Introduction
It is well known that many medicinal plants are
beneficial sources of minerals, vitamins, dietary fiber and various
phytochemicals (1). Certain herbs
are popular at present since their ingredients may not only
regulate body homeostasis but also prevent several degenerative
diseases (2,3). More than 60 species of the genus
Angelica are established plant sources of vitamin B
complexes, vitamin C, chlorophylls and minerals (4). The highly potent antioxidant
properties of the fresh leaves make these functional food
ingredients. Additionally, members of this group of plants have
traditionally been used as anti-inflammatory agents, as well as
remedies for colds, flu, hepatitis, arthritis, indigestion,
coughing, chronic bronchitis, fever, cancer and bacterial
infections (5–7) due to the group’s flavonoid, saponin
and coumarin content. Further studies have revealed that oils from
these plants are able to inhibit the growth of PANC-1 pancreatic
cancer cells (8).
Angelica keiskei has been widely used as an
alternative medicine for treating irritable bowel syndrome,
arthritis and immune diseases (9).
It has also been demonstrated that this plant reduces inflammation
in vivo in a chronic ethanol-induced test (10). The administration of Angelica
keiskei extracts to ICR mice at 10, 25 and 50 mg/kg per
os (p.o.; by mouth) improves alcohol-induced hepatotoxicity,
suggesting that these extracts indirectly protect the liver against
free radical attack (10).
However, limited scientific information has prevented the use of
Angelica keiskei for treating various degenerative
disorders. Previously, the anti-asthmatic activities of an aqueous
extract in an ovalbumin-induced animal model was investigated in
our laboratory (11).
Angelica extract was orally administered to
ovalbumin-sensitized mice and their lungs were analyzed to compare
IL-4 and IL-13 cytokine expression levels in the tissues using
immunohistochemistry. The extract was revealed to have potent
anti-asthmatic effects capable of controlling CD4+ cell
populations, IL-4 and IL-13 expression and asthma-associated
biomarkers in the lungs (11).
Previously, several alkylated chalcones obtained from
Angelica have been observed to inhibit influenza virus
neuraminidase (12). Other
preventive approaches against various degenerative diseases may
ameliorate the opportunistic damage and/or causes (13,14).
The Korea Food and Drug Administration (KFDA) has
suggested that guidelines (in vitro 3T3 NRU phototoxicity
test and local lymph node assay) should be established for
evaluating functional cosmetic ingredients [http://www.kfda.go.kr/search/search/search.kfda
(In Korean)]. Plant extracts with pungent scents appear to cause
skin irritation. Unwanted reactions to cosmetics are common in
patients with allergic dermatitis (15). Since various side-effects may be
caused by acute or chronic toxicity, irritation or sensitization,
various in vivo animal models, as well as in vitro,
semi in vivo and ex vivo models, should be used in
further toxicity studies, although they are modified tests
(16). If a cosmetic component or
constituent is demonstrated to be non-toxic to the skin in animals
or clinical trials, its use should be approved. Although cosmetic
ingredients have rarely caused serious damage, no studies have
conclusively demonstrated that these substances actively protect
the skin or promote tissue regeneration.
Previously, our group published the results of a
study demonstrating the effects of fractions of the plant
Angelica keiskei on eye mucosa irritancy (17). In the present study, acute skin
irritation and phototoxicity tests were performed using animal
models to analyze the in vivo effects of the Angelica
keiskei leaf. Various parameters were measured by comparing the
acute toxicity tests with calculated degrees to ascertain whether
the Angelica extracts may potentially be used for cosmetic
applications without damaging the skin.
Materials and methods
Animal care and use
New Zealand white (NZW) rabbits (9-week-old males
weighing between 2.1 and 2.4 kg) and guinea pigs (Hartley,
7-week-old males weighing between 319.6 and 372.9 g) were purchased
from Samtaco Korea (Osan, Korea) and used for the skin irritancy
and phototoxicity tests, respectively. The animals were fed a
commercial diet (Purina Korea, Seoul, Korea) and water ad
libitum throughout all the experiments. The study protocols
complied with the guidelines of the International Association for
the Study of Pain Committee for Research and Ethical Issues
(18) and strictly observed the
internal guidelines of the University Animal Ethics Committee. All
animals were acclimated to the laboratory environment for at least
one week prior to the commencement of the experiments.
