Introduction
Worldwide, approximately 1 in 3 adults are smokers
(1–3). In developed countries, smoking is the
main cause of early mortality in adults. Approximately half of the
individuals that have been smoking since adolescence succumb to
smoking-related diseases (4). Many
simply consider smoking as a bad habit. In fact, it is a type of
disease. The World Health Organization (WHO) defines smoking as a
chronic addiction disease, and smokers are patients with a chronic
disease.
Nicotine is the main component of tobacco. It
exhibits pharmacological effects by interacting with nicotinic
acetylcholine receptors (nAChRs). Animal experiments and clinical
studies have demonstrated that nicotine has analgesic effects
(5–7). However, studying the physiological
changes in the body following nicotine withdrawal has more
practical significance than simply studying its analgesic effect.
Nicotine withdrawal causes a number of responses, including
convulsion, tremor, bradycardia and depression, as well as neural
adaptive changes in glutamate and dopamine receptors and
desensitization of nAChRs (8). In
a rat model of chronic pain with sciatic nerve injury, the pain
sensitivity to mechanical stimulation in nicotine-dependent rats
increases, with phosphorylation of cyclic adenosine monophosphate
(cAMP)-response element binding protein (CREB) in dorsal horn
neurons, activation of microglia and an increase in interleukin
(IL)-1β (9,10). In clinical practice, there is a
high incidence of back pain in long-term smokers. At 48 h after
coronary artery bypass grafting, the dose of opioid drugs for
smoking patients is 33% greater than that for non-smoking patients.
Female smokers take more opioid drugs for analgesia than
non-smokers following gynecological surgery (11). The increased postoperative pain
sensitivity for smokers may be related to nicotine dependence
caused by long-term smoking and perioperative nicotine withdrawal.
However, the mechanism remains unclear. Therefore, the
establishment of an animal model of postoperative pain with
nicotine dependence and withdrawal to study the mechanism of
increased pain sensitivity is extremely important.
Malin et al (12–14)
established a rat model of nicotine dependence and withdrawal by
subcutaneously embedding automatic ALZET® osmotic mini
pumps. However, this model has the following problems: i) although
a stable plasma nicotine concentration is maintained by the mini
pumps, there are no continuous smokers in real life, and ii) the
mini pump is expensive. In the present study, a rat model of
plantar incisional pain was established based on the previous rat
model of nicotine dependence and withdrawal. The changes in pain
sensitivity to mechanical and thermal stimulation were observed.
This study provides a basis for further investigation of the
mechanisms of increased postoperative sensitivity to pain in
smokers after quitting smoking.
Materials and methods
Animal grouping
Clean healthy male Wistar rats (150–200 g) were
provided by the Pharmaceutical Industry Research Institute of
Shandong Province, China [SCXK (Lu) 20080002]. This study was
carried out in strict accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health. The animal use protocol has been reviewed and
approved by the Institutional Animal Care and Use Committee (IACUC)
of Shandong Province-owned Hospital Affiliated to Shandong
University. Twelve healthy male Wistar rats were randomly divided
into a control and withdrawal group, with 6 rats in each group. In
the control group, the rats were raised in normal conditions (room
temperature 24±2°C, 50% humidity, circadian rhythm alternation,
free food and water) without any treatment. Then, the plantar
incisional pain model was created in the right lower extremity and
changes in the healthy and operative side plantar mechanical
withdrawal threshold (MWT) and thermal withdrawal latency (TWL)
were observed for 7 successive days. In the withdrawal group, the
rats were fed in normal conditions and treated with a subcutaneous
injection of 3 mg/kg pure nicotine (Sigma-Aldrich, St. Louis, MO,
USA) 3 times a day (7:00, 15:00 and 23:00) for 7 days. Mecamylamine
is a nicotinic antagonist and precipitates nicotine abstinence
syndrome in rats. The plantar incisional pain model was then
created in the right lower extremity, and the changes in bilateral
plantar MWT and TWL were observed for 7 days.
Establishment of the incisional pain
model
The rat model of incisional pain was established
according to the method of Brennan et al (15). Following anesthetic induction with
2% isoflurane, the anesthesia was maintained using 1.4% isoflurane.
