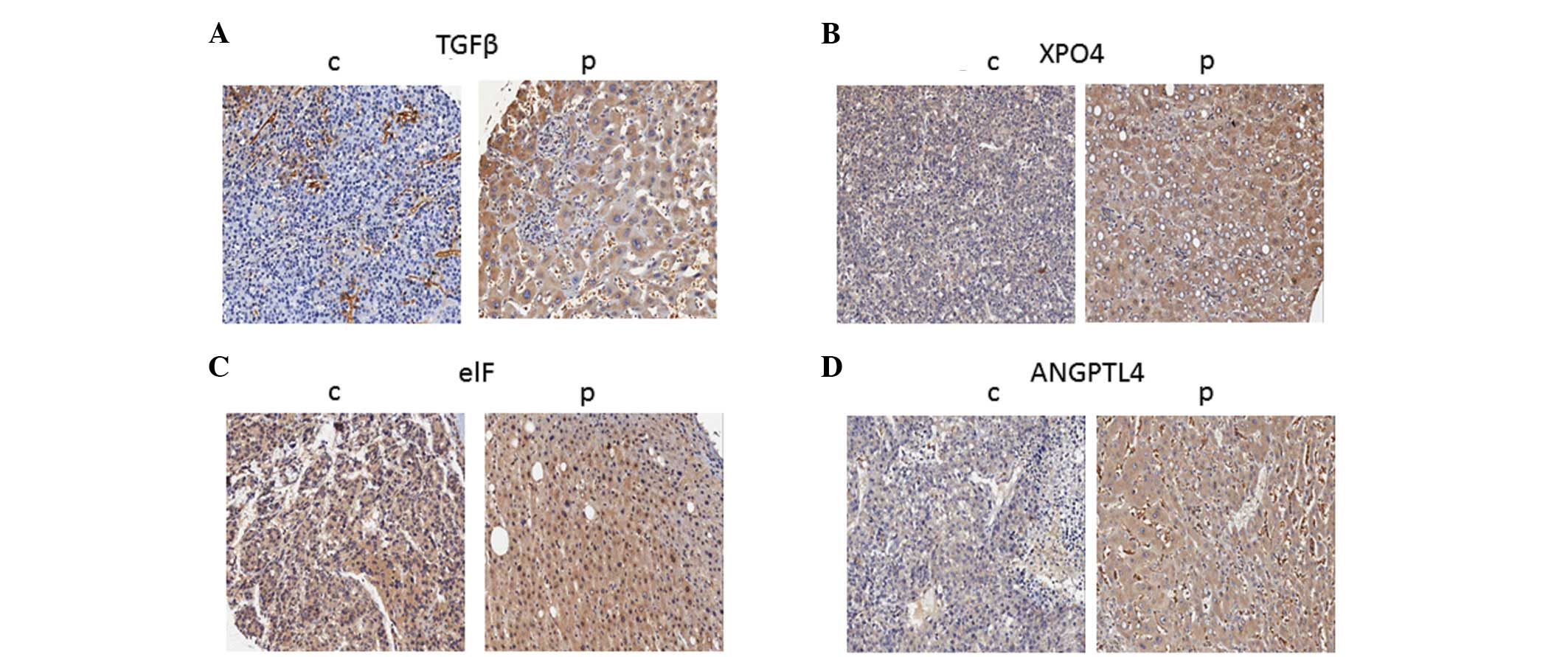
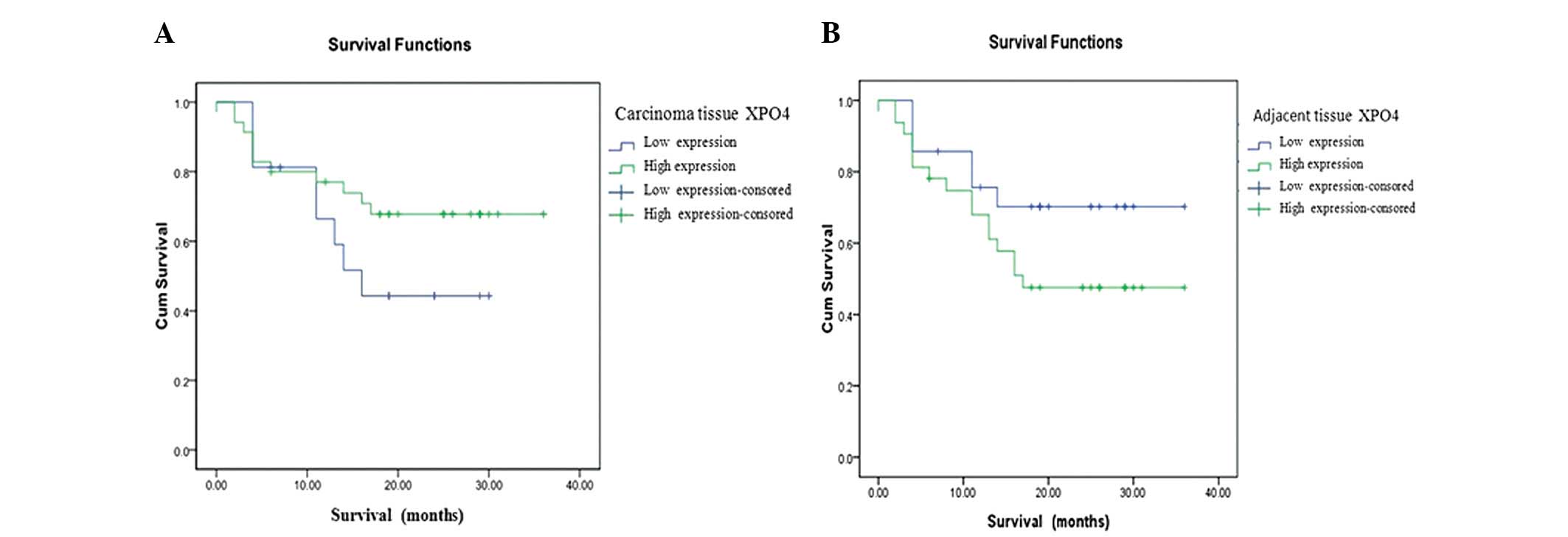
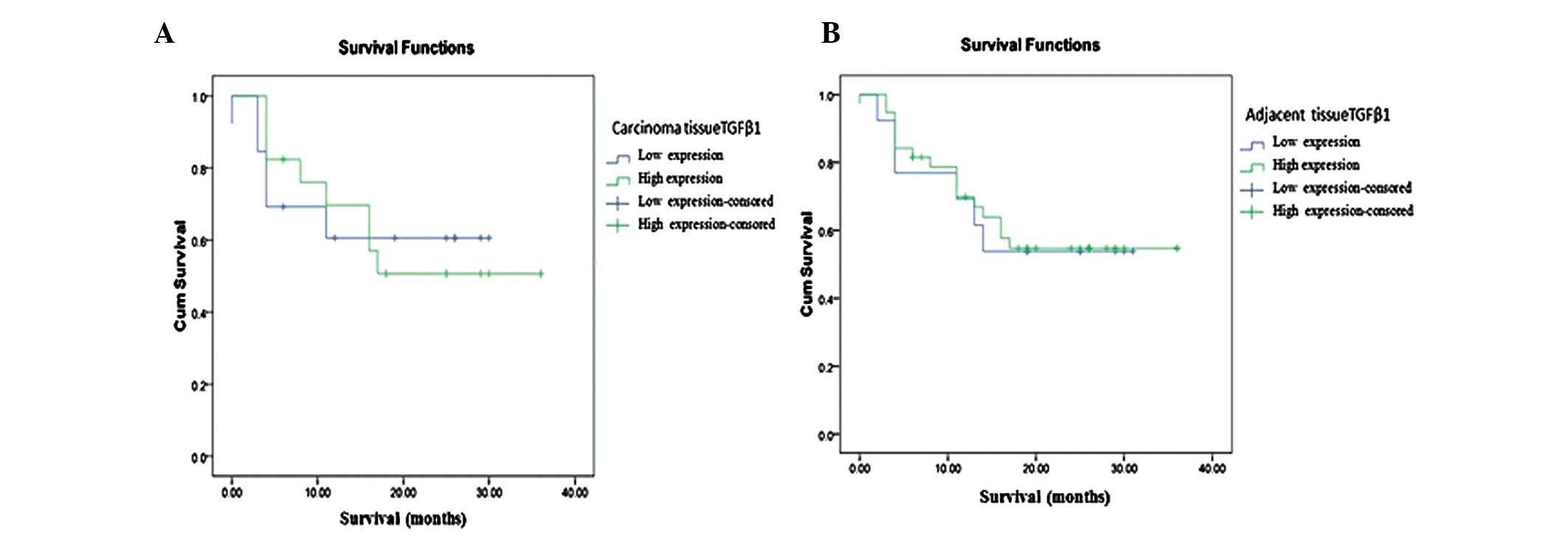
|
1
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001.PubMed/NCBI
|
|
2
|
Fabregat I: Dysregulation of apoptosis in
hepatocellular carcinoma cells. World J Gastroenterol. 15:513–520.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dong ZZ, Yao DF, Yao M, et al: Clinical
impact of plasma TGF-beta1 and circulating TGF-beta1 mRNA in
diagnosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis
Int. 7:288–295. 2008.PubMed/NCBI
|
|
4
|
Mishra L, Derynck R and Mishra B:
Transforming growth factor-beta signaling in stem cells and cancer.