Sample preparation
Angelica keiskei leaves purchased from
Myung-il Farm Co. (Eumsung, Korea) were used throughout the
experiments. Sample preparation was carried out as previously
described (19). The slice-dried
leaves were pulverized with a homogenizer (20,000 rpm for 15 min;
Shin-Il, Seoul, Korea) to obtain aqueous and ethanol fractions of
Angelica keiskei leaves and powder. Voucher specimens of the
Angelica keiskei leaf and powder were deposited in the
Laboratory of Food Enzyme Biotechnology, Kyungpook National
University (#2010-Ak; Daegu, Korea).
Skin irritancy test
In order to determine whether the Angelica
keiskei fractions have toxic effects on the middle back skin of
male 9-week-old NZW rabbits (2.1–2.4 kg), several toxicity
parameters were evaluated. NZW rabbits are widely used for safety
testing. Since a large amount of data for NZW rabbits has been
accumulated over a long period of time, it is relatively simple to
interpret data from experiments using these animals. The aqueous
and ethanol fractions were solubilized in propylene glycol at a
concentration of 10 mg/ml. Approximately 24 h prior to the
administration of the test samples, the rabbit fur was carefully
removed with an electric haircutter. The skin of the shaved back
area was divided into four compartments (2.5×2.5 cm); two
compartments served as the control areas and two were the test
areas. Each compartment was diagonally located from its matching
group member in the wound or non-wound group. In the wound group,
each site was scratched with an 18-G needle so that only the
epithelial tissues were damaged without drawing blood and a #
symbol was scratched into the skin. The test sample was applied to
each compartment of the skin on the back (90.5 ml/site) using
3-fold gauze (2.5×2.5 cm), then covered with squares of gauze
(10×10 cm) and fixed with tape in order to prevent leakage and
evaporation. The test substance was removed by carefully removing
the gauze squares after 24 h. Draize skin reactions (20) were evaluated and scored by
observing skin erythema, crust formation and edema following the
administration of the test samples (24, 48 or 72 h). The average
score was calculated by adding the scores for edema formation
according to the primary skin irritation index (primary irritation
index, PII) as shown in Table
I.
 | Table ITotal scores from the phototoxicity
test evaluating the effects of aqueous and ethanol fractions
obtained from Angelica keiskei leaves. |
Table I
Total scores from the phototoxicity
test evaluating the effects of aqueous and ethanol fractions
obtained from Angelica keiskei leaves.
| Criteria | Total scores | Aqueous | 50% Ethanol | 100% Ethanol | 0.1% 8-MOP |
|---|
| Non-irritating | 0.0–0.5 | Yes | Yes | Yes | - |
| Minimally
irritating | 0.6–1.2 | - | - | - | - |
| Severely
irritating | 1.3–2.5 | - | - | - | Yes |
| Extremely
irritating | 2.6–5.0 | - | - | - | - |
Phototoxicity test
A phototoxicity test was conducted using Hartley
guinea pigs. The animals were divided as follows: untreated group,
two experimental (aqueous and ethanol fractions) groups and a
positive control group treated with 8-methoxypsoralen (8-MOP). Each
group contained five guinea pigs (seven-week-old males weighing
between 319.6 and 372.9 g). The untreated group was exposed to
propylene glycol. For the two experimental groups, 0.5 ml/site of
the aqueous or ethanol fraction were applied. The treated skin was
then irradiated with ultraviolet (UV) light at a distance of 10 cm
for 10 min using a UV irradiation apparatus (UVITEC LF-206. LS,
Strasburg, France) with a UV lamp (365 nm). The left site was
designated as the light irradiation site, whereas the right site
was not irradiated. After 2, 4 and 24 h of irradiation, skin
erythema, eschar and swelling was scored relative to the control.
