Introduction
Radiation delivery to the head and neck is a common
treatment modality for malignancies. Salivary glands in the
radiation field are severely damaged and patients experiencing
reduced salivary flow suffer from considerable morbidity, including
xerostomia, dental caries, mucosal infection, dysphagia and
extensive discomfort (1,2). Although significant improvements,
including the introduction of intensity modulated radiotherapy
(IMRT), have been made for targeting radiation more precisely to
the tumor and sparing normal tissues (3), the occurrence of radiation-induced
sialadentitis is still inevitable (4). Therefore, it is important to increase
the tolerance of normal tissues by using a radioprotector to
improve patients’ quality of life.
Previously, human clinical trials and animal studies
have revealed amifostine as a promising radioprotective agent that
may reduce xerostomia in patients (5) and preserve salivary gland function,
particularly that of the parotid glands (6,7).
However, it also has clinically undesirable manifestations,
including nausea, vomiting, hypotension, allergic reaction,
thrombocytopenia and leucopenia (8,9).
Junn et al also reported that varying doses of amifostine
had no evident cytoprotective effects in three groups of cancer
patients treated with primary chemoradiation (10). Amifostine imposes a high level of
physical discomfort on patients and may lead to treatment
interruption (10). Therefore, due
to its high toxicity and the possibility of it protecting tumors
(11), alternatives to amifostine
should be explored.
It is known that the water-secretory function of the
salivary gland is regulated by α-adrenoceptors and muscarinic
receptors. In order to prevent xerostomia and improve the secretive
function of the salivary gland following irradiation, one study
pretreated rat parotid glands with cyclocytidine (an α-adrenoceptor
agonist) and pilocarpine (a muscarinic receptor agonist) (12). Data revealed that cyclocytidine
effectively protected the parotid gland against weight loss and
flow rate reduction at early and late phases, while pilocarpine
caused no significant change in any of the glandular parameters
(12). Furthermore, phenylephrine,
an α1-adrenoceptor agonist, has also shown efficacy in
cytoprotection against early phase irradiation damage in the
parotid gland (13). However, the
exact mechanism remains unknown. The aim of this study was to
investigate the molecular mechanism of cytoprotection by
phenylephrine pretreatment in rat submandibular glands following
irradiation.
Materials and methods
Animals
Male Wistar rats, weighing 230–250 g were used. They
were kept in polycarbonate cages under an alternating 12 h
1ight/dark cycle. The animals were maintained on laboratory chow
and water ad libitum. All experimental procedures were
approved by the Animal Care and Use Committee and were in
accordance with the Guidance of the Ministry of Public Health for
the care and use of laboratory animals.
The rats were randomly divided into three groups as
follows: i) the control group (n=9); ii) the irradiation-only group
(n=9); and iii) the phenylephrine pretreatment group (n=9).
Phenylephrine (5 mg/kg) was injected intraperitoneally 30 min prior
to irradiation. The control and irradiation-only groups were
administered the same volume of saline. The submandibular glands
were removed on day 7 post-irradiation under standard
anesthesia.
Irradiation
Prior to irradiation, rats were anesthetized by an
intraperitoneal injection of ketamine (130 mg/kg), weighed and then
firmly immobilized in a box shielded with 3 mm thickness of lead,
such that only the head and neck regions were exposed. The rats
were locally irradiated in the region of the head and neck with a
single dose of 20 Gy. We used a radiation dose that has been used
in previous studies and that was expected to cause significant
gland impairment (14).
Irradiation was carried out with 6 MV X-rays from a Varian 23 EX
linac linear accelerator (Varian Medical Systems, Palo Alto, CA,
USA) at a dose rate of 5 Gy/min. The irradiation field was an area
of ∼3x24 cm2 and the distance from the source was 100
cm. Four rats were irradiated simultaneously. Control animals were
anesthetized and sham-radiated. All irradiation was carried out
from 11:00 to 13:00.
Reagents and antibodies
Phenylephrine was purchased from Sigma (St. Louis,
MO, USA). Antibodies against proliferation cell nuclear antigen
(PCNA) and biotin-conjugated anti-goat immunoglobulin secondary
antibody were purchased from Santa Cruz Biotechnology Inc. (Santa
Cruz, CA, USA). The in situ cell death detection kit was
purchased from Roche Applied Science (Penzberg, Germany). Other
chemicals and reagents were of analytical grade.
