Introduction
The liver plays a central role in whole-body lipid
metabolism by governing the synthesis, oxidization, transport and
excretion of lipids. Unfolded protein response (UPR) was identified
as a signal transduction system that is activated by endoplasmic
reticulum (ER) stress (1). ER
stress and activation of UPR have been linked to numerous human
disorders, including obesity, type 2 diabetes and cancer (2). In addition, Rutkowski et al
showed that unresolved ER stress contributes to metabolic
dysfunction and hepatic steatosis (3).
UPR is a signaling system emanating from ER that is
activated when ER protein folding is disturbed (4). Previous studies have revealed novel
diverse functions of mammalian UPR, including its role in hepatic
lipid metabolism (3). UPR
activation has been observed in fatty liver diseases, suggesting
the induction of ER stress in these conditions (5). Previous studies have demonstrated
that ER stress activates the sterol regulatory element-binding
proteins (SREBPs), transcription factors involved in de novo
lipid biosynthesis (6). SREBPs
play a significant role in cholesterol metabolism and LDL receptor
expression (SREBP-2), as well as fatty acid and triglyceride
biosynthesis (SREBP-1) (7). We
have previously reported that transgenic mice expressing nuclear
SREBP-1c (nSREBP-1c) in adipose tissue under the control of the aP2
promoter, an inherited lipodystrophic model with insulin resistance
and fatty acids, spontaneously develop steatohepatitis (8).
Adiponectin is a hormone mainly produced by adipose
tissue (9). Experimental studies
have suggested that adiponectin plays a major role in the
pathophysiology of insulin resistance and metabolic lipid storage
and lipolysis in insulin-sensitive tissues, which may induce an
increased flux of free fatty acids from adipose tissue to the liver
and cause steatosis (10).
Adiponectin plays a major role in the pathophysiology of insulin
resistance and metabolic syndrome.
We have previously reported that the
nSREBP-1c/adiponectin double-transgenic mice show hepatic
adiponectin transgenically expressed in the liver, and nonalcoholic
steatohepatitis (NASH)-like hepatic lesions were markedly
attenuated in age-matched double-transgenic mice (11). In addition, Awazawa et al
showed that adiponectin suppresses hepatic SREBP1c expression in an
adiponectin receptor (AdipoR)1/LKB1/AMPK-dependent pathway
(12). In this study, we examined
whether adiponectin suppresses ER stress in NASH.
Materials and methods
Preparation of nSREBP-1c transgenic mice
and nSREBP-1c/adiponectin double-transgenic mice
Transgenic mice (C57BL/6 background) expressing
nSREBP-1c in adipose tissue (13)
were purchased from Jackson Laboratory (Bar Harbor, ME, USA), and
bred in our laboratory by crossing with wild-type C57BL/6 mice
(Nippon Clea, Shizuoka, Japan). Generation of transgenic mice
expressing full-length human adiponectin in the liver of C57BL/6
background mice was previously described (14). Female mice that expressed nSREBP-1c
in adipose tissue were crossed with adiponectin-expressing male
mice to produce a nSREBP-1c/adiponectin double-transgenic line. The
double-transgenic mice were identified by polymerase chain reaction
(PCR) of tail DNA using nSREBP-1c-specific primers (5′-CTA
CATTCGCTTTCTGCAAC-3′ and 5′-ATAGAAGGACACC TAGTCAG-3′) and human
adiponectin transgenic specific primers
(5′-TGAATTCGGGCTCAGGATGCTGTTGCT-3′ and
5′-AGGATCCTGATCAGTTGGTGTCATGGTA-3′). Male mice heterozygous for
nSREBP-1c and human adiponectin were used in the following
experiments. All mice were fed standard mouse chow (347 kcal/100g,
protein 24.9 g/100 g, fat 4.6 g/100 g; Nippon Clea) and water ad
libitum. Body weight was measured prior to sacrifice. All
procedures were approved by the Ethics Review Committee for Animal
Experimentation of Kurume University School of Medicine.
Biochemical assays
Aspartate aminotransferase (AST), alanine
aminotransferase (ALT), triglyceride and total cholesterol levels
were determined by spectrophotometric enzyme assays using
peroxidase, lipoprotein lipase and cholesterol oxidase,
respectively (Wako Ltd., Osaka, Japan). Glucose tolerance was
assessed using an intraperitoneal glucose tolerance test (IPGTT).
The IPGTT was performed by injecting glucose (1 g/kg in 10%
solution) intraperitoneally in overnight-fasted mice. Glucose
levels in blood obtained from the tail veins were measured by
glucose dehydrogenase methods using Free Style (Nipro, Osaka,
Japan) at 0, 30, 60 and 120 min following glucose injection. Serum
levels of mouse leptin and adiponectin were measured with
enzyme-linked immunosorbent assay kits from R&D (Oxon, United
Kingdom) and AdipoGen (Seoul, Korea), respectively.
Light microscopy
Paraffin-embedded sections of the liver were stained
with hematoxylin and eosin for standard microscopy or the
Azan-Mallory stain to observe the location of the extracellular
matrix in the liver tissues. The specimens were reviewed by a
hepatopathologist. Each specimen was assigned to one of the
following histological subgroups for the purposes of comparative
analysis: type 1, fatty liver alone, which was predominantly
macrovesicular in more than 33% of the lobules; type 2, fat
accumulation and lobular inflammation; type 3, fat accumulation and
ballooning hepatocytes; type 4, fat accumulation, ballooning
hepatocytes, and either Mallory’s hyaline or fibrosis. We dealt
with types 3 and 4 as NASH, as previously described by Matteoni
et al (5).
Electron microscopy
Passaged and cryopreserved HSCs were fixed in 1%
glutaraldehyde for 1 h at 4°C, and post-fixed in 1% osmic acid for
1 h at 4°C, dehydrated with ethanol and embedded. They were then
sectioned and stained with lead citrate. The samples were then
observed under an electron microscope (H-7650, Hitachi, Tokyo,
Japan).
