Introduction
In 1959, Summerskill and Walshe first reported
benign recurrent intrahepatic cholestasis (BRIC) as a rare disease
that did not progress to cirrhosis despite recurrent jaundice of an
unknown origin (1). BRIC is an
autosomal recessive disorder (2)
caused by the mutation of two hepatic transporter genes: the
ATP8B1 gene, coding for familial intrahepatic cholestasis-1
(FIC1; BRIC type 1) and the ABCB11 gene, coding for the bile
salt export pump (BSEP; BRIC type 2) (3,4).
Pruritus and jaundice are the only subjective symptoms and
cholestasis generally improves within a few months. Although the
level of serum alkaline phosphatase increases significantly, the
level of γ-glutamyltranspeptidase remains within the normal
range.
Unlike progressive familial intrahepatic cholestasis
(PFIC), which is caused by the same genes and progresses to chronic
intrahepatic cholestasis, BRIC has a fair prognosis without any
progression to cirrhosis (2). In
Japan, only 20 cases of BRIC type 1 have been reported thus far
(5).
In the present study a difficult case of BRIC with
prolonged jaundice was reported. Endoscopic nasal biliary drainage
(ENBD) was performed to discharge the bile outside the body, which
immediately improved the jaundice and pruritus.
Case report
A 66-year-old male developed pruritus of an
unprecedented intensity and also experienced general malaise and
white stool in early May 2012. The patient presented to the Tokyo
Rosai Hospital after his wife remarked on his yellow eyes. The
patient’s alcohol consumption was 3 gō of Japanese sake (equivalent
to 66 g ethanol) per day, 5 days per week until he stopped drinking
in April 2012. There was no history of smoking. His prior medical
history included cerebral infarction at age 48 and a
cholecystectomy for cholecystitis at age 62. There was no
significant family history reported. The patient was taking the
following oral medication: pitavastatin 1 mg/day, olmesartan 20
mg/day, aspirin 81 mg/day, cilnidipine 15 mg/day, nicergoline 15