Light microscopic observation
The submandibular gland tissues were fixed in 10%
neutral-buffered formalin and processed for paraffin embedding
according to a standard procedure. The submandibular gland sections
were stained with hematoxylin and eosin (H&E) to evaluate the
morphological changes by light microscopy.
Immunohistochemistry
The submandibular gland tissues were fixed in 10%
neutral buffered formalin and embedded in paraffin. The sections (4
μm thick) were rinsed several times in phosphate-buffered
saline (PBS) and then blocked with 3% hydrogen peroxide
(H2O2) and 3% normal goat serum to eliminate
non-specific staining. Then, the sections were incubated with goat
polyclonal antibody against PCNA (1:100) overnight at 4°C.
Biotin-conjugated anti-goat immunoglobulin secondary antibody was
applied for 2 h at room temperature. Following incubation with
streptavidin-horseradish peroxidase substrate, the slides were
counterstained with hematoxylin. Negative controls were incubated
with goat IgG in place of the primary antibody. The PCNA-positive
cells were counted in 10 different fields in each section under
×400 magnification.
Terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate-biotin nick end
labeling (TUNEL) staining
To detect apoptotic cells, the TUNEL method was
performed using the in situ cell death detection kit, POD.
The detection procedure was performed according to the
manufacturer’s instructions. Briefly, the tissue sections were
incubated with terminal deoxynucleotidyl transferase in a
humidified chamber at 37°C for 1 h. A mixture of
antidigoxigenin-peroxidase and substrate-chromagen was used for
visualization and the sections were counterstained with
hematoxylin. The nuclei of apoptotic cells were stained dark brown
and counted in 10 different fields in each section under a light
microscope at ×400 magnification.
Statistical analysis
Data are expressed as mean ± standard error of the
mean (SEM). Comparison of means was performed by one-way analysis
of variance (ANOVA) followed by the Bonferroni test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Histopathological alterations of the
submandibular gland post-irradiation
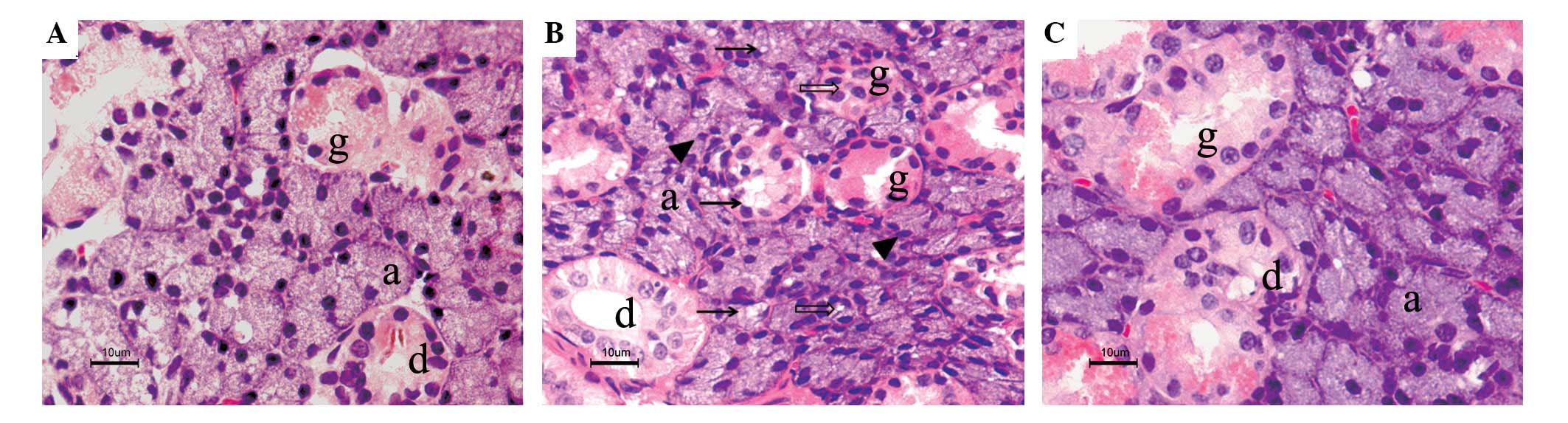
Normal acinar and ductal cells were observed in the
control submandibular glands under a light microscope (Fig. 1A). In the irradiated submandibular
glands, pathohistological changes were expressed as vacuolization
of acinar cells, pyknotic nuclei and lysis of entire acini and
granular convoluted tubules (Fig.