Real-time PCR analysis
For real-time quantitative PCR, the messenger
ribonucleic acid (mRNA) levels of mouse adiponectin and AdipoR1 and
2, and human adiponectin were assayed using the 7000 sequence
detection system ABI Prism sequence detector (Applied Biosystems,
Tokyo, Japan), and the double-strand specific dye SYBR-Green
(Applied Biosystems). The PCR conditions and cycles were as
follows: initial DNA denaturations for 10 min at 95°C, followed by
40 cycles of denaturation at 95°C for 15 sec, followed by an
annealing step and then extension at 60°C for 1 min. Each point was
performed in triplicate. To ensure that the primers produced a
single and specific PCR amplification product, a dissociation curve
was generated during the PCR cycle and only primers with a unique
dissociation peak were selected, followed by migration on a 2%
agarose gel to ensure that the PCR product was unique. The
amplification efficiency for each primer pair was calculated. The
expression level of each gene was adjusted by the level of GAPDH
and expressed as the ratio to GAPDH.
Western blot analysis
To determine whether there was any synergy between
human and mouse adiponectins, TNF receptor 1 (TNFR1), pNFκB,
acetyl-CoA carboxylase (pACC) and ER stress-related agents, such as
X-box-binding protein-1 (XBP-1) and activating transcription
factor-4 (ATF-4) and ATF-6, we performed Western blot analysis of
whole cell protein extracts. Cells were lysed in RIPA buffer. Equal
amounts of protein were separated on 10% SDS-polyacrylamide gel,
and then blotted on a PVDF membrane. The membrane was
immunoblotted. Immunoreactive bands were visualized using ECL
detection reagents. The cells were incubated in a RIPA lysis buffer
for 20 min. Following determination of protein concentrations using
a BCA protein assay kit (Pierce, Rockford, IL, USA), the samples
were separated by 10% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis, and electroblotted onto nitrocellulose membranes.
First they were reacted with primary antibodies and then with
peroxidase-conjugated secondary antibody (dilution 1:1000) (GE
Healthcare, Buckinghamshire, UK). Antigens were visualized by
enhanced chemiluminescence using the ECL Western blotting detection
system (Amersham, San Francisco, CA, USA).
Statistical analysis
Numerical data were expressed as the means ± SD.
Unpaired Student’s t-test was performed to assess statistical
significance between groups. A value of P<0.05 was considered to
be statistically significant.
Results
Biochemical assays
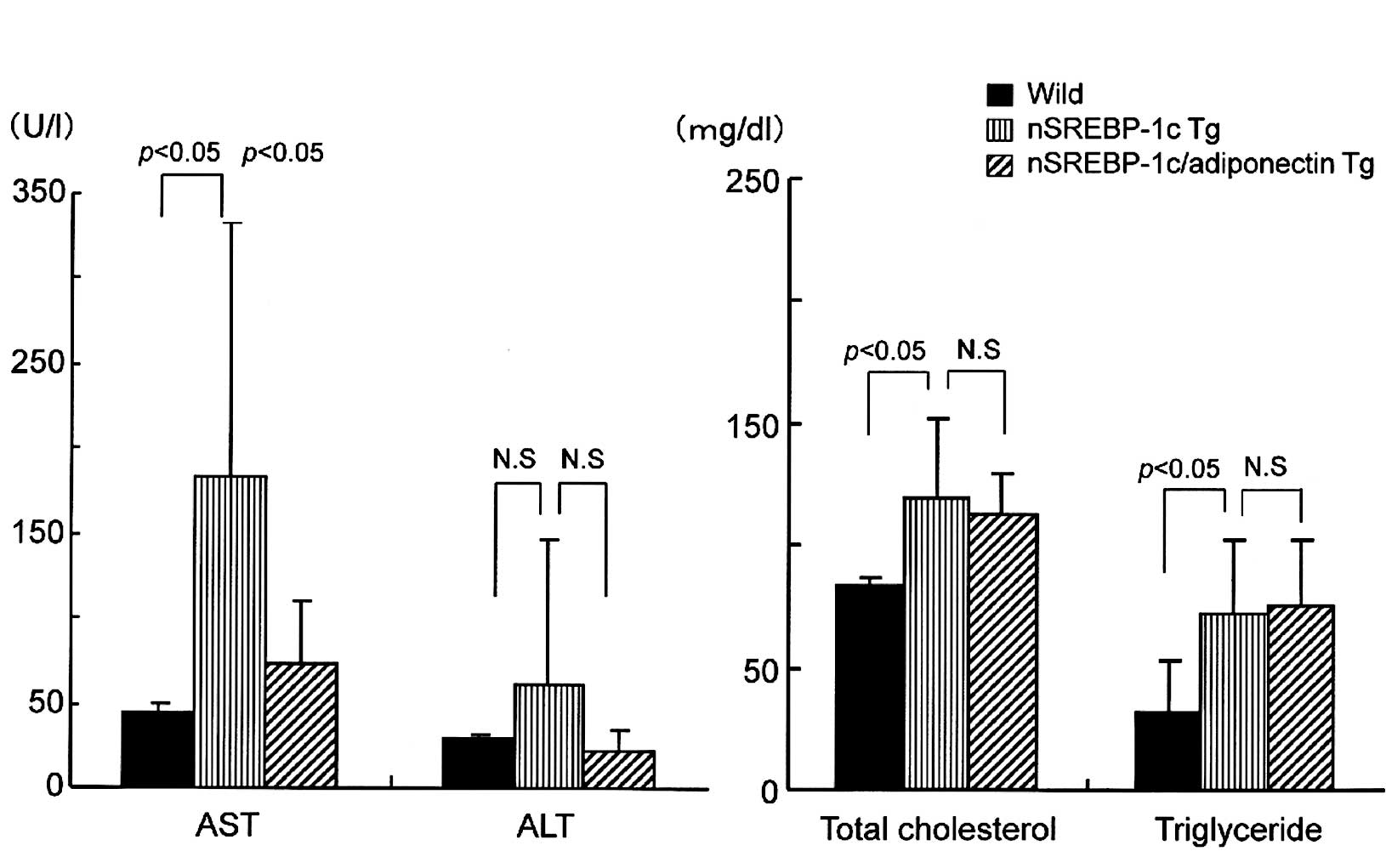
At 20 weeks of age, serum AST and ALT levels were
elevated in the nSREBP-1c transgenic mice compared with the
wild-type and nSREBP-1c/adiponectin transgenic mice. AST levels
were highest in the nSREBP-1c transgenic mice, and were
significantly reduced in the nSREBP-1c/adiponectin transgenic mice.