mg/day and famotidine 40 mg/day. At presentation, he had a clear
sensorium, with a blood pressure of 138/90 mmHg, a pulse rate of 60
beats/min (non-arrhythmic) and a body temperature of 36.0°C. The
palpebral conjunctiva was not anemic while the bulbar conjunctiva
was yellow. Heart and breath sounds were clear. The abdomen was
flat and soft with no tenderness or rebound tenderness. The liver
and spleen were impalpable. Laboratory findings at presentation
included increased bilirubin levels with a direct dominant
fraction, a T-Bil of 12.6 mg/dl and a D-Bil of 9.7 mg/dl, an
increased total bile acid level of 101.5 μmol/l and mild hepatic
impairment as indicated by an AST of 29 IU/l and an ALT of 49 IU/l
(Table I).
 | Table I:Laboratory test results on
admission |
Table I:
Laboratory test results on
admission
| Diagnostic blood
tests | Results |
|---|
| Biochemistry | |
| CRP | 0.4 mg/dl |
| Na | 138 mEq/l |
| K | 3.7 mEq/l |
| Cl | 105 mEq/l |
| TP | 7.1 g/dl |
| Alb | 4.4 g/dl |
| T-Bil | 12.6 mg/dl |
| D-Bil | 9.7 mg/dl |
| AST | 29 IU/l |
| ALT | 49 IU/l |
| LDH | 174 IU/l |
| ALP | 446 IU/l |
| GGT | 154 IU/l |
| T-Cho | 197 mg/dl |
| TG | 466 mg/dl |
| BUN | 12 mg/dl |
| Cr | 0.56 mg/dl |
| BS | 136 mg/dl |
| HbA1c | 6.0% |
| PT% | 119% |
| PT-INR | 0.93 |
| Hematology | |
| WBC | 8100/μl |
| RBC |
453×104/μl |
| Hgb | 14.6 mg/dl |
| Hct | 41.9% |
| PLT |
26.3×104/μl |
| Serology | |
| anti-HCV | (−) |
| HBsAg | (−) |
| anti-HBs | (−) |
| ANA | (−) |
| AMA | (−) |
| p-ANCA | (−) |
| IgG | 981 mg/dl |
| IgA | 263 mg/dl |
| IgM | 97 mg/dl |
| TBA | 101.5 μmol/l |
The patient was subsequently admitted for a detailed
examination into the causes of the jaundice. Abdominal
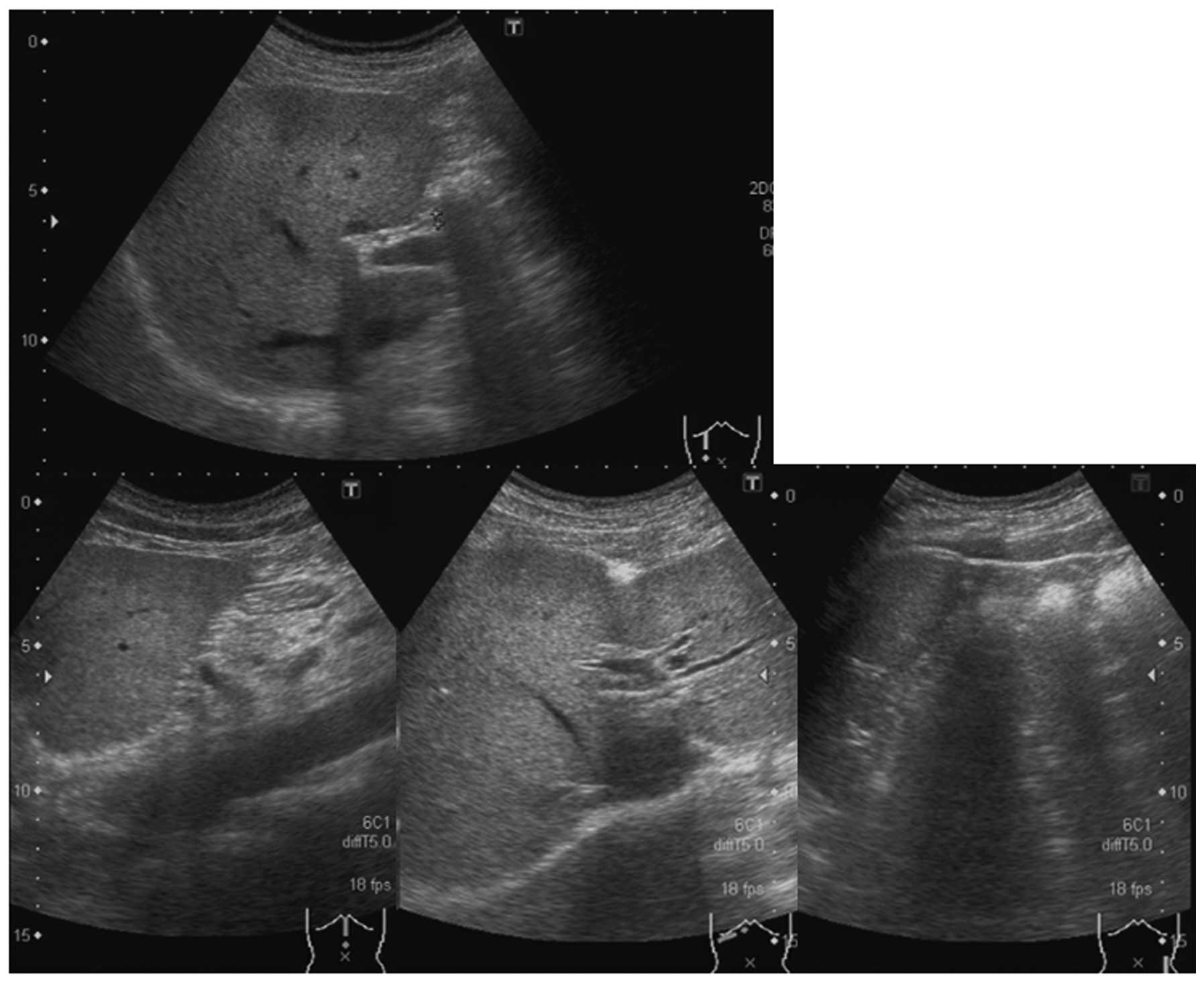
ultrasonography (US) performed on hospital day 2 showed a fatty
liver-like parenchyma with patchy bright areas. Neither
hepatomegaly nor splenomegaly was observed and no mass lesions were
observed in the liver. No ascites or distension of the intrahepatic
or common bile ducts were apparent (Fig. 1). Abdominal computed tomography