Following disinfection with diluted iodine tincture, a 1-cm
longitudinal incision (from the plantar proximal end to the toe)
was made. The subcutaneous muscle was picked up, followed by
longitudinal cutting. The muscle starting site and attachment point
were kept intact. After gently pressing for hemostasis, the
successive layers of incision were sutured. Wound infection was
prevented by the administration of 40,000 units penicillin. The
rats were then raised in a quiet and warm environment. Following
surgery, there was no dyskinesia in the postoperative foot;
however, the rats were unwilling to touch the ground with the foot
and licking behavior appeared. This indicated that the incisional
pain model was successfully established.
Determination of MWT
Determination of MWT was performed at 8:00–12:00
using a BME-404 electronic mechanical stimulator (Institute of
Biomedical Engineering, Chinese Academy of Medical Sciences,
Tianjin, China). The main technical parameters of this equipment
were as follows: end face diameter of test needle, 0.6 mm; pressure
measurement range, 0.1–50 g; and pressure measurement resolution,
0.05 g. An organic glass box (26×20×14 cm) was placed on the sieve
of the metal frame. The rat was placed into the box for 30-min
adaptation. The lower extremity plantar surface was touched with
the test needle until the escaping behavior appeared. The duration
of measurement was 1 min. The pressure value was automatically
recorded. The measurement was conducted 5 times for each rat
(interval, ≥5 min) and the mean was calculated as MWT for this
measurement.
Determination of TWL
Following MWT measurement, determination of TWL was
performed using a BME-410C automatic heat pain stimulator
(Institute of Biomedical Engineering). The main technical
parameters were as follows: 12 V/10 W halogen lamp; area of
stimulating light, <20 mm2; timing accuracy, 10 msec;
and stimulation temperature, 45–65°C (adjustable). The host
equipment was placed on the desktop, with a lamp stand under the
