Introduction
Hepatic alveolar echinococcosis (HAE) is a malignant
parasitic disease of the liver (1).
HAE is prevalent in Asia, Africa, Europe and North America
(2), and has become a serious global
problem. In particular, a high incidence has been reported in
Qinghai province in China (3). The
current treatment strategy for HAE is with orally administered
targeted drugs, which include albendazole tablets (4). However, the number of blood vessels is
reduced in HAE, which results in poor drug delivery and treatment
efficacy (5). Therefore, further
research is required in order to understand the characteristics of
vascular invasion and blood supply in HAE to provide a theoretical
basis for development of novel drug administration routes and
targets, as well as increasing the awareness of HAE.
Surgery is also an effective method for treating
this disease (6–7). However, radical resection may be
difficult, as the parasite exhibits an invasive growth and tends to
invade intrahepatic vessels in particular (8). Thus, accurate pre-operative assessment
of a patient's vascular invasion status is key to successful
surgery (9). Relatively few studies
have focused on the vascular invasion status, and even fewer have
addressed the physiological characteristics of vascular invasion.
Furthermore, the current knowledge regarding the vascular invasion
characteristics is currently insufficient and its potential
significance in guiding clinical treatment remains unexplored.
Magnetic resonance imaging (MRI) is an important
imaging technique that is able to display the structural
characteristics of blood vessels and bile ducts in HAE, as well as
their invasion status (10).
Therefore, MRI of HAE lesions for analysis of vascular invasion
characteristics and lesion growth patterns has an important value
in increasing the accuracy of evaluation and the development of
novel treatment methods. The objective of the present study was to
provide a basis for personalized treatment of intermediate and
advanced HAE by elucidating the characteristics of vascular
invasion and lesion growth by using MRI, as well as intra-operative
and pathological observations.
Patients and methods
Patients
A total of 160 HAE patients treated at Affiliated
Hospital of Qinghai University (Xining, China) between January 2014
and February 2017 were recruited for the present study. All of the
subjects had been diagnosed with HAE by 3.0-T MRI with confirmation
by post-operative pathology and had at least one lesion measuring
>5.0 cm in one dimension. The cohort included 79 males and 91
females. The ethnological composition of the cohort was 154
Tibetans and 6 Han Chinese individuals. The age of the patients
ranged from 10 to 71 years, and their mean age was 36.17±12.15
years. All patients underwent evaluation, analysis and surgery,
with the analysis including 3.0-T MR scans.
Inclusion and exclusion criteria
The present study included patients who had i) a