1B). However, in the phenylephrine-pretreated submandibular
glands, the levels of acinar cellular atrophy and degeneration were
much less than those in the irradiated glands and the morphologic
manifestation was much closer to that of the control glands
(Fig. 1C).
Proliferation in the submandibular gland
post-irradiation
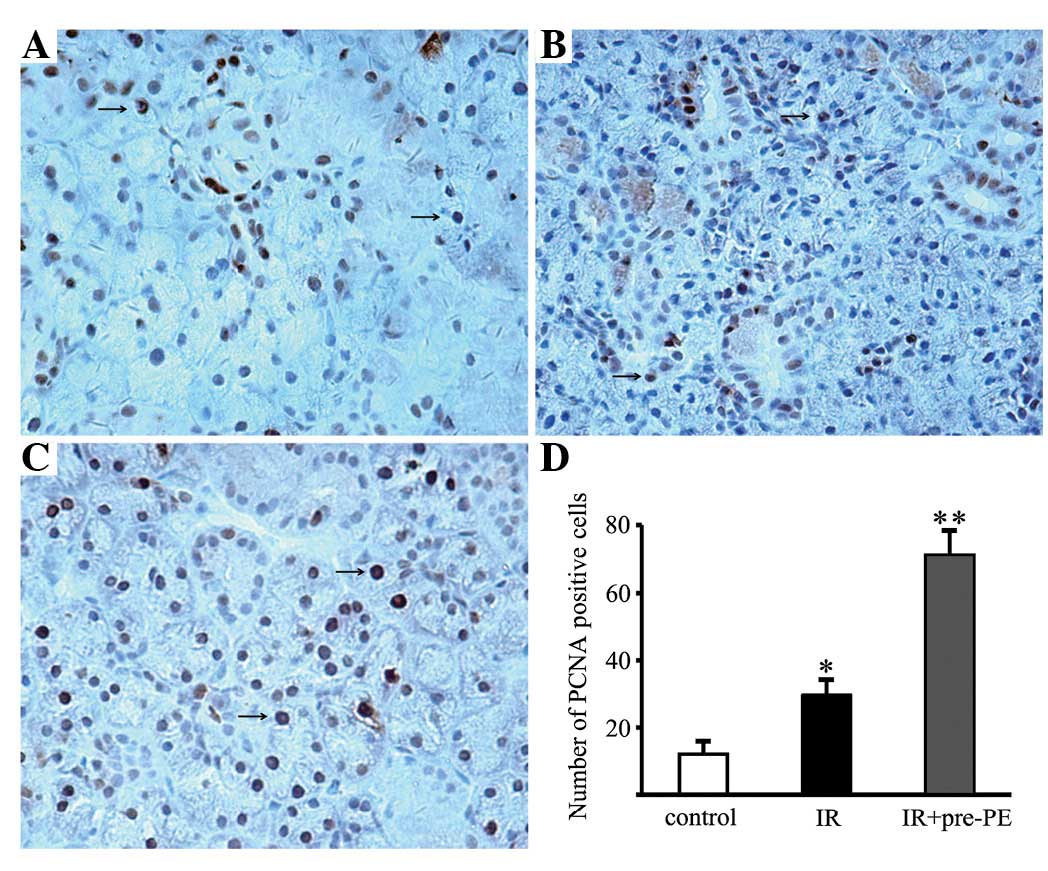
A number of brown-nucleus PCNA-positive cells were
identified in the ductal cells in the control submandibular gland
(Fig. 2A). The numbers of
PCNA-positive cells were increased in the acinar cells, granular
convoluted tubules and ductal cells of the irradiated glands
(Fig. 2B). They were further
increased in the phenylephrine-pretreated glands (Fig. 2C). The numbers of PCNA-positive
cells in the control glands, irradiated glands and
phenylephrine-pretreated glands were 12.11±3.66, 29.56±4.45 and
71.22±7.17 per high-power field, respectively (Fig. 2D).
