Introduction
Osteoarthritis (OA) is one of the most common types
of arthritis, affecting 15% of the population worldwide, which
leads to a reduced quality of life and poses a substantial health
threat (1–3). To the best of our knowledge, effective
early treatment of OA remains a challenge. Despite many etiological
factors having been shown to be involved in the progression of OA,
the underlying mechanism remains to be fully elucidated. Oxidative
stress is known to serve a critical role in the progression of OA.
Our previous studies confirmed that a lack of nuclear factor
(erythroid-derived 2)-like 2 (Nrf2), a key transcription factor
that regulates the antioxidant defense system, can result in
cartilage degradation (4).
Therefore, the aim of the present study was to establish novel
effective strategies by targeting oxidative stress.
7,8-Dihydroxyflavone (7,8-DHF) is a natural flavone, which is
widely distributed in plants. 7,8-DHF was first reported to have a
therapeutic effect on various central nervous system diseases,
including the tropomyosin receptor kinase B (TrkB) agonist
(5). Previous studies have reported
that 7,8-DHF can independently confer neuroprotection through its
antioxidant activity (5–7). Further studies have reported that
7,8-DHF protected C2C12 myoblasts and hamster lung fibroblasts from
oxidative stress via ERK/Nrf2/heme oxygenase-1 signaling pathway
activation (8,9). The aim of the present study was to
examine the regulatory role of 7,8-DHF on the Nrf2 signaling
pathway in primary articular chondrocytes, and investigate whether
7,8-DHF can delay the progression of articular cartilage
destruction in an animal model of OA.
Materials and methods
Chemicals and reagents
7,8-DHF (purity >99%) was procured from
Sigma-Aldrich; Merck KGaA. Anti-Nrf2 (cat. no. BS1258) and
anti-HO-1 (cat. no. BS6626) antibodies were supplied by Bioworld
Technology at a dilution of 1:1,000. Anti-actin (cat. no. 4970) and
anti-lamin B (cat. no. 13435) antibodies were obtained from Cell
Signaling Technology, Inc. at a dilution of 1:5,000. Secondary
HRP-conjugated goat anti-rabbit (cat. no. BS13278) antibody was
purchased from Bioworld Technology, Inc. and used at a dilution of
1:5,000. The Cell Counting Kit-8 (CCK-8) and nuclear protein
extraction kit were purchased from Beyotime Institute of
Biotechnology. The superoxidase dismutase (SOD) and malondialdehyde
(MDA) assay kits were supplied by Nanjing Jiancheng Bioengineering
Institute. The fluorescent probe 2′,7′-dichlorodihydrofluorescein
diacetate (H2DCFDA) was obtained from Molecular Probes; Thermo
Fisher Scientific, Inc.
Cell viability assay
Cell viability was evaluated using a CCK-8 assay.
Briefly, chondrocytes were seeded in 96-well plates at a density of
5×103 cells/well and cultured with various
concentrations of 7,8-DHF (0, 1, 2, 4, 6, 8 and 10 µM) for 8 h at
37°C. Following incubation, 10 µl of CCK-8 solution was added to
each well and the cells were further incubated for 1 h at 37°C. The
absorbance was measured at a wavelength of 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc.).
Analysis of antioxidant enzyme
activities
The primary mouse chondrocytes pre-treated with
various doses of 7,8-DHF (0,1,3 and 9 µM) for 8 h were cultured in
40 ng/ml H2O2 for 24 h at 37°C. The cells
were washed in ice-cold phosphate buffer and total proteins were
extracted with 100 µl RIPA for 15 min. The lysates were homogenized
using an ultrasonic homogenizer and centrifuged at 13,000 × g for 5
min at 4°C. The MDA contents in the supernatant were measured by
monitoring thiobarbituric acid reacting substances. Using a
spectrophotometer, the activities of SOD were measured at a
wavelength of 532 nm using the assay kit (Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
protocols.
Measurement of intracellular reactive
oxygen species (ROS)
The generation of ROS was detected using the
fluorescent probe, H2DCFDA. Following incubation with various
concentrations of 7,8-DHF for 8 h, the primary mouse chondrocytes
were treated with/without H2O2 for 24 h. The
cells were incubated with H2DCFDA (10 µM) for 30 min at room
temperature in the dark. The production of ROS in the cells was
monitored using a flow cytometer (BD Biosciences) with CellQuest
Pro software, version 5.0 (BD Biosciences).
Western blot analysis
The proteins lysed from the primary mouse
chondrocytes and the cartilage tissue extracted from the knee
joints were measured and normalized for western blot analysis, as
previously described (10). Each
experimental unit for the mice consisted of a pool of two cartilage
compartments. Nuclear extraction was performed according to the
manufacturer's protocols of the nuclear protein extraction kit
(Beyotime Institute of Biotechnology). Briefly, normalized volumes
of samples (20 µg protein) were separated by 10% SDS-PAGE at 100 V
for 100 min in Tris-glycine-SDS running buffer (Thermo Fisher
Scientific, Inc.). The gel was transferred for 90 min at 300 mA to
nitrocellulose membranes (GE Healthcare, Chicago, IL, USA).
Membranes were then incubated for 2 h with blocking buffer (5%
skimmed milk in TBST) at room temperature, and incubated overnight
with the anti-Nrf2 (1:1,000; cat. no. BS1258; Bioworld Technology,
Inc.), anti-HO-1 (1:1,000; cat. no. BS6626; Bioworld Technology,
Inc.) antibodies and anti-actin (1:5,000; cat. no. 4970; Cell
Signaling Technology, Inc.) antibodies at 4°C. Subsequently, the
membranes were washed three times in TBST, then incubated with
secondary HRP-conjugated goat anti-rabbit (1:5,000 cat. no.
BS13278; Bioworld Technology, Inc.) at room temperature for 1 h and
finally washed an additional three times in TTBS. The membranes
were visualized using the enhanced chemiluminescence assay (Pierce;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Densitometry of protein bands was performed for western
blot analysis. Bands were semi-quantified by reverse image scanning
densitometry with Photoshop (version 6.0; Adobe Systems, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RNA was extracted from the knee cartilage of mice
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
following homogenization. The cartilage, which was snap-frozen in
liquid nitrogen and stored at −80°C until RNA extraction, was
ground with a pestle and liquid nitrogen-chilled mortar prior to
TRIzol extraction. Each experimental unit consisted of a pool of
1–2 cartilage compartments and, when pooling was performed, the
experimental unit was regarded as a single unit. The purity and
yield of the RNA was determined using a NanoDrop ND-1000
spectrophotometer (Nanodrop Technologies; Thermo Fisher Scientific,
Inc.). cDNA was synthesized from the RNA with PrimeScript RT Master
mix (Takara Bio, Inc.). Gene expression was measured using a 7500
real-time PCR system in the presence of SYBR Premix Ex Taq
reagents, according to the manufacturer's protocol. The
thermocycling conditions were as follows: Denaturation at 95°C for
10 min, followed by 45 cycles of denaturation at 95°C for 5 sec,
followed by an annealing/extension step at 60°C for 30 sec. The
primer sequences are presented in Table
I. Relative quantification was defined as 2−ΔΔCq
(11). qPCR was performed in
triplicate for each sample.
 | Table I.G ene-specific primer sequences for
qPCR. |
Table I.