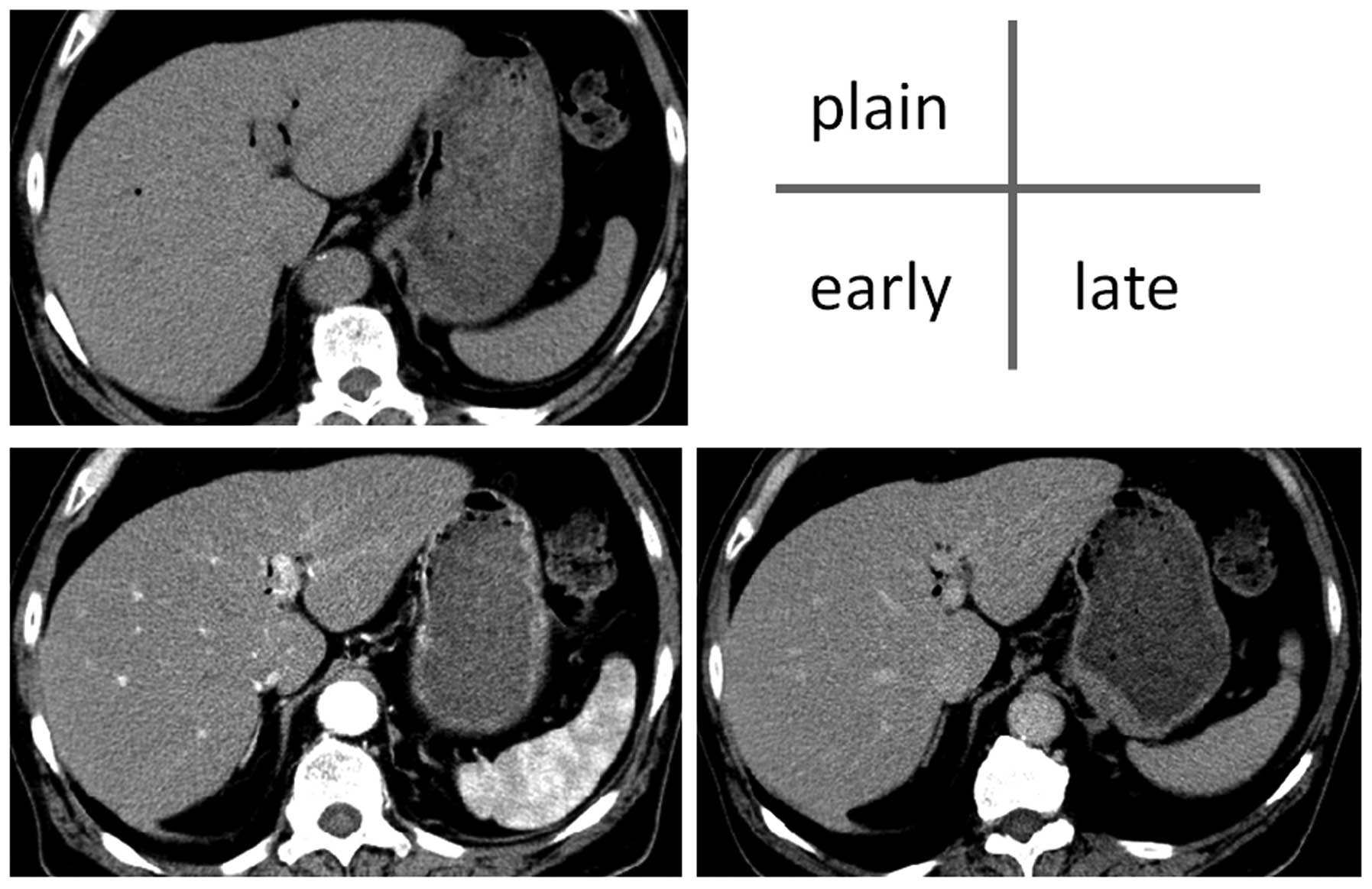
(CT) performed on hospital day 7 also revealed no hepatomegaly,
splenomegaly, distension of the intrahepatic or common bile ducts
or ascites (Fig. 2). Following a
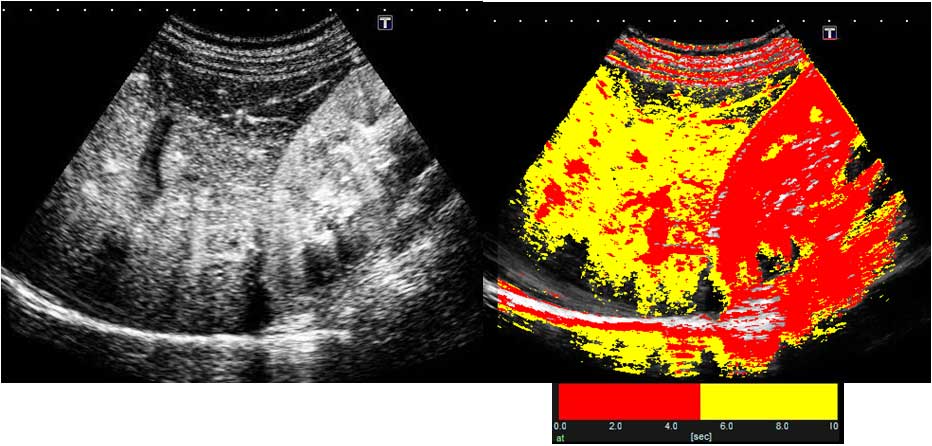
further increase in bilirubin levels confirmed by blood testing,
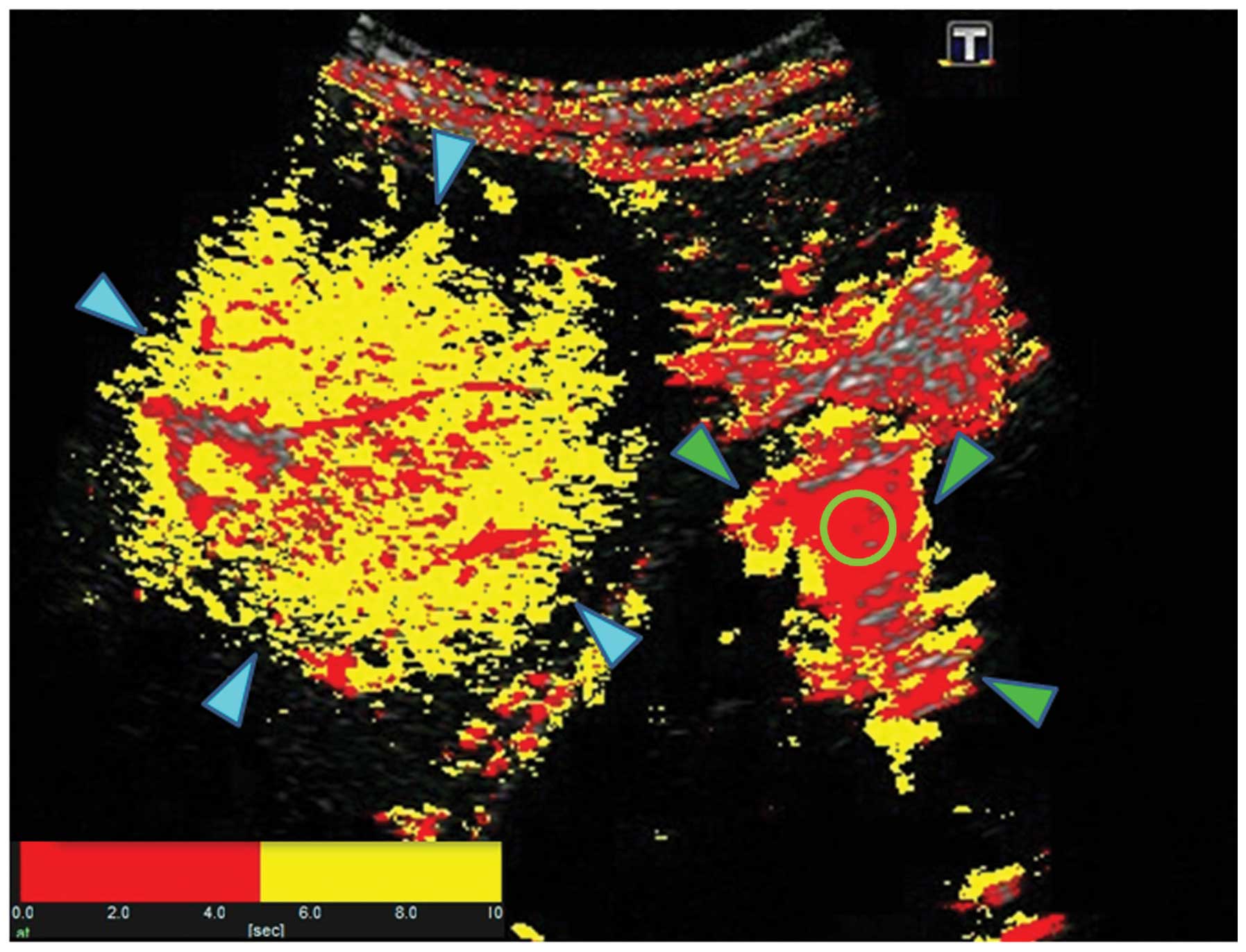
Sonozoid-enhanced US with arrival time parametric imaging (At-PI),
an imaging technique that has been shown to be effective in
evaluating the degree of progression of hepatic lesions (3), was performed on hospital day 9.
Contrast-enhanced US and At-PI
The Toshiba SSA-790A (AplioXG; Toshiba Medical
Systems, Otawara, Japan) US system was used with a 3.75-MHz convex
probe (PVT-375BT). Imaging was performed with a mechanical index of
0.21. Images showing the liver parenchyma from the right
inter-costal space to the S6 region of the right hepatic lobe and
the right kidney all on a single screen were used for analysis. The
focus was set at a depth of 8 cm to visualize the kidney. After
imaging conditions were set, Sonazoid (perfluorobutane; GE
Healthcare, Oslo, Norway) was infused at the recommended dose of
0.015 ml/kg via the median cubital vein. Images captured
immediately after the 40-sec Sonazoid infusion were saved as raw
data on the system’s hard disk drive. The At-PI calculation and
drawing were performed with the saved movie data, using software
provided with the system, as described previously (6). Briefly, subsequent to the region of
interest (ROI) being set in the kidney parenchyma, the movie was
played and arrival times were sequentially calculated for each
liver parenchymal pixel, with the time point at which 80% of the
ROI was enhanced by contrast medium defined as time 0. A color map
was then automatically superposed on a B-mode image. The display
colors were set according to user preference, and in the present
study, color mapping was configured to display the arrival times of
0–5 sec in red and those of 5–10 sec in yellow (Figs. 3 and 4).