Transdermal administration was performed by removing the fur in a
4×6-cm area with an electric hair cutter and applying the test
sample to two regions (each 2×2 cm). The test group samples were
0.5 ml at a concentration of 10 mg/ml, while 0.5 ml of a 0.1%
solution of 8-MOP was applied at each side of the test site as a
positive control (21). The
non-irradiated site was shielded using aluminum tape.
Analysis of parameters
Lesions were examined at 24, 48 and 72 h during the
skin irritancy test and 2, 4 and 24 h after applying of the test
fractions to evaluate phototoxicity. The designated criteria were
strictly observed. Skin irritation and phototoxic effects were
evaluated by measuring irritancy, edema or inflammation by trained
examiners under the supervision of a veterinary pathologist from
the Center for the Care and Use of Laboratory Animals, Kyungpook
National University (Daegu, Korea).
Results and discussion
In a previous study by our laboratory, it was
determined that aqueous and ethanol fractions of Angelica
keiskei leaf have potent whitening and anti-atopic activities
at a concentration of 100 mg/ml (19).
The Angelica keiskei fractions were
previously reported not to be toxic. During an eye irritancy test,
no hazing, swelling, redness or emissions from the eye mucosa were
observed and the fractions were revealed to have potential use in
the cosmetic industry or other associated purposes (17). To first determine whether the
fractions were cytotoxic, RAW264.7 cells were used for an in
vitro assay. The results from this assay demonstrated that the
aqueous and ethanol fractions were not cytotoxic to the cells at a
concentration of 300 mg/ml (data not shown).
Currently, there are numerous plants used for
industrial applications due to their beneficial properties. The
Angelica sp. is a valuable herb used in Korea, Japan and
other Far Eastern countries for its antioxidant and
immunomodulatory activities (4).
Angelica leaves contain various nutrients, including
minerals, vitamins, flavonoids and other polyphenol compounds
(8,9). This plant also has a significant
potential for other purposes, including utilization as a cosmetic
ingredient. To ensure the safe use of this and other ingredients,
only animal data should be submitted, such as results from Draize
eye mucosal irritancy, skin irritancy and phototoxicity tests.
These tests involve applying reagents/substances to rabbit/guinea
pig eyes or skin. When assessing the safety of the ingredients,
guidelines for the use of a test material should be based on data
from several skin toxicity tests.
Major factors correlated with toxicity are
associated with amines, nitrous compounds or detrimental substances
which may be produced during plant growth, storage, preservation,
processing or cooking. However, there are a number of studies
describing the antitumor, antidiabetic and anti-inflammatory
activities of extracts from the Angelica sp. (2,3). To
examine the biological activities of the Angelica sp., our
group previously performed a study to determine whether these
extracts had anti-atopic dermatitis or anti-asthmatic properties
(22). These types of ingredients
may be extracted from raw fruits, vegetables and medicinal herbs.
Subsequently, the ingredients may be converted into more potent
food biomaterials using various techniques, such as fermentation,
biotransformation or chemical modification. The final products may
be converted into a cosmetic, cosmeceutical, nutraceutical or
pharmaceutical compound. To investigate this point we first
performed tests to obtain a biological profile for the
anti-melanogenesis and anti-asthmatic activity of Angelica
keiskei(19,22).