diagnosis of HAE based on clinical information and
imaging/laboratory tests, ii) post-operative pathological tests
confirming the diagnosis and iii) at least one lesion measuring
>5.0 cm in one dimension. Patient were excluded if they had i)
comorbidities of other liver diseases, ii) severe liver or kidney
impairment, iii) an allergy to the contrast agent (Ultravist), iv)
concomitant conditions including pregnancy or aplastic anemia
and/or v) a history of autologous liver transplantation.
MRI techniques
A Philips Achieva 3.0-T TX MR scanner (Achieva MRI;
Philips, Eindhoven, the Netherlands) was used for the plain and
enhanced scans using a 6-channel phased-array having two coils
placed anterior to the body and a 3-channel phased-array having two
coils placed posterior to the spine. Prior to scanning, patients
were fasted for 4 h, trained to hold their breathe during the scan
and were requested to drink 1 l of water over 30 min to distend
their stomach.
MRI protocols with the following pulse sequences was
used for the present study: Enhanced T1-weighted High Resolution
Isotropic Volume Excitation [repetition time (TR)/echo time (TE),
3.9/1.87 msec; matrix size, 236×234; number of excitations (NEX),
1; contrast agent, magnevist (2 ml/kg of body weight with the flow
rate of 0. 2 ml, followed by normal saline flush; Chengdu, China)];
T2-weighted Spectral Attenuated Inversion Recovery (T2WI-SPAIR;
TR/TE, 446/80 msec; matrix size, 268×140; section thickness/gap,