Apoptosis in the submandibular gland
post-irradiation
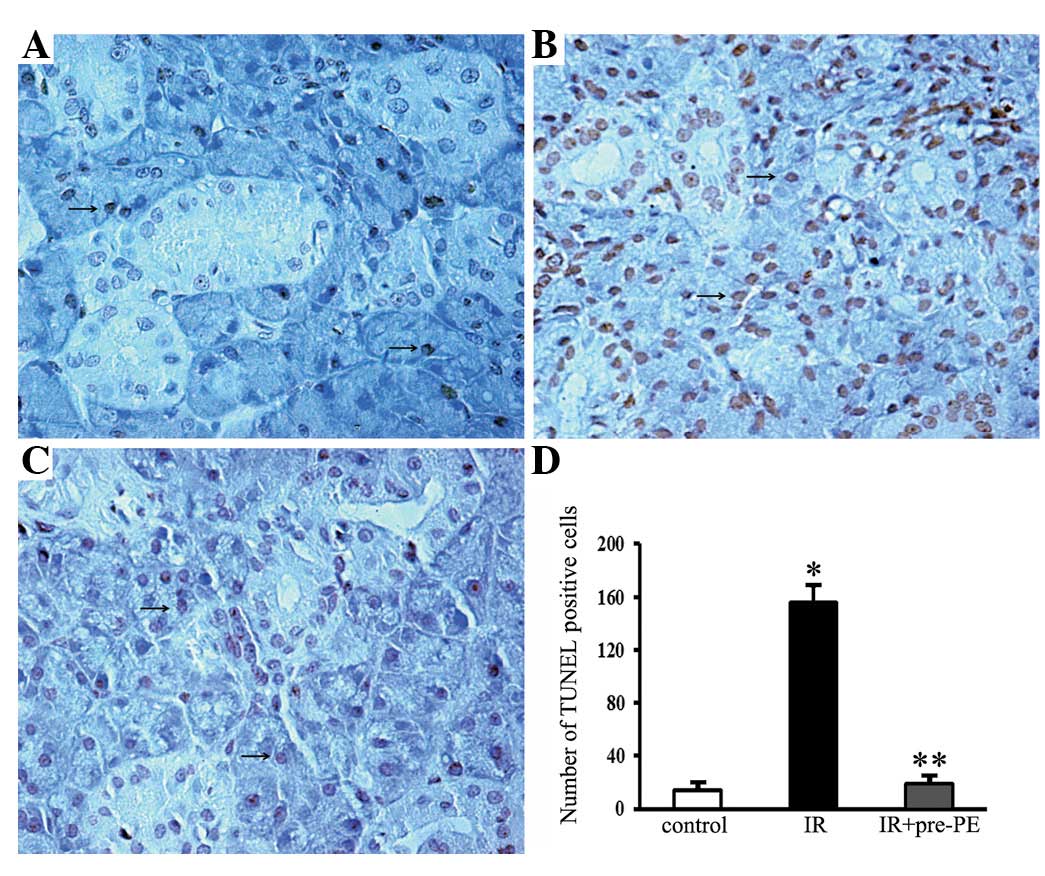
The control submandibular gland revealed very few
apoptotic cells (Fig. 3A).
Apoptotic activity was markedly increased in the acinar cells,
intercalated cells and granular convoluted tubule cells following
irradiation. TUNEL-positive cells with shrunken cell bodies and
condensed nuclei were observed in the irradiated glands (Fig. 3B). Conversely, only a few
TUNEL-positive cells were detected in the phenylephrine-pretreated
glands (Fig. 3C). The number of
TUNEL-positive cells in the control glands, irradiated glands and
phenylephrine-pretreated irradiated glands were 13.67±3.39,
155.44±12.71 and 19.11±2.99 per high-power field, respectively
(Fig. 3D).
Discussion
Elucidation of the potential mechanisms underlying
salivary gland radio-sensitivity has been approached by functional
animal studies as well as from a molecular perspective. The most
consistent observations in all animal models include significant
reductions in flow rate, loss of glandular weight and loss of
acinar cells (14–16). A study in rats reported a 40%
reduction in salivary flow rates with single doses of 5 or 10 Gy
and a 60% reduction following 15 or 20 Gy, three days after
treatment (14). Additionally,
studies have indicated that α-adrenoceptor agonists have the
potential to be used as radio-protectants (12,13).
Although the entity of the protective effect has been described and
studied extensively, its underlying mechanism remains enigmatic.
The results of the present study demonstrated the mechanisms
underlying the cytoprotective effect of phenylephrine, which may be
related to the improvement of cell proliferation and inhibition of
apoptosis.
