Introduction
Human periodontal diseases are predominantly caused
by infections with gram-negative bacteria, such as Porphyromonas
gingivalis and Bacteroides forsythus(1). A complex interaction between these
bacteria and the host immune system may induce inflammatory
conditions that result in the loss of the collagenous structures
that support the teeth (2,3). A number of inflammatory mediators,
such as interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor-α
(TNF-α), prostaglandins and matrix metalloproteinases (MMPs) are
involved in periodontal diseases (4,5).
These mediators may affect the activities of leukocytes,
osteoblasts and osteoclasts and promote the tissue remodeling
process systemically and locally (6–8). The
collagenolytic enzymes, including MMPs, are mediated by a variety
of inflammatory cytokines, such as IL-1, IL-6, IL-8 and TNF-α
(9,10).
IL-6, a multifunctional cytokine, has a number of
biological activities, including B-lymphocyte differentiation,
T-lymphocyte proliferation and the stimulation of immunoglobulin
(Ig) secretion by B-lymphocytes (11). In particular, IL-6 induces bone
resorption by itself and in conjunction with other bone-resorbing
agents (12). IL-8, formerly known
as neutrophil-activating peptide-1 (NAP-1), is important in the
initiation and development of inflammatory processes through its
capacity to attract and activate neutrophils (13). IL-8-mediated chemotactic and
activation effects on neutrophils in the inflamed gingiva may
contribute to the periodontal tissue destruction (14). TNF-α, secreted predominantly by
monocytes and macrophages, is a potent inflammatory cytokine that
upregulates the production of collagenases, prostaglandin (PG) E2,
chemokines and cytokines, cell adhesion molecules and bone
resorption-related factors (10,14).
The levels of IL-6, IL-8 and TNF-α have been
observed to be elevated in chronically inflamed gingival tissues,
as well as in the gingival crevicular fluid from patients with
periodontitis (8,10,14–18).
It has been suggested that the protein expression levels of IL-6,
IL-8 and TNF-α may be clinical parameters of gingival and
periodontal inflammatory conditions. The aim of the current study
was to quantify IL-6, IL-8 and TNF-α levels and assess the
correlation of these inflammatory markers in the human gingival
tissues of patients with periodontitis.
Subjects and methods
Study subjects and Institutional Review
Board (IRB)
This study was conducted according to the guidelines
of the Declaration of Helsinki and approved by the IRB of the Kyung
Hee University Dental Hospital (Reg. no. 2009-1), Seoul, Korea. The
participants voluntarily provided written informed consent.
Nineteen patients with periodontitis, aged 25–70 years old
(49.2±12.7 years, mean age ± standard deviation) were enrolled for
the study, which was performed using the gingival tissues of the 19
patients. Samples were obtained whilst the patients underwent
periodontal surgery. The tissues of the periodontitis lesions were
collected by periodontology specialists in the Department of
Periodontology of the Kyung Hee University Dental Hospital.
Total protein extraction from the tissues
of patients with periodontitis
Each sample was homogenized in 500 ml of
phosphate-buffered saline (PBS; 137 mM NaCl, 10 mM
Na2HPO4 and 2.7 mM KCl; pH 7.3) with a
protease inhibitor cocktail (Roche Korea, Seoul, Korea). The
samples were centrifuged at 16,000 × g for 15 min at 4°C, and the
supernatant was refrigerated at −70°C until tested. A Bio-Rad
protein assay (Bio-Rad Life Science Group, Hercules, CA, USA) was
used to quantify the protein concentration.
Enzyme-linked immunosorbent assay
(ELISA)
The assessment of the IL-6, IL-8 and TNF-α levels in
the tissues was performed by ELISA, using a human IL-6, IL-8 and
TNF-α ELISA Max™ Set Deluxe (BioLegend, Inc., San Diego, CA, USA),
in accordance with the manufacturer's instructions. Briefly, one
day prior to running the assay, 96-well plates were coated with the
capture antibody. Following 18 h incubation at 4°C, the plates were
washed with PBS containing 0.05% Tween-20 (Sigma-Aldrich, St.
Louis, MO, USA) and then incubated for 1 h at room temperature with
a diluent buffer to block nonspecific binding. After washing, 100
ml sample (100 mg) was added to each well and then incubated for 2
h at room temperature. After washing of the plates, 100 ml
biotinylated detection antibody was added to each well. The plates
were then incubated for 1 h, prior to further washing. Following
this, 100 ml avidin-horseradish peroxidase (HRP) was added to each
well followed by incubation for 30 min at room temperature. After
further washing, 3,3′,5,5′-tetramethylbenzidine (TMB) substrate
solution was added and the plates were incubated in the dark for 15
min. The reaction was stopped by the addition of 100 ml 2 N
sulfuric acid, and the absorbance at 450 nm and 570 nm was
measured.
