Introduction
Superficial bladder cancer is mainly treated by
transurethral resection of the bladder tumor (TUR-BT). Yet, the
intravesical recurrence rate of bladder cancer after TUR-BT is
reported to be 50–70% (1–3). Therefore, prophylaxis of the high
frequency of recurrence after TUR-BT is important. Bacillus
Calmette-Guérin (BCG), doxorubicin, mitomycin, epirubicin, thiotepa
and pirarubicin are currently used as intravesical instillation
chemotherapy agents to reduce the recurrence rate of bladder cancer
(4–13).
Pirarubicin (Fig.
1), an anthracycline drug which has been shown to have a high
affinity to bladder tumor cells or tissues (14–16),
is a drug used widely for intravesical instillation chemotherapy.
The concentration of pirarubicin in the tumor tissue becomes
markedly high in a relatively short time after administration into
the bladder (15). It is reported
that the intravesical instillation with pirarubicin produces an
antitumor effect against bladder cancer, and that the prophylactic
effect of recurrence in the bladder after intravesical instillation
chemotherapy with pirarubicin is higher than that after TUR-BT only
(17). Moreover, short-time
intravesical instillation chemotherapy is performed to obtain a
greater antitumor effect and to prevent an increase in side
effects, as this method has the advantage of a higher penetration
of pirarubicin into bladder tumor tissues (18).
Conditions for optimal injection of pirarubicin, a
concentration-dependent anticancer drug, has yet to be established.
The role involving the exposure time of the bladder to pirarubicin
in the prophylactic effect against recurrence of bladder cancer has
been extensively studied (17–19),
whereas the contribution of the concentration of pirarubicin in
bladder tumor tissue to the prophylactic effect against recurrence
of bladder cancer remains unclear.
As part of a program for the development of
guidelines for the safe use of pirarubicin and effective
intravesical chemotherapy with pirarubicin for the prevention of
recurrence of bladder cancer, the present study investigated the
relationship between the concentration of pirarubicin in bladder
tumor tissues and its exposure time in the bladder or therapeutic
effect after intravesical instillation chemotherapy with
pirarubicin.
Materials and methods
Patients
Characteristics of the patients enrolled in this
study are summarized in Table I.
Twenty-two patients with superficial bladder cancer, who were
hospitalized in the Department of Urology of Aichi Medical
University Hospital, Tokoname Municipal Hospital and Sakashita
Hospital, between August 2008 and March 2010, participated in this
study. The patients included 21 males and 1 female, aged 51–92
years (mean 70). All patients were diagnosed with transitional cell
carcinoma with papillary and initial and primary tumors with
superficial (Ta, T1) tumors and a non-invasive tumor. From
histopathological findings, the tumor grade was found to be G1 in 5
patients and G2 in 17 patients. The tumor stage was shown to be pT1
in 2 patients and pTa in 20 patients. Solitary and multiple tumors
were found in 17 and 5 patients, respectively. The study was
approved by the Institutional Review Board of Aichi Medical
University School of Medicine, and written informed consent was
obtained for all participants prior to enrollment in the study.
 | Table I.Characteristics of the patients
enrolled in this study. |
Table I.
Characteristics of the patients
enrolled in this study.
| Patients | |
| Males | 21 |
| Females | 1 |
| Age, in years | |
| Range | 51–92 |
| Mean | 70.0 |
| Tumors | |
| Solitary | 17 |
| Multiple (2) | 5 |
| Grade | |
| G1 | 5 |
| G2 | 17 |
| Stage | |
| pTa | 20 |
| pT1 | 2 |
Drugs
Pirarubicin injection (therarubicin, 10 mg/20 mg of
pirarubicin per injection; Meiji Seika, Tokyo, Japan) and
pirarubicin hydrochloride (Wako Chemicals, Tokyo, Japan) were used
in this study. Pirarubicin (30 mg) was dissolved in 30 ml of
distilled water for the injection (1 mg/ml). All other reagents
were commercially available, of analytical grade, and were used
without further purification.