In the present study, the skin irritancy potential
of the Angelica keiskei leaf aqueous and ethanol fractions
(100 mg/dose) was investigated by applying these compounds to the
skin of rabbits. When the animal skin was wounded with an 18-G
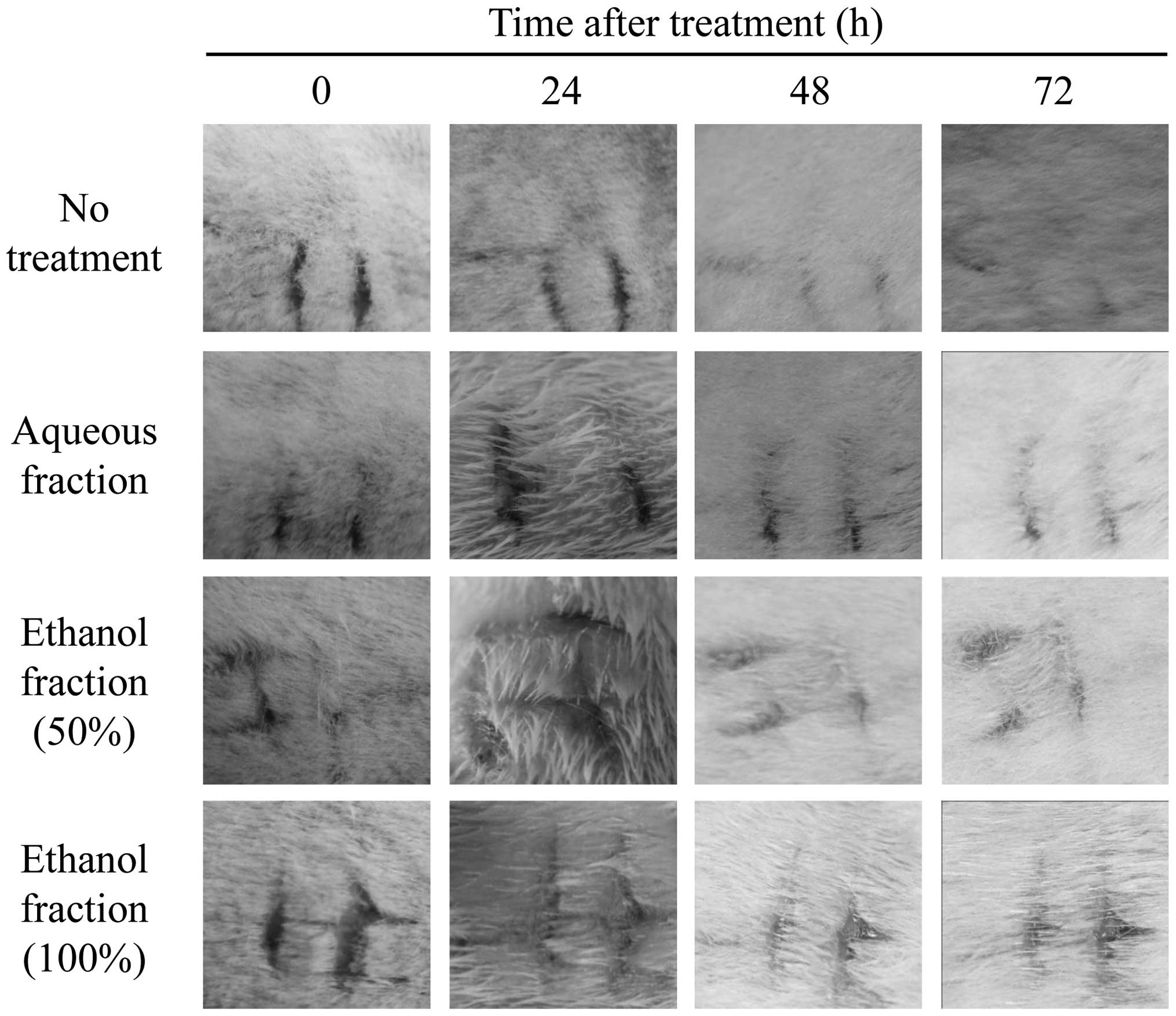
needle in the designated area, all skin symptoms appeared in the
same pattern (Fig. 1). After 24,
48 and 72 h, wound healing proceeded naturally in the control group
(Fig. 1, 1st row), while wound
healing also progressed naturally in the test groups (2nd, 3rd and
4th rows). Since the Draize skin irritancy test strictly evaluates
phenotypic characteristics and does not fully reflect the degree of