In the present study, histological staining
experiments revealed that notable atrophy and degeneration occurred
in the submandibular gland following irradiation, including
vacuolization of acinar cells, pyknotic nuclei and lysis of entire
acini and granular convoluted tubules. By contrast, the atrophy and
degeneration were markedly reduced by the phenylephrine
pretreatment. Our data was supported by another study which
suggested that the secretory granules of acinar cells are damaged
by radiation-induced lipid peroxidation, which leads to the lysis
of these cells (17).
Administration of α-adrenergic agonists prior to radiation has been
shown to maintain partial salivary flow in rats and mice (12,17–19).
Loss of serous acinar cells has been linked to reductions in
salivary flow since these cells are responsible for water and
protein secretion (20). Notably,
there are other studies that have confirmed the protective effects
of α-adrenergic agonist administration, whose results indicated
that the secretory granules do not play the important role
previously assumed in affecting the radio-sensitivity of the
salivary glands (13). These
studies suggest that the underlying mechanism of the observed
improvement in salivary gland function may involve a secondary
messenger-induced increase in the proliferation of salivary gland
cells, resulting in the recovery of tissue following irradiation
(13). The complexity of salivary
gland morphology suggests the involvement of multiple pathways in
the dysfunction following irradiation. Therefore, it is valuable to
uncover the regulatory events and functional repair mechanisms in
the cellular response of the salivary gland to irradiation.
Enhanced cell proliferation following irradiation is
commonly considered as a sign of initiation of regeneration of the
gland tissue (21). It is known
that the activation of the α1-adrenoceptors by
phenylephrine plays an important role in promoting cell survival
and DNA repair, growth and proliferation (22,23).
In order to determine the mechanism of cytoprotection, we examined
the effect of phenylephrine pretreatment on cell proliferation in
irradiated submandibular glands. In this study, the number of
PCNA-positive cells increased in irradiated glands compared with
the controls. The data was consistent with an earlier study which
reported increases in proliferation in all gland compartments,
reaching a maximum level at day 6 post-irradiation under 15 Gy
(21). Our data also demonstrated
that the number of PCNA-positive cells further increased in
phenylephrine-pretreated glands. The results from our study
demonstrate that the regenerative capacity of irradiated glands may
be promoted by phenylephrine, resulting in improved tissue renewal
following irradiation. A number of individuals have permanent
salivary gland hypofunction (24),
which has been attributed to the attrition of acinar cells followed
by replacement with fibrotic tissue (25). Further investigation is required to
determine whether phenylephrine inhibits mesenchymal fibrosis in
irradiated salivary glands.
The major cause of significant acinar cell loss
across animal models following irradiation has been widely debated.
A study in rats quantified radiation-induced apoptosis by counting
condensed nuclei and reported 2–3% apoptotic cells 6 h after
treatment with a broad range of doses (2.5–25 Gy) (26). The extent of apoptosis was not
dose-dependent and the authors concluded that the magnitude of
apoptosis did not explain the significant loss of function
(26). Conversely,
radiation-induced apoptosis is dose-dependent in the parotid glands
of mice, with significantly higher levels detected by
immunohistochemistry against activated caspase-3 (27,28).
Mouse parotid glands are ∼30% apoptotic 24 h after a single 5-Gy
exposure (27,28). One study reported that 5–8% of
murine submandibular gland acinar cells are apoptotic 3 days after
exposure to 7.5 and 15 Gy (21),
while another study observed only 2% apoptosis 24 h after
irradiation with 5 Gy (28). To
understand the cytoprotective mechanism of phenylephrine on the
irradiated submandibular gland, we examined the level of apoptosis
using TUNEL staining. An increase in the number of TUNEL-positive
cells was detected in the irradiated group compared with the
controls, which accords with the studies that reported apoptosis in
irradiated submandibular glands (21,29,30).
We also identified that the pre-administration of phenylephrine
resulted in a marked decrease in the number of TUNEL-positive cells
in the treated group. Previous studies have suggested that
phenylephrine protects cells against apoptosis triggered by certain
stressors, including injury induced by ischemia/reperfusion in the
rabbit submandibular gland and hypoxia and serum deprivation in
neonatal rat cardiomyocytes (31,32).
In the current study, we further proved that the cytoprotective
mechanism of phenylephrine in irradiated submandibular glands may
be related to its anti-apoptotic efficacy.
Multiple pathways, including p53 and protein kinase
C-δ (PKC-δ) regulation of apoptosis, may lead to salivary gland
dysfunction following irradiation (27,28).