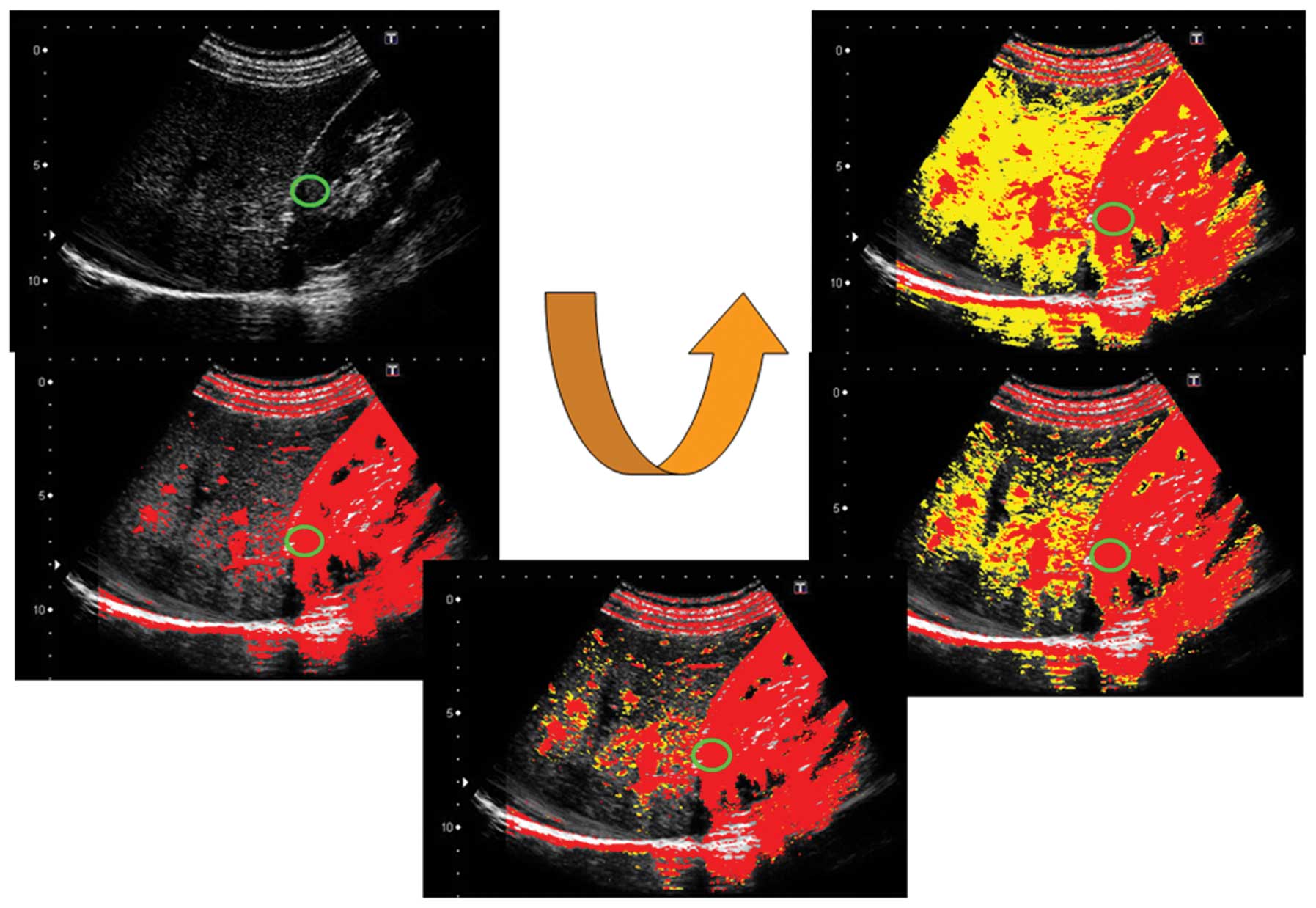
Calculation of the ratio of red (ROR)
area to the entire contrast-enhanced area
For a quantitative evaluation of the At-PI data
obtained, the ratio of the area of red pixels with shorter arrival
times to the entire contrast-enhanced area was calculated as the
ROR using image analysis software ImageJ (version 1.42. Wayne
Rasband, National Institutes of Health, Bethesda, MD, USA; Fig. 5). The ROR during an episode of
jaundice (hospital day 9; T-Bil 19.3 mg/dl, D-Bil 15.2 mg/dl) was
calculated as 15.7% (Fig. 6).
Clinical course following admission
As the jaundice had not been improved by the oral
administration of ursodeoxycholic acid (300 mg/day) that had been
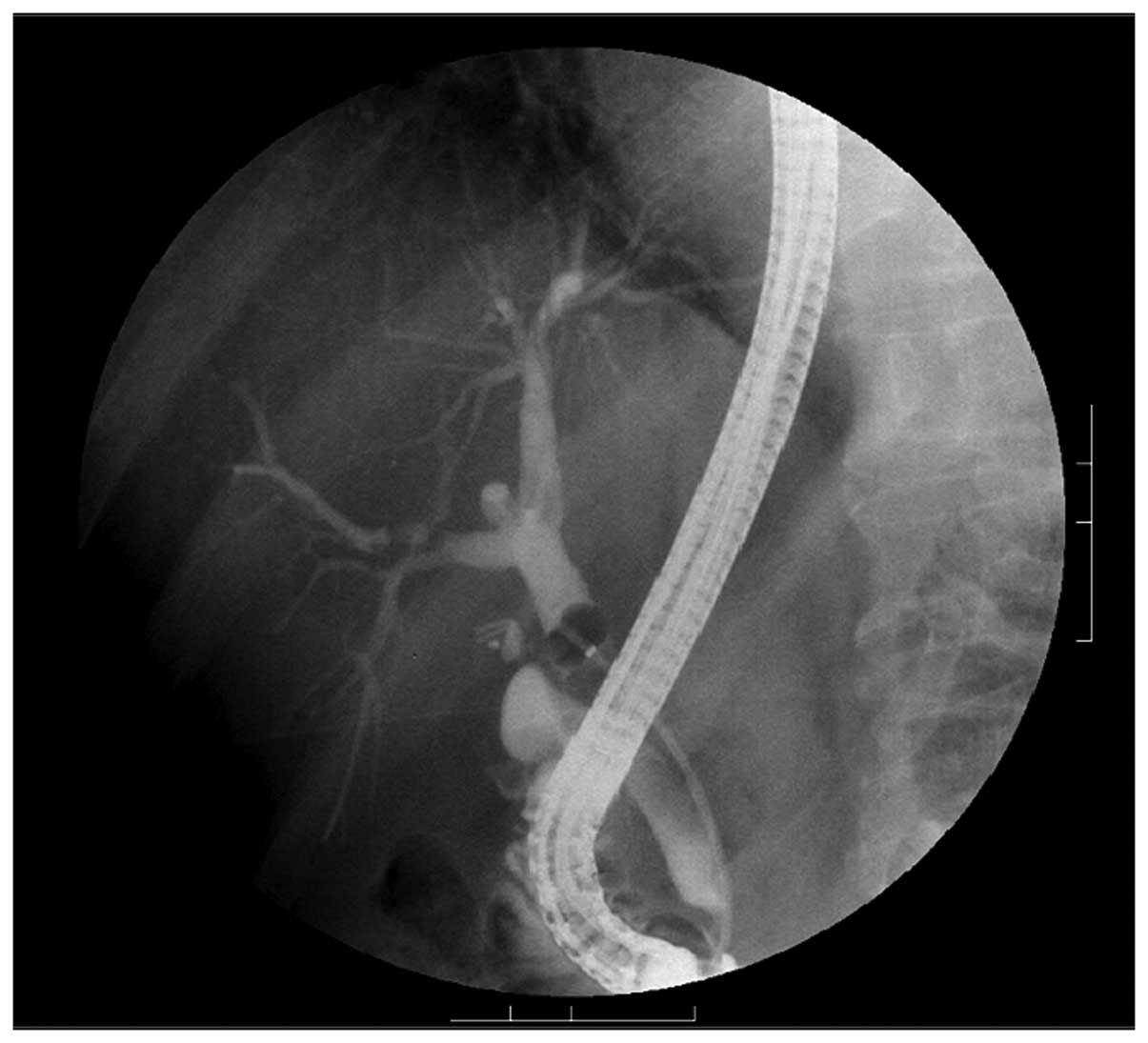