4/0.4 mm; NEX, 1); dual-Fast Field Echo (TR/TE, 4050/120 msec;
matrix size, 288×174; section thickness/gap, 4/0.4 mm; NEX, 4);
diffusion-weighted imaging (TR/TE, 1204/650 msec; matrix size,
256×205; NEX, 1); MR cholangiopancreatography (TR/TE, 4050/120
msec; matrix size, 288×174; section thickness/gap, 4/0.4 mm; NEX,
4). All MRI images were interpreted on a picture archiving and
communications system (PACS) workstation (IntelliSpace PACS V7.0;
Philips, The Netherlands)
Image processing and analysis
The raw data were transferred to the PACS and two
attending physicians (WL and HL) were responsible for observing the
various stages of vascular change and imaging status. They also
evaluated the invasion status of i) the inferior vena cava; ii) the
left, middle and right hepatic vein; iii) the trunk of the hepatic
portal veins, as well as its branches; iv) the hepatic arteries;
and v) the primary and secondary porta hepatis. On the basis of the
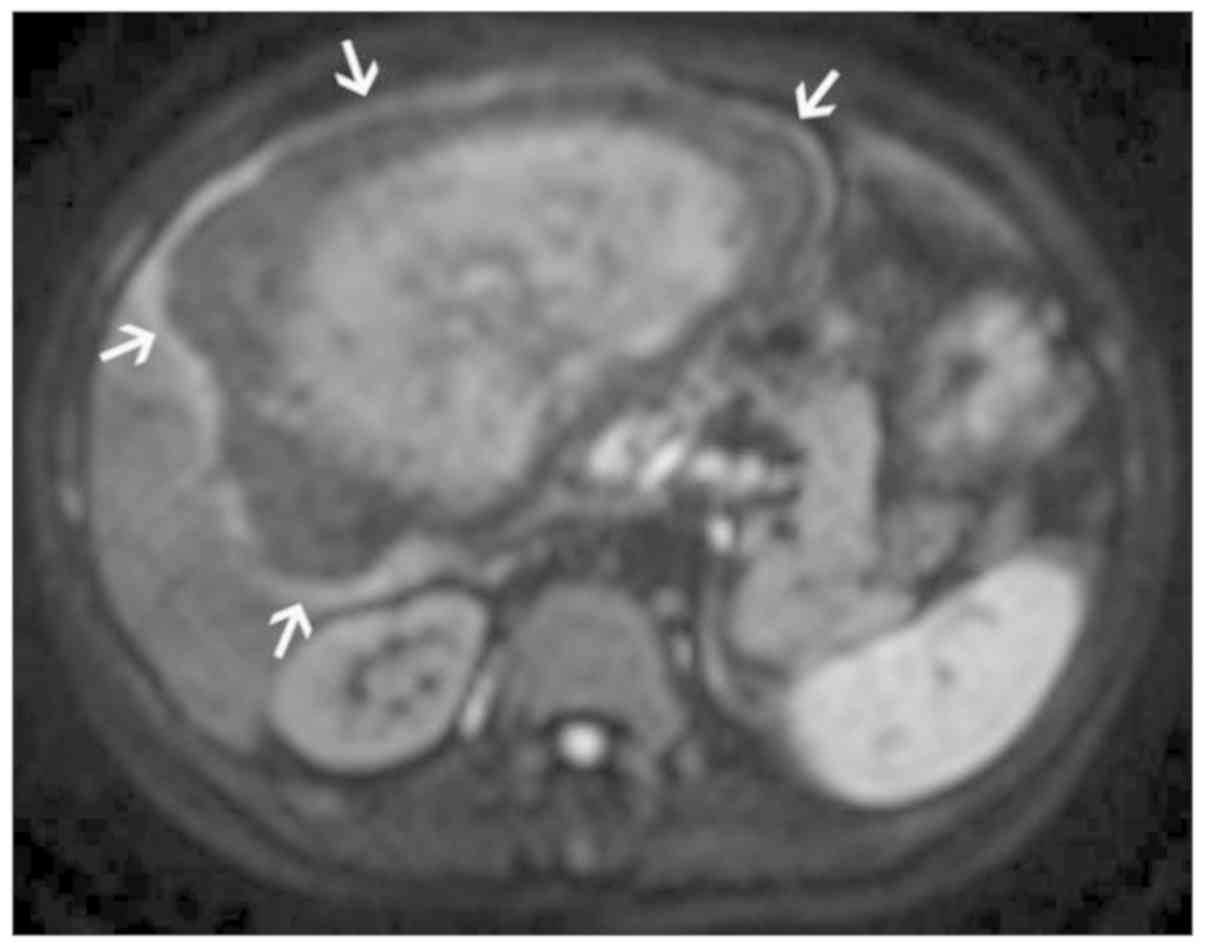
MRI observations, vascular invasion was defined as follows: i)
Incomplete low vascular wall signal in the enhanced T1WI-SPAIR
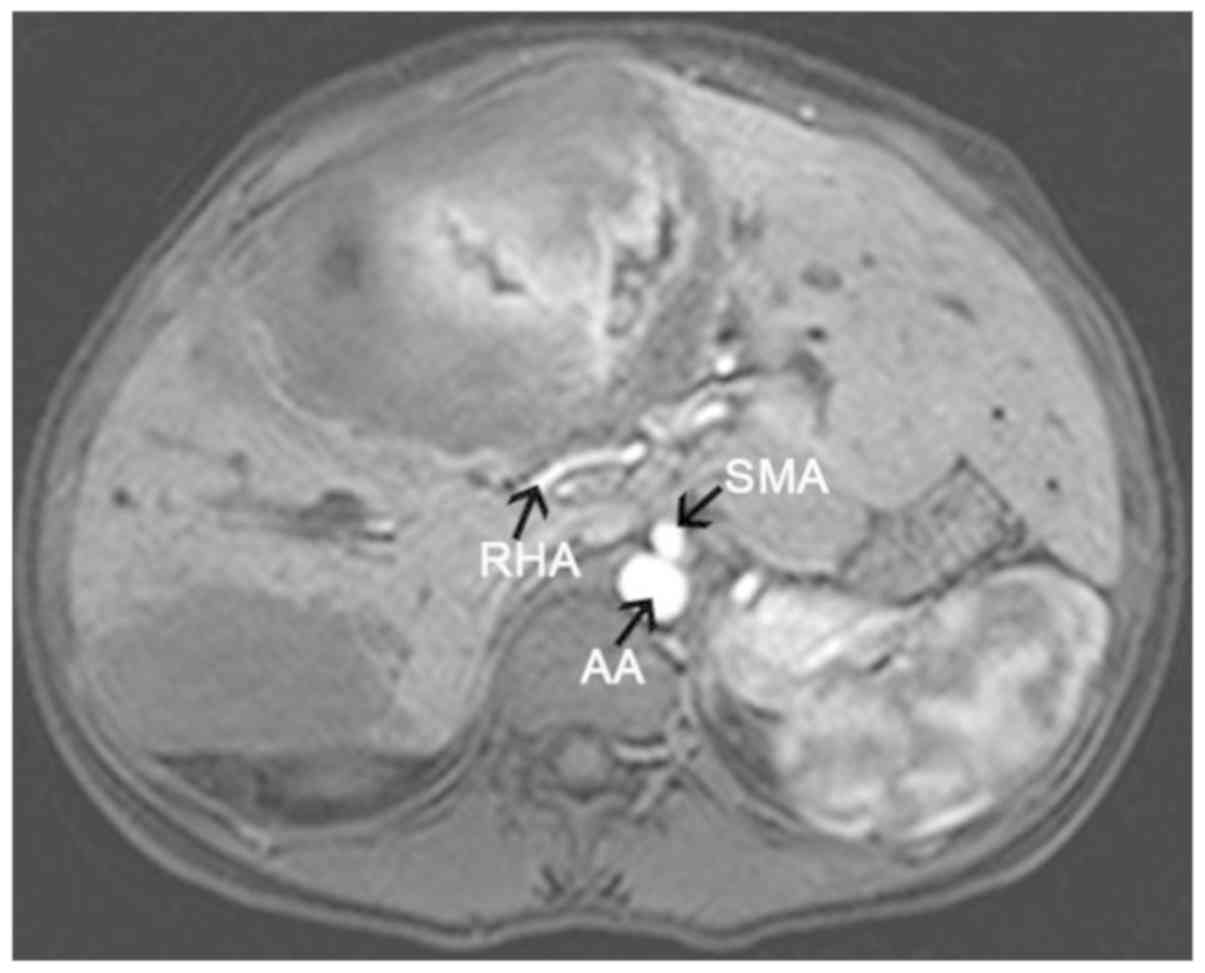
sequence (Fig. 1); ii) hepatic
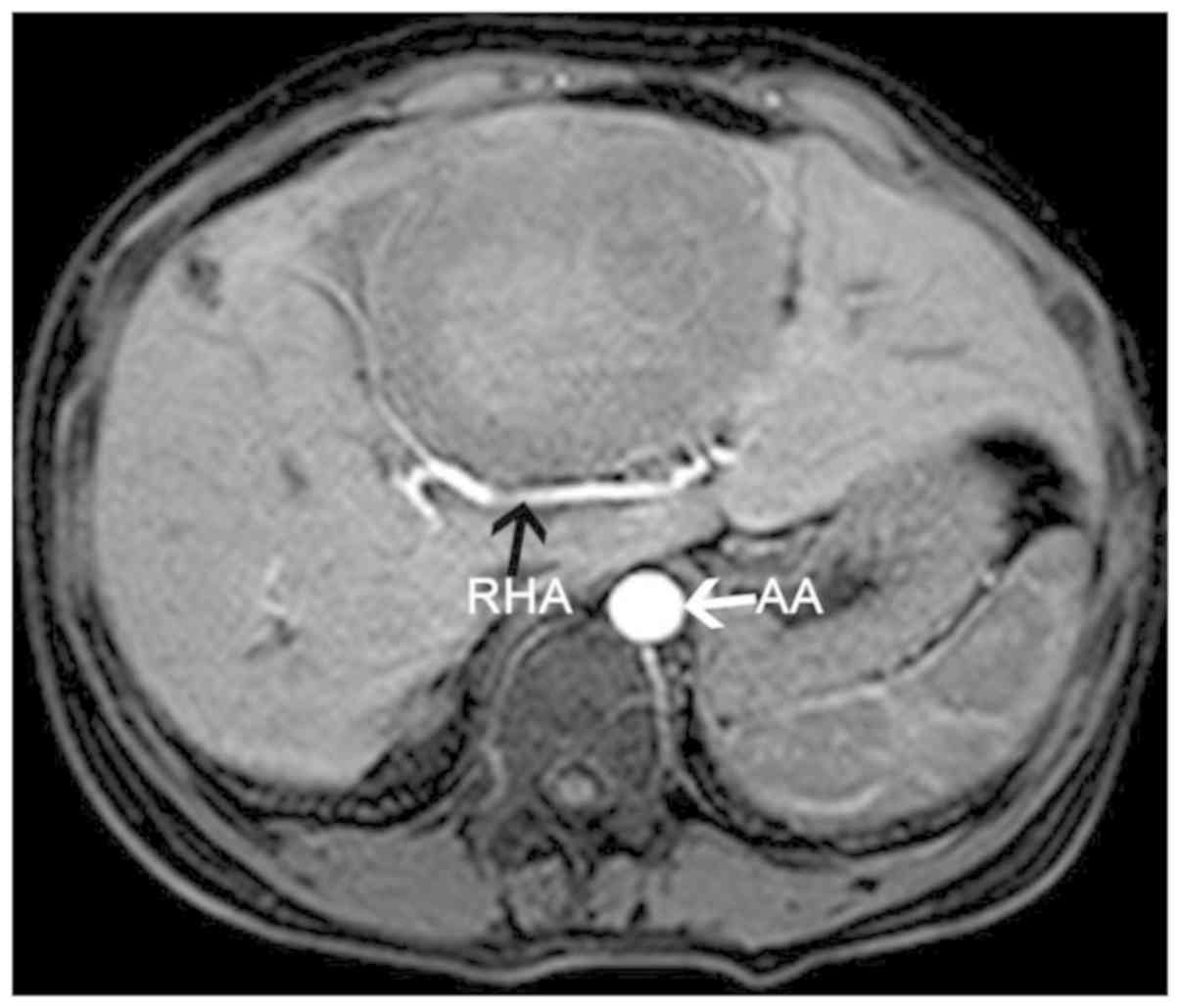
artery stenosis, becoming tortuous and finer as it passed through
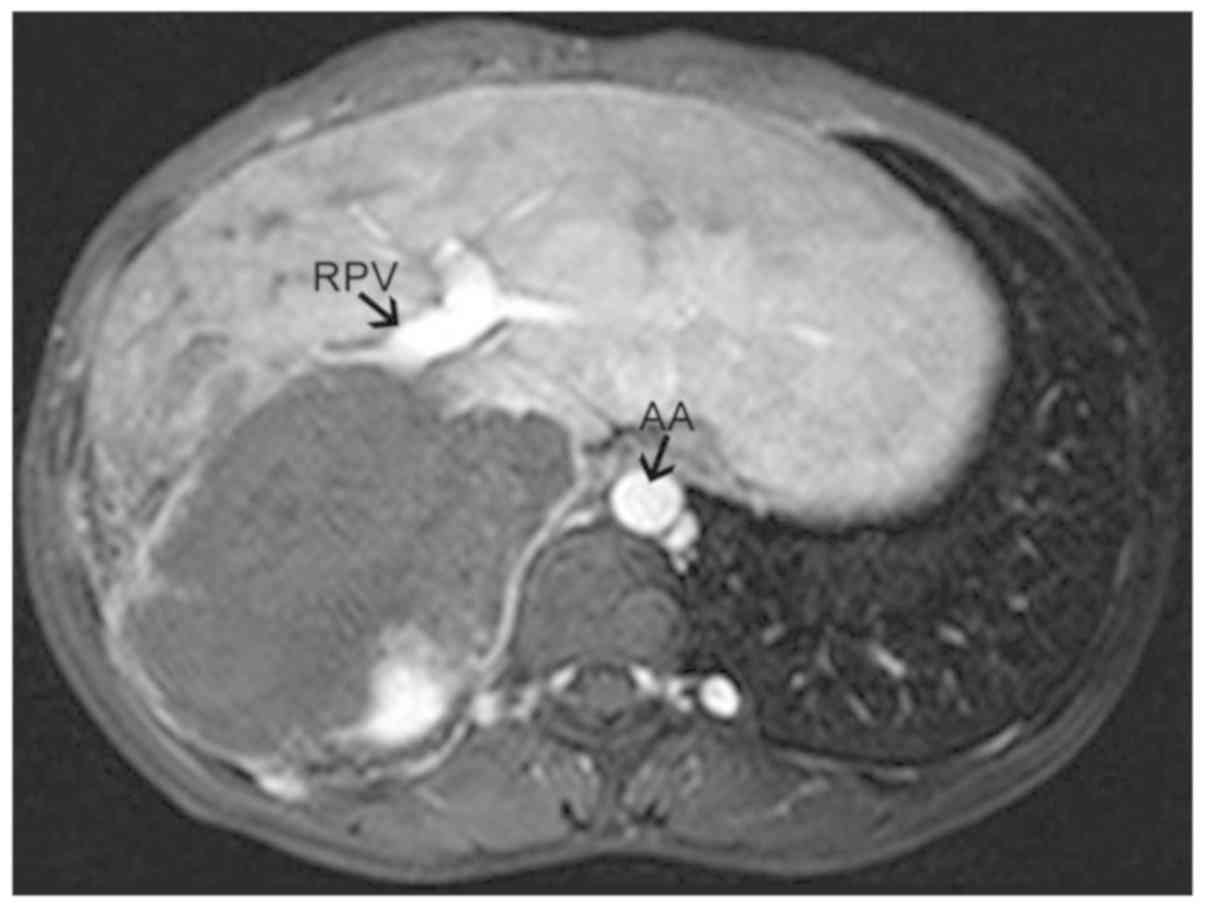
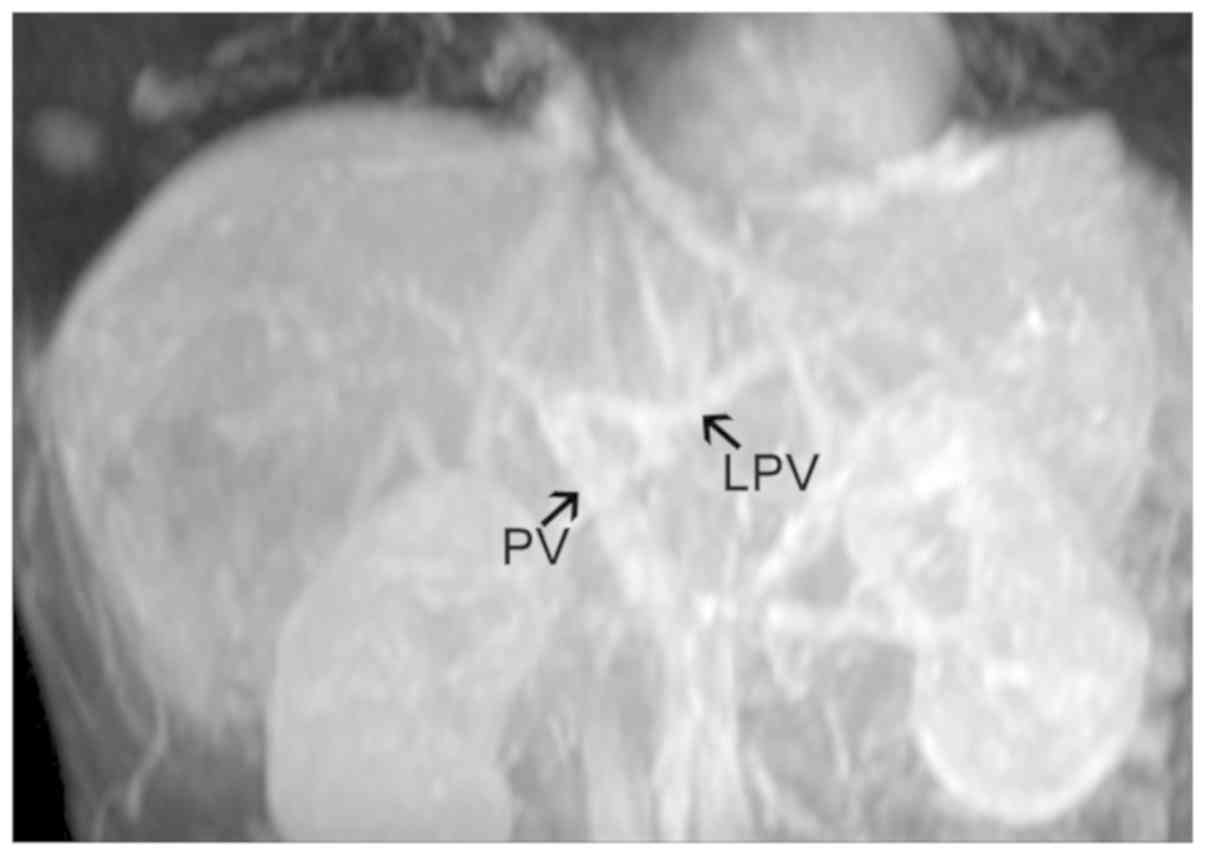
the lesion; iii) truncation of the hepatic portal vein trunk and