Drug administration
Pirarubicin (30 mg) was administered transurethrally
for 8 min in 1 patient, 9 min in 1 patient, 10 min in 1 patient, 15
min in 3 patients, 20 min in 2 patients, 25 min in 1 patient, 30
min in 10 patients and 35 min in 1 patient (Table II). Bladder cancer tissue and blood
samples were collected at the designated intervals described above.
The removed bladder cancer tissues were washed immediately with
ice-cold saline five times. The washed tissue and serum samples
obtained from the patients were stored at −80°C until analysis.
 | Table II.Characteristics of the patients that
received single intravesical therapy with pirarubicin. |
Table II.
Characteristics of the patients that
received single intravesical therapy with pirarubicin.
| Patient | Gender | Age (years) | Grade | Stage | No. of tumors | Size (mm) | Retention time
(min) | Concentration (μg/g
tissue) | Recurrence | Follow-up
(months) |
|---|
| 1 | M | 66 | G2 | pTa | 1 | 50 | 8 | 10.6 | + | 3 |
| 2 | M | 85 | G2 | pT1 | 1 | 24 | 9 | 44.4 | − | 16 |
| 3 | M | 67 | G1 | pTa | 2 | 20 | 9 | 5.6 | − | 17 |
| 4 | M | 75 | G2 | pTa | 1 | 39 | 10 | 7.7 | x | x |
| 5 | M | 74 | G1 | pTa | 1 | 15 | 12 | 16.9 | − | 13 |
| 6 | M | 70 | G2 | pTa | 2 | 19 | 15 | 65.6 | x | x |
| 7 | M | 72 | G2 | pTa | 1 | 15 | 15 | 20.4 | − | 14 |
| 8 | M | 54 | G2 | pTa | 1 | 5 | 15 | 13.9 | − | 20 |
| 9 | M | 53 | G2 | pTa | 2 | 24 | 20 | 4.9 | − | 14 |
| 10 | F | 58 | G2 | pTa | 1 | 20 | 20 | 17.9 | x | x |
| 11 | M | 67 | G2 | pTa | 1 | 17 | 25 | 23.0 | − | 13 |
| 12 | M | 92 | G2 | pTa | 1 | 15 | 30 | 13.8 | − | 29 |
| 13 | M | 51 | G2 | pTa | 1 | 30 | 30 | 2.3 | − | 29 |
| 14 | M | 71 | G2 | pT1 | 2 | 34 | 30 | 47.7 | + | 5 |
| 15 | M | 80 | G2 | pTa | 1 | 15 | 30 | 4.7 | − | 29 |
| 16 | M | 87 | G2 | pTa | 1 | 23 | 30 | 125.0 | x | x |
| 17 | M | 73 | G1 | pTa | 1 | 10 | 30 | 79.6 | − | 26 |
| 18 | M | 61 | G1 | pTa | 1 | 15 | 30 | 11.9 | − | 14 |
| 19 | M | 65 | G2 | pTa | 1 | 13 | 30 | 26.7 | − | 26 |
| 20 | M | 81 | G2 | pTa | 2 | 8 | 30 | 16.3 | x | x |
| 21 | M | 71 | G1 | pTa | 1 | 5 | 30 | 125.0 | − | 20 |
| 22 | M | 67 | G2 | pTa | 1 | 6 | 35 | 45.9 | − | 10 |
Drug analysis
The pirarubicin concentrations in the plasma and
tissue were determined by high-performance liquid chromatography
(HPLC). The apparatus used for HPLC was a Shimadzu LC-10A system
(Kyoto, Japan) equipped with a fluorescence detector (RF-10AXL;
Shimadzu) (excitation, 273 nm; emission, 464 nm) consisting of an
LC-10A liquid pump and an SIL-10A autoinjector. The conditions were
as follows: column, a Cosmocil 5C18 column (4.6 by 150 mm; Nacalai
Tesque, Kyoto, Japan); mobile phase, 20 mM potassium dihydrogen
phosphate-acetonitrile [1:1 (vol/vol)] solution; column temperature
(CTO-10AC; Shimadzu), 50°C; flow rate, 1.0 ml/min.