skin irritancy, the toxicity test is considered imprecise and
unreliable. However, this test is currently the most accurate for
analyzing animal models. Therefore, the following two tests were
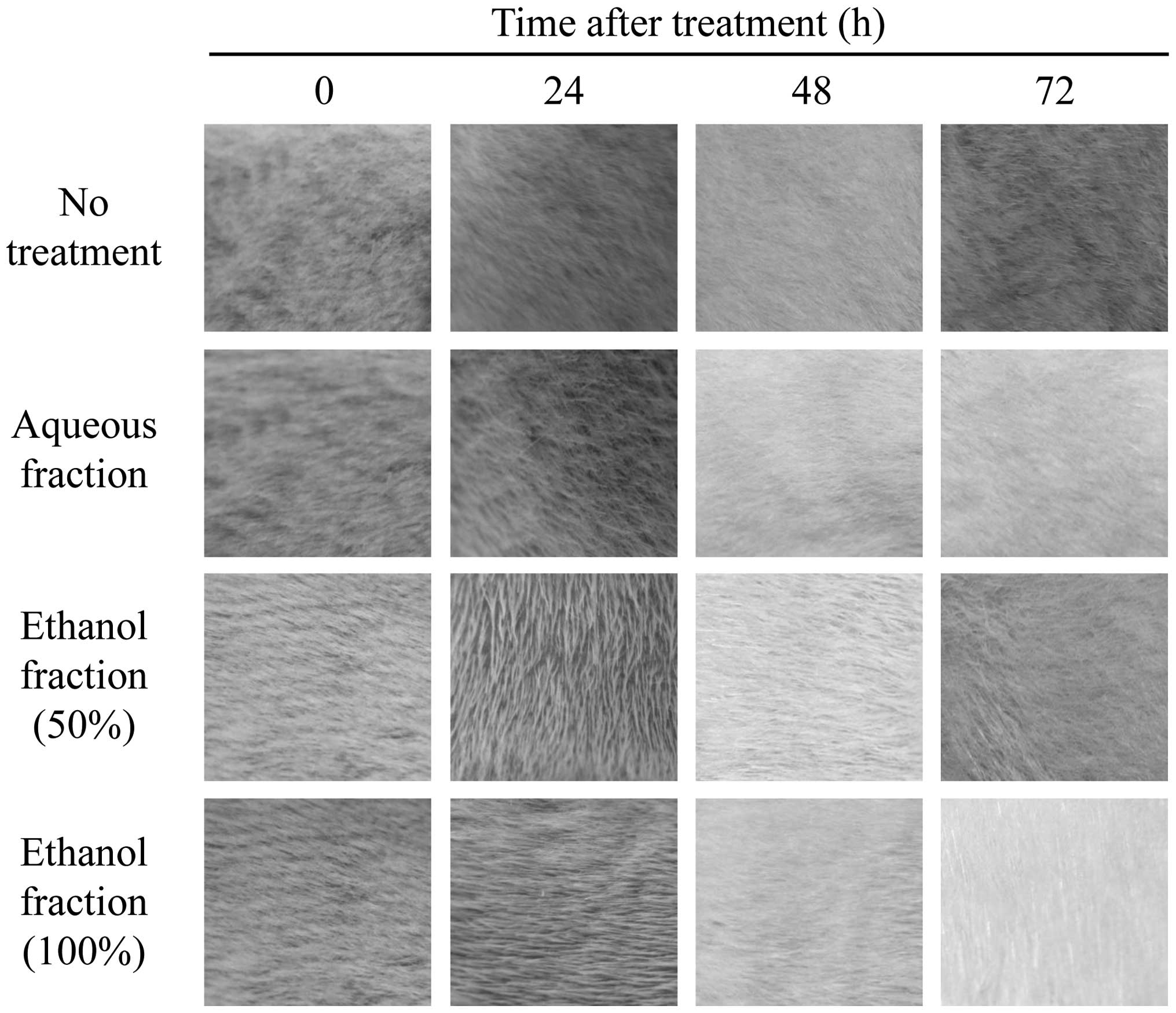
used to accurately evaluate toxic symptoms with (Fig. 1) or without (Fig. 2) skin excoriation. Normally, wounds
heal naturally via the homeostasis system of the body. To assess
whether wound healing in skin treated with fractions was affected,
the skin of the rabbits was damaged by inflicting abrasions and the
healing process was monitored. Skin abrasions with or without the
application of aqueous or ethanol fractions (Figs. 1 and 2) were examined over time and compared.