Ionizing radiation (5 Gy) induced p53 transcriptional activation
and apoptosis of mouse salivary acinar cells in vitro and
in vivo(27).
PKC-δ-deficient mice exhibited significantly lowered levels of
radiation-induced apoptosis (1 and 5 Gy) (28). Importantly, our previous study
demonstrated that phenylephrine increases the expression of
phospho-PKC-ζ to improve cell survival in submandibular glands that
have been damaged by ischemia/reperfusion injury (23). Since the response of the salivary
glands to irradiation is complex and presumably multi-factorial, it
will be important to investigate the α1-adrenoceptor
signaling pathway to improve our understanding of
irradiation-induced salivary gland dysfunction and possibly
contribute to the development of new treatment strategies.
In conclusion, our findings provide the first
evidence that the mechanism of the protective effect of
phenylephrine is related to the improvement of cell proliferation
and inhibition of apoptosis in irradiated submandibular glands.
Future research into the molecular mechanism may lead to new
therapeutic interventions to improve the quality of life for
patients undergoing irradiation therapy for head and neck
malignancies.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (Nos.
30973335 and 81170978).
References
|
1.
|
Nagler RM and Baum BJ: Prophylactic
treatment reduces the severity of xerostomia following radiation
therapy for oral cavity cancer. Arch Otolaryngol Head Neck Surg.
129:247–250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Vissink A, Jansma J, Spijkervet FK,
Burlage FR and Coppes RP: Oral sequelae of head and neck
radiotherapy. Crit Rev Oral Biol Med. 14:199–212. 2003. View Article : Google Scholar
|
|
3.
|
Braam PM, Terhaard CH, Roesink JM and
Raaijmakers CP: Intensity modulated radiotherapy significantly
reduces xerostomia compared with conventional radiotherapy. Int J
Radiat Oncol Biol Phys. 66:975–980. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bhide SA, Newbold KL, Harrington KJ and
Nutting CM: Clinical evaluation of intensity-modulated radiotherapy
for head and neck cancers. Br J Radiol. 85:487–494. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Brizel DM, Wasserman TH, Henke M, et al:
Phase III randomized trial of amifostine as a radioprotector in
head and neck cancer. J Clin Oncol. 18:3339–3345. 2000.PubMed/NCBI
|
|
6.
|
McDonald S, Meyerowitz C, Smudzin T and
Rubin P: Preliminary results of a pilot study using WR-2721 before
fractionated irradiation of the head and neck to reduce salivary
gland dysfunction. Int J Radiat Oncol Biol Phys. 29:747–754. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Rudat V, Münter M, Rades D, et al: The
effect of amifostine or IMRT to preserve the parotid function after
radiotherapy of the head and neck region measured by quantitative
salivary gland scintigraphy. Radiother Oncol. 89:71–80. 2008.
View Article : Google Scholar
|
|
8.
|
Rades D, Fehlauer F, Bajrovic A, Mahlmann
B, Richter E and Alberti W: Serious adverse effects of amifostine
during radio-therapy in head and neck cancer patients. Radiother
Oncol. 70:261–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Büntzel J, Küttner K, Fröhlich D and
Glatzel M: Selective cyto-protection with amifostine in concurrent
radiochemotherapy for head and neck cancer. Ann Oncol. 9:505–509.
1998.PubMed/NCBI
|
|
10.
|
Junn JC, Sciubba JJ, Bishop JA, et al: The
effect of amifostine on submandibular gland histology after
radiation. Int J Otolaryngol. 2012:5082792012.PubMed/NCBI
|
|
11.
|
Lindegaard JC and Grau C: Has the outlook
improved for amifostine as a clinical radioprotector? Radiother
Oncol. 57:113–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Coppes RP, Zeilstra LJ, Kampinga HH and
Konings AW: Early to late sparing of radiation damage to the
parotid gland by adrenergic and muscarinic receptor agonists. Br J
Cancer. 85:1055–1063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Nagler RM and Laufer D: Protection against
irradiation-induced damage to salivary glands by adrenergic agonist
administration. Int J Radiat Oncol Biol Phys. 40:477–481. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Vissink A, ‘s-Gravenmade EJ, Ligeon EE and
Konings WT: A functional and chemical study of radiation effects on
rat parotid and submandibular/sublingual glands. Radiat Res.