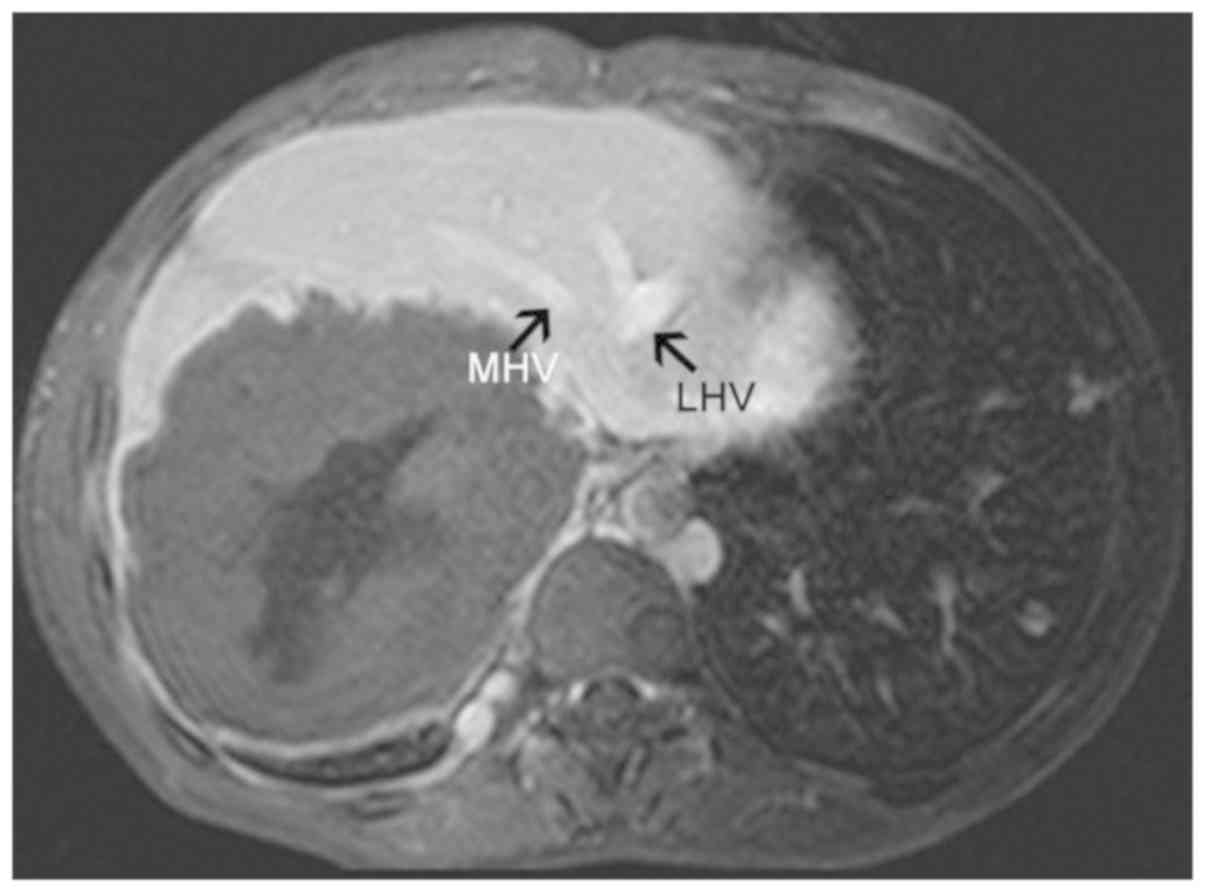
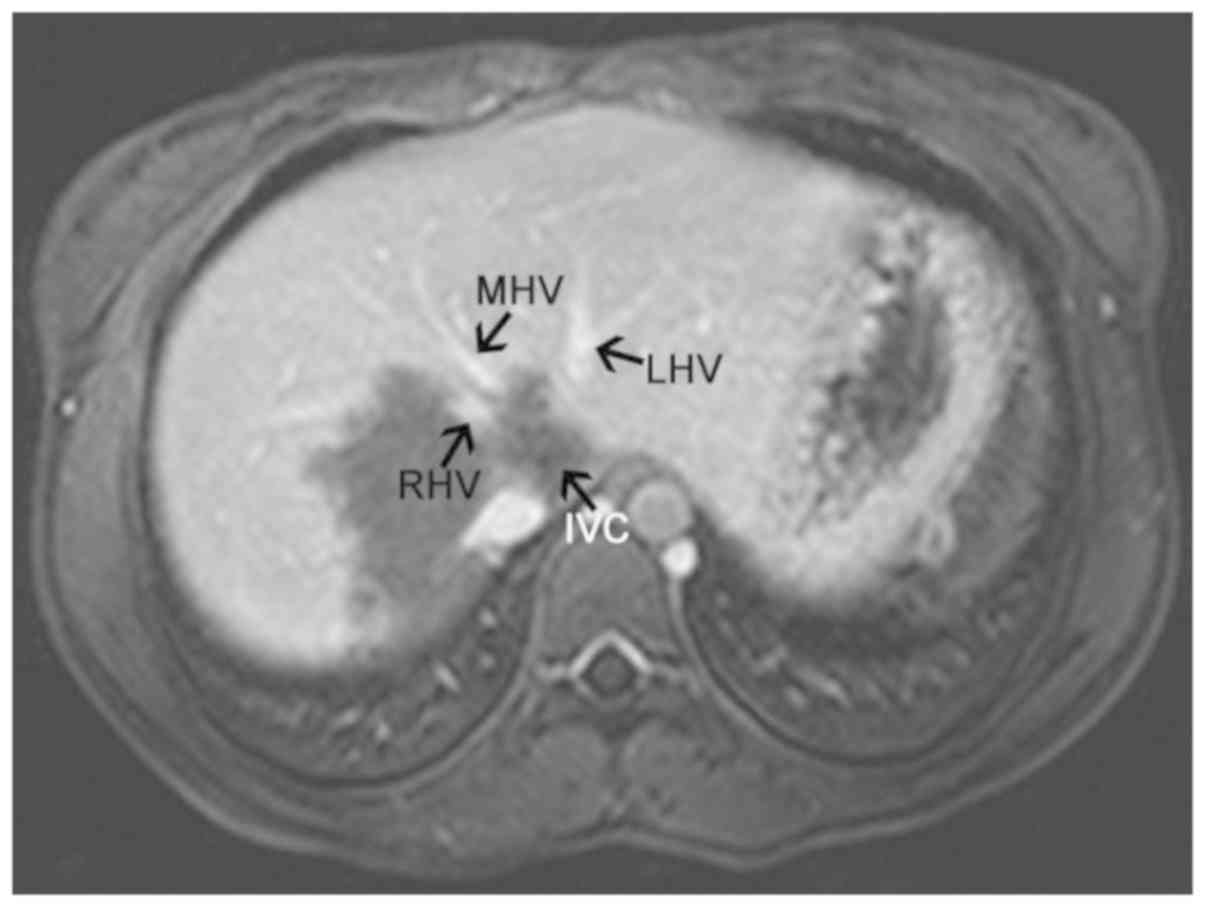
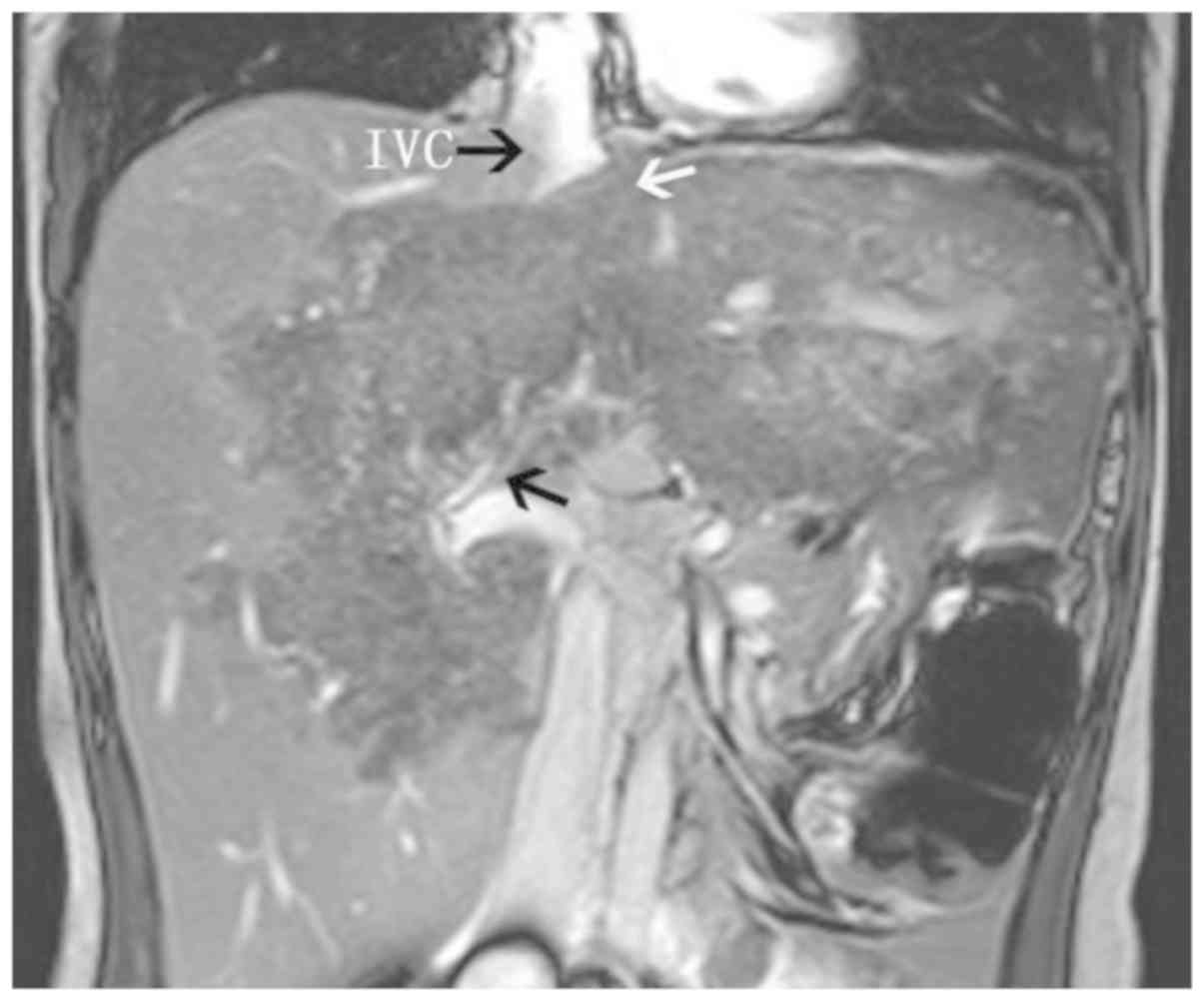
its branches (failure to appear on the scan) (Fig. 2); iv) hepatic veins and inferior vena
cava compressed and exhibiting signs of flattening, narrowing and
failure to appear on the scan (Fig.
3); v) partial or complete envelopment and stenosis of blood
vessels in the primary and secondary porta hepatis (Figs. 4–6)
combined with the appearance of filling defects in certain blood
vessels; and vi) MR venography indicating lesions in the lumen that
exhibit eccentric, irregular stenosis or occlusion (Fig. 7). Patients were excluded if they had
blood vessel compression or displacement by the lesion, and if
their vessels had a complete low vascular wall signal (Fig. 8).
Evaluation of pathological
results
The surgeon combined MRI evaluation, intra-operative
observations and gross pathology to determine the invasion status
of the blood vessels prior to suturing, annotation and sending of
specimens for pathological examination. The vascular walls of
arteries and veins consist of (from the inside to the outside) the
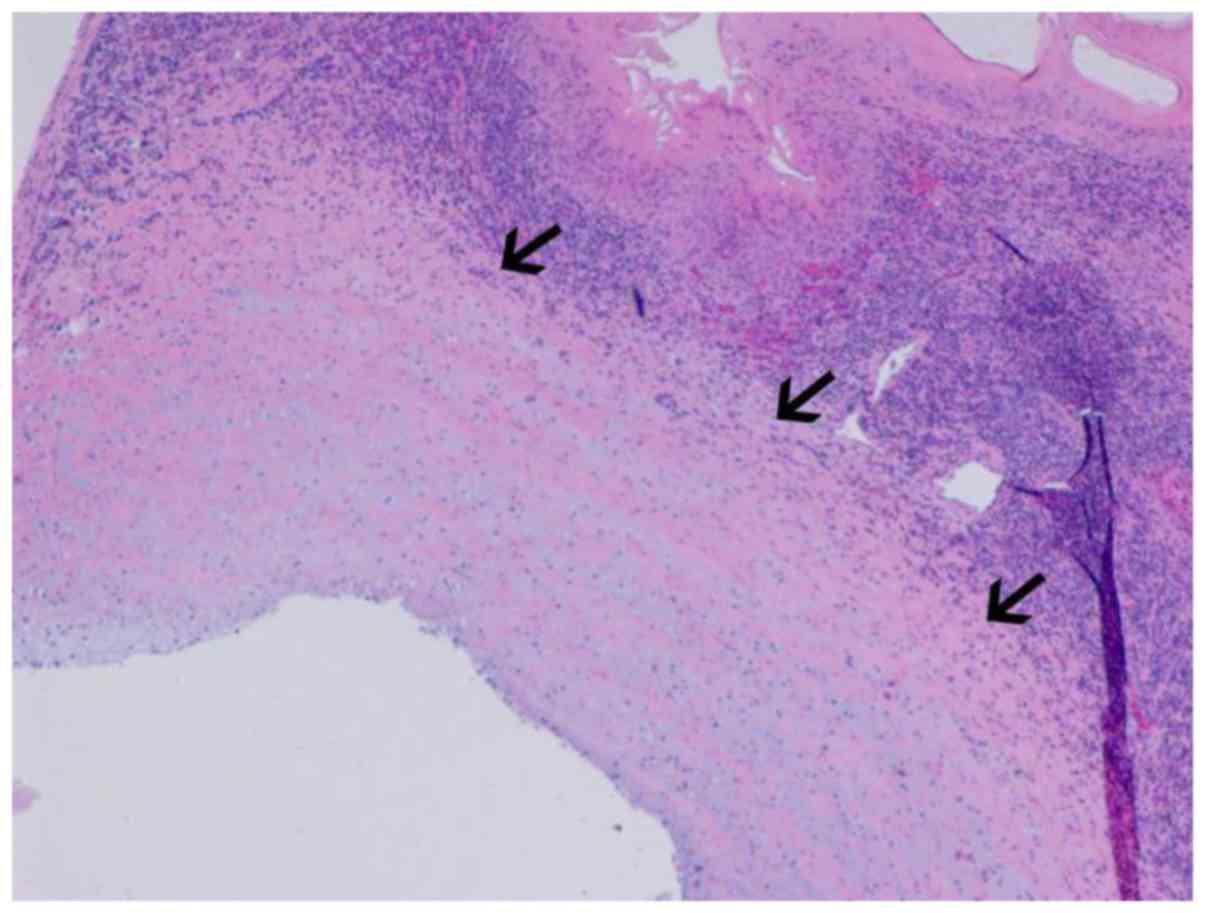
tunica intima, tunica media and tunica externa. On the basis of
microscopic observations, vascular invasion is considered when the
endothelial cells of the blood vessel are surrounded by lesion
cells or covered by muscular walls with elastic membranes (Fig. 9) (11,12).