started immediately after hospital admission, endoscopic retrograde
cholangiopancreatography (ERCP) was performed on hospital day 14
for direct scrutiny of the bile ducts. This again revealed no
abnormalities, including distension or narrowing of the
common or intrahepatic bile ducts (Fig. 7). After considering its potential
effects for improving jaundice, ENBD was performed on the same day
and was followed by an immediate improvement in jaundice and skin
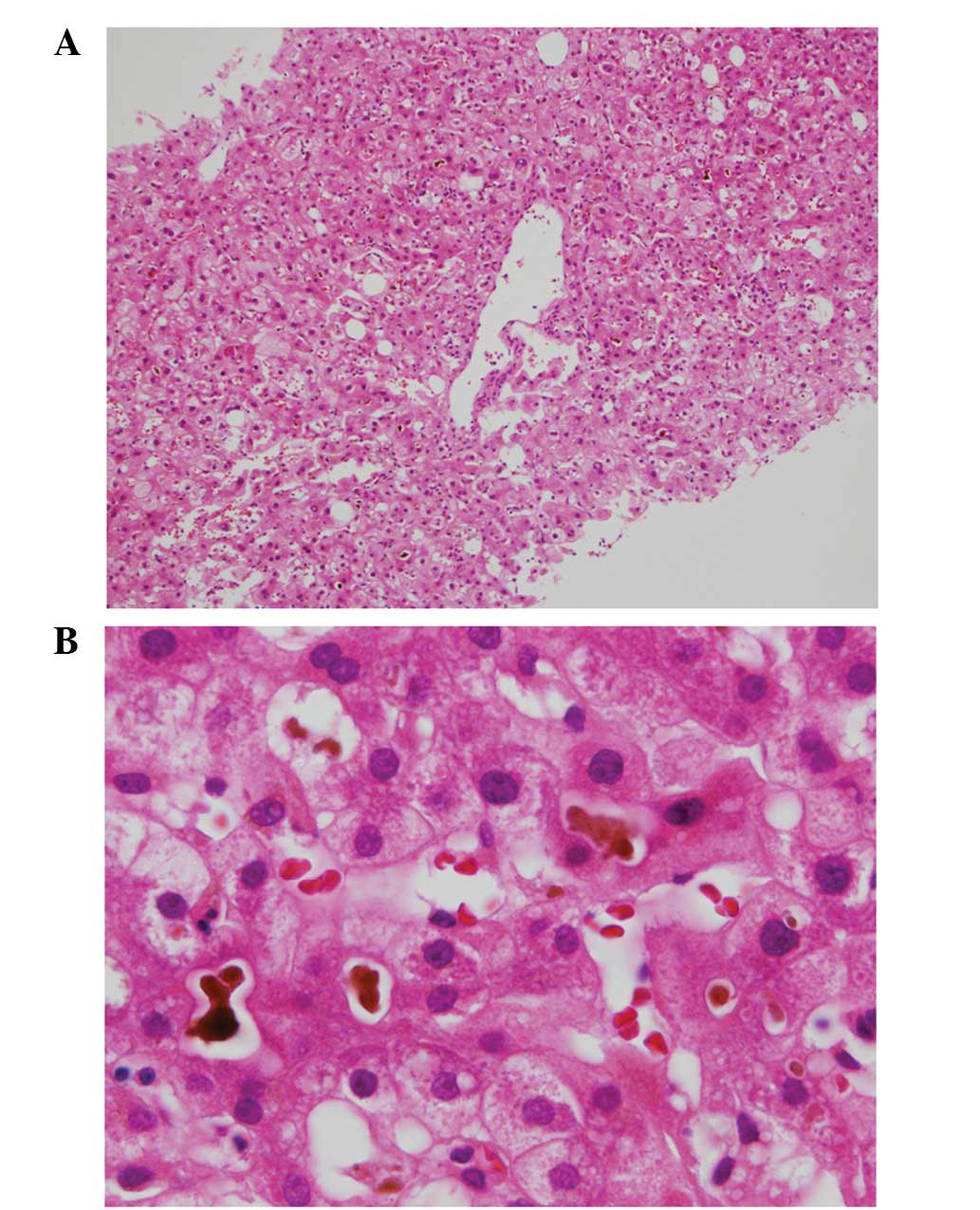
pruritus. Subsequently, a liver biopsy was performed on hospital
day 27 for further examination of the liver. The biopsy revealed
bile deposition in the centrilobular hepatocytes and bile thrombus
formation, with minimal inflammatory and fibrotic findings
(Fig. 8).