Each bladder tissue sample (0.002–0.215 g) was
weighed and homogenized with ice-cold saline using a tight
homogenizer (20 strokes up and down) and adjusted to 0.5, 1 or 2 ml
by saline according to each tumor weight. Each sample (100 μl) of
serum and diluted tissue homogenates, 50 μl of 0.1 M
NaHCO3-saturated NaCl solution and 150 μl of
acetonitrile were mixed vigorously and centrifuged at 21.880 × g
for 10 min at 4°C. After centrifugation, the supernatant (50 μl)
was injected into the column.
The standard curves for this assay were shown to be
linear for the concentrations measured (500, 1,000, 2,000 and 4,000
ng/ml) with a correlation coefficient of 0.999. The within-day and
between-day coefficients of variation (CV) for this assay were
<8%. No interference with the peak of pirarubicin was observed
in any samples. The quantitative limit of this assay was 500
ng/ml.
Clinical evaluation
Adverse events, such as frequent urination, pain on
urination, hematuria and a feeling of residual urine were observed.
The frequency and severity of these events were also investigated.
The therapeutic effect was evaluated between the date of surgery
and January 2011 for the non-recurrence period. Cystoscopy and
urinary cytology in all patients were performed at 3-month
intervals during the first year.
Statistical analysis
Data are represented as observed values. Statistical
analysis was performed using Stat View (Abacus Concept, Barkeley,
CA, USA).
Results
Various clinical data for each patient receiving a
single intravesical therapy with pirarubicin are summarized in
Table II. Concentrations of
pirarubicin in bladder cancer tissues showed a wide range from 2.3
to 125 μg/g tissue, whereas no serum concentrations of pirarubicin
were observed in any of the patients.
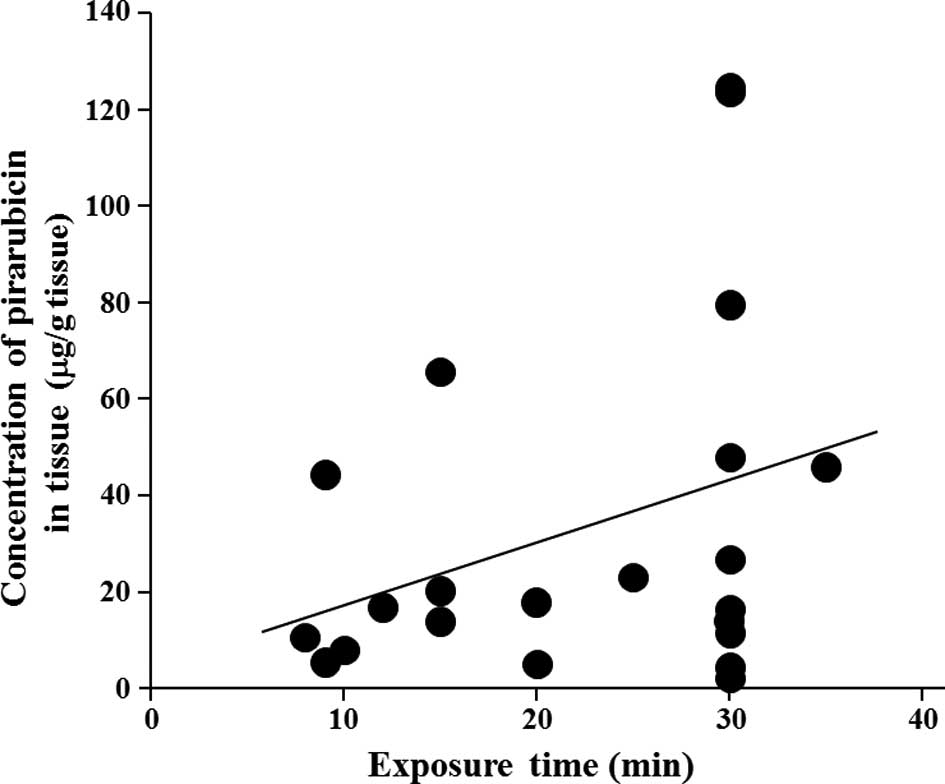
Weak relationships, not statistically significant,
were observed between pirarubicin tissue concentrations and
exposure time of pirarubicin in the bladder or tumor size. As shown
in Fig. 2A, the concentration of
pirarubicin in tumor tissues tended to be higher as the exposure
time was prolonged, whereas the concentration tended to be lower as
the tumor size increased (Fig.