G ene-specific primer sequences for
qPCR.
| Gene | qPCR primer
(5′-3′) |
|---|
| IL-1β | Forward:
ATGGCAGAAGTACCTAAGCTCGC |
| IL-1β | Reverse:
ACACAAATTGCATGGTGAAGTCAGTT |
| IL-6 | Forward:
ACACACTGGTTCTGAGGGAC |
| IL-6 | Reverse:
TACCACAAGGTTGGCAGGTG |
| TNF-α | Forward:
ATGAGCACAGAAAGCATGATCCGC |
| TNF-α | Reverse:
CCAAAGTAGACCTGCCCGGACTC |
| MMP-1 | Forward:
GCCACAAAGTTGATGCAGTT |
| MMP-1 | Reverse:
GCAGTTGAACCAGCTATTAG |
| MMP-3 | Forward:
ATGAAAATGAAGGGTCTTCCGG |
| MMP-3 | Forward:
GCAGAAGCTCCATACCAGCA |
| MMP-13 | Forward:
ATGCATTCAGCTATCCTGGCCA |
| MMP-13 | Reverse:
AAGATTGCATTTCTCGGAGCCTG |
| HO-1 | Forward:
ACATCGACAGCCCCACCAAGTTCAA |
| HO-1 | Reverse:
CTGACGAAGTGACGCCATCTGTGAG |
| Nrf2 | Forward:
TCTCCTCGCTGGAAAAAGAA |
| Nrf2 | Reverse:
AATGTGCTGGCTGTGCTTTA |
| β-actin | Forward:
TGACGGGGTCACCCACACTGTGCCCATCTA |
| β-actin | Reverse:
CTAGAAGCATTTGCGGTGGACGATGGAGGG |
Experimental OA and 7,8-DHF
treatment
A total of 92 wild-type B6 mice (8–10 weeks old)
were used in the experiments and were housed in standard mouse
cages (10 animals per cage) in specific-pathogen-free conditions
under a light-dark cycle of 12:12 h at 25±2°C with standard mouse
food (RM3; Special Dietary Systems) and water ad libitum.
Animal health and behavior were monitored every 12 h. When
pain/distress were observed, the animals were treated with Buprenex
(Reckitt & Colman Pharmaceuticals, Inc.; 0.1–2.0 mg/kg), in
addition to crushed or wet food. If pain or distress continued, the
mouse was sacrificed regardless of the scheduled endpoints. The
criteria that determined discomfort/distress/pain were any three of
the following signs: Abnormal posture, a slow, careful or abnormal
(waddling) gait, low activity levels, slow eating, cowering or
vocalizing on handling, a change in eye or coat appearance, and
weight loss. Sacrifice was confirmed by one of the following
criteria: Cervical dislocation following no response to tail or toe
pinch, no respiration or heartbeat following continuous monitoring
for 30 sec, or rigor mortis. All procedures were performed in
accordance with the Nanjing Medical University Institutional Animal
Care and Use Committee (Nanjing, China) guidelines. The mice were
anesthetized by inhalation of ether during OA surgery. OA was
induced following sectioning of the medial meniscotibial ligament,
also known as the coronary ligament, which connects the medial
meniscus to the periphery of the tibial plateau, and mice
undergoing sham surgery were used as a control (12,13).
7,8-DHF (Sigma-Aldrich; Merck KGaA) was subsequently dissolved in
ethanol and phosphate-buffered saline (PBS). 7,8-DHF was
administered by intraperitoneal injections (5 mg/kg) once a week
postoperatively. The knee joints of the mice were harvested 4 and 8
weeks postoperatively, and were stained with Safranin O/Fast Green
for assessment using the OARSI scoring system for histologic
alterations (14). The mounted
slides were placed in 70% ethyl alcohol for 15 min and then stained
with 0.04% safranin O/sodium acetate buffer (pH 4.0), for 10 min at
22°C.
Cell culture
Murine knee articular cartilage was obtained from
5-day-old B6 mice and digested with collagenase D, as previously
described (15). The chondrocytes
isolated from articular cartilage of one litter were seeded onto a
10-cm dish at a density of 5×105 cells/per dish. On
reaching 80% confluency, the cells were detached and randomly
plated in six-well plates (105/well), and cultured in
Ham's F-12 (DMEM/F12; Gibco; Thermo Fisher Scientific, Inc.)
containing 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA), 100
IU/ml penicillin (Sigma-Aldrich; Merck KGaA), 5% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 2 mM L-glutamine at 37°C. Only the
first passage cells were used for 7,8-DHF treatment, as indicated
below.
Luciferase assays
293 cells (Shanghai Institutes for Biological
Sciences, China) were maintained in Dulbecco's modified Eagle's
medium (DMEM; Hyclone; GE Healthcare, Chicago, IL, USA) + 200 µM
geneticin (G-418; Gibco; Thermo Fisher Scientific, Inc.),
supplemented with 10% FBS (Sigma-Aldrich; Merck KGaA) at 37°C with
5% CO2. The HO-1 promoter was amplified by PCR from RAW
cell genome DNA using the following primer sequences: HO-1, forward
5′-GGAAGATCTCTGCAGAGCCCCACTGGAG-3′ and reverse
5′-CCCAAGCTTGGAACAGCAACGCTGT-3′. The PCR product was subsequently
inserted into the pGL3 vector at the HindIII and
Bg1II sites. A non-specific oligo was used to construct a
control plasmid. The 293 cells (3×105 cells/well) seeded
in 24-well plates were transfected with the
HO-1-ARE-promoter-driven luciferase plasmid (Beyotime Institute of
Biotechnology). Transfection was performed using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). After 24 h of
transfection, the cells were treated with 1, 3 and 9 µM of 7,8-DHF
for 8 h at 37°C. The relative luciferase activity was measured by
normalizing HO-1-ARE-promoter-driven firefly luciferase activity to
Renilla luciferase activity with a luciferase assay system (Promega
Corporation).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Data following Gaussian distribution were further
analyzed using Student's t-test and data without Gaussian
distribution was analyzed via the Mann-Whitney U test. Differences
between groups were analyzed using one-way analysis of variance and
Duncan's post hoc test. P<0.05 was considered to indicate a
statistically significant difference. All data were analyzed using
SPSS 16.0 (SPSS, Inc.) software.
Results
7,8-DHF activates the Nrf2 signaling
pathway in primary mouse chondrocytes
Primary mouse chondrocytes were treated with various
doses of 7,8-DHF for 8 h (9). The
cytotoxicity of 7,8-DHF was analyzed in primary mouse chondrocytes
and non-toxic concentrations were used in the subsequent
experiments (Fig. 1A). To examine
whether 7,8-DHF can activate Nrf2 signaling in chondrocytes, the
effect of 7,8-DHF on the expression of the Nrf2 downstream protein
in primary mouse chondrocytes was examined. The results indicated
that the expression of HO-1 was upregulated by 7,8-DHF in a dose-
and time-dependent manner (Fig.