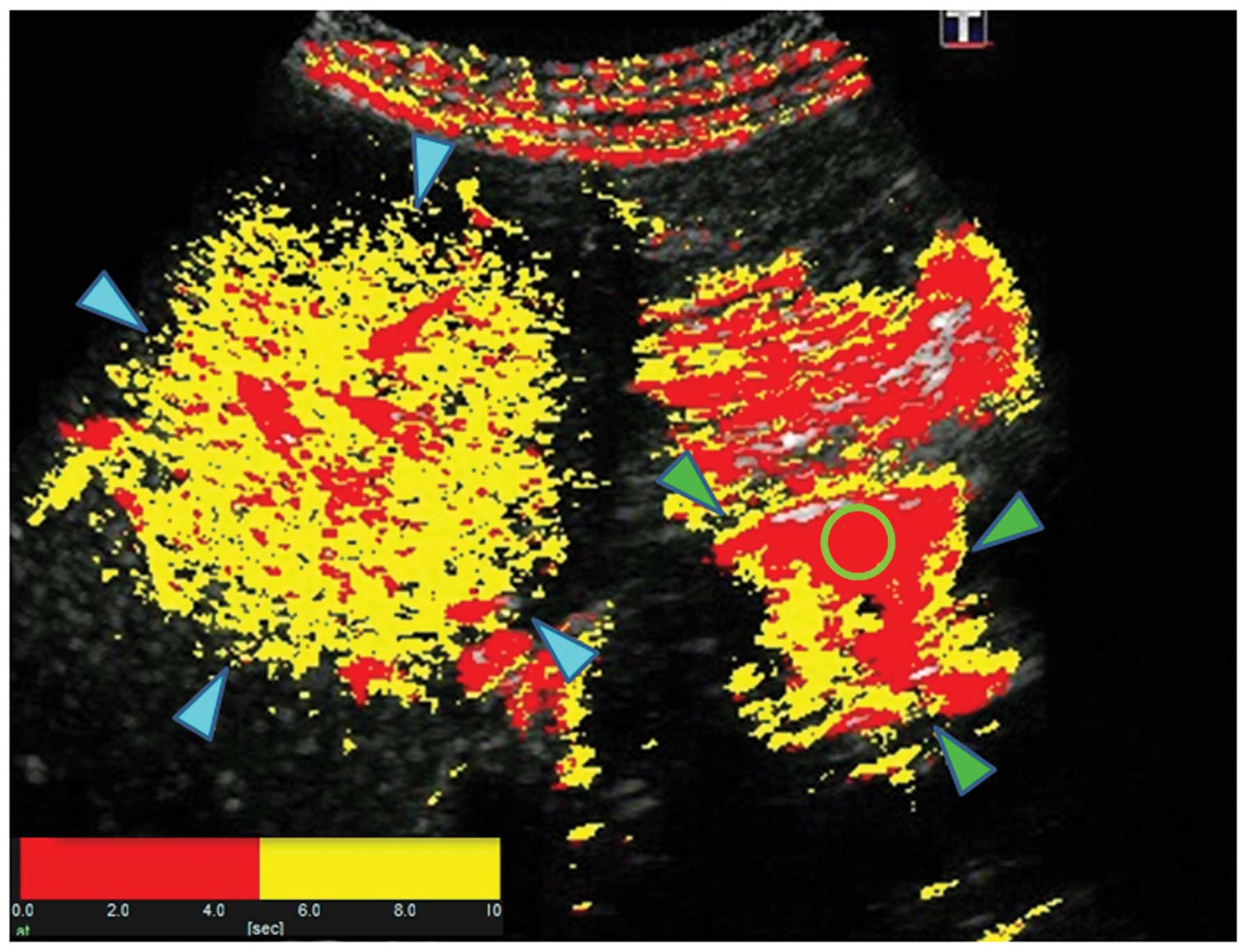
Sonazoid-enhanced US with At-PI was performed as the
patient was recovering from jaundice (hospital day 40: T-Bil, 3.1
mg/dl, D-Bil, 2.5 mg/dl). The ROR was calculated as 11.6%, which
was not significantly different from the value obtained when the
patient was jaundiced (Fig. 9).
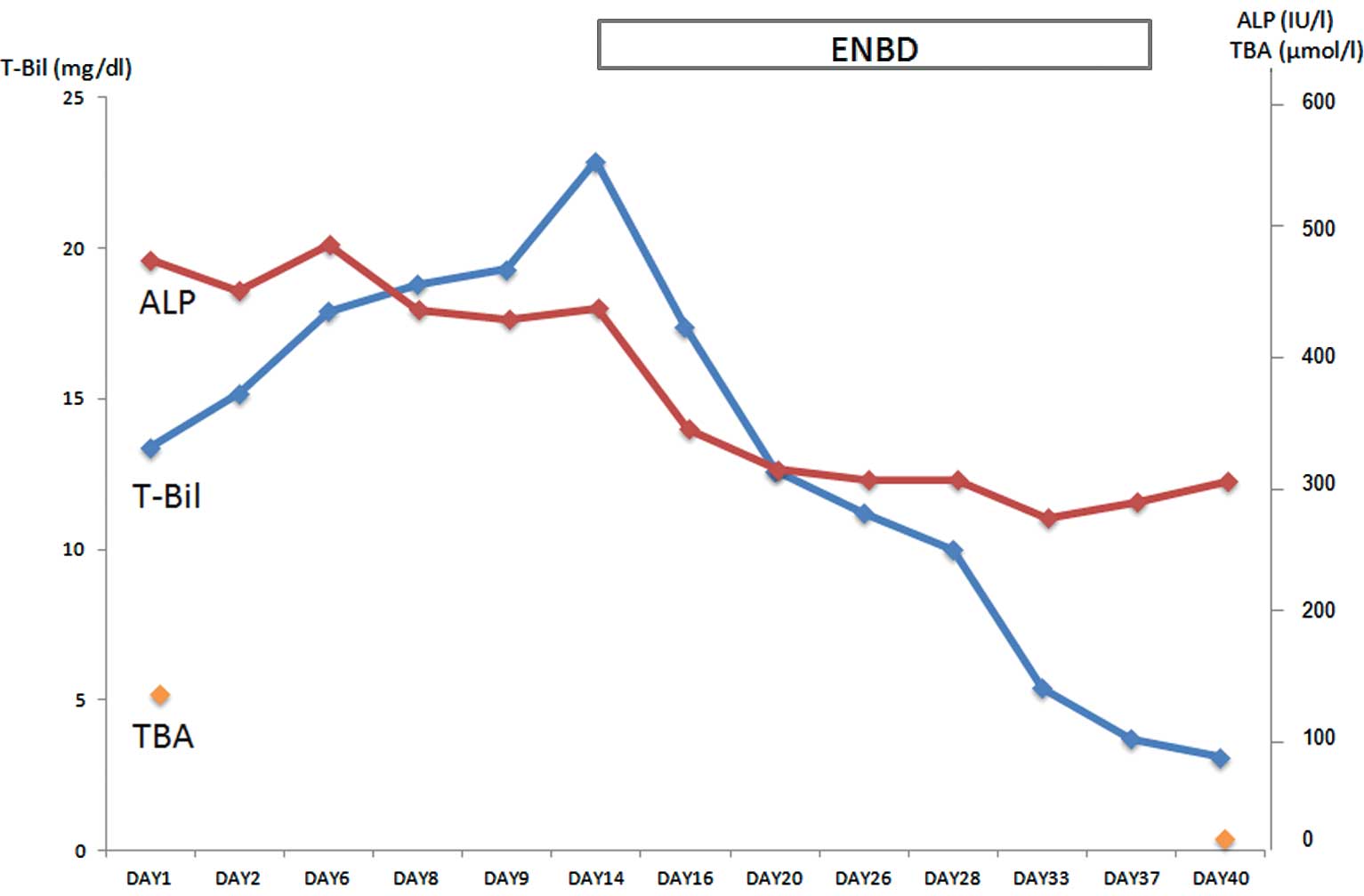
The patient was discharged well on hospital day 41. The patient’s
clinical course following admission is shown in Fig. 10. Written informed patient consent
was obtained from the patient. This study was performed with
approval of the Ethics Committee at Tokyo Rosai Hospital (Tokyo,
Japan).