Statistical analysis
Statistical analysis for the correlation line was
performed using SPSS 20.0 statistical software (SPSS, Inc.,
Chicago, IL, USA). The Pearson correlation coefficient (r) and
associated probability (P) were calculated for the data sets of
each combination of the three cytokine expressions. P<0.05 was
considered to indicate a statistically significant difference
(19).
Results
The study employed human gingival tissue samples
from 19 patients (male, n=14; female, n=5) with periodontitis. The
protein expression levels of IL-6, IL-8 and TNF-α were determined
by ELISA. As shown in Table I, the
protein expression of the three target cytokines was detected in
all the gingival tissue samples, with particularly high levels of
IL-8 observed, compared with those of the other two cytokines.
 | Table IDemographic and laboratory data of the
19 patients with periodontitis. |
Table I
Demographic and laboratory data of the
19 patients with periodontitis.
| No. | Gender | Age (years) | IL-6 (pg/ml) | IL-8 (pg/ml) | TNF-α (pg/ml) |
|---|
| 1 | M | 49 | 3.53±0.42 | 20.49±2.24 | 21.42±1.74 |
| 2 | M | 54 | 10.21±0.07 | 44.06±1.17 | 20.87±0.32 |
| 3 | M | 56 | 53.08±0.45 | 97.04±0.01 | 20.28±0.01 |
| 4 | M | 70 | 3.47±0.02 | 19.98±0.33 | 20.51±0.23 |
| 5 | M | 31 | 7.80±0.03 | 23.54±0.37 | 20.32±0.17 |
| 6 | M | 64 | 3.56±0.03 | 22.85±0.67 | 20.87±0.35 |
| 7 | M | 54 | 12.34±0.15 | 49.50±1.64 | 20.50±0.13 |
| 8 | M | 42 | 7.02±0.24 | 30.40±2.70 | 20.96±0.18 |
| 9 | M | 47 | 4.72±0.04 | 38.13±1.06 | 21.24±0.86 |
| 10 | M | 47 | 3.84±0.01 | 26.88±0.29 | 21.09±0.39 |
| 11 | M | 63 | 10.86±0.75 | 42.94±0.01 | 20.05±0.01 |
| 12 | M | 53 | 3.60±0.01 | 26.71±0.58 | 20.78±0.09 |
| 13 | M | 39 | 3.31±0.01 | 19.53±0.63 | 20.98±2.86 |
| 14 | M | 63 | 8.35±0.23 | 41.06±5.41 | 21.07±2.08 |
| 15 | F | 42 | 3.41±0.15 | 21.40±0.01 | 20.09±0.01 |
| 16 | F | 65 | 7.12±0.01 | 28.99±2.51 | 21.52±0.77 |