2B). However, there was no relationship between pirarubicin
tissue concentrations and prophylactic effect against recurrence of
bladder cancer after TUR-BT.
No severe adverse events caused by treatment with
pirarubicin were noted in all patients.
Discussion
There is no detailed information regarding the
effect of the exposure time of pirarubicin in the bladder on tissue
concentrations in patients with superficial bladder cancer. In the
present study, to evaluate this issue, the concentrations of
pirarubicin were measured in the tumor tissues and serum at
different collection times after a single intravesical
administration of pirarubicin in 22 patients with superficial
bladder cancer.
In the present study, a weak relationship between
pirarubicin tissue concentrations and exposure time of pirarubicin
in the bladder or tumor size was observed. Unexpectedly, the
concentrations of pirarubicin in the tumor tissues had a tendency
to be lower as the tumor size increased, indicating that the uptake
of pirarubicin into tumor tissue decreases as the tumor size
increases. We postulated the possibility that concentrations of
pirarubicin in the bladder did not reach a steady state. The
present study found that in the 2 patients who presented with a
recurrence of bladder cancer the tumor size was larger than 30 mm.
These results corroborate those of Kanayama et al (20), who reported that a higher incidence
of the recurrence of bladder cancer was observed in patients having
5 or more tumors, or in patients with a tumor size larger than 30
mm. Therefore, tumor size should be considered when intravesical
chemotherapy with pirarubicin against superficial bladder cancer is
conducted. Assuming that pirarubicin is distributed into tumor
tissues by a passive diffusion system, we should consider the
presence of an efflux pump in cases of tissues of small tumors
exhibiting lower concentrations of pirarubicin. The susceptibility
of pirarubicin to tumor tissues of each patient should also be
considered.
There are several reports regarding the involvement
of certain efflux drug transporters in bladder cancer (21–25).
It is well known that one of the drug transporters, ABCB1/
P-glycoprotein, an ATP-binding cassette transporter protein, acts
as an efflux pump for various drugs, such as Vinca alkaloid
and anthracycline anticancer drugs. This transporter is expressed
in anticancer drug-resistant tumor cells. In the present study,
relatively low concentrations of pirarubicin in bladder tumor
tissues were observed in several patients who were administered
well-known P-glycoprotein substrates, such as atorvastatin,
fexofenadine, diltiazem and itraconazole. Considering that
pirarubicin is a typical P-glycoprotein substrate, the observed low
concentrations of pirarubicin in bladder tissues may be explained
by efflux of pirarubicin from tumor tissues overexpressing
P-glycoprotein or by efflux of pirarubicin by competitive
inhibition of P-glycoprotein substrates. These observations suggest
the need to measure the expression levels of P-glycoprotein in the
tumor tissues of each patient before initiating intravesical
chemotherapy with pirarubicin.
The present findings are, at least in part,
supported by the results of Maruyama et al (26), who reported that the growth of T-24
cells in vitro was inhibited by the presence of pirarubicin
in a dose- and time-dependent manner. The present results
corroborate those of Yamamoto et al (27), who reported that pirarubicin was
not detected in the plasma after single intravesical administration
of pirarubicin, and by a report by Okamura et al (17), who reported that there were no
severe local toxicities after a single dose of pirarubicin for 60
min.
In conclusion, the results obtained from the present
study suggest that the concentration of pirarubicin in bladder
cancer tissue appears to be dependent on the exposure time of
pirarubicin in the bladder after single intravesical
administration. Considering that intravesical administration of
anticancer drugs is useful for the therapy of superficial bladder
cancer with considerably high recurrence, further studies are
required to investigate the optimal dosage regimen for intravesical
administration of pirarubicin using a large number of patients with
superficial bladder cancer.