Discussion
BRIC is a rare condition that is clinically
diagnosed according to five criteria: multiple episodes of jaundice
with severe pruritus and laboratory findings suggestive of
cholestasis; absence of factors known to be associated with
cholestasis, including drugs and pregnancy; normal intrahepatic and
extrahepatic bile ducts confirmed by direct cholangiography; liver
biopsy demonstrating bile thrombus formation; and a symptom-free
interval lasting several months to years (7). BRIC-1 is an autosomal recessive
disorder caused by a mutation in the ATP8B1 gene on
chromosome 18q21 that encodes the FIC1 protein, a P-type ATPase.
The gene is also known to be responsible for the development of
PFIC. The FIC1 protein is localized to the cell membranes of the
hepatocytes that form the bile canaliculi (8). Although the functions of the FIC1
protein have not been fully elucidated, one of its suggested
functions is to flip phospholipids present in the outer layer of
the lipid bilayer of cell membrane, such as phosphatidylserine,
into the inner layer. Lack of the FIC1 protein leads to disruption
of the lipid arrangement in the cell membrane exposed to the bile
canaliculus and increases susceptibility to damage caused by
hydrophobic bile acid, resulting in cholestasis (9). Recently, a new form of BRIC was
reported with a mutation not in the ATP8B1 gene but in the
ABCB11 gene on chromosome 2q24 (4). This has become known as BRIC-2. The
ABCB11 gene encodes the BSEP protein whose mutation is also
known to cause PFIC-2. BSEP protein is localized to the cell
membrane of hepatocytes exposed to the bile canaliculi and
functions to transport bile acids from the hepatocytes into the
canaliculi. Thus, it is difficult to clinically distinguish between
the two types of BRIC associated with mutations in different genes.
Moreover, although BRIC shares the same causative gene with PFIC,
PFIC is characterized by persistent cholestasis that may progress
to cirrhosis, whereas BRIC is characterized by recurrent but
transient cholestasis that does not cause permanent damage to the
liver.
BRIC is also characterized by non-age-related
occurrences, recurrent episodes of pruritus and increased levels of
bilirubin with a dominant direct fraction and blood TBA. Episodes
may last from several weeks to months and usually resolve
spontaneously. The severity of jaundice varies from mild to severe
and the interval between the jaundice episodes varies from months
to a decade or more (10,11). In the present study, the patient
was under considerable stress from pressure at work and began
suffering from malaise around April 2012. He then experienced
pruritus of an unprecedented intensity and had white stool in early
May 2012. The patient was informed by his wife that his eyes had
become yellow. In the majority of BRIC cases, the initial clinical
manifestation is of severe pruritus followed by an awareness of
jaundice and white stool a few days later (12), which is similar to the present
case. Another characteristic finding is a mild increase in ALT/AST
(<3 times the normal level). In the present study, only mild
increases were observed in these parameters, with an ALT of 49 IU/l
and an AST of 29 IU/l. The absence of bile duct distension is
another characteristic finding of BRIC. US, CT and ERCP were
performed in the present study due to increased bilirubin levels
with a dominant direct fraction on the blood tests at admission,
but there were no abnormal findings that indicated bile duct
narrowing. Detailed examinations to identify the cause of the
jaundice were performed although viral hepatitis and autoimmune
hepatitis were suspected. The patient was identified as negative
for anti-HCV, anti-HBs, HBsAb, anti-nuclear antibodies (ANA) and
anti-mitochondiral antibodies (AMA), indicating that hepatitis C/B
and autoimmune hepatitis were unlikely. Drug-induced hepatitis was
also suspected, but again ruled out as there had been no changes in
the patient’s oral medication in the past year. Due to the
persistent and progressive increase in bilirubin even after
admission, a liver biopsy was also performed. This procedure
revealed bile deposition in the centrilobular hepatocytes and bile
thrombus formation, with minimal inflammatory and fibrotic
findings. These findings were consistent with the characteristic
features of BRIC (13).
The patient had not experienced symptoms of jaundice
and pruritus prior to this occurrence and, although genetic
analysis was not performed, the diagnosis of BRIC was made based on
the clinical course and histological findings. Sonazoid-enhanced US
with At-PI was also performed. This procedure allows for the degree
of progression of a hepatic lesion to be evaluated by analyzing
blood flow balance between the hepatic artery and portal vein, the
two vessels supplying the liver. In the present study, the ROR was
calculated as 15.7% during an episode of jaundice which was
comparable to that of the normal liver (13.7%) as reported
previously (6). This also
suggested the absence of a progressive lesion in the liver and
supported the diagnosis of BRIC, rather than PFIC. Moreover, the
fact that there was no significant difference between the ROR
calculated during the episode of jaundice and that calculated while
the patient was recovering from jaundice also suggests that
cholestasis in BRIC does not affect the portal vein/hepatic artery
blood flow balance.
BRIC is associated with recurrent cholestasis but
does not cause permanent damage to the liver, it therefore differs
from PFIC which is associated with persistent cholestasis and
progression to cirrhosis. This means that monitoring for the
presence or absence of progression to cirrhosis is essential for
the differential diagnosis between the two conditions.
Sonazoid-enhanced US with At-PI may be performed non-invasively and
repeatedly, unlike liver biopsies, and thus is useful in monitoring
and identifying patients with early BRIC or PFIC.
Ursodeoxycholic acid is used as the first-line
treatment for jaundice and pruritus in most cases of BRIC, but it
is not necessarily effective (14). The efficacy of rifampicin against
cholestasis (15) and the
usefulness of cholestyramine have also been documented, but certain
studies suggest that these agents have a transient effect (16). While a study has suggested that
medical therapy for BRIC has insufficient effects, the usefulness
of ENBD has been demonstrated by Stapelbroek et al(17), who performed ENBD for bile drainage
in 3 patients with BRIC and reported that blood TBA levels (100–200
μmol/l pretreatment) decreased to almost normal levels 3 days after
the insertion of an ENBD tube. A resolution of pruritus was also
reported in all patients. The present study also showed significant
decreases in the patient’s TBA and total bilirubin levels in ∼1
week following ENBD tube insertion and additionally that these
levels were maintained almost within the normal ranges even
subsequent to tube removal. ENBD may therefore be considered as
highly effective in the treatment of jaundice and pruritus in BRIC.