| 17 | F | 25 | 3.40±0.04 | 24.23±0.01 | 20.06±0.01 |
| 18 | F | 32 | 5.89±0.03 | 42.53±0.20 | 20.02±0.09 |
| 19 | F | 40 | 4.20±0.25 | 26.00±0.92 | 20.66±0.06 |
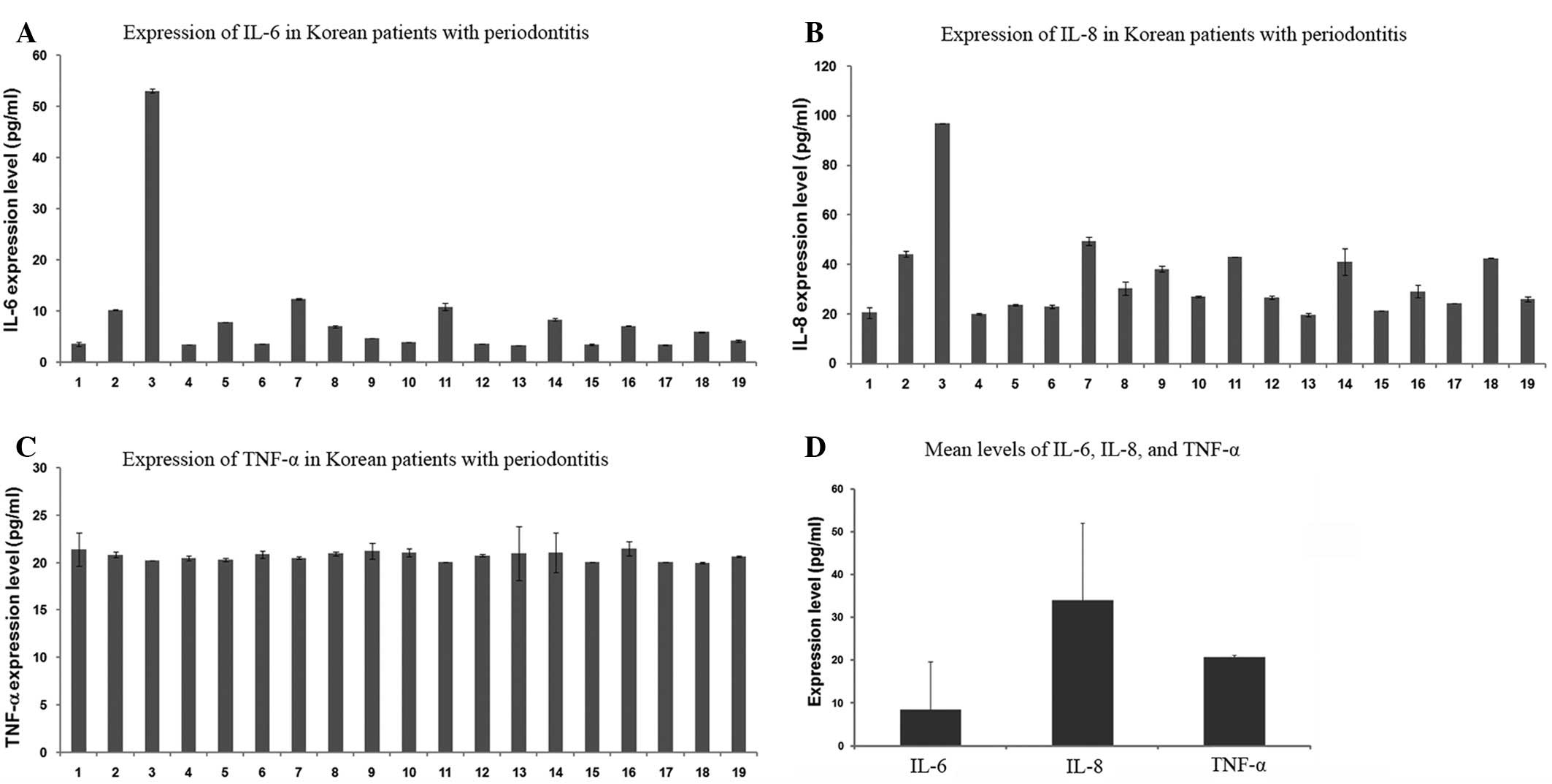
In Table I and
Fig. 1, the concentrations of
IL-6, IL-8 and TNF-α in the gingival tissue samples from the 19
patients with periodontitis are presented. The IL-6 levels of the
majority of the patients were observed to measure between 3 and 13
pg/ml, with the exception of one patient (No. 3, 53.08±0.45 pg/ml;
Fig. 1A). Similarly, the IL-8
levels were detected to be between 19 and 50 pg/ml, with the
exception of one patient (No. 3, 97.04±0.01 pg/ml; Fig. 1B). However, the TNF-α levels in all
samples were between 20 and 22 pg/ml (Fig. 1C). Interindividual fluctuations
were observed in the IL-6 and IL-8 levels among the 19 patients,
while there was little difference in the TNF-α levels (Table I). As shown in Fig. 1D, the mean levels of IL-6, IL-8 and
TNF-α were 8.41±0.25, 34.01±1.09 and 20.70±0.31 pg/ml,
respectively. In a comparison of the three tested cytokines, IL-8
was highly expressed, whereas IL-6 was detected at low levels and
TNF-α was moderately expressed. The mean levels of IL-6, IL-8 and
TNF-α in the 14 male patients were 9.69, 35.93 and 20.78 pg/ml,
respectively, and in the five female patients were 4.80, 28.63 and
20.47 pg/ml, respectively (data not shown). Although the female
sample number was small, the mean levels of IL-6 and IL-8 in the
five female patients appeared to be lower than those in the 19
patients as a whole, while the mean level of TNF-α in the five
female patients appeared consistent with that of the 19 patients.
The mean level of IL-6 in the five female patients (4.80 pg/ml) was
~49.5% of that in the 14 male patients (9.69 pg/ml), which was due
to the fact that the interindividual fluctuations of the IL-6
levels in the female patients were relatively small, and the levels
remained between 3 and 8 pg/ml.