References
|
1.
|
Lutzeyer W, Rubben H and Dahm H:
Prognostic parameters in superficial bladder cancer: an analysis of
315 cases. J Urol. 127:250–252. 1982.PubMed/NCBI
|
|
2.
|
Herr HW, Laudone VP and Whitmore WF: An
overview of intravesical therapy for superficial bladder tumors. J
Urol. 138:1363–1368. 1987.PubMed/NCBI
|
|
3.
|
Miki T, Nonomura N, Kojima Y, et al: A
randomized study on intravesical pirarubicin (THP) chemoprophylaxis
of recurrence after transurethral resection of superficial bladder
cancer. Hinyokika Kiyo. 43:907–912. 1997.(In Japanese).
|
|
4.
|
Sylvester RJ, van der MA and Lamm DL:
Intravesical bacillus Calmette-Guerin reduces the risk of
progression in patients with superficial bladder cancer: a
meta-analysis of the published results of randomized clinical
trials. J Urol. 168:1964–1970. 2002. View Article : Google Scholar
|
|
5.
|
Lamm DL, Blumenstein BA, Crawford ED, et
al: A randomized trial of intravesical doxorubicin and
immunotherapy with bacille Calmette-Guérin for transitional-cell
carcinoma of the bladder. N Engl J Med. 325:1205–1209.
1991.PubMed/NCBI
|
|
6.
|
Witjes JA, Meijden AP, Sylvester LC,
Debruyne FM, van Aubel A and Withes WP: Long-term follow-up of an
EORTC randomized prospective trial comparing intravesical bacilli
Calmette-Guerin-RIVM and mitomycin C in superficial bladder cancer.
EORTC GU Group and the Dutch South East Cooperative Urological
Group European Organisation for Research and Treatment of Cancer
Genito-Urinary Tract Cancer Collaborative Group. Urology.
52:403–410. 1998.
|
|
7.
|
Soloway MS, Sofer M and Vaidya A:
Contemporary management of stage T1 transitional cell carcinoma of
the bladder. J Urol. 167:1573–1583. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Oosterlinck W, Kurth KH, Schröder F,
Bultinck J, Hammond B and Sylvester R; Members of the European
Organization for Research and Treatment of Cancer Genitourinary
Group: A prospective European organization for research and
treatment of cancer genitourinary group randomized trial comparing
transurethral resection followed by a single intravesical
instillation of epirubicin or water in single stage Ta, T1
papillary carcinoma of the bladder. J Urol. 149:749–752. 1993.
|
|
9.
|
Tolley DA, Parmar MK, Grigor KM, et al:
The effect of intravesical mitomycin C on recurrence of newly
diagnosed superficial bladder cancer: a further report with 7 years
of follow-up. J Urol. 155:1233–1238. 1996.PubMed/NCBI
|
|
10.
|
Rajala P, Liukkonen T, Raitanen M, Rintala
E, Kaasinen E, Helle M and Lukkarinen O: Transurethral resection
with perioperative instillation of interferon-α or epirubicin for
the prophylaxis of recurrent primary superficial bladder cancer: a
prospective randomized multicenter study-Finnbladder III. J Urol.
161:1133–1136. 1999.
|
|
11.
|
Solsona E, Iborra I, Ricós JV, Monrós L,
Casanova J and Dumont R: Effectiveness of a single immediate
mitomycin C instillation in patients with low risk superficial
bladder cancer: short and long-term followup. J Urol.
161:1120–1123. 1999. View Article : Google Scholar
|
|
12.
|
Zincke H, Utz DC, Taylor WF, Myers RP and
Leary FJ: Influence of thiotepa and doxorubicin instillation at
time of transurethral surgical treatment of bladder cancer on tumor
recurrence: a prospective, randomized double-blind, controlled
trial. J Urol. 129:505–509. 1983.
|
|
13.
|
Prout GR, Koontz WW, Coomms LJ, Hawkins IR
and Friedell GH: Long-term fate of 90 patients with superficial
bladder cancer randomly assigned to receive or not to receive
thiotepa. J Urol. 130:677–680. 1983.PubMed/NCBI
|
|
14.
|
Fukushima T, Ueda T and Nakamura T:
Pharmacokinetics and action mechanism of anthracyclines. Gan To
Kagaku Ryoho. 19:445–450. 1992.(In Japanese).
|
|
15.
|
Akaza H, Niijima T, Hisamatsu T and
Fujigaki M: Comparative investigation on use of
(2′′R)-4′-O-tetrahydropyranyl-adriamycin and adriamycin as
intravesical chemotherapy for superficial bladder tumors. Urology.