Science. 310:68–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SH, Ahn S and Park CK: Smad3 and its
phosphoisoforms are prognostic predictors of hepatocellular
carcinoma after curative hepatectomy. Hepatobiliary Pancreat Dis
Int. 11:51–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coulouarn C, Factor VM and Thorgeirsson
SS: Transforming growth factor-beta gene expression signature in
mouse hepatocytes predicts clinical outcome in human cancer.
Hepatology. 47:2059–2067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zender L, Xue W, Zuber J, et al: An
oncogenomics-based in vivo RNAi screen identifies tumor suppressors
in liver cancer. Cell. 135:852–864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurisaki A, Kurisaki K, Kowanetz M, et al:
The mechanism of nuclear export of Smad3 involves exportin 4 and
Ran. Mol Cell Biol. 26:1318–1332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang XT, Pan K, Chen MS, et al: Decreased
expression of XPO4 is associated with poor prognosis in
hepatocellular carcinoma. J Gastroenterol Hepatol. 26:544–549.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fransvea E, Mazzocca A, Antonaci S and
Giannelli G: Targeting transforming growth factor (TGF)-betaRI
inhibits activation of beta1 integrin and blocks vascular invasion
in hepatocellular carcinoma. Hepatology. 49:839–850. 2009.
View Article : Google Scholar
|
|
11
|
Perdiguero EG, Galaup A, Durand M, et al:
Alteration of developmental and pathological retinal angiogenesis
in angptl4-deficient mice. J Biol Chem. 286:36841–36851. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Ge C, Zhao F, et al:
Hypoxia-inducible factor 1 alpha-activated angiopoietin-like
protein 4 contributes to tumor metastasis via vascular cell
adhesion molecule-1/integrin β1 signaling in human hepatocellular
carcinoma. Hepatology. 54:910–919. 2011.PubMed/NCBI
|
|
13
|
Huang RL, Teo Z, Chong HC, et al: ANGPTL4
modulates vascular junction integrity by integrin signaling and
disruption of intercellular VE-cadherin and claudin-5 clusters.
Blood. 118:3990–4002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Padua D, Zhang XH, Wang Q, et al: TGFbeta
primes breast tumors for lung metastasis seeding through
angiopoietin-like 4. Cell. 133:66–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao FY, Ferrell L, Bass NM, et al: Liver
transplantation for hepatocellular carcinoma: expansion of the
tumor size limits does not adversely impact survival. Hepatology.
33:1394–1403. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Q, Qiu SJ, Fan J, et al: Intratumoral
balance of regulatory and cytotoxic T cells is associated with
prognosis of hepatocellular carcinoma after resection. J Clin
Oncol. 25:2586–2593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Battifora H: The multitumor (sausage)
tissue block: novel method for immunohistochemical antibody
testing. Lab Invest. 55:244–248. 1986.PubMed/NCBI
|
|
18
|
Kononen J, Bubendorf L, Kallioniemi A, et
al: Tissue microarrays for high-throughput molecular profiling of
tumor specimens. Nat Med. 4:844–847. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiang ZL, Zeng ZC, Tang ZY, et al:
Potential prognostic biomarkers for bone metastasis from
hepatocellular carcinoma. Oncologist. 16:1028–1039. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giannelli G, Mazzocca A, Fransvea E, Lahn
M and Antonaci S: Inhibiting TGFβ signaling in hepatocellular
carcinoma. Biochim Biophys Acta. 1815:214–223. 2011.
|
|
21
|
Picon A, Gold LI, Wang J, Cohen A and
Friedman E: A subset of metastatic human colon cancers expresses
elevated levels of transforming growth factor beta1. Cancer
Epidemiol Biomarkers Prev. 7:497–504. 1998.PubMed/NCBI
|
|
22
|
Mishra L, Banker T, Murray J, et al: Liver
stem cells and hepatocellular carcinoma. Hepatology. 49:318–329.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baek HJ, Lim SC, Kitisin K, et al:
Hepatocellular cancer arises from loss of transforming growth
factor beta signaling adaptor protein embryonic liver fodrin
through abnormal angiogenesis. Hepatology. 48:1128–1137. 2008.
View Article : Google Scholar
|
|
24
|
Cazes A, Galaup A, Chomel C, et al:
Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial
cell adhesion, migration, and sprouting and alters actin
cytoskeleton. Circ Res. 99:1207–1215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hasita H, Komohara Y, Okabe H, et al:
Significance of alternatively activated macrophages in patients
with intrahepatic cholangiocarcinoma. Cancer Sci. 101:1913–1919.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shibata K, Nakayama T, Hirakawa H, Hidaka
S and Nagayasu T: Clinicopathological significance of
angiopoietin-like protein 4 expression in oesophageal squamous cell
carcinoma. J Clin Pathol. 63:1054–1058. 2010. View Article : Google Scholar : PubMed/NCBI
|