1E-J). Furthermore, 7,8-DHF treatment caused the nuclear
translocation of Nrf2 and activated HO-1 promoter transactivation
activity (Fig. 1B-D). Therefore,
these findings suggested that 7,8-DHF effectively activated Nrf2
signaling in primary chondrocytes.
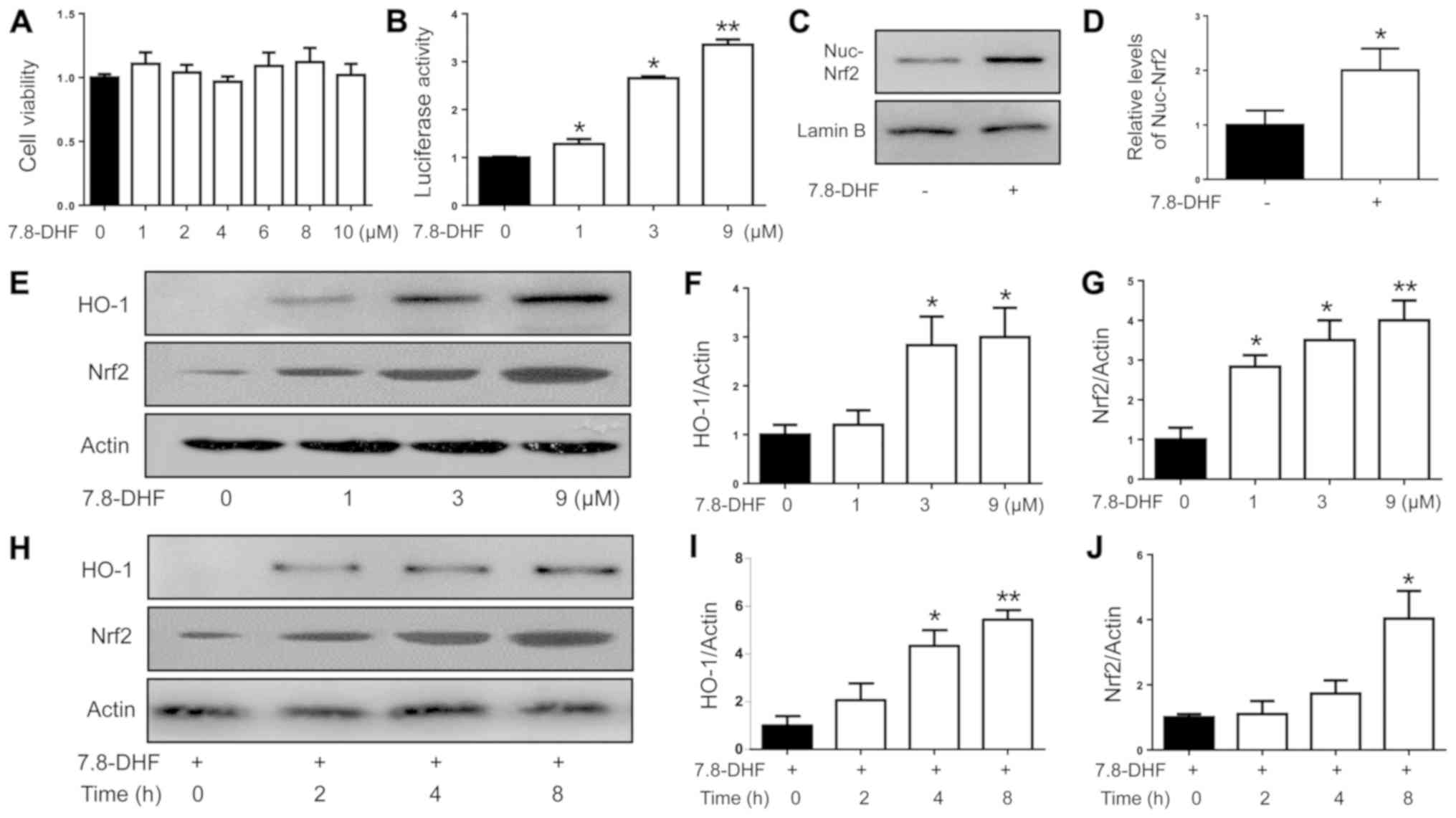 | Figure 1.Induction of the expression of Nrf2
and HO-1 with 7,8-DHF treatment in primary mouse chondrocytes. (A)
Cell viability assay. Primary mouse chondrocytes were treated with
various doses of 7,8-DHF for 8 h. A Cell Counting Kit-8 assay was
used to measure the viable cells in triplicate. (B) 293 cells were
transiently transfected with HO-1 promoter reporter plasmid and a
Renilla luciferase plasmid for 24 h. The cells were treated
with various doses of 7,8-DHF for 8 h. The luciferase activity was
measured and normalized to Renilla luciferase activity.
*P<0.05 and **P<0.01 compared with the untreated control. (C)
Primary mouse chondrocytes were treated with 7,8-DHF (9 µM) for 8
h. The cell nuclear fractions were isolated to be assayed for Nrf2,
with lamin B serving as a control. (D) Quantitative analysis of
nuclear expression levels of Nrf2. *P<0.05 compared with the
untreated controls. (E) Primary mouse chondrocytes were treated
with various doses of 7,8-DHF for 8 h. Quantitative analysis of the
expression levels of (F) HO-1 and (G) Nrf2. *P<0.05 and
**P<0.01 compared with untreated control. (H) Primary mouse
chondrocytes were treated with 7,8-DHF (9 µM) at different time
points and HO-1 and Nrf2 were assayed by western blot analysis.
Quantitative analysis of the expression levels of (I) HO-1 and (J)
Nrf2. *P<0.05 and **P<0.01 compared with the untreated
controls. 7,8-DHF, 7,8-dihydroxyflavone; Nrf2, nuclear factor
(erythroid-derived 2)-like 2; HO-1, heme oxygenase-1. |
7,8-DHF regulates the activities of
antioxidant markers
To investigate whether 7,8-DHF affects oxidative
stress damage, the level of intracellular ROS, the content of MDA
and the activity of SOD were measured. The
H2O2-induced ROS fluorescence intensity was
markedly weakened in the chondrocytes pretreated with 7,8-DHF
(Fig. 2A). 7,8-DHF treatment
suppressed the expression level of MDA (Fig. 2B) and increasing the activity of SOD
(Fig. 2C). The results suggested
that 7,8-DHF attenuated H2O2-induced
oxidative stress in primary chondrocytes.