table. An organic glass box was placed on the glass plate
(thickness, 3 mm). The rat was placed into the box for 30-min
adaptation. The lower extremity plantar surface was stimulated with
light irradiation until the withdrawal response appeared. The pain
latency time was recorded. The measurement was repeated 5 times for
each rat (interval, ≥5 min) and the mean was calculated as TWL for
this measurement.
Statistical analysis
Data were expressed as the means ± standard
deviation (SD). Statistical analyses were performed using SPSS 13.0
statistical software (SPSS Inc., Chicago, IL, USA). The repeated
measures analysis of variance (ANOVA) was performed for comparisons
within the groups and a least significant difference (LSD) test was
used for comparisons between the two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
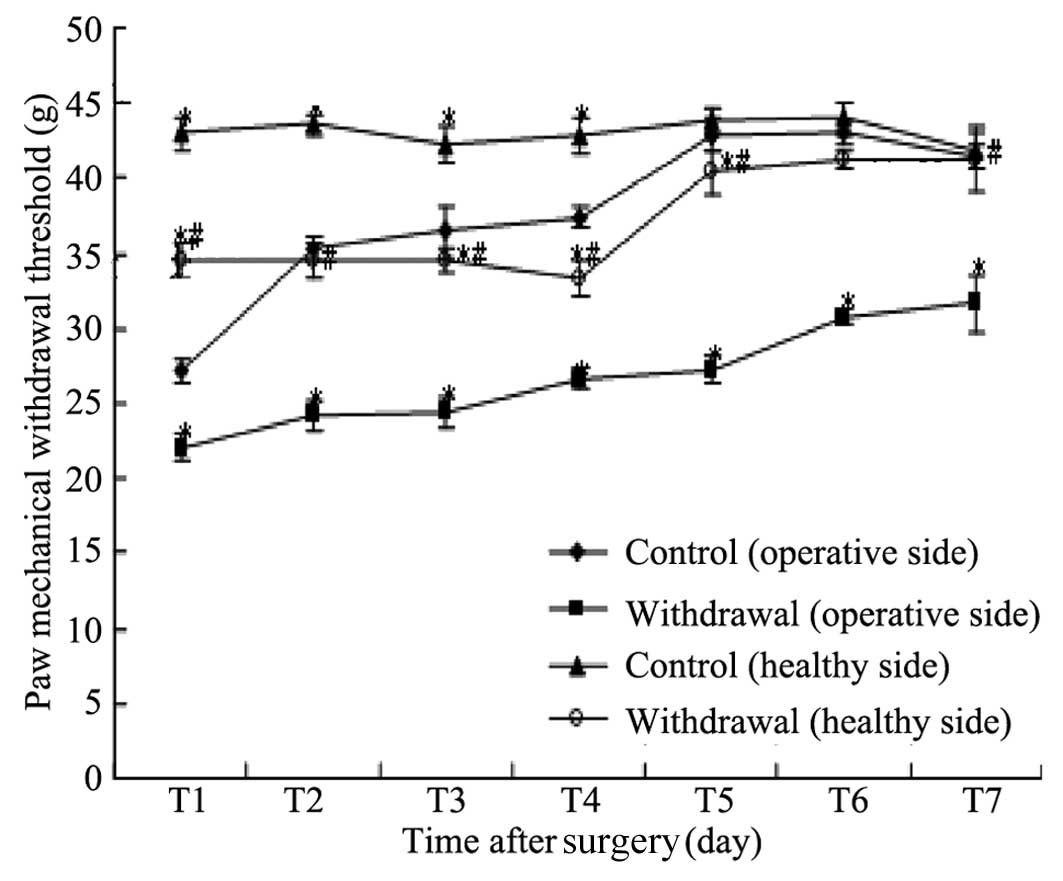
Variations of MWT in the two groups
As shown in Table I
and Fig. 1, compared with the
operative side plantar MWT in the control group, the operative side
plantar MWT in the withdrawal group was significantly lower on
postoperative days 1–7, respectively (P<0.05) and the healthy
side plantar MWT in the control group was significantly higher on
postoperative days 1–4, respectively (P<0.05), with no
significant difference in postoperative days 5–7 (P>0.05).
Compared with the healthy side plantar MWT in the control group,
the healthy side plantar MWT in the withdrawal group was
significantly reduced on postoperative days 1–7, respectively
(P<0.05).
 | Table IMWT in the two groups on different
postoperative days. |
Table I
MWT in the two groups on different
postoperative days.
| Group | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
|---|
| Control (operative
side) | 27.25±0.78 | 35.24±0.81 | 36.42±1.77 | 37.38±0.74 | 42.93±1.09 | 43.10±0.80 | 41.46±0.73 |
| Withdrawal (operative
side) | 22.09±0.89a | 24.25±1.00a | 24.48±1.00a | 26.66±0.74a | 27.25±0.89a | 30.83±0.49a | 31.69±1.91a |
| Control (healthy