The aqueous and ethanol (50% and 100%) fractions did not affect the
skin damage (Fig. 1), suggesting
that these fractions are not toxic. Similar results were also
obtained from skin that had not been wounded (Fig. 2), indicating that the fractions
tested have no effects on the skin. The final results demonstrated
that the skin irritation index score was 0. Therefore, the findings
indicate that neither the aqueous nor ethanol fraction irritated
the skin, suggesting that the fractions caused no prick erythema or
eschar on the skin and no additional symptoms.
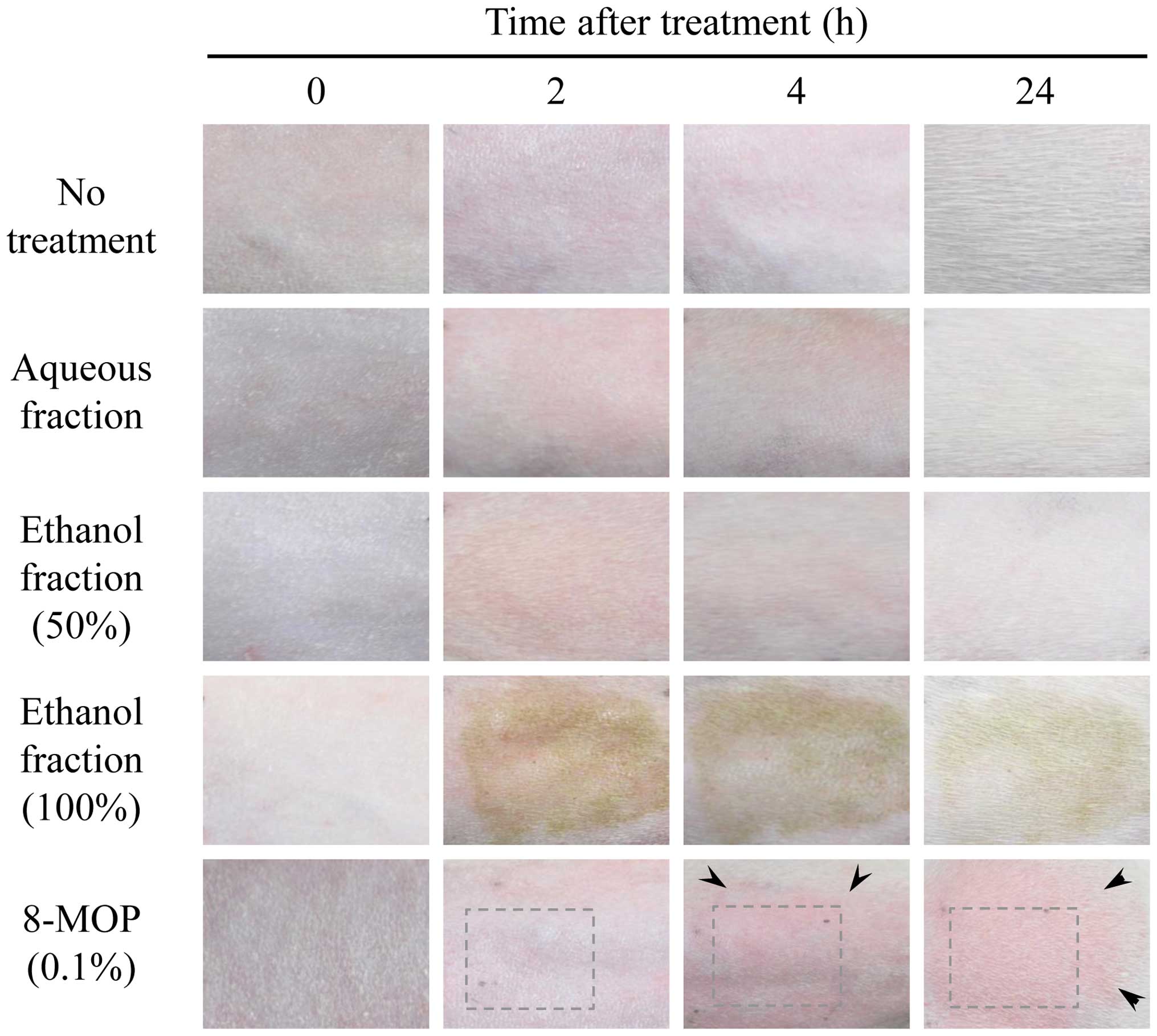
Phototoxicity was subsequently evaluated by
analyzing skin exposed to UV irradiation. After removing the fur,
guinea pig skin was treated with the aqueous and ethanol fractions
and 8-MOP. In this test, the degree of erythema was determined on
the following scale: 0 for no erythema, 1 for slight erythema, 2
for well-defined erythema, 3 for moderate to severe erythema and 4
for severe erythema to slight eschar formation. Similar erythema
symptoms were observed up to 4 h after UV irradiation. After 24 h,
the fraction-treated groups exhibited no symptoms of toxicity in
the skin, unlike the positive control. Treatment with the fractions
did not lead to erythema or eschar, while 8-MOP (0.1% as a positive
control) caused moderate to severe erythema (Fig. 3). To measure edema, the following
scale was used: 0 for no edema, 1 for slight edema, 2 for
well-defined edema, 3 for moderate to severe edema and 4 for severe
edema. The results demonstrated that the fractions did not cause
erythema or eschar, whereas 8-MOP administration resulted in slight
edema (Fig. 3). The final score
was determined by assessing the average of the total values of
erythema, edema and crust formation as follows: 0.0–0.5 for almost
no phototoxicity, 0.6–1.2 for slightly phototoxic, 1.3–2.5 for
clearly and highly phototoxic and 2.6–5.0 for severely phototoxic.
As shown in Table I, all three
samples (aqueous, 50% ethanol and 100% ethanol fractions) had
scores of only 0.0–0.5, suggesting that the fractions tested in the
experiment were non-irritating. However, 8-MOP was observed to be
an irritating compound that caused erythema, eschar and edema
(Fig. 3). Following 2 to 4 h of UV
irradiation, slight redness was observed in all fraction-treated
groups but this redness disappeared after 24 h. By contrast, groups
treated with 8-MOP developed erythema and edema, indicating that