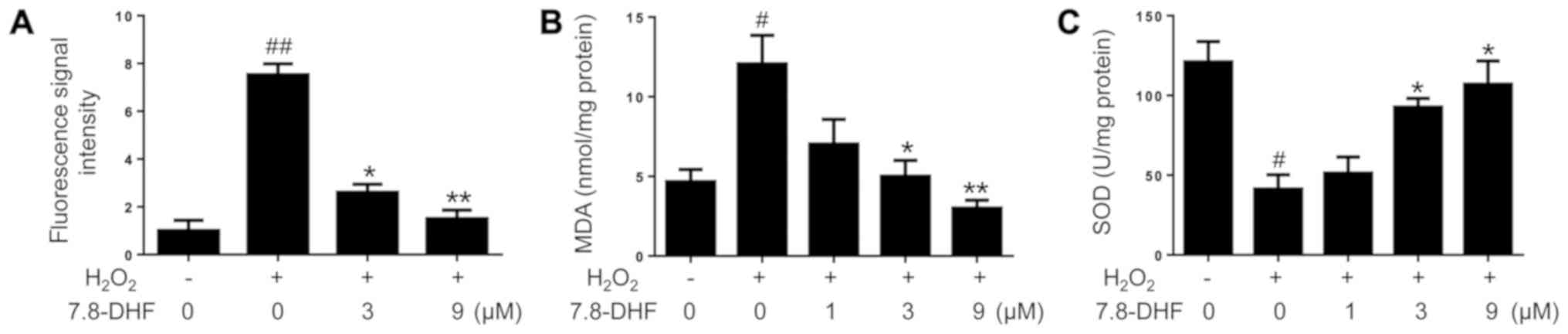 | Figure 2.7,8-DHF ameliorates oxidative stress
in primary mouse-treated chondrocytes. Chondrocytes were pretreated
with different concentrations of 7,8-DHF for 8 h and incubated with
40 ng/ml H2O2 for 24 h. (A) Effect of 7,8-DHF
treatment on the level of intracellular ROS. (B) Effects of 7,8-DHF
on MDA content. (C) Effects of 7,8-DHF on SOD activities.
#P<0.05 and ##P<0.01 compared with the
untreated control. *P<0.05, **P<0.01 compared with
H2O2-treated group. SOD, superoxidase
dismutase; MDA, malondialdehyde; 7,8-DHF, 7,8-dihydroxyflavone;
ROS, reactive oxygen species. |
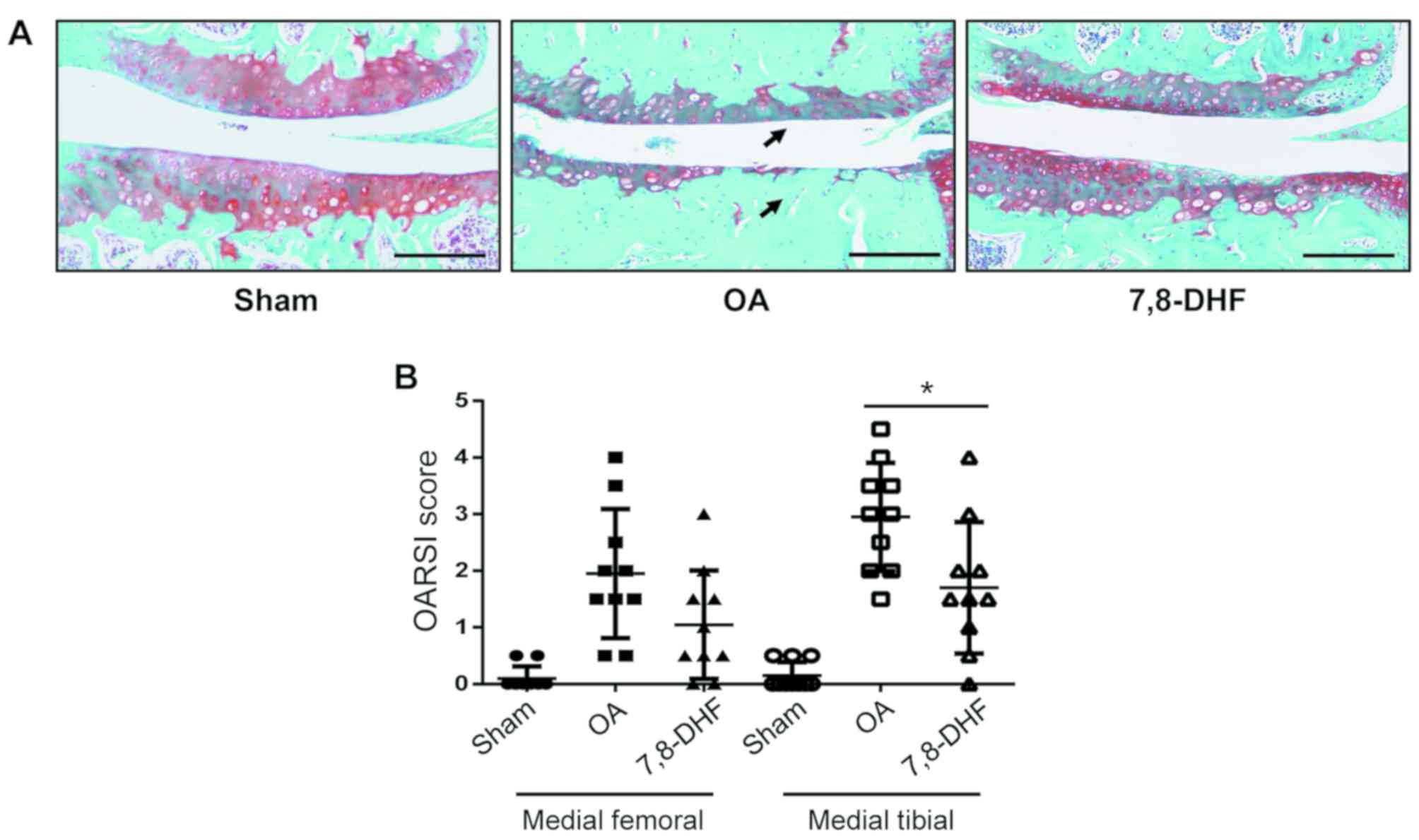
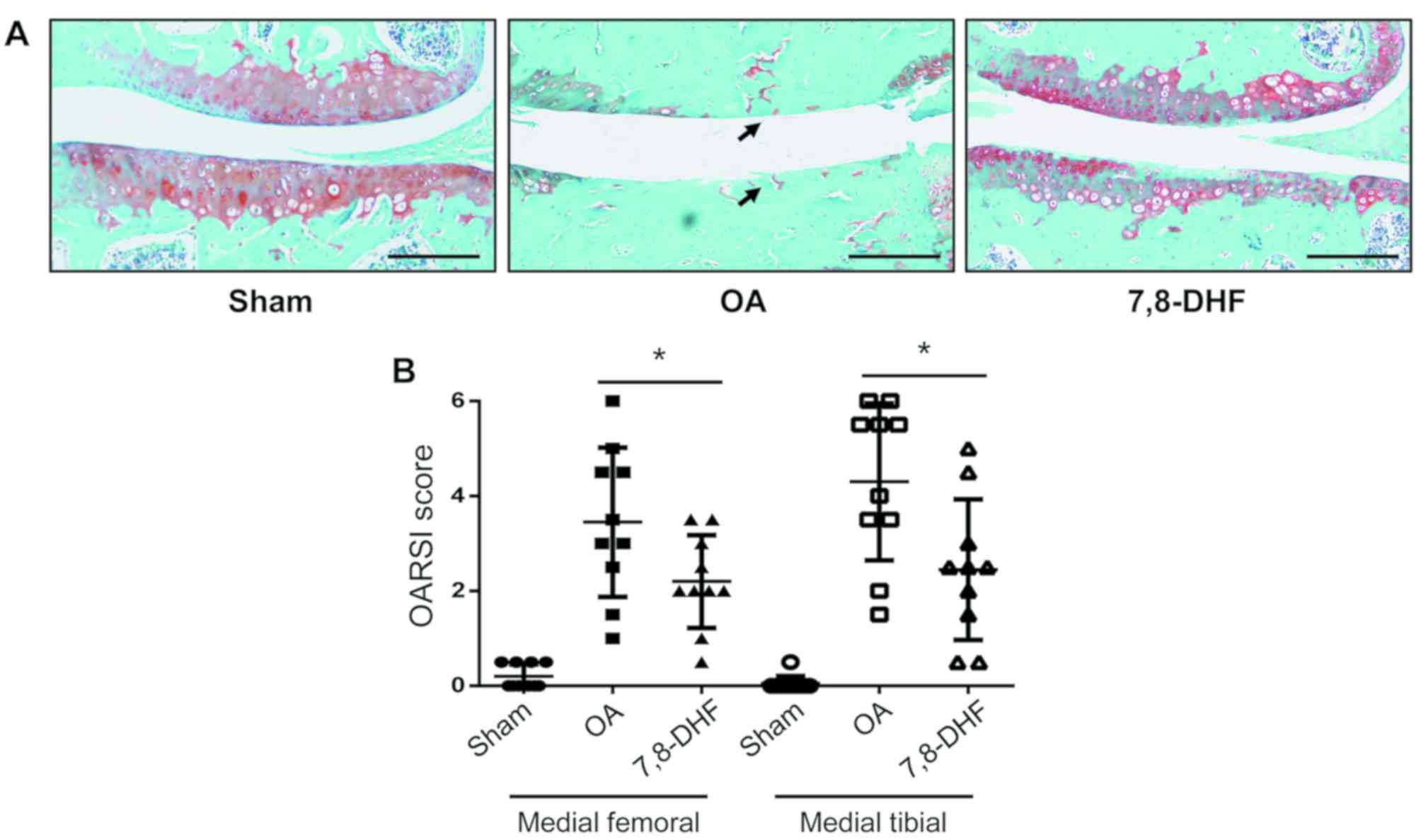
7,8-DHF alleviates OA-associated
cartilage damage
The vehicle-treated OA mice exhibited a loss of knee
joint cartilage with exposed subchondral bone, whereas the
7,8-DHF-treated group exhibited a greater extent of cartilage
damage alleviation (Fig. 3A). The
7,8-DHF-treated group exhibited a lower staining score of 1.70±1.10
for the tibia (P<0.05) 4 weeks following surgery (Fig. 3B). In the 7,8-DHF-treatment group,
the stained sections of the joints harvested at 8 weeks had lower
scores of 2.20±0.93 for the femur and 2.45±1.40 for the tibia,
indicating that 7,8-DHF significantly protected the cartilage from
damage in the femoral condyle and medial tibial plateau (Fig. 4A and B). Therefore, 7,8-DHF had a
protective effect on the cartilage of the OA mouse model.
7,8-DHF attenuates OA-associated mRNA
expression
The cartilage of the knee joints was used to examine