ALT elevation was attenuated to normal levels in the
nSREBP-1c/adiponectin transgenic mice, although the difference was
not statistically significant (Fig.
1A). No significant difference was observed in total
cholesterol or triglyceride levels between the nSREBP-1c transgenic
mice and the nSREBP-1c/adiponectin transgenic mice (Fig. 1B).
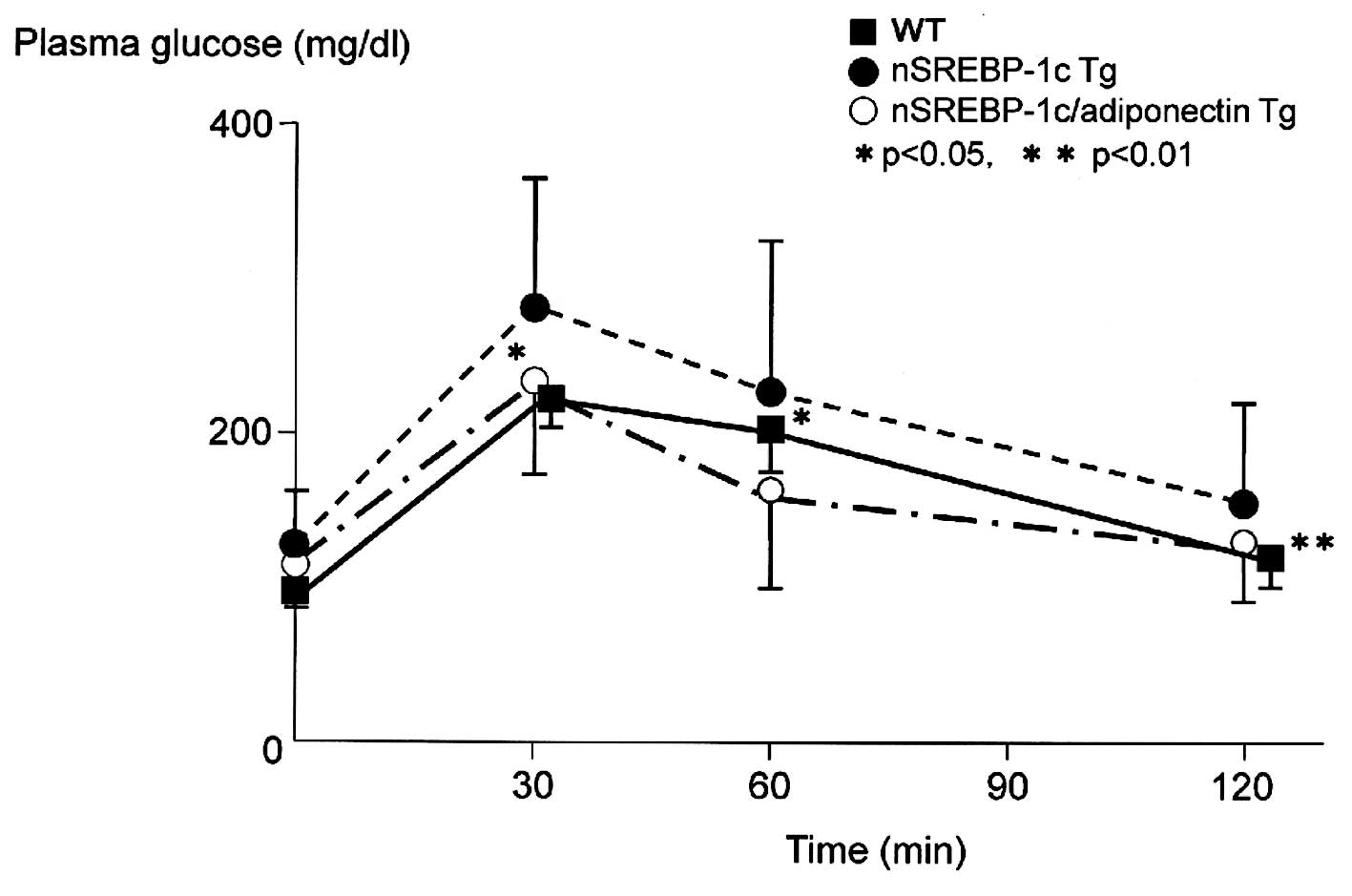
The intraperitoneal glucose (1 g/kg in 10% solution)
tolerance test performed at 20 weeks of age showed significantly
lower plasma glucose levels in the nSREBP-1c/adiponectin transgenic
mice than in the nSREBP-1c transgenic mice at 30 and 60 min after
glucose load (Fig. 2).