Statistical analysis
The data were processed using SPSS software (version
19.0; IBM Corp., Armonk, NY, USA). All measurement data with a
normal distribution were expressed as the mean ± standard
deviation, while those with a skewed distribution were presented as
the median and interquartile range. The Kruskal-Wallis test was
used for comparison between three groups, and the Mann-Whitney U
test with Bonferroni correction was used for comparison between
each group. Data were expressed as invasion rates and analyzed
using χ2 partitioning methods, with a test level of
α=0.05, followed by Bonferroni correction. P<0.05 was considered
to indicate a statistically significant difference.
Results
Characteristics of lesions in patients
with HAE
The boundary of the lesion is usually very clear
(Fig. 10). In the 160 patients, a
total of 181 lesions were larger than 5.0 cm in one dimension; the
largest of these was 25.6×14.4×6.8 cm and the smallest was
5.3×4.5×3.2 cm. A total of 71 lesions (39.23%) had a maximum
diameter of 5–10 cm, 76 had a maximum diameter of 10–15 cm (41.99%)
and 34 had a maximum diameter of >15 cm (18.78%). A total of 78
patients (48.75%) had lesions in the S5-8 segment/partial right
liver lobe, 21 patients (13.13%) had lesions involving the S2-4
segment/partial left liver lobe and 61 patients (38.13%) had
lesions that transcended the left and right liver lobes.
Histologic analysis
Pathological examination revealed that the vascular
invasion rate in the hepatic arteries, intrahepatic veins and
hepatic portal veins was 26.87, 43.28 and 51.88%, respectively. The
cohort comprised 128 patients (80.00%) with liver hilum invasion,
71 patients (55.47%) featured invasion of the primary porta
hepatis, 11 patients (8.59%) had invasion of the secondary porta
hepatis and 46 patients (35.94%) exhibited invasion of the primary
and secondary porta hepatis.
Data analysis results
The pathological results were regarded as the gold
standard. No significant difference in age and sex was observed in
either vascular invasion or hepatic hilum invasion groups (data not
shown). Furthermore, no significant difference in the size of the
lesions was identified between the types of hepatic vessels
affected (P>0.05; Table I).
However, a significant difference was detected in the size of the
lesions between the different porta hepatis invasion groups
(P<0.05; Table II). A pairwise
comparison of invasion rates between several hepatic blood vessels
was performed using χ2-partitioning methods (α=0.05;
Table II). A new test level
α'=0.0167 was obtained and the difference was statistically
significant (P<0.01). It was revealed that the invasion rate is
highest in the hepatic portal veins (Table II). The maximum lesion diameter in
the primary and secondary porta hepatis invasion group was
significantly larger than that in the primary porta hepatis
invasion group and the secondary porta hepatis invasion group,
respectively (P<0.001; Table
III). A new test level α'=0.0167 was obtained and the
difference was statistically significant, P<0.01. However, there
was no significant difference in the maximum lesion diameter
detected between the primary porta hepatis invasion group and the
secondary porta hepatis invasion group (P>0.05; Table III). A pairwise comparison of
invasion rates between porta hepatis was performed using
χ2-partitioning methods (α=0.05; Table IV). A new test level α'=0.0167 was
obtained and the difference was statistically significant
(P<0.01).
 | Table I.Comparison of the maximum diameter of
the lesions in the intrahepatic veins, hepatic portal veins and
hepatic arteries. |
Table I.
Comparison of the maximum diameter of
the lesions in the intrahepatic veins, hepatic portal veins and
hepatic arteries.
| Vessel type | Invaded vessels
(n) | Maximum lesion
diameter (cm) | χ2 | P-value |
|---|
| Intrahepatic
veins | 277 | 12.30
(10.35–15.25) |
|
|
| Hepatic portal
veins | 166 | 11.65
(8.98–14.60) | 3.84 | 0.147 |
| Hepatic arteries | 86 | 12.50
(9.88–15.10) |
|
|
 | Table II.Pairwise comparison of invasion rates
in the intrahepatic veins, hepatic portal veins and hepatic
arteries. |
Table II.
Pairwise comparison of invasion rates
in the intrahepatic veins, hepatic portal veins and hepatic
arteries.
| Vessel type | Vessels (n) | Invaded vessels, n
(%) | χ2 | P-value |
|---|
| Intrahepatic
veins | 640 | 277 (43.28) | – | – |
| Hepatic portal
veins | 320 | 166
(51.88)a | – | <0.05 |
| Hepatic arteries | 320 | 86
(26.87)a,b | – | <0.05 |
| Total | 1,280 | 529 (41.33) | 43.25 | <0.001 |
 | Table III.Pairwise comparison of the maximum
diameter of lesions in the hepatic hilum. |
Table III.
Pairwise comparison of the maximum
diameter of lesions in the hepatic hilum.
| Areas affected | Cases of invasion, n
(%) | Maximum lesion
diameter (cm) | χ2 | P-value |
|---|
| Primary porta
hepatis | 71 (55.47) | 11.90
(10.00–15.00) |
|
|
| Secondary porta
hepatis | 11 (8.60) | 10.90
(10.30–11.80) | 25.463 | <0.001 |
| Primary and
secondary porta hepatis | 46 (35.94) | 14.90
(12.73–16.65)a,b |
|
|
 | Table IV.Pairwise comparison of invasion rates
in the hepatic hilum. |
Table IV.
Pairwise comparison of invasion rates
in the hepatic hilum.
| Site | Total cases
(n) | Cases of invasion,
n (%) | χ2 | P-value |
|---|
| Primary porta
hepatis | 160 | 71 (44.38) | – | – |
| Secondary porta
hepatis | 160 | 11
(6.88)a | – | <0.01 |
| Primary and
secondary porta hepatis | 160 | 46
(28.75)a,b | – | <0.01 |
| Total | 480 | 128 (26.67) | 58.06 | <0.001 |
Discussion
HAE is able to invade the intrahepatic vasculature
and infest tissues and organs. Late-stage HAE may result in
cirrhosis, jaundice and liver failure (13), as well as metastasize to organs
including the lungs and brain (14).
The management of this disease is based on drugs (15), while surgery (including radical
resection, lesion reduction or liver transplantation) is the major
treatment method applied (16,17).
However, HAE lesions tend to invade blood vessels, resulting in a
low success rate. Thus, simple diagnosis by imaging no longer
fulfills clinical requirements, and researchers are now focusing on
pre-operative vascular evaluation. In this regard, 3.0-T
multi-stage enhanced MR scanning has high temporal and spatial
resolution capabilities and is able to clearly display the
boundaries of the lesions. This may shorten the blood T1 time and
improve blood signals so that the contrast between blood vessels
and surrounding tissues is enhanced and the blood vessel structure
is clearly displayed. This allows clinicians to accurately evaluate
the vascular invasion status and to design personalized treatment
regimens (18–20). Wang et al (21) investigated vascular involvement in
cystic echinococcosis. However, HAE reproduces by exogenous budding
or infiltrative growth, and has malignant invasion characteristics
(1). In this way, the disease
differs from cystic echinococcosis with regard to vascular
involvement. One study reported that MR angiography and MR
venography are able to indicate the association between the lesion
and the degree of vascular stenosis (22). In addition, according to Li et
al (10), MRI is more accurate
than CT with this regard. Therefore, clinicians may use MRI to
examine the vascular invasion characteristics and lesion growth
patterns.