changes in the OA-associated mRNA expression levels via RT-qPCR
analysis. Consistent with the histopathological results, the
cartilage of the OA group exhibited significantly higher mRNA
expression levels of TNF-α, IL-1β, IL-6, MMP-1, MMP-3 and MMP-13
compared with that in the sham group, and the upregulated gene
expression levels were suppressed by 7,8-DHF (Fig. 5A-F).
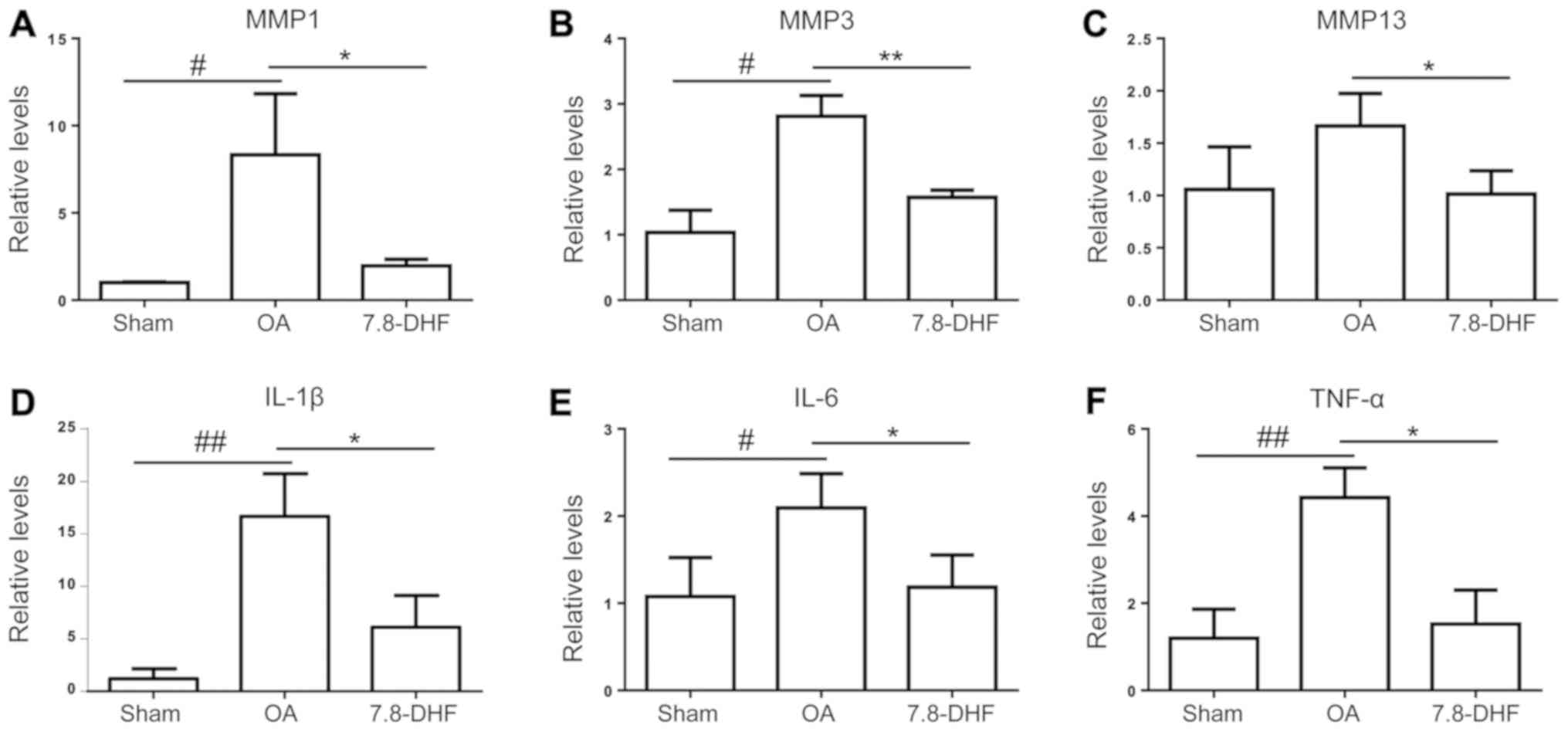 | Figure 5.Effects of 7,8-DHF on OA-associated
mRNA expression. Relative gene expression levels of (A) MMP-1, (B)
MMP-3, (C) MMP-13, (D) IL-1β, (E) IL-6 and (F) TNF-α in the knee
joint cartilage of mice. #P<0.05 and
##P<0.01 compared with the sham group. *P<0.05 and
**P<0.01 compared with the OA group. 7,8-DHF,
7,8-dihydroxyflavone; OA, osteoarthritis; MMP, matrix
metalloproteinases; IL, interleukin; TNF-α, tumor necrosis factor
α. |
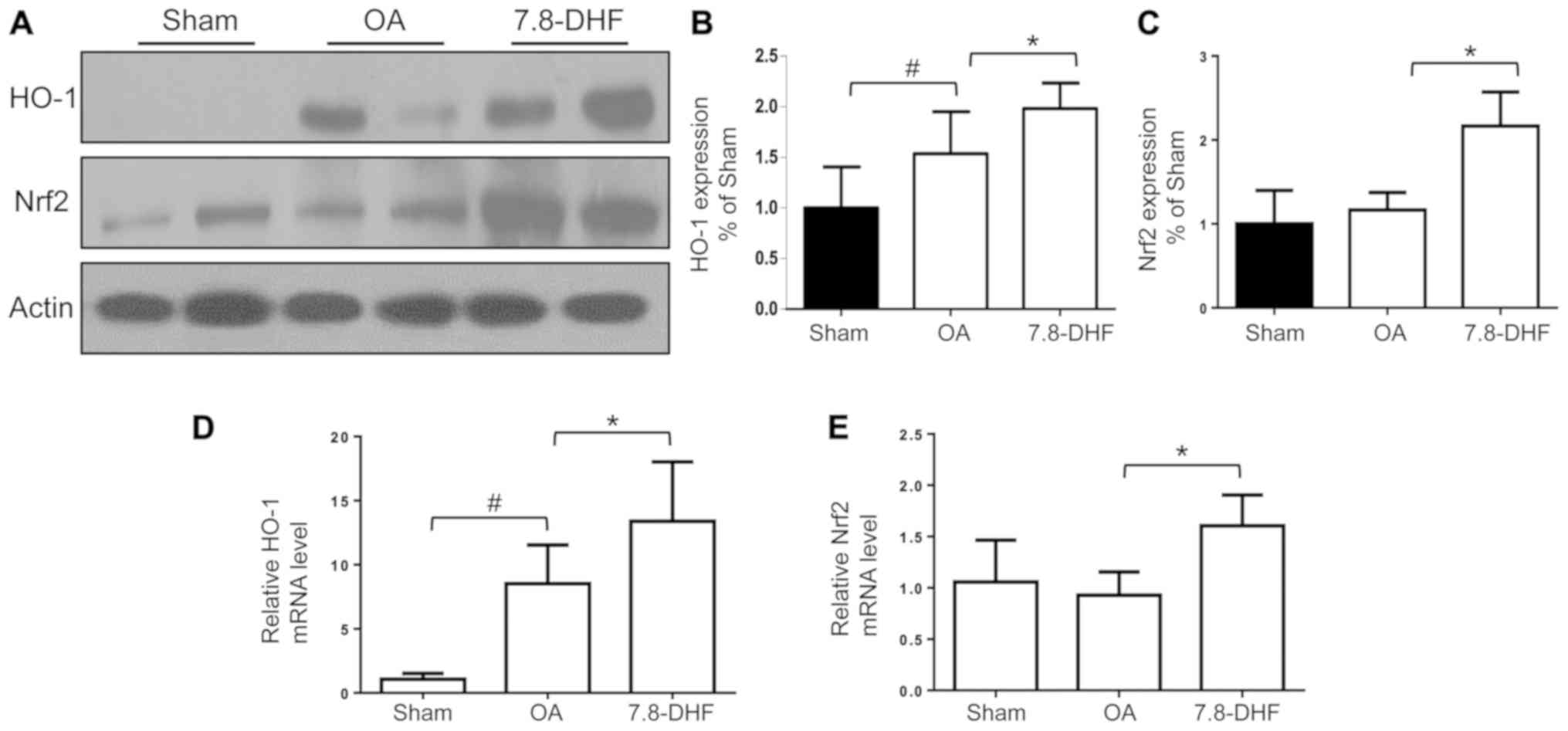
7,8-DHF increases the expression of
HO-1 and Nrf2 in vivo
The expression of HO-1 and Nrf2 in the knee
cartilage obtained from mice was further examined, in order to
investigate whether Nrf2-HO-1 was substantially upregulated in the
7,8-DHF-treatment group. The results of the present study confirmed
that the protein and gene expression levels of HO-1 and Nrf2
increased following 7,8-DHF treatment (Fig. 6A-E). This was consistent with the
data in vitro, indicating that 7,8-DHF may serve a
protective role in cartilage erosion in OA mice via Nrf2 signaling
pathway activation.
Discussion
Excessive oxidative stress is a common pathological