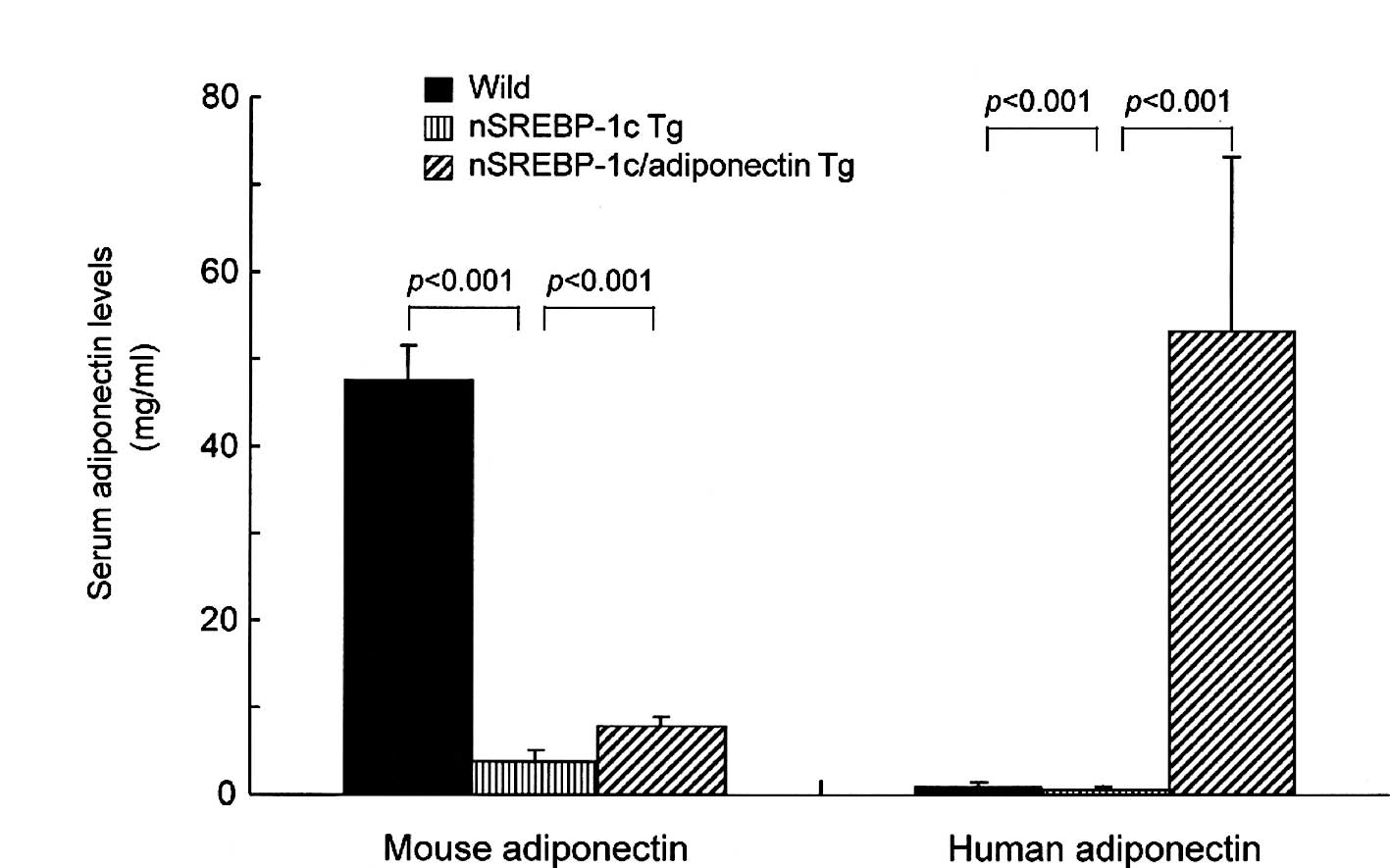
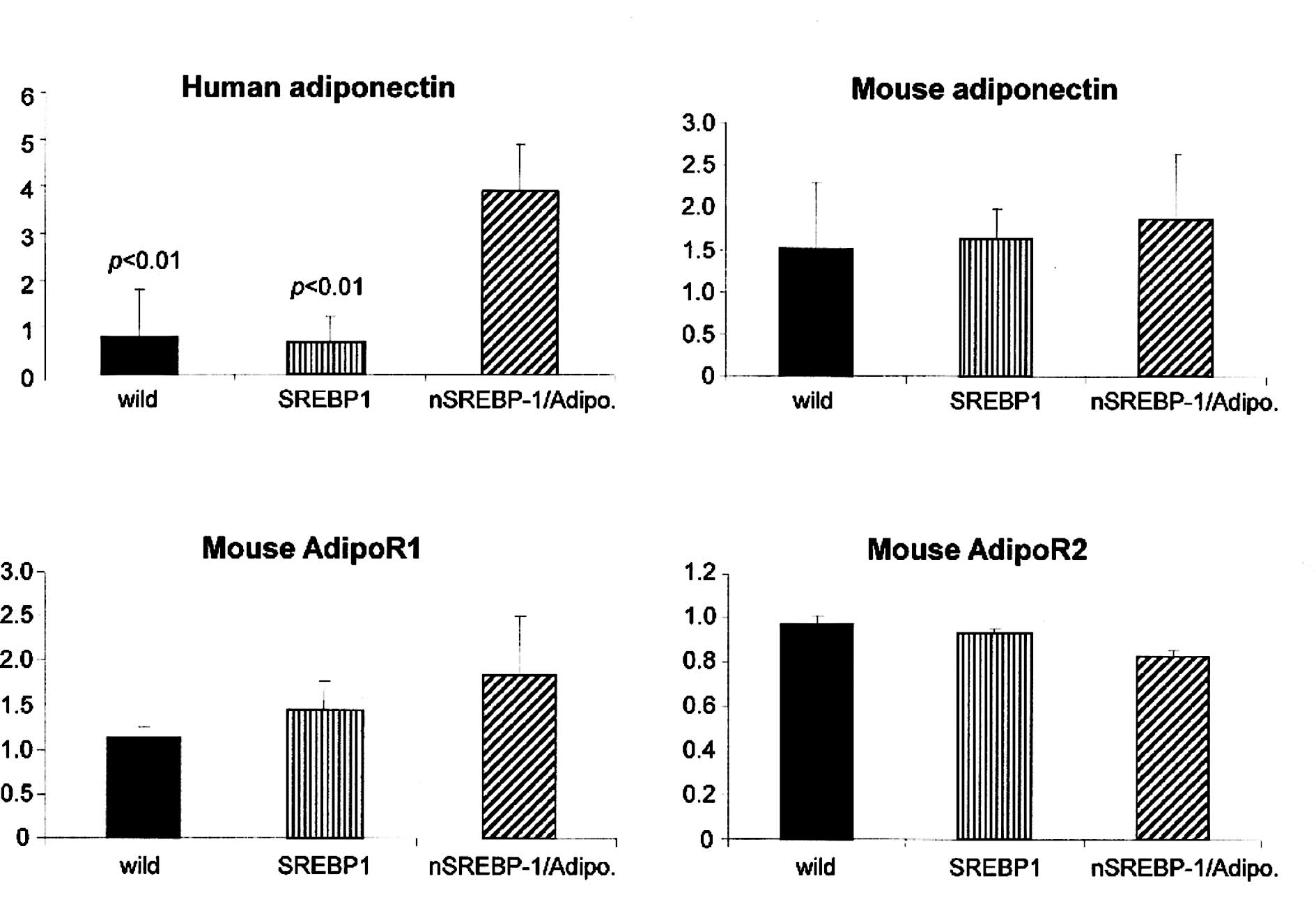
Circulating endogenous mouse adiponectin levels were
slightly but significantly higher in the nSREBP‐1c/adiponectin
transgenic mice than in the nSREBP-1c transgenic mice (Fig. 3A). Human adiponectin levels in the
nSREBP-1c/adiponectin transgenic mice at 20 weeks of age were
significantly higher than in the other two groups (Fig. 3B).