the results of the phototoxicity test were achieved normally. Taken
together, the findings suggest that while 8-MOP induced erythema,
edema and/or eschar, no experimental samples caused moderate to
severe toxicity. Based on this information, we determined that all
fractions tested (aqueous, 50% ethanol and 100% ethanol fractions)
did not exhibit skin irritation caused by UV irradiation, whereas
8-MOP was associated with toxicity which caused skin
irritation.
In conclusion, the present study investigated
whether fractions of Angelica keiskei extract potentially
cause skin irritation and phototoxicity. None of the fractions was
found to irritate the skin or to be phototoxic, indicating that
these fractions may be useful in the cosmetic or cosmeceutical
industry and for other applications. Although the fractions were
derived from an edible plant, their potential phototoxicity
requires further evaluation and additional skin irritancy tests
must be performed.
Acknowledgements
This study was supported by a research
fund (Technology Transfer Project) from Kyungpook National
University Technopark, Daegu, Korea (S.-H.L). The author would like
to thank Hyeong-U Son, Min-A Kim, Yong-Soo Cha and Dong-Yoon Nam
for their technical assistance.
References
|
1
|
Sarker SD and Nahar L: Natural medicine:
the genus Angelica. Curr Med Chem. 11:1479–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan MH, Lai CS, Dushenkov S and Ho CT:
Modulation of inflammatory genes by natural dietary bioactive
compounds. J Agric Food Chem. 57:4467–4477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pan MH, Ghai G and Ho CT: Food bioactives,
apoptosis, and cancer. Mol Nutr Food Res. 52:43–52. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwon D, Yoon S, Carter O, Bailey GS and
Dashwood RH: Antioxidant and antigenotoxic activities of
Angelica keiskei, Oenanthe javanica and Brassica
oleracea in the Salmonella mutagenicity assay and in
HCT116 human colon cancer cells. Biofactors. 26:231–244. 2006.
|
|
5
|
Lee MY, Lee JA, Seo CS, Ha H, Lee H, Son
JK and Shin HK: Anti-inflammatory activity of Angelica
dahurica ethanolic extract on RAW264.7 cells via upregulation
of heme oxygenase-1. Food Chem Toxicol. 49:1047–1055. 2011.
|
|
6
|
Kimura Y: New anticancer agents: in vitro
and in vivo evaluation of the antitumor and antimetastatic actions
of various compounds isolated from medicinal plants. In Vivo.