mechanism, which has been demonstrated to be involved in the
progression of OA (16–18). Excessive oxidative stress can
increase the secretion of inflammatory cytokines and alter the
function of multiple signaling pathways in chondrocytes, causing
cell dysfunction, extracellular matrix degradation and the
programmed cell death or necrosis of chondrocytes during OA
(19–21). Nrf2 is known to regulate antioxidant
protein expression, which protects against the oxidative damage
induced by inflammation and injury. Usually, Nrf2 resides in the
cytoplasm and translocates to the nucleus during conditions of
stress. Nrf2 binds to the antioxidant response elements, which are
located in the promoter region, and one of the target genes is
HO-1, which counteracts the oxidative damage in various tissue
injuries (22–24). The expression of HO-1 has been
reported to be upregulated in numerous inflammatory diseases, as a
physiological response to oxidant damage in the aforementioned
conditions. Maicas et al reported that HO-1 was expressed at
a high level in mice with K/BxN serum-induced rheumatoid arthritis
(RA) (25). Although Kobayashi et
al demonstrated that the expression of HO-1 was enhanced in the
joint tissues of patients with RA compared with that in patients
with OA (26), the present study
demonstrated that the level of HO-1 was higher in cartilage with OA
compared with that in normal mice cartilage, which indicated that
activation of the Nrf2/HO-1 signaling pathway may have a
cytoprotective effect in the cartilage once OA develops.