Light microscopy
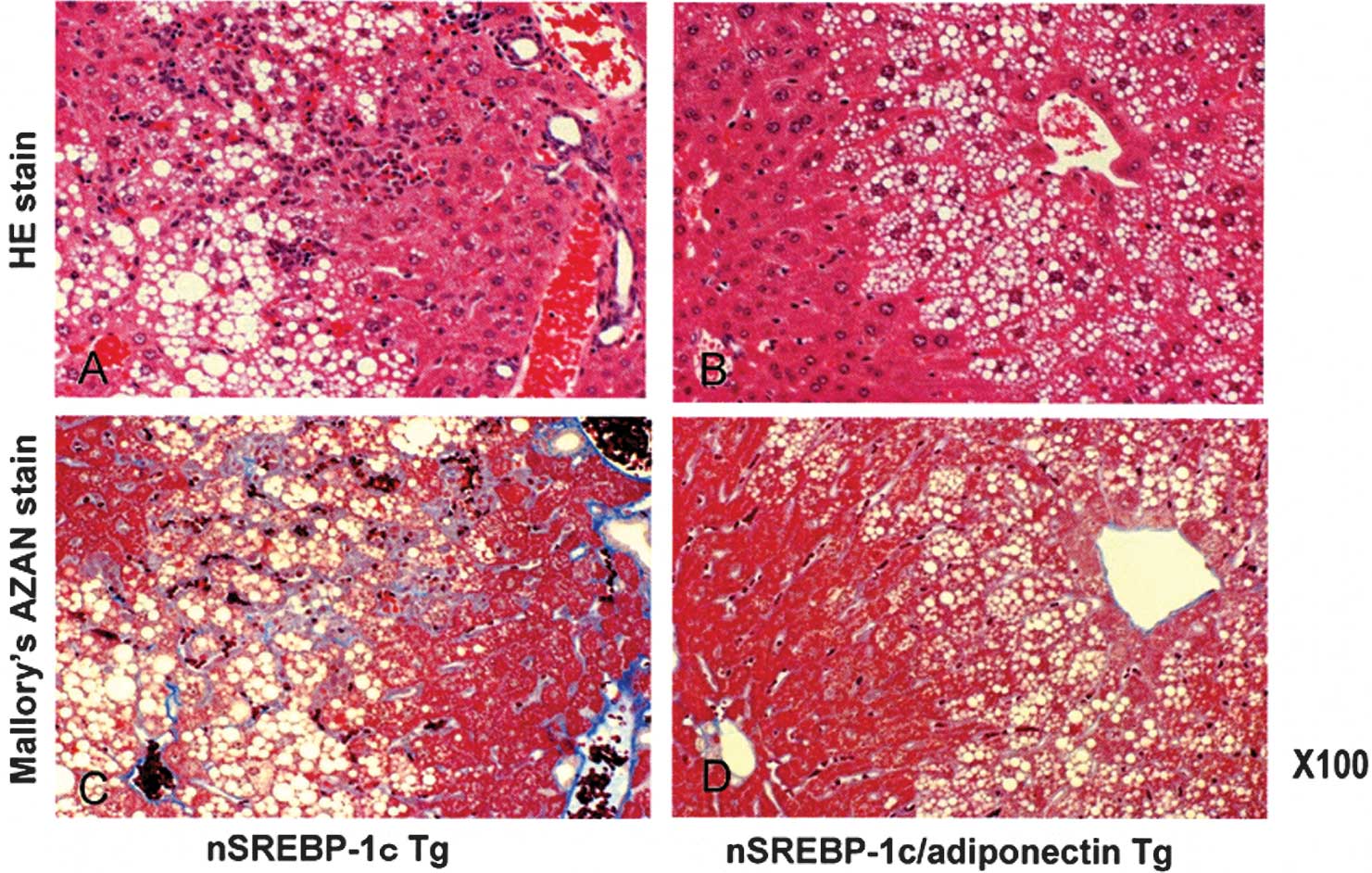
For light microscopy, sections were stained with
hematoxylin and eosin staining or Mallory’s Azan. Fat droplets,
cell infiltration and pericellular fibrosis were decreased in the
nSREBP-1c/adiponectin transgenic mice (Fig. 4A and B) compared with the nSREBP-1c
transgenic mice (Fig. 4C and
D).
Electron microscopy
The livers of SREBP-1c transgenic mice contain
hepatocytes with degenerated nuclei, and small and large fat
droplets. Enlarged ERs are visible in the magnified image (Fig. 5A). However, hepatocytes in the
livers of the nSREBP-1c and adiponectin double-transgenic mice
contain a few small fat droplets in the cytoplasm. However, the
nucleus and organelles, such as mitochondria and ER, are similar to
those of normal hepatocytes. These findings show that the ER stress
was decreased in the double-transgenic mice (Fig. 5B).