124:259–265. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Nagler RM: Short- and long-term functional
vs morphometrical salivary effects of irradiation in a rodent
model. Anticancer Res. 18:315–320. 1998.PubMed/NCBI
|
|
16.
|
Li J, Shan Z, Ou G, Liu X, Zhang C, Baum
BJ and Wang S: Structural and functional characteristics of
irradiation damage to parotid glands in the miniature pig. Int J
Radiat Oncol Biol Phys. 62:1510–1516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Nagler R, Marmary Y, Fox PC, Baum BJ,
Har-El R and Chevion M: Irradiation-induced damage to the salivary
glands: the role of redox-active iron and copper. Radiat Res.
147:468–476. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Coppes RP, Roffel AF, Zeilstra LJ, Vissink
A and Konings AW: Early radiation effects on muscarinic
receptor-induced secretory responsiveness of the parotid gland in
the freely moving rat. Radiat Res. 153:339–346. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Takakura K, Takaki S, Takeda I, Hanaue N,
Kizu Y, Tonogi M and Yamane GY: Effect of cevimeline on
radiation-induced salivary gland dysfunction and AQP5 in
submandibular gland in mice. Bull Tokyo Dent Coll. 48:47–56. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Turner RJ: Mechanisms of fluid secretion
by salivary glands. Ann NY Acad Sci. 694:24–35. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Bralic M, Muhvic-Urek M, Stemberga V, et
al: Cell death and cell proliferation in mouse submandibular gland
during early post-irradiation phase. Acta Med Okayama. 59:153–159.
2005.PubMed/NCBI
|
|
22.
|
Alexandrov A, Keffel S, Goepel M and
Michel MC: Stimulation of alpha1A-adrenoceptors in Rat-1 cells
inhibits extracellular signal-regulated kinase by activating p38
mitogen-activated protein kinase. Mol Pharmacol. 54:755–760.
1998.
|
|
23.
|
Xiang B, Zhang Y, Li YM, Zhang K, Zhang
YY, Wu LL and Yu GY: Effects of phenylephrine on transplanted
submandibular gland. J Dent Res. 85:1106–1111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Li Y, Taylor JM, Ten Haken RK and Eisbruch
A: The impact of dose on parotid salivary recovery in head and neck
cancer patients treated with radiation therapy. Int J Radiat Oncol
Biol Phys. 67:660–669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Radfar L and Sirois DA: Structural and
functional injury in minipig salivary glands following fractionated
exposure to 70 Gy of ionizing radiation: an animal model for human
radiation-induced salivary gland injury. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 96:267–274. 2003. View Article : Google Scholar
|
|
26.
|
Paardekooper GM, Cammelli S, Zeilstra LJ,
Coppes RP and Konings AW: Radiation-induced apoptosis in relation
to acute impairment of rat salivary gland function. Int J Radiat
Biol. 73:641–648. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Limesand KH, Schwertfeger KL and Anderson
SM: MDM2 is required for suppression of apoptosis by activated Akt1
in salivary acinar cells. Mol Cell Biol. 26:8840–8856. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Humphries MJ, Limesand KH, Schneider JC,
Nakayama KI, Anderson SM and Reyland ME: Suppression of apoptosis
in the protein kinase Cδ null mouse in vivo. J Biol Chem.
281:9728–9737. 2006.
|
|
29.
|
Muhvic-Urek M, Bralic M, Curic S,
Pezelj-Ribaric S, Borcic J and Tomac J: Imbalance between apoptosis
and proliferation causes late radiation damage of salivary gland in
mouse. Physiol Res. 55:89–95. 2006.PubMed/NCBI
|
|
30.
|
Avila JL, Grundmann O, Burd R and Limesand
KH: Radiation-induced salivary gland dysfunction results from
p53-dependent apoptosis. Int J Radiat Oncol Biol Phys. 73:523–529.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Xiang B, Zhang Y, Li YM, Gao Y, Gan YH, Wu
LL and Yu GY: Phenylephrine protects autotransplanted rabbit
submandibular gland from apoptosis. Biochem Biophys Res Commun.
377:210–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Zhu H, McElwee-Witmer S, Perrone M, Clark
KL and Zilberstein A: Phenylephrine protects neonatal rat
cardiomyocytes from hypoxia and serum deprivation-induced
apoptosis. Cell Death Differ. 7:773–784. 2000. View Article : Google Scholar : PubMed/NCBI
|