 22:3803–3815. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scutera S, Zucca M and Savoia D: Novel
approaches for the design and discovery of quorum-sensing
inhibitors. Expert Opin Drug Discov. 9:353–366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sztajer H, Lemme A, Vilchez R, Schulz S,
Geffers R, Yip CY, Levesque CM, Cvitkovitch DG and Wagner-Döbler I:
Autoinducer-2-regulated genes in Streptococcus mutans UA159
and global metabolic effect of the luxS mutation. J Bacteriol.
190:401–415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Auger S, Krin E, Aymerich S and Gohar M:
Autoinducer 2 affects biofilm formation by Bacillus cereus.
Appl Environ Microbiol. 72:937–941. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bachtiar EW, Bachtiar BM, Jarosz LM, Amir
LR, Sunarto H, Ganin H, Meijler MM and Krom BP: AI-2 of
Aggregatibacter actinomycetemcomitans inhibits Candida
albicans biofilm formation. Front Cell Infect Microbiol.
4:942014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Azakami H, Teramura I, Matsunaga T,
Akimichi H, Noiri Y, Ebisu S and Kato A: Characterization of
autoinducer 2 signal in Eikenella corrodens and its role in
biofilm formation. J Biosci Bioeng. 102:110–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
González Barrios AF, Zuo R, Hashimoto Y,
Yang L, Bentley WE and Wood TK: Autoinducer 2 controls biofilm
formation in Escherichia coli through a novel motility
quorum-sensing regulator (MqsR, B3022). J Bacteriol. 188:305–316.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Z, Meric G, Liu Z, Ma R, Tang Z and
Lejeune P: luxS-based quorum-sensing signaling affects Biofilm
formation in Streptococcus mutans. J Mol Microbiol
Biotechnol. 17:12–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Geier H, Mostowy S, Cangelosi GA, Behr MA
and Ford TE: Autoinducer-2 triggers the oxidative stress response
in mycobacterium avium, leading to biofilm formation. Appl Environ
Microbiol. 74:1798–1804. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh SY, Kwon HC, Kim SH, Jang JS, Kim MC,
Kim KH, Han JY, Kim CO, Kim SJ, Jeong JS and Kim HJ:
Clinicopathologic significance of HIF-1alpha, p53, and VEGF
expression and preoperative serum VEGF level in gastric cancer. BMC
Cancer. 8:1232008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cane G, Ginouvès A, Marchetti S, Buscà R,
Pouysségur J, Berra E, Hofman P and Vouret-Craviari V: HIF-1alpha
mediates the induction of IL-8 and VEGF expression on infection
with Afa/Dr diffusely adhering E. coli and promotes EMT-like
behaviour. Cell Microbiol. 12:640–653. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang M, Jing L, Wang J, Yu Y, Cao L, Zhang
L, Zhou X and Sun Z: Macrophages participate in local and systemic
inflammation induced by amorphous silica nanoparticles through
intratracheal instillation. Int J Nanomedicine. 11:6217–6228. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cuffini AM, Carlone NA, Xerri L and
Pizzoglio MF: Synergy of ceftazidime and human macrophages on
phagocytosis and killing of Staphylococcus aureus and
Pseudomonas aeruginosa. J Antimicrob Chemother. 20:261–271.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brubaker AL, Rendon JL, Ramirez L,
Choudhry MA and Kovacs EJ: Reduced neutrophil chemotaxis and
infiltration contributes to delayed resolution of cutaneous wound
infection with advanced age. J Immunol. 190:1746–1757. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gander S: Bacterial biofilms: Resistance
to antimicrobial agents. J Antimicrob Chemother. 37:1047–1050.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghigo JM: Natural conjugative plasmids
induce bacterial biofilm development. Nature. 412:442–445. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chew SC, Yam JKH, Matysik A, Seng ZJ,
Klebensberger J, Givskov M, Doyle P, Rice SA, Yang L and Kjelleberg
S: Matrix polysaccharides and SiaD diguanylate cyclase alter
community structure and competitiveness of Pseudomonas
aeruginosa during dual-species biofilm development with
Staphylococcus aureus. MBio. 9(pii): e00585–18.
2018.PubMed/NCBI
|