32:141–145. 1988.
|
|
16.
|
Kunimoto S, Miura K, Takahashi Y, Takeuchi
T and Umezawa H: Rapid uptake by cultured tumor cells and
intracellular behavior of 4-O-tetrahydropyranyladriamycin. J
Antibiot. 36:3123–3117. 1983.(In Japanese).
|
|
17.
|
Okamura K, Ono Y, Kinukawa T, et al:
Nagoya University Urological Oncology Group. Randomized study of
single early instillation of
(2′′R)-4-O-tetrahydropyranyl-doxorubicin for a single superficial
bladder carcinoma. Cancer. 94:2363–2368. 2002.PubMed/NCBI
|
|
18.
|
Nakagawa S, Kojima M, Takeda H, Sugimoto
K, Mikami K and Watanabe H: Short-duration bladder instillation
therapy with pirarubicin for superficial bladder tumor based on
pharmacodynamic study. Gan To Kagaku Ryoho. 19:1837–1877. 1992.(In
Japanese).
|
|
19.
|
Sugano O, Hatakeyama T and Kato H:
Investigation of retention time of intravesical instillation
therapy with pirarubicin (THP). Gan To Kagaku Ryoho. 23:1169–1174.
1996.(In Japanese).
|
|
20.
|
Kanayama H, Yokota K, Kurokawa Y, et al:
Postoperative prophylactic intravesical instillation of
tetrahydropyranyl-adriamycin (THP) for superficial bladder cancer.
Gan To Kagaku Ryoho. 26:651–655. 1999.(In Japanese).
|
|
21.
|
Kuwano M, Toh S, Uchiumi T, Takano H,
Kohno K and Wada M: Multidrug resistance-associated protein
subfamily transporters and drug resistance. Anticancer Drug Des.
14:123–131. 1999.PubMed/NCBI
|
|
22.
|
Hasegawa S, Abe T, Naito S, et al:
Expression of multidrug resistance-associated protein (MRP), MDR1
and DNA topoisomerase II in human multidrug-resistant bladder
cancer cell lines. Br J Cancer. 71:907–913. 1995. View Article : Google Scholar
|
|
23.
|
Kimiya K, Naito S, Soejima T, Sakamoto N,
Kotoh S, Kumazawa J and Tsuruo T: Establishment and
characterization of doxorubicin-resistant human bladder cancer cell
line, KK47/ ADM. J Urol. 148:441–445. 1992.PubMed/NCBI
|
|
24.
|
Naito S, Kotoh S, Omoto T, et al: The
Kyushu University Urological Oncology Group: Prophylactic
intravesical instillation chemotherapy against recurrence after a
transurethral resection of superficial bladder cancer: a randomized
controlled trial of doxorubicin plus verapamil versus doxorubicin
alone. Cancer Chemother Pharmacol. 42:367–372. 1998.
|
|
25.
|
Tada Y, Wada M, Migita T, et al: Inceased
expression of multidrug resistance-associated proteins in bladder
cancer drug clinical course and drug resistance to doxorubicin. Int
J Cancer. 98:630–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Maruyama T, Higuchi Y, Suzuki T, Qiu J,
Yamamoto S and Shima H: Double short-time exposure to pirarubicin
produces higher cytotoxicity against T24 bladder cancer cells. J
Infect Chemother. 17:11–16. 2010. View Article : Google Scholar
|
|
27.
|
Yamamoto Y, Nasu Y, Saika T, Aaeda T,
Tsushima T and Kumon H: The absorption of pirarubicin instilled
intravesicaly immediately after transurethral resection of
superficial bladder cancer. BJU Int. 86:802–804. 2000. View Article : Google Scholar : PubMed/NCBI
|