In the present study, a pairwise comparison of
invasion rates in the intrahepatic veins, hepatic portal veins and
hepatic arteries was performed. It was revealed that the invasion
rate is highest in the hepatic portal veins, followed by that in
intrahepatic veins and hepatic arteries. The possible reasons for
this are as follows: i) The liver has a dual blood supply, with the
hepatic portal veins supplying 75% of the blood, while the hepatic
arteries supply 25%. Thus, the tendency of the hepatic portal veins
to be invaded is increased due to the ratio of blood supply to the
liver (23). ii) In the present
study, lesions in the right hemiliver accounted for 49.38%, while
those in the left hemiliver accounted for 13.13% of all total
cases. In the S-2 and S-3 segments of the left hemiliver and the
S-7 and S-8 segments of the right hemiliver, the blood flow in the
hepatic vein and inferior vena cava were more abundant. However, in
the S-5 and S-6 segments of the right hemiliver and the S4b segment
of the left hemiliver, the left and right branches of the hepatic
portal vein and its tributaries contained more blood vessels.
Therefore, the hepatic portal vein and its tributaries tend to be
invaded more frequently. iii) Arteries are thicker than veins and
have more smooth muscle and elastic fibers within their vascular
walls, with smaller lumens compared with veins. Veins have thinner
walls with large lumens, and are less elastic compared with
arterial vascular walls. Therefore, veins are more likely to be
invaded than arteries (24). iv) HAE
larvae enter the liver through the mesenteric veins and hepatic
portal veins. They then invade the liver tissues and vasculature,
causing vesicles and cyst fluid to enter the blood vessels and
metastasize to the lung, brain and other organs via hepatic venous
drainage into the inferior vena cava. The transmission and
dissemination route require passage through the portal venous
system and are intimately associated with the tendency towards
venous invasion (25). v) Baheti
et al (26) reported that the
invasion rate of liver cancer was greater in the hepatic portal
veins than in the intrahepatic veins, and greater in the
intrahepatic veins than in the hepatic arteries. They also
suggested that liver cancer is mainly supplied by blood from the
hepatic arteries, with the hepatic portal veins supplying only a
small percentage of the blood. Conversely, Fan et al
(27) and Ren and Xiao (28) indicated that the major blood supply
in HAE is from the hepatic arteries, while the hepatic portal veins
only provide a minor amount of blood. The vascular invasion and
blood supply observed in patients with HAE appear to be similar to
that of patients with liver cancer, and the vascular invasion
characteristics also exhibit certain similarities.
The present study also investigated invasion in the
hepatic hilum. The ratio of invasion in this type of tissue was
ranked as follows: Primary porta hepatis > primary and secondary
porta hepatis > secondary porta hepatis. The possible reasons
for this are as follows: i) The left, middle and right hepatic vein
drain into the inferior vena cava through the secondary porta
hepatis, while the hepatic arteries, hepatic portal veins and
hepatic ducts enter the liver through the primary porta hepatis.
The bifurcation of the hepatic arteries is furthest away from the
hepatic hilum, while the hepatic portal vein bifurcation is
slightly further from the secondary porta hepatis than the hepatic
duct bifurcation. When an echinococcosis larva enters the liver
through the hepatic portal vein, it is blocked by liver sinusoids
and grows near the primary porta hepatis. As HAE easily invades the
hepatic portal veins, a localized lesion is most likely to invade
the primary porta hepatis, while the secondary porta hepatis is
only invaded if the lesion grows. ii) In the present study, most
lesions were situated in the right hemiliver, with the S-4 and S-5
segments being the most common sites. Lesions in this location are
anatomically nearer to the primary porta hepatis and the extent of
invasion is greater; iii) HAE lesions receive blood from the
hepatic arteries, as well as from the hepatic portal veins. The
primary porta hepatis receives the most blood from the hepatic
portal veins and hepatic arteries. At the secondary porta hepatis,
the hepatic veins exit the liver and contain fewer nutrients; iv)
Follow-up examinations indicated that the lesion first invades the
primary porta hepatis prior to growing upwards through the inferior
vena cava and finally invading the secondary porta hepatis. Only in
a few cases, the secondary porta hepatis was involved. Thus, the
barrier effects of the liver sinusoids resulted in fewer cases
where echinococcosis larvae first resided in the S-7 and S-8
segments. Conversely, the intensity of lesion activity was
associated with lesion growth, which was in turn supported by
portosystemic anastomosis in the hepatic portal veins and
intrahepatic veins (data not shown).
The current study demonstrated that HAE has
vein-targeting characteristics. Therefore, on the basis of the
initial diagnosis, depending on whether the lesion is situated
close to veins, clinicians can usually predict growth trends and
possible factors that may affect surgical resection. These
predictions may be considered to guide the selection of treatment
options, including the earlier use of drugs to suppress lesion
growth or direct resection. Furthermore, in patients who are
treated with oral drugs, the route of administration may be changed
to intravenous injection, nanoparticle implantation or targeted
delivery to the hepatic portal veins, so that the drugs act rapidly
on the lesions. This approach may eliminate the disadvantages
associated with oral drugs, reducing the drug dosage and systemic
toxicity, and increasing the therapeutic efficacy.
In case of flattened and narrowed hepatic portal
veins, hepatic veins and inferior vena cava appearing on MRI
examination and during surgery, the surgeon would usually perform a
hemihepatectomy to dissect blood vessels from the lesions. If the
hepatic artery appears narrowed and the contrast agent development
of the blood vessel is partially interrupted, the possibility of
invasion is greater. The secondary and tertiary blood vessels of
corresponding liver segments may be resected during a hemiliver
resection, reducing the intra-operative risk. Extensive involvement
is indicated if the lesion has invaded the primary porta hepatis,
and irregular stenosis and signs of blockade appear in the MRI of
the hepatic portal vein. In such cases, the lesion is difficult to
dissect during surgery. In such cases, extended hemihepatectomy is
commonly peformed, whereby the liver segment is resected along with
the walls of certain blood vessels, and the vessels are then
repaired using patches or direct suturing. In case of invasion of
the primary porta hepatis as well as the secondary porta hepatis,
wrapping of the inferior vena cava and hepatic vein root is usually
performed. In such cases, it is difficult to completely excise the
lesion during surgery, and surgeons usually opt for palliative
lesion reduction surgery, wherein certain parts of the lesion are
left behind.
In conclusion, the growth pattern of intermediate
and advanced HAE is determined by the location, blood supply and
activity of the lesion. Considering its vein-targeting feature,
lesions tend to invade the intrahepatic venous system and porta
hepatis.
Acknowledgements
The authors wish to thank Dr Xingjian Guo
(Department of Pathology, Qinghai University Affiliated Hospital,
Xining, China) for helping with the histopathological analysis of
the vascular invasion.
Funding
The current study was supported by a grant from the
Qinghai Provincial Science and Technology Planning Project of
Qinghai University Affiliated Hospital (grant no. 2017-SF-158).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY and YK analyzed and interpreted all patient data
regarding the status of HAE. HB designed the project and also
reviewed the manuscript. HL and WL examined the degree of vascular
invasion and also reviewed the manuscript. JC performed the
histological examinations on hepatic tissue samples. YQ acquired
imaging in patients. YQ and XY prepared the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Qinghai University
(approval no. P-SL-2017053). Written informed consent was obtained
from the individual participants or their guardians, and all
procedures conformed to the ethical standards of the China Human
Experimentation Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HAE
|
hepatic alveolar echinococcosis
|
|
RHA
|
right hepatic artery
|
|
SMA
|
superior mesenteric artery
|
|
AA
|
abdominal aorta
|
|
PV
|
portal vein
|
|
RPV
|
right hepatic portal vein
|
|
LPV
|
left hepatic portal vein
|
|
MHV
|
middle hepatic vein
|
|
LHV
|
left hepatic vein
|
|
RHV
|
right hepatic vein
|
|
IVC
|
inferior vena cava
|
References
|
1
|
Carmena D and Cardona GA: Echinococcosis
in wild carnivorous species: Epidemiology, genotypic diversity, and
implications for veterinary public health. Vet Parasitol.