19:37–60. 2005.PubMed/NCBI
|
|
7
|
Tan BK and Vanitha J: Immunomodulatory and
antimicrobial effects of some traditional chinese medicinal herbs:
a review. Curr Med Chem. 11:1423–1430. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sigurdsson S, Ogmundsdottir HM,
Hallgrimsson J and Gudbjarnason S: Antitumour activity of
Angelica keiskei leaf extract. In Vivo. 19:191–194.
2005.
|
|
9
|
Sigurdsson S, Ogmundsdottir HM and
Gudbjarnason S: The cytotoxic effect of two chemotypes of essential
oils from the fruits of Angelica keiskei L. Anticancer Res.
25:1877–1880. 2005.PubMed/NCBI
|
|
10
|
Yeh ML, Liu CF, Huang CF and Huang TC:
Hepatoprotective effect of Angelica archangelica in
chronically ethanol-treated mice. Pharmacology. 68:70–73. 2003.
|
|
11
|
Heo JC and Lee SH: Amelioration of
asthmatic-related symptoms by an aqueous extract of Angelica
archangelica L. J Life Sci. 18:1336–1341. 2008.(In Korean).
|
|
12
|
Park JY, Jeong HJ, Kim YM, Park SJ, Rho
MC, Park KH, Ryu YB and Lee WS: Characteristic of alkylated
chalcones from Angelica keiskei on influenza virus
neuraminidase inhibition. Bioorg Med Chem Lett. 21:5602–5604. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Corvera CU, Dunnican WJ, Blumgart LH and
D’Angelica M: Recurrent invasive intraductal papillary mucinous
carcinoma of the pancreas mimicking pott disease: review of the
literature. Pancreas. 32:321–324. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang SH, Lin CM and Chiang BH: Protective
effects of Angelica sinensis extract on amyloid
beta-peptide-induced neurotoxicity. Phytomedicine. 15:710–721.
2008.
|
|
15
|
Nigam PK: Adverse reactions to cosmetics
and methods of testing. Indian J Dermatol Venereol Leprol.
75:10–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Draize JH, Woodard G and Calvery HO:
Methods for the study of irritation and toxicity of substances
applied topically to the skin and mucous membranes. J Pharmacol Exp
Ther. 82:377–390. 1944.
|
|
17
|
Son HU, Yoon EK, Cha YS, Kim MA, Shin YK,
Kim JM, Choi YH and Lee SH: Comparison of the toxicity of aqueous
and ethanol fractions of Angelica keiskei leaf using the eye
irritancy test. Exp Ther Med. 4:820–824. 2012.PubMed/NCBI
|
|
18
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Son HU, Nam DY, Kim MA, Cha YS, Kim JM,
Shin YK and Lee SH: Comparison of anti-melanogenesis activity in
aqueous and ethanol fractions of Angelica keiskei leaf. Kor
J Food Preserv. 17:998–1001. 2011.(In Korean).
|
|
20
|
Tardiff RG, Hubner RP and Graves CG:
Harmonization of thresholds for primary skin irritation from
results of human repeated insult patch tests and laboratory animal
skin irritation tests. J Appl Toxicol. 23:279–281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neumann NJ, Blotz A, Wasinska-Kempka G,
Rosenbruch M, Lehmann P, Ahr HJ and Vohr HW: Evaluation of
phototoxic and photoallergic potentials of 13 compounds by
different in vitro and in vivo methods. J Photochem Photobiol B.
79:25–34. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee S-H, Heo JC and Park JY: Extract of
Angelica archangelica having anti-asthmatic activity. KR
Patent 10-825869. Filed July 6, 2006; issued April 22, 2008.
|