MMPs and proinflammatory cytokines are considered to
promote cartilage OA injuries (27–29). In
accordance with previous studies, the present study indicated that
the increased expression of MMP-1, MMP-3 and MMP-13 in the
cartilage of mice with OA was suppressed following 7,8-DHF
treatment. The findings reported by Choi et al (30) indicated that 7,8-DHF inhibited the
expression of MMP-1 in Hs68 cells. Therefore, the suppressive
effect of 7,8-DHF on MMPs depends on the cell type. Proinflammatory
cytokines also serve major roles in the pathogenesis of OA. qPCR
analysis indicated that 7,8-DHF decreased the expression of IL-1β,
IL-6 and TNF-α in the arthritic cartilage. This result was
consistent with the results of a previous study that the
lipopolysaccharide (LPS)-induced production of TNF-α, prostaglandin
E2, NO and IL-6 was inhibited by 7,8-DHF in RAW264.7 cells
(31). Park et al (32), further reported that 7,8-DHF may
reduce the generation of pro-inflammatory mediators and cytokines
by inhibiting the MAPK and nuclear factor (NF)-κB signaling
pathways in LPS-stimulated BV2 microglial cells.
In addition to the antioxidant benefits, HO-1 serves
an important regulatory role in the inflammation and catabolism in
chondrocytes. Associated studies have confirmed that HO-1 is
involved in the production of MMPs and proinflammatory cytokines.
The upregulation of HO-1, which is induced by BTB domain and CNC
homology 1 deficiency, decreased the gene expression of MMP-13 and
A disintegrin and metalloproteinase with thrombospondin motifs 5 in
response to cytokine treatment in primary mouse articular
chondrocytes (33). Rousset et
al also reported that the increased expression of HO-1 caused a
significant decrease in the expression of MMP-1 stimulated by IL-1β
in human C-20/A4 chondrocytes (34).
As an inducible isoform of HO, HO-1 degrades heme to carbon
monoxide, biliverdin and Fe2+. Carbon monoxide can
suppress the synthesis of inflammatory mediators, including
pro-inflammatory cytokines and nitric oxide (35). Previous studies have reported that
the overexpression of HO-1 inhibited the generation of IL-6 and
activation of the NF-κB signaling pathway in cultured human
tracheal smooth muscle cells (36).
7,8-DHF is an occurring flavone found in natural
plants. It has been reported to act as a potent and selective
small-molecule agonist of TrkB. The protective effects of 7,8-DHF
on cells by scavenging intracellular ROS has been reported in HT-22
hippocampal cells and PC12 pheochromocytoma cells (37,38).
7,8-DHF has also been demonstrated to protect lung fibroblasts
against oxidative damage by activating the ERK/Nrf2/HO-1 and
PI3K/Akt signaling pathways (9). Our
previous study indicated that Nrf2 deficiency caused more severe
cartilage degradation in the knee joints of mice with OA, however,
the damage was reduced by activating the Nrf2/HO-1 signaling
pathway. The results of the present study indicated that 7,8-DHF
enhanced the expression and translocation of Nrf2 into the nucleus,
leading to an enhanced expression of HO-1 in primary mouse
chondrocytes. The expression of HO-1 in the knee cartilage of mice
with OA was also upregulated following 7,8-DHF treatment by
administering once-a-week intraperitoneal injections
postoperatively. These were in line with the findings in
vitro. These results suggested that 7,8-DHF may protect against
oxidative damage in OA cartilage.
OA is an age-associated joint disease and is
characterized by the slow progression of cartilage degradation.
Therefore, mice were sacrificed at different time points, in order
to properly mimic this stepwise development of OA. The histological
results obtained 4 and 8 weeks following surgery demonstrated that
7,8-DHF effectively reduced cartilage damage in the cartilage of OA
mice. As 7,8-DHF was administered to mice at the same time of the
onset of OA in the study, whether 7,8-DHF is protective in
pre-arthritic knees remains to be elucidated.
In conclusion, the present study demonstrated for
the firstςtime, to the best of our knowledge, the protective effect
of 7,8-DHF treatment against cartilage degradation in the
development of OA. This effect was associated with activation of
the Nrf2-HO-1 signaling pathways. Therefore, these results suggests
that 7,8-DHF can be considered as a potential therapeutic option in
the treatment of human OA.
Acknowledgements
Not applicable.
Funding
The present study was subsidized by a grant from the
Natural Science Foundation of the Jiangsu Higher Education
Institutions of China (grant mo. 18KJB320009) for financial
support.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DWC, WF and JQ conceived and designed the study.
DWC, WF, JL, LHJ, SCC, TBY, CY, HX and DWG performed the
experiments. DWC, WF wrote the manuscript. JQ reviewed and edited
the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
All experiments related to the use of animals were
approved by the Institutional Animal Care and Use Committee of The
Sir Run Run Hospital of Nanjing Medical University (Nanjing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rezende MU, Hernandez AJ, Oliveira CR and
Bolliger Neto R: Experimental osteoarthritis model by means of
medial meniscectomy in rats and effects of diacerein administration
and hyaluronic acid injection. Sao Paulo Med J. 133:4–12. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Y, Han C, Zhao L and Ren Y: Is local
platelet-rich plasma injection clinically superior to hyaluronic
acid for treatment of knee osteoarthritis? A systematic review of
randomized controlled trials. Arthritis Res Ther. 20:1282018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai D, Yin S, Yang J, Jiang Q and Cao W:
Histone deacetylase inhibition activates Nrf2 and protects against
osteoarthritis. Arthritis Res Ther. 17:2692015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jang SW, Liu X, Yepes M, Shepherd KR,
Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE and Ye K: A
selective TrkB agonist with potent neurotrophic activities by 7,
8-dihydroxyflavone. Proc Natl Acad Sci USA. 107:2687–2692. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Devi L and Ohno M: 7,8-dihydroxyflavone, a
small-molecule TrkB agonist, reverses memory deficits and BACE1
elevation in a mouse model of Alzheimer's disease.
Neuropsychopharmacology. 37:434–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castello NA, Nguyen MH, Tran JD, Cheng D,
Green KN and LaFerla FM: 7,8-Dihydroxyflavone, a small molecule
TrkB agonist, improves spatial memory and increases thin spine
density in a mouse model of Alzheimer disease-like neuronal loss.
PLoS One. 9:e914532014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang JS, Choi IW, Han MH, Kim GY, Hong SH,
Park C, Hwang HJ, Kim CM, Kim BW and Choi YH: The cytoprotective
effects of 7,8-dihydroxyflavone against oxidative stress are
mediated by the upregulation of Nrf2-dependent HO-1 expression
through the activation of the PI3K/Akt and ERK pathways in C2C12
myoblasts. Int J Mol Med. 36:501–510. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ryu MJ, Kang KA, Piao MJ, Kim KC, Zheng J,
Yao CW, Cha JW, Hyun CL, Chung HS, Park JC, et al: Effect of
7,8-dihydroxyflavone on the up-regulation of Nrf2-mediated heme
oxygenase-1 expression in hamster lung fibroblasts. In Vitro Cell
Dev Biol Anim. 50:549–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gardiner MD, Vincent TL, Driscoll C,
Burleigh A, Bou-Gharios G, Saklatvala J, Nagase H and Chanalaris A:
Transcriptional analysis of micro-dissected articular cartilage in
post-traumatic murine osteoarthritis. Osteoarthritis Cartilage.