side) | 42.96±1.01a | 43.58±0.72a | 42.26±1.27a | 42.90±1.19a | 43.92±0.81 | 43.96±1.08 | 41.88±1.19 |
| Withdrawal (healthy
side) |
34.58±1.05a,b | 34.57±1.19b |
34.52±0.75a,b |
33.31±1.24a,b |
40.41±1.53a,b |
41.23±0.65a,b | 41.28±2.16b |
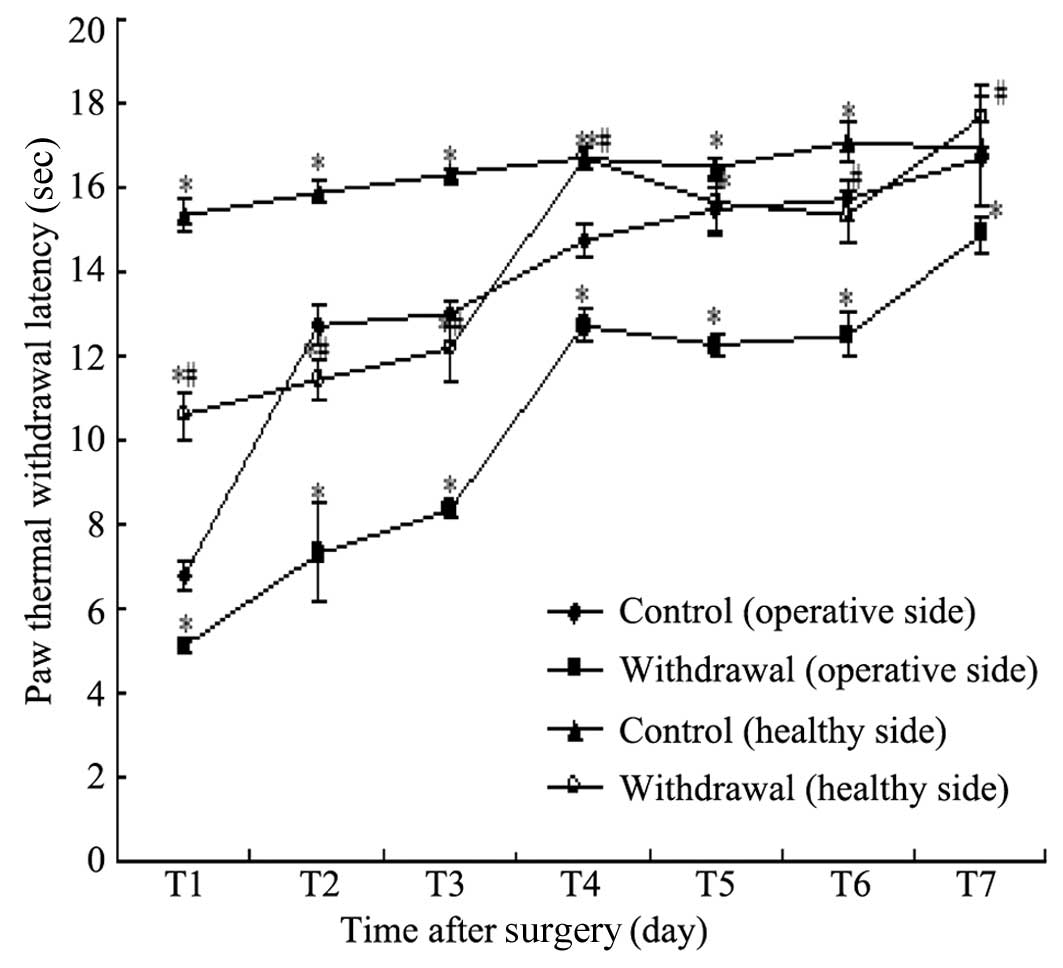
Variations of TWL in the two groups
TWL in the two groups on different postoperative
days are shown in Table II and
Fig. 2. Compared with the
operative side in the control group, the operative side plantar TWL
in the withdrawal group was significantly lower on days 1–7,
respectively (P<0.05) and the healthy plantar TWL in the control
group was significantly higher on days 1–6 (P<0.05). Compared
with the healthy side plantar TWL in the control group, the healthy
plantar TWL in the withdrawal group was significantly reduced on
days 1, 2, 3, 5 and 6 (P<0.05).
 | Table IITWL in the two groups on different
postoperative days. |
Table II
TWL in the two groups on different
postoperative days.
| Group | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
|---|
| Control (operative
side) | 6.76±0.38 | 12.74±0.52 | 13.00±0.32 | 14.74±0.38 | 15.49±0.53 | 15.69±0.51 | 16.72±0.85 |
| Withdrawal (operative
side) | 5.12±0.16a | 7.31±1.17a | 8.33±0.14a | 12.69±0.37a | 12.22±0.27a | 12.49±0.54a | 14.85±0.41a |
| Control (healthy
side) | 15.34±0.40a | 15.89±0.28a | 16.33±0.13a | 16.68±0.25a | 16.45±0.25a | 17.09±0.47a | 16.89±1.34 |
| Withdrawal (healthy
side) |
10.57±0.56a,b |
11.43±0.47a,b |
12.20±0.83a,b | 16.68±0.24a | 15.67±0.75b | 15.32±0.65b | 17.67±0.74 |
Discussion
This study identified that the pain sensitivities of
the operative and healthy sides to mechanical and thermal
stimulation significantly increase in rat models of incisional pain
with nicotine dependence and withdrawal. This is consistent with a
previous study reporting the increase of postoperative pain
sensitivity in patients after quitting smoking (11). However, the exact
pathophysiological mechanism of pain modulation by smoking remains
unclear.