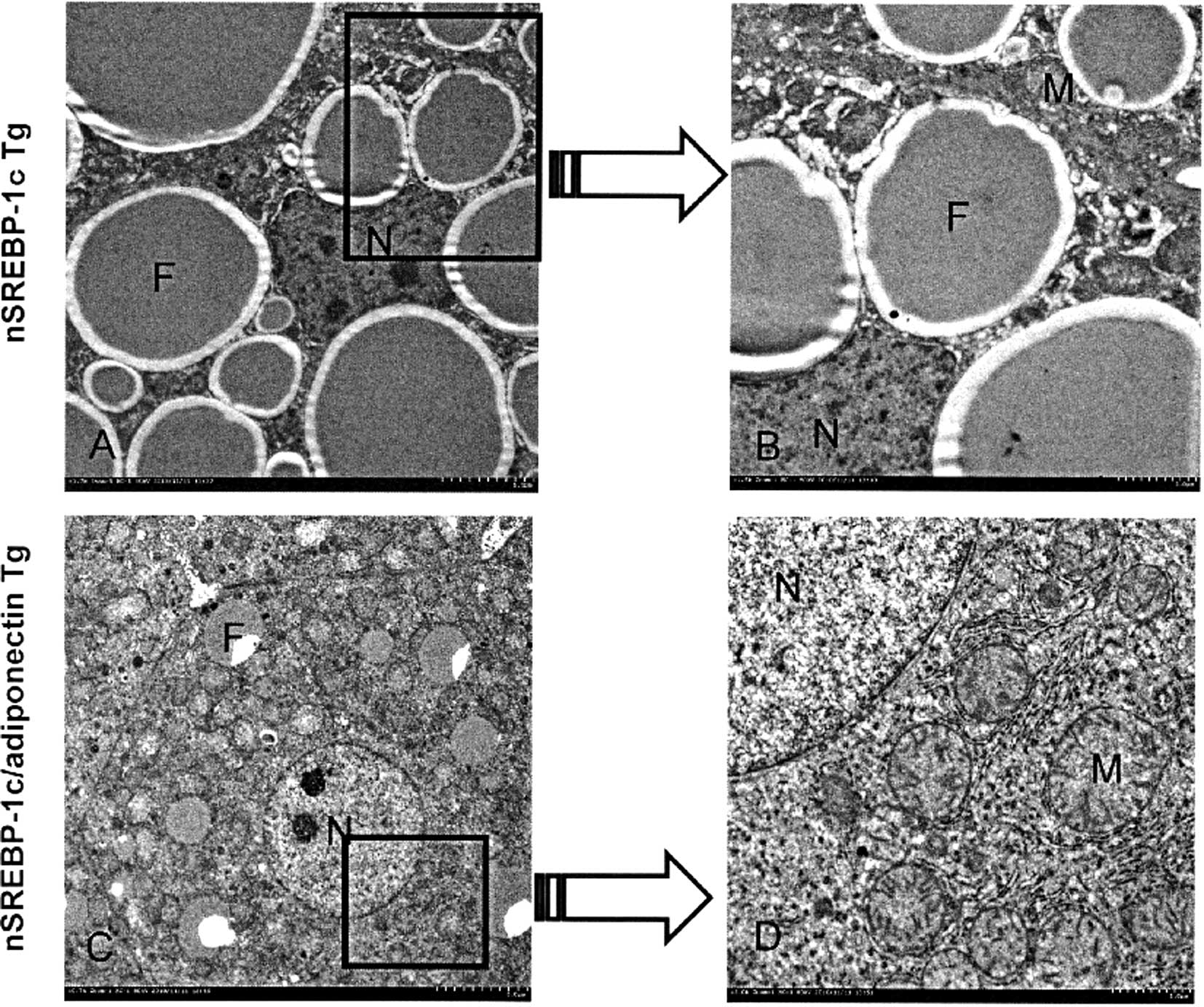 | Figure 5.Electron microscopy findings. (A and
B) In the liver of the SREBP-1c transgenic mice, hepatocytes with
degenerated nuclei, small and large fat droplets, and, in the
magnified image, enlarged endoplasmic reticula are visible. (C and
D) However, in the liver of the nSREBP-1c and adiponectin
double-transgenic mouse, only a few small fat droplets are located
in the cytoplasm of the hepatocytes. However, the nuclei and
organelles, such as mitochondria and ER, are similar to those of
normal hepatocytes. |
Real-time PCR analysis
Real-time PCR analysis showed that human adiponectin
expression was significantly increased in the liver of
nSREBP-1c/adiponectin transgenic mice compared with the wild-type
and nSREBP-1c transgenic mice (Fig.
6A). However, there was no significant difference in the mouse
adiponectin mRNA expression between the wild-type, nSREBP-1c
transgenic mice and nSREBP-1c/adiponectin transgenic mice (Fig. 6B).
We observed no significant difference between the
nSREBP-1c transgenic mice and nSREBP-1c/adiponectin transgenic mice
in mouse AdipoR1 and 2 expression levels (Fig. 6C and D).