23:616–628. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Glasson SS, Blanchet TJ and Morris EA: The
surgical destabilization of the medial meniscus (DMM) model of
osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage.
15:1061–1069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Little CB and Hunter DJ: Post-traumatic
osteoarthritis: From mouse models to clinical trials. Nat Rev
Rheumatol. 9:485–497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Glasson SS, Chambers MG, Van Den Berg WB
and Little CB: The OARSI histopathology initiative-recommendations
for histological assessments of osteoarthritis in the mouse.
Osteoarthritis Cartilage. 18 (Suppl 3):S17–S23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gosset M, Berenbaum F, Thirion S and
Jacques C: Primary culture and phenotyping of murine chondrocytes.
Nat Protoc. 3:1253–1260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Legrand C, Ahmed U, Anwar A, Rajpoot K,
Pasha S, Lambert C, Davidson RK, Clark IM, Thornalley PJ, Henrotin
Y and Rabbani N: Glycation marker glucosepane increases with the
progression of osteoarthritis and correlates with morphological and
functional changes of cartilage in vivo. Arthritis Res Ther.
20:1312018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yudoh K, Nguyen vT, Nakamura H,
Hongo-Masuko K, Kato T and Nishioka K: Potential involvement of
oxidative stress in cartilage senescence and development of
osteoarthritis: Oxidative stress induces chondrocyte telomere
instability and downregulation of chondrocyte function. Arthritis
Res Ther. 7:R380–R391. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dalle-Donne I, Rossi R, Colombo R,
Giustarini D and Milzani A: Biomarkers of oxidative damage in human
disease. Clin Chem. 52:601–623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davies CM, Guilak F, Weinberg JB and
Fermor B: Reactive nitrogen and oxygen species in
interleukin-1-mediated DNA damage associated with osteoarthritis.
Osteoarthritis Cartilage. 16:624–630. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Loeser RF: Aging and osteoarthritis: The
role of chondrocyte senescence and aging changes in the cartilage
matrix. Osteoarthritis Cartilage. 17:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Loboda A, Damulewicz M, Pyza E, Jozkowicz
A and Dulak J: Role of Nrf2/HO-1 system in development, oxidative
stress response and diseases: An evolutionarily conserved
mechanism. Cell Mol Life Sci. 73:3221–3247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Biswas C, Shah N, Muthu M, La P, Fernando
AP, Sengupta S, Yang G and Dennery PA: Nuclear heme oxygenase-1
(HO-1) modulates subcellular distribution and activation of Nrf2
impacting metabolic and anti-oxidant defenses. J Biol Chem.
289:26882–26894. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maicas N, Ferrándiz ML, Brines R, Ibáñez
L, Cuadrado A, Koenders MI, van den Berg WB and Alcaraz MJ:
Deficiency of Nrf2 accelerates the effector phase of arthritis and
aggravates joint disease. Antioxid Redox Signal. 15:889–901. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kobayashi H, Takeno M, Saito T, Takeda Y,
Kirino Y, Noyori K, Hayashi T, Ueda A and Ishigatsubo Y: Regulatory
role of heme oxygenase 1 in inflammation of rheumatoid arthritis.
Arthritis Rheum. 54:1132–1142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Wang D, Yuan Y and Min J: New
insights on the MMP-13 regulatory network in the pathogenesis of
early osteoarthritis. Arthritis Res Ther. 19:2482017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shanmugaapriya S, van Caam A, de Kroon L,
Vitters EL, Walgreen B, van Beuningen H, Davidson EB and van der
Kraan PM: Expression of TGF-β signaling regulator RBPMS
(RNA-binding protein with multiple splicing) is regulated by IL-1β
and TGF-β superfamily members, and decreased in aged and
osteoarthritic cartilage. Cartilage. 7:333–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abdullah M, Mahowald ML, Frizelle SP,
Dorman CW, Funkenbusch SC and Krug HE: The effect of
intra-articular vanilloid receptor agonists on pain behavior
measures in a murine model of acute monoarthritis. J Pain Res.
9:563–570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi JW, Lee J and Park YI:
7,8-Dihydroxyflavone attenuates TNF-α-induced skin aging in Hs68
human dermal fibroblast cells via down-regulation of the MAPKs/Akt
signaling pathways. Biomed Pharmacother. 95:1580–1587. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park HY, Kim GY, Hyun JW, Hwang HJ, Kim
ND, Kim BW and Choi YH: 7,8-Dihydroxyflavone exhibits
anti-inflammatory properties by downregulating the NF-κB and MAPK
signaling pathways in lipopolysaccharide-treated RAW264. 7 cells.
Int J Mol Med. 29:1146–1152. 2012.PubMed/NCBI
|
|
32
|
Park HY, Park C, Hwang HJ, Kim BW, Kim GY,
Kim CM, Kim ND and Choi YH: 7,8-Dihydroxyflavone attenuates the
release of pro-inflammatory mediators and cytokines in
lipopolysaccharide-stimulated BV2 microglial cells through the
suppression of the NF-κB and MAPK signaling pathways. Int J Mol
Med. 33:1027–1034. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takada T, Miyaki S, Ishitobi H, Hirai Y,
Nakasa T, Igarashi K, Lotz MK and Ochi M: Bach1 deficiency reduces
severity of osteoarthritis through upregulation of heme
oxygenase-1. Arthritis Res Ther. 17:2852015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rousset F, Nguyen MV, Grange L, Morel F
and Lardy B: Heme oxygenase-1 regulates matrix metalloproteinase
MMP-1 secretion and chondrocyte cell death via Nox4 NADPH oxidase
activity in chondrocytes. PLoS One. 8:e664782013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee TS and Chau LY: Heme oxygenase-1
mediates the anti-inflammatory effect of interleukin-10 in mice.
Nat Med. 8:240–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee IT, Luo SF, Lee CW, Wang SW, Lin CC,
Chang CC, Chen YL, Chau LY and Yang CM: Overexpression of HO-1
protects against TNF-alpha-mediated airway inflammation by
down-regulation of TNFR1-dependent oxidative stress. Am J Pathol.
175:519–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen J, Chua KW, Chua CC, Yu H, Pei A,
Chua BH, Hamdy RC, Xu X and Liu CF: Antioxidant activity of
7,8-dihydroxyflavone provides neuroprotection against
glutamate-induced toxicity. Neurosci Lett. 499:181–185. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han X, Zhu S, Wang B, Chen L, Li R, Yao W
and Qu Z: Antioxidant action of 7,8-dihydroxyflavone protects PC12
cells against 6-hydroxydopamine-induced cytotoxicity. Neurochem
Int. 64:18–23. 2014. View Article : Google Scholar : PubMed/NCBI
|