The effectiveness of ENBD against cholestasis in BRIC may be
explained by evidence showing that it forces bile drainage, blocks
the enterohepatic circulation and that subsequent reduction in the
bile acid pool results in restoring the function of bile excretion
transporters. In obstructive jaundice, an internal fistula is
usually created by bile duct stenting. In cases of BRIC, however,
bile duct stenting is ineffective as there is no narrowing or other
abnormality in the intra- or extrahepatic bile ducts and bile
drainage cannot be achieved by an internal fistula. It remains
unclear as to how transporter function is restored by ENBD.
Stapelbroek et al(17)
noted increased levels of phospholipids other than
phosphatidylcholine, particularly sphingomyelin, in the bile
drained by ENBD and suggested that a disrupted phospholipid
gradient may have been restored as a result of the bile acid pool
being reduced.
In conclusion, although a rare condition, the
possibility of BRIC should be considered and an appropriate workup
should be performed when patients present with severe pruritus, an
increase in direct bilirubin and a mild increase in transaminase
levels. The use of ENBD should be considered for the treatment of
BRIC if jaundice is not improved by the first-line treatment of
ursodeoxycholic acid.
In suspected cases of early BRIC or PFIC,
Sonazoid-enhanced US with At-PI may be useful in distinguishing
between the two conditions as it is minimally invasive and may be
performed repeatedly.
Abbreviations:
|
At-PI
|
arrival time parametric imaging
|
|
US
|
ultrasonography
|
|
CT
|
computed tomography
|
|
AMA
|
anti-mitochondrial antibody
|
|
ANA
|
anti-nuclear antibody
|
|
p-ANCA
|
perinuclear anti-neutrophil cytoplasm
autoantibodies
|
|
TBA
|
total bile acids
|
|
BRIC
|
benign recurrent intrahepatic
cholestasis
|
|
ENBD
|
endoscopic nasobiliary drainage
|
|
ROR
|
ratio of red
|
|
ROI
|
region of interest
|
|
PFIC
|
progressive familial intrahepatic
cholestasis
|
|
BSEP
|
bile salt export pump
|
References
|
1.
|
Summerskill WH and Walshe JM: Benign
recurrent intrahepatic ‘obstructive’ jaundice. Lancet. 31:686–690.
1959.
|
|
2.
|
Luketic VA and Shiffman ML: Benign
recurrent intrahepatic cholestasis. Clin Liver Dis. 8:133–149.
2004. View Article : Google Scholar
|
|
3.
|
Bull LN, van Eijk MJ, Pawlikowska L, et
al: A gene encoding a P-type ATPase mutated in two forms of
hereditary cholestasis. Nat Genet. 18:219–224. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
van Mil SW, van der Woerd WL, van der
Brugge G, et al: Benign recurrent intrahepatic cholestasis type 2
is caused by mutations in ABCB11. Gastroenterology. 127:379–384.
2004.PubMed/NCBI
|
|
5.
|
Takahashi T, Miura T, Nakamura J, Yamada S
and Yanagi M: A case of benign recurrent intrahepatic cholestasis
type 1 in that laparoscopy with liver biopsy was crucial for
diagnosis. Gastroenterol Endosc. 51:2723–2727. 2009.(In
Japanese).
|
|
6.
|
Wakui N, Takayama R, Kanekawa T, et al:
Usefulness of arrival time parametric imaging in evaluating the
degree of liver disease progression in chronic hepatitis C
infection. J Ultrasound Med. 31:373–382. 2012.PubMed/NCBI
|
|
7.
|
Tygstrup N and Jensen B: Intermittent
intrahepatic cholestasis of unknown etiology in five young males
from the Faroe Islands. Acta Med Scand. 185:523–530. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Verhulst PM, van der Velden LM, Oorschot
V, et al: A flippase-independent function of ATP8B1, the protein
affected in familial intrahepatic cholestasis type 1, is required
for apical protein expression and microvillus formation in
polarized epithelial cells. Hepatology. 51:2049–2060. 2010.
View Article : Google Scholar
|
|
9.
|
Cai SY, Gautam S, Nguyen T, et al: ATP8B1
deficiency disrupts the bile canalicular membrane bilayer structure
in hepatocytes, but FXR expression and activity are maintained.
Gastroenterology. 136:1060–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
de Pagter AG, van Berge Henegouwen GP, ten
Bokkel Huinink JA and Brandt KH: Familial benign recurrent
intrahepatic cholestasis. Interrelation with intrahepatic
cholestasis of pregnancy and from oral contraceptives?
Gastroenterology. 71:202–207. 1976.PubMed/NCBI
|
|
11.
|
Brenard R, Geubel AP and Benhamou JP:
Benign recurrent intrahepatic cholestasis. A report of 26 cases. J
Clin Gastroenterol. 11:546–551. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Friedman JR, Russo P, Flick J, Mamula P
and Piccoli DA: Cases in pediatric gastroenterology from the
Children’s Hospital of Philadelphia: a 14-year-old boy with
jaundice and pruritus. Med Gen Med. 6:612004.
|
|
13.
|
Tygstrup N, Steig BA, Juijn JA, Bull LN
and Houwen RH: Recurrent familial intrahepatic cholestasis in the
Faeroe Islands. Phenotypic heterogeneity but genetic homogeneity.
Hepatology. 29:506–508. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
van der Woerd WL, van Mil SW, Stapelbroek
JM, et al: Familial cholestasis: progressive familial intrahepatic
cholestasis, benign recurrent intrahepatic cholestasis and
intrahepatic cholestasis of pregnancy. Best Pract Res Clin
Gastroenterol. 24:541–553. 2010.
|
|
15.
|
Cançado EL, Leitão RM, Carrilho FJ and
Laudanna AA: Unexpected clinical remission of cholestasis after
rifampicin therapy in patients with normal or slightly increased
levels of gamma-glutamyl transpeptidase. Am J Gastroenterol.
93:1510–1517. 1998.
|
|
16.
|
Uegaki S, Tanaka A, Mori Y, et al:
Successful treatment with colestimide for a bout of cholestasis in
a Japanese patient with benign recurrent intrahepatic cholestasis
caused by ATP8B1 mutation. Intern Med. 47:599–602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Stapelbroek JM, van Erpecum KJ, Klomp LW,
et al: Nasobiliary drainage induces long-lasting remission in
benign recurrent intrahepatic cholestasis. Hepatology. 43:51–53.
2006. View Article : Google Scholar : PubMed/NCBI
|