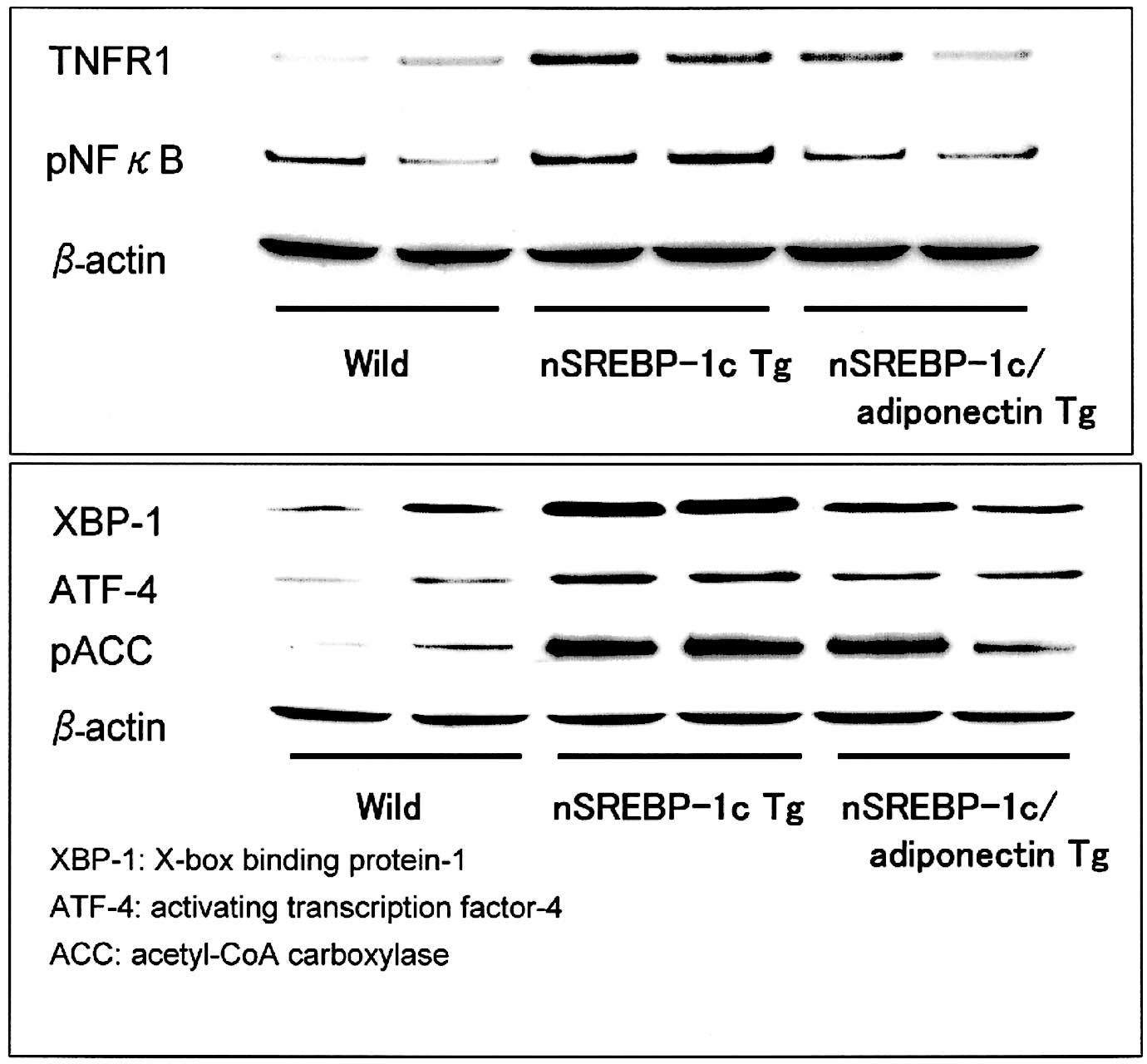
Western blot analysis
In Western blot analysis, TNFR1 and phosphorylated
NFκB were decreased in the liver of the nSREBP-1c/adiponectin
transgenic mice compared with the nSREBP-1c transgenic mice. It can
be assumed that adiponectin suppresses the inflammation through the
TNFR1 and NFκB pathway in the liver of the nSREBP-1c/adiponectin
transgenic mice (Fig. 7A).
In addition, the protein expressions of XBP-1, ATF-4
and phosphorylated ACC were decreased in the liver of the
nSREBP-1c/adiponectin transgenic mice compared with nSREBP-1c
transgenic mice. However, ATF-6 protein expression in the three
groups was almost the same (Fig.
7B).
Discussion
Our study shows that ER stress is enhanced in the
liver of nSREBP-1c transgenic NASH model mice. Our findings also
indicate that adiponectin suppresses ER stress through the XBP-1,
ATF-4 and ACC pathway in the liver of the nSREBP-1c/adiponectin
transgenic mice. Adiponectin is known to suppress ER stress in
obesity and diabetes (16,17). Recent studies have linked ER stress
to obesity, insulin action and type 2 diabetes (18,19),
and the activation of the UPR is governed by three ER transmembrane
proteins: inositol-requiring kinase-1 (IRE1), protein kinase-like
ER kinase (PERK) and activating transcription factor-6 (ATF6). Upon
ER stress, IRE1 endonuclease facilitates the splicing of XBP-1
mRNA, leading to the translation of an activated version of the
transcription factor XBP-1. XBP-1’s binding of one of the PI3K
regulatory subunits, p85α or p85β, enhances nuclear entry of this
transcription factor and induction of UPR target genes (20,21).
The p85 subunit does not directly modulate other arms of the UPR,
including the association of IRE1 with TRAF2 and the subsequent
activation of JNK and PERK phosphorylation of eIF2 (p-eIF2), which
represses protein synthesis coincident with preferential
translation of the UPR transcriptional activator ATF4. However, the
ATF6 arm of the UPR, which is involved in proteolytic cleavage and
release of the transcription factor into the nucleus, may be
activated through association with p85 (22).
Although hypoadiponectinemia has been implicated in
the development of human nonalcoholic fatty liver disease (NAFLD)
and NASH (23), there was no
direct evidence that adiponectin has preventive effects on
spontaneously occurring NASH. In this study, the double-transgenic
mice producing adiponectin in the liver showed normal or slightly
elevated serum levels of adiponectin. The double-transgenic mice
normally expressed the two subtypes of AdipoRs, that is, AdipoR1
and 2, in the liver. Adiponectin expressed in the liver stimulates
adenosine monophosphate-activated protein kinase activation and
PPARα signaling pathways through AdipoR1 and 2, respectively.
Adiponectin may also exert a protective effect
through its anti-inflammatory action (9). Adiponectin is an adipocytokine
secreted by mature adipocytes, and its receptors are widely
distributed in a number of tissues, including the liver.
Adiponectin has direct actions in the liver with prominent roles in
improving hepatic insulin sensitivity, increasing fatty acid
oxidation and decreasing inflammation. Adiponectin-null mice
exhibit impaired liver regeneration and increased hepatic steatosis
(24). Yoshiuchi et al
directly evaluated the effects of the diabetic agent pioglitazone
on in vivo ER stress under diabetic conditions. Pioglitazone
treatment reduced the accumulation of fat droplets in the liver and
attenuated the development of insulin resistance, that is,
pioglitazone suppresses ER stress in the liver (25). Consequently, adiponectin may
directly improve the pathogenesis of NAFLD, including NASH.
In conclusion, the transgenic mouse expressing
nSREBP-1c in adipose tissue may serve as a unique model of NASH.
The transgene of adiponectin in the liver of the nSREBP-1c
transgenic mice induced the improvement of insulin resistance, ER
stress, and intralobular inflammation and fibrosis. These
observations may lead to the development of novel therapies for
NASH in humans.