Over 4,000 different chemical substances have been
identified in tobacco. Animal experiments and clinical studies
suggest that nicotine is the main component involved in pain
modulation (6,16). Exposure to nicotine has significant
effects on the central and peripheral nervous systems by combining
with nAChRs, which pass through the Na+, Ca2+
and K+ channels. Activation of post-synaptic nAChRs
directly acts on excitatory neurons through these cation channels.
The activation of presynaptic nAChRs affects the release of other
neurotransmitters, including dopamine, glutamate, γ-aminobutyric
acid (GABA), 5-hydroxytryptamine (5-HT), histamine and
noradrenaline (17). These effects
are related to the anti-nociceptive effect of nicotine and the
mechanisms involved. Studying the mechanism of pain modulation
following nicotine withdrawal has greater clinical significance.
Nicotine withdrawal causes numerous emotional responses. In the
clinic, various types of chronic pain, including back pain, carpal
tunnel syndrome and complex regional pain syndrome, are aggravated
in long-term smokers (18–20). After quitting smoking, the
postoperative pain is more severe than in non-smoking patients, so
more painkillers are required (21,22).
These phenomena may be associated with the anti-nociceptive effect
of nicotine and the increased pain sensitivity in long-term smoking
patients after quitting smoking.
The incisional pain rat model, in which
postoperative pain is simulated, was proposed by Brennan et
al (15). In this model, rats
present spontaneous pain, allodynia and thermal hyperalgesia
following incisional surgery. At 30 min after surgery, allodynia
appears in the foot on the operative side. The allodynia reaches a
peak at 2 h and continues for 4–7 days, while the non-operative
side is not affected. These pain behaviors and durations of time
are similar to clinical intraoperative and postoperative pain. In
the current study, the changes in pain behavior in the healthy and
operative side planta in the control group are consistent with the
above results. The pain sensitivities to mechanical and thermal
stimulation in incisional pain rat models with nicotine dependence
and withdrawal are significantly higher than in the control. This
is consistent with the clinical increase of postoperative pain
sensitivity in smokers after quitting smoking.
Long-term exposure to nicotine induces the
upregulation and inactivation of nAChRs, resulting in a decrease of
inhibitory neurotransmitter release. For patients with acute
smoking cessation, the increased pain sensitivity in the first week
is related to the elevated utilization of β2*-nAChR in
the thalamus (23). It was
identified that microglia are the responder cells that respond the
fastest to injuries, including trauma, ischemia and inflammation.
They interact with neurons through chemokines, proinflammatory
cytokines, inflammatory mediators, nutritional factors and
autoreceptors, participating in the occurrence and development of
pain (24). In addition, nAChRs
play an important role in the cholinergic anti-inflammatory pathway
(25–27). Further studies are required to
investigate the correlation of increased pain sensitivity to
mechanical and thermal stimulation with activation of microglia in
incisional pain rat models with nicotine dependence and withdrawal,
as well as the changes in inflammatory mediators in the incision
site.
In conclusion, the pain sensitivity to mechanical
and thermal stimulation significantly increases in incisional pain
rat models with nicotine dependence and withdrawal. This is
consistent with the clinical increase of postoperative pain in
smokers after quitting smoking and has provided a basis for further
investigation into the related pathophysiological mechanisms.
References
|
1
|
Peto R, Lopez AD, Boreham J, Thun M, Heath
C Jr and Doll R: Mortality from smoking worldwide. British Med
Bull. 52:12–21. 1996. View Article : Google Scholar
|
|
2
|
Benowitz NL: Clinical pharmacology of
nicotine: implications for understanding, preventing, and treating
tobacco addiction. Clin Pharmacol Ther. 83:531–541. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mathers CD and Loncar D: Projections of
global mortality and burden of disease from 2002 to 2030. PLoS Med.
3:e4422006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schultz H: Tobacco or health: A global
status report. Ann Saudi Med. 18:1951998.PubMed/NCBI
|
|
5
|
Jankowski CJ, Weingarten TN, Martin DP, et
al: Randomised trial of intranasal nicotine and postoperative pain,
nausea and vomiting in non-smoking women. Eur J Anaesthesiol.