202:69–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farrokh D, Zandi B, Pezeshki Rad M and
Tavakoli M: Hepatic alveolar echinococcosis. Arch Iran Med.
18:199–202. 2015.PubMed/NCBI
|
|
3
|
Ren L, Zhang LQ, Zhou FS, Fan HN, Deng Y,
Wang HJ, Ma J, Wang Z and Luo SDW: Epidemiological investigation on
hepatic hydatid disease in Banma county of Guoluo tibetan
autonomous prefecture of Qinghai province. Chin J Dis Control Prev.
20:1032–1035. 2016.(In Chinese).
|
|
4
|
Mihmanli M, Idiz UO, Kaya C, Demir U,
Bostanci O, Omeroglu S and Bozkurt E: Current status of diagnosis
and treatment of hepatic echinococcosis. World J Hepatol.
28:1169–1181. 2016. View Article : Google Scholar
|
|
5
|
Jiang W, Wang J, Xiao H, Li TT, Liu H and
Liu W: Evaluation of blood supply distribution of hepatic
echinococcosis by dual-source CT energy imaging: correlation
between iodine concentration and histopathology. J Xinjiang Med
Univ. 38:1207–1212. 2015.(In Chinese).
|
|
6
|
Fang D, Chen ZY, Zeng Y, Li B, Wen TF,
Wang WT, Wu H, Xu MQ, Yang JY, Wei YG, et al: Surgical treatment of
hepatic alveolar echinococcosis. Chin J Bases Clin in General Surg.
23:521–525. 2016.(In Chinese).
|
|
7
|
Chen KF, Tang YY, Wang R, Fang D, Chen JH,
Zeng Y, Li B, Wen TF, Wang WT, Wu H, et al: The choose of different
surgical therapies of hepatic alveolar echinococcosis: A
single-center retrospective case-control study. Medicine
(Baltimore). 97:e00332018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang SJ, Zhang L, Zhao JQ, Zhang YG, Wu
XW, Peng XY, Cao YW, Yang HQ, Lv HL, Sun H and Chen XP: Inhibition
of invasive growth and metastasis of hepatic alveolar
echinococcosis by anti-osteopontin antibody. Chin J Parasitol
Parasit Dis. 29:410–414. 2011.(In Chinese).
|
|
9
|
He YB, Bai L, Aji T, Jiang Y, Zhao JM,
Zhang JH, Shao YM, Liu WY and Wen H: Application of 3D
reconstruction for surgical treatment of hepatic alveolar
echinococcosis. World J Gastroenterol. 35:10200–10207. 2015.
View Article : Google Scholar
|
|
10
|
Li HL, Hou LC, Ren L, Fan HN, Bao HH, Wen
SB and Li WX: Significance of magnetic resonance imaging in
preoperative evaluation for patients with hepatic alveolar
echinococcosis. Chin J Bases Clin in General Surg. 23:535–538.
2016.(In Chinese).
|
|
11
|
Sanaat Z, Khalili R, Almasi S, Aliparasti
MR, Tavangar SM, Movasaghpoor A, Kazemi F and Davani A: Does
chemotherapy change expression of VEGF A&C and MVD in acute
myeloid leukemia? Int J Hematol Oncol Stem Cell Res. 8:24–29.
2014.PubMed/NCBI
|
|
12
|
Ramnefjell M, Aamelfot C, Helgeland L and
Akslen LA: Vascular invasion is an adverse prognostic factor in
resected non-small-cell lung cancer. APMIS. 125:197–206. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geramizadeh B, Attaran Y, Malek-Hosseini
SA, Kaviani MJ and Hossieni-Asl K: Photoclinic. Alveolar hydatid
cyst of the liver. Arch Iran Med. 14:211–212. 2011.PubMed/NCBI
|
|
14
|
Piarroux M, Piarroux R, Giorgi R, Knapp J,
Bardonnet K, Sudre B, Watelet J, Dumortier J, Gérard A, Beytout J,
et al: Clinical features and evolution of alveolar echinococcosis
in France from 1982 to 2007: Results of a survey in 387 patients. J
Hepatol. 55:1025–1033. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vuitton DA and Bresson-Hadni S: Alveolar
echinococcosis: Evaluation of therapeutic strategies. J Expert Opin
Orphan Drugs. 2:67–86. 2014. View Article : Google Scholar
|
|
16
|
Nunnari G, Pinzone MR, Gruttadauria S,
Celesia BM, Madeddu G, Malaguarnera G, Pavone P, Cappellani A and
Cacopardo B: Hepatic echinococcosis: Clinical and therapeutic
aspects. World J Gastroenterol. 18:1448–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wen H, Dong JH, Zhang JH, Zhao JM, Shao
YM, Liang YR and Ji XW: Ex-vivo liver resection combined liver
autotransplantation for the treatment of hepatic alveolar
echinococcosis. Chin J Dig Surg. 10:148–149. 2011.
|
|
18
|
Qian NS, Liao YH, Cai SW, Rautd V and Dong
JH: Comprehensive application of modern technologies in precise
liver resection. Hepatobiliary Pancreat Dis Int. 12:244–250. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thian YL, Riddell AM and Koh DM:
Liver-specific agents for contrast-enhanced MRI: Role in
oncological imaging. Cancer Imaging. 13:567–579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun YQ, Zhang YH, Yang M, Wang JJ and
Zhang QX: Application of CT and MRI in Ex-vivo autologous liver
transplantation of advanced hepatic alveolar echinococcosis. Chin J
Med Imaging. 23:610–613. 2015.(In Chinese).
|
|
21
|
Wang J, Huang Y, Zhao YP, Liu WY, Wang J
and Liu XL: Preoperative multi-slice spiral CT evaluation of
involvement of vessels and biliary ducts in hepatic cystic
echinococcosis. Chin J Radiol. 44:397–400. 2010.
|
|
22
|
Wu JW, Qu XL, Gao H, Lv MG, Lu L and Wang
Xuan: Application of LAVA CE-MRA in hepatectomy for huge hepatic
tumor. Chin J Prac Surg. 33:960–963. 2013.
|
|
23
|
Goceri E: Automatic labeling of portal and
hepatic veins from MR images prior to liver transplantation. Int J
Comput Assist Radiol Surg. 11:2153–2161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Q, Lai SL, Zhang W, Jin GQ, Wang C, Su
DK and Xie D: Radiologic hepatic capsular invasion on msct in
prediction of microvascular invasion of hepatocellular carcinoma. J
Clin Radiol. 6:838–840. 2017.
|
|
25
|
Conraths FJ, Probst C, Possenti A, Boufana
B, Saulle R, La Torre G, Busani L and Casulli A: Potential risk
factors associated with human alveolar echinococcosis: Systematic
review and meta-analysis. PLoS Negl Trop Dis. 11:e00058012017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baheti AD, Dunham GM, Ingraham CR, Moshiri
M, Lall C, Park JO, Li D, Katz DS, Madoff DC and Bhargava P:
Clinical implications for imaging of vascular invasion in
hepatocellular carcinoma. Abdom Radiol (NY). 41:1800–1810. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan YX, Ren WX, Bawudun D, Ji WZ, Gu JP,
Xu XD and Zhang HX: Digital subtraction angiographic observation on
blood supply of rat's hepatic alveolar echinococcosis. Chin J
Interv Imaging Ther. 9:37–40. 2012.
|
|
28
|
Ren WX and Xiao XS: The blood supply of
portal vein in experimentalhepatic alveolar echinococcosis. Chin J
Interv Imaging Ther. 4:142–147. 2007.
|