References
|
1.
|
Lee AH and Glimcher LH: Intersection of
the unfolded protein response and hepatic lipid metabolism. Cell
Mol Life Sci. 66:2835–2850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Basseri S and Austin RC: ER stress and
lipogenesis: a slippery slope toward hepatic steatosis. Dev Cell.
15:795–796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Rutkowski DT, Wu J, Back SH, Callaghan MU,
Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze
MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GD and Kaufman
RJ: UPR pathways combine to prevent hepatic steatosis caused by ER
stress-mediated suppression of transcriptional master regulators.
Dev Cell. 15:829–840. 2008. View Article : Google Scholar
|
|
4.
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ji C: Dissection of endoplasmic reticulum
stress signaling in alcoholic and non-alcoholic liver injury. J
Gastroenterol Hepatol. 23:S16–S24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Colgan SM, Tang D, Werstuck GH and Austin
RC: Endoplasmic reticulum stress causes the activation of sterol
regulatory element binding protein-2. Int J Biochem Cell Biol.
39:1843–1851. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Goldstein JL, DeBose-Boyd RA and Brown MS:
Protein sensors for membrane sterols. Cell. 124:35–46. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Nakayama H, Otabe S, Ueno T, Hirota N,
Yuan X, Fukutani T, Hashinaga T, Wada N and Yamada K: Transgenic
mice expressing nuclear sterol regulatory element-binding protein
1c in adipose tissue exhibit liver histology similar to
nonalcoholic steatohepatitis. Metabolism. 56:470–475. 2007.
View Article : Google Scholar
|
|
9.
|
Mendez-Sanchez N, Chavez-Tapia NC,
Zamora-Valdes and Uribe M: Adiponectin, structure, function and
pathophysiological implications in non-alcoholic fatty liver
disease. Mini Rev Med Chem. 6:651–656. 2006. View Article : Google Scholar
|
|
10.
|
Yamauchi T, Kamon J, Waki H, Terauchi Y,
Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N,
et al: The fat-derived hormone adiponectin reverses insulin
resistance associated with both lipoatrophy and obesity. Nat Med.
7:941–946. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Nakayama H, Otabe S, Yuan X, Ueno T,
Hirota N, Fukutano T, Wada N, Hashinaga T and Yamada K: Effects of
adiponectin transgenic expression in liver of nonalcoholic
steatohepatitis model mice. Metabolism. 58:901–908. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Awazawa M, Ueki K, Inabe K, Yamauchi T,
Kaneko K, Okazaki Y, Bardeesy N, Ohnishi S, Nagai R and Kadowaki T:
Adiponectin suppresses hepatic SREBP1c expression in an
AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun.
382:51–56. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shimomura I, Hammer RE, Richardson JA,
Ikemoto S, Bashmakov Y, Goldstein JH, Goldstein JL and Brown MS:
Insulin resistance and diabetes mellitus in transgenic mice
expressing nuclear SREBP-1c in adipose tissue: model for congenital
generalized lipodystrophy. Genes Dev. 12:3182–3194. 1998.
View Article : Google Scholar
|
|
14.
|
Otabe S, Yuan X, Fukutani T, Wada N,
Hashinaga T, Nakayama H, Hirota N, Kojima M and Yamada K:
Overexpression of human adiponectin in transgenic mice results in
suppression of fat accumulation and prevention of premature death
by high-calorie diet. Am J Physiol Endocrinol Metab. 293:E210–E218.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Matteoni CA, Younossi ZM, Gramlich T,
Boparai N, Liu YC and McCullough AJ: Nonalcoholic fatty liver
disease: a spectrum of clinical and pathological severity.
Gastroenterology. 116:1413–1419. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hosogai N, Fukuhara A, Oshima K, Miyata Y,
Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M and
Shimomura I: Adipose tissue hypoxia in obesity and its impact on
adipocytokine dysregulation. Diabetes. 56:901–911. 2007. View Article : Google Scholar
|
|
17.
|
Frizzell N, Lima M and Baynes JW:
Succination of proteins in diabetes. Free Radic Res. 45:101–109.
2011. View Article : Google Scholar
|
|
18.
|
Ozcan U, Cao Q, Yilman E, Lee AH, Iwakoshi
NN, Ozdelen E, Görgün C, Glimcher LH and Hotamisligil GS:
Endoplasmic reticulum stress links obesity, insulin action, and
type 2 diabetes. Science. 306:457–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wek RC and Anthony TG: Obesity: stressing
about unfolded proteins. Nat Med. 16:374–376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung
J, Ueki K and Ozcan U: The regulatory subunits of PI3K, p85α and
p85β, interact with XBP-1 and increase its nuclear translocation.
Nat Med. 16:429–437. 2010.
|
|
21.
|
Winnay JH, Boucher J, Mori MA, Ueki K and
Kahn R: A regulatory subunit of phosphoinositide 3-kinase increases
the nuclear accumulation of X-box-binding protein-1 to moderate the
unfolded protein response. Nat Med. 16:438–445. 2010. View Article : Google Scholar
|
|
22.
|
Van Herpen NA and Schrauwen-Hinderling VB:
Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol
Behav. 94:231–241. 2008.PubMed/NCBI
|
|
23.
|
Bugianesi E, Pagotto U, Manini R, Vanni E,
Gastaldelli A, de Lasio R, Pasquali R, Cassio A, Cicognani A and
Cacciari E: Plasma adiponectin in nonalcoholic fatty liver is
related to hepatic insulin resistance and hepatic fat content, not
to liver disease severity. J Clin Endocrinol Metab. 90:3498–3504.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Shu RZ, Zhang F, Wang F, Feng DC, Li XH,
Ren WH, Wu XL, Yang X, Liao XD, Huang L and Wang ZG: Adiponectin
deficiency impairs liver regeneration through attenuating STAT3
phosphorylation in mice. Lab Invset. 89:1043–1052. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Yoshiuchi K, Kaneko H, Matsuoka T, Kasami
R, Kohono K, Iwawaki T, Nakatani Y, Yamasaki Y, Shimomura I and
Matsuhisa M: Pioglitazone reduces ER stress in the liver: direct
monitoring of in vivo ER stress using ER stress-activated indicator
transgenic mice. Endocr J. 56:1103–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|