28:585–591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flood P and Daniel D: Intranasal nicotine
for postoperative pain treatment. Anesthesiology. 101:1417–1421.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anderson KL, Pinkerton KE, Uyeminami D,
Simons CT, Carstens MI and Carstens E: Antinociception induced by
chronic exposure of rats to cigarette smoke. Neurosci Lett.
366:86–91. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dani JA, Jenson D, Broussard JI and De
Biasi M: Neurophysiology of nicotine addiction. J Addict Res Ther.
S12011.PubMed/NCBI
|
|
9
|
Brett K, Parker R, Wittenauer S, Hayashida
K, Young T and Vincler M: Impact of chronic nicotine on sciatic
nerve injury in the rat. J Neuroimmunol. 186:37–44. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Josiah DT and Vincler MA: Impact of
chronic nicotine on the development and maintenance of neuropathic
hypersensitivity in the rat. Psychopharmacology (Berl).
188:152–161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi Y, Weingarten TN, Mantilla CB, Hooten
WM and Warner DO: Smoking and pain: pathophysiology and clinical
implications. Anesthesiology. 113:977–992. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malin DH: Nicotine dependence: studies
with a laboratory model. Pharmacol Biochem Behav. 70:551–559. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malin DH, Lake JR, Carter VA, et al: The
nicotinic antagonist mecamylamine precipitates nicotine abstinence
syndrome in the rat. Psychopharmacology (Berl). 115:180–184. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malin DH, Lake JR, Newlin-Maultsby P, et
al: Rodent model of nicotine abstinence syndrome. Pharmacol Biochem
Behav. 43:779–784. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brennan TJ, Vandermeulen EP and Gebhart
GF: Characterization of a rat model of incisional pain. Pain.
64:493–501. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simons CT, Cuellar JM, Moore JA, et al:
Nicotinic receptor involvement in antinociception induced by
exposure to cigarette smoke. Neurosci Lett. 389:71–76. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
LeSage MG, Keyler DE, Shoeman D, Raphael
D, Collins G and Pentel PR: Continuous nicotine infusion reduces
nicotine self-administration in rats with 23-h/day access to
nicotine. Pharmacol Biochem Behav. 72:279–289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nicotine and caffeine intake in complex
regional pain syndrome. J Back Musculoskelet Rehabil. 16:33–38.
2002.PubMed/NCBI
|
|
19
|
Nathan PA, Meadows KD and Istvan JA:
Predictors of carpal tunnel syndrome: an 11-year study of
industrial workers. J Hand Surg Am. 27:644–651. 2002.PubMed/NCBI
|
|
20
|
Nyiendo J, Haas M, Goldberg B and Sexton
G: Pain, disability, and satisfaction outcomes and predictors of
outcomes: a practice-based study of chronic low back pain patients
attending primary care and chiropractic physicians. J Manipulative
Physiol Ther. 24:433–439. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Z, Arheart KL, Morris R, et al:
CYP2D6 poor metabolizer genotype and smoking predict severe
postoperative pain in female patients on arrival to the recovery
room. Pain Med. 13:604–609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Woodside JR: Female smokers have increased
postoperative narcotic requirements. J Addict Dis. 19:1–10. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cosgrove KP, Esterlis I, McKee S, et al:
Beta2* nicotinic acetylcholine receptors modulate pain
sensitivity in acutely abstinent tobacco smokers. Nicotine Tob Res.
12:535–539. 2010.
|
|
24
|
Kreutzberg GW: Microglia: a sensor for
pathological events in the CNS. Trends Neurosci. 19:312–318. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Simone R, Ajmone-Cat MA, Carnevale D
and Minghetti L: Activation of alpha7 nicotinic acetylcholine
receptor by nicotine selectively up-regulates cyclooxygenase-2 and
prostaglandin E2 in rat microglial cultures. J Neuroinflammation.
2:42005.PubMed/NCBI
|
|
26
|
Libert C: Inflammation: A nervous
connection. Nature. 421:328–329. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang H, Yu M, Ochani M, et al: Nicotinic
acetylcholine receptor alpha7 subunit is an essential regulator of
inflammation. Nature. 421:384–388. 2003. View Article : Google Scholar : PubMed/NCBI
|