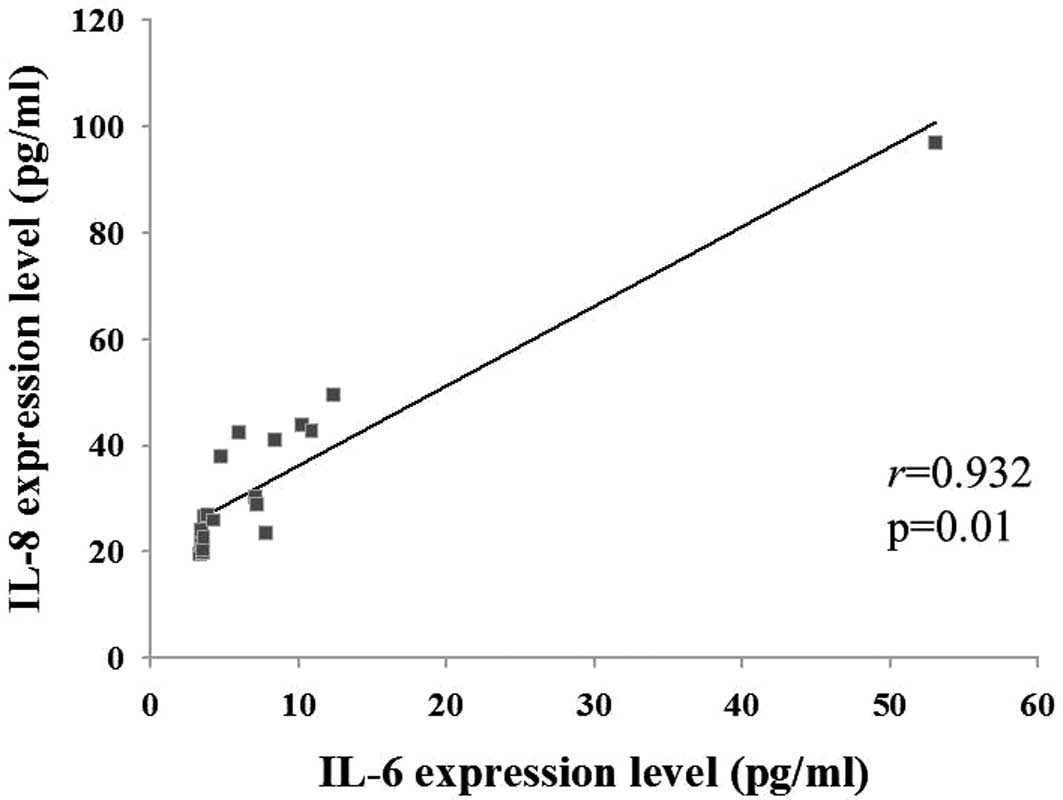
As shown in Fig. 2,
the Pearson correlation coefficient (r) and associated probability
(P) were assessed for the levels of the three cytokines. The IL-6
and IL-8 levels were positively correlated with each other
(r=0.932, P=0.01); however, the TNF-α level was not correlated with
the IL-6 or IL-8 levels (data not shown).
Discussion
Periodontal diseases may lead to chronic
inflammatory conditions and the destruction of the supporting
structures of the dentition. Periodontal diseases are caused by
gram-negative bacterial infections, which produce
lipopolysaccharides (LPS) (1). The
bacterial infection and inhabitation is the etiological factor of
periodontitis, due to the fact that the host response and immune
reaction to such infectious organisms mediate the development of
the periodontitis, rather than infection itself (1). Previous studies have revealed the
roles of TNF-α (5), IL-1β
(14) and IL-6 and IL-8 (20) as proinflammatory cytokines.
Consistent with other inflammatory diseases, proinflammatory
cytokines, including IL-6, IL-8 and TNF-α, appear to be major
mediators in periodontitis (14).
Therefore, in the current study, the levels of IL-6, IL-8 and TNF-α
expressed in the human gingival tissues of patients with
periodontitis were investigated.
IL-6 is produced by a number of different cell
types, such as monocytes, macrophages, fibroblasts, endothelial
cells, epithelial cells, T- and B-cells and keratinocytes. IL-6 is
also expressed in a variety of situations involving host immune
responses and inflammatory reactions (10). With regard to periodontal diseases,
an immunohistochemical study observed that a higher level of IL-6
was expressed in inflamed gingival tissues than in healthy control
tissues (21). Moreover, previous
data obtained using a reverse transcription polymerase chain
reaction (RT-PCR) and ELISA showed that the mRNA expression, as
well as protein expression, of IL-6 was increased in patients with
periodontal diseases (22,23). The data from the present study
revealed that IL-6 was expressed in the gingival tissue of each of
the 19 patients with periodontitis. The results from the present
study for the IL-6 levels, obtained using ELISA, were consistent
with previous findings.
IL-8 is an established chemotactic protein and is
released in inflamed human gingival tissues (10,24).
Gingival fibroblasts affected by infectious organisms express
higher levels of IL-8 mRNA in comparison with the baseline
expression (23). Our data showed
that IL-8 was highly expressed in the gingival tissues of all the
patients with periodontitis. The present results were consistent
with those from previous studies. However, the mean level of IL-8
was higher than that of IL-6 or TNF-α. Moreover, as shown in
Fig. 2, the IL-8 level was
positively correlated with the IL-6 level in each sample. The
results suggested that IL-8 may be important in the identification
of patients with periodontitis.
TNF-α is considered to be a major cytokine involved
in the pathogenesis of periodontal disease, affecting the
consequences of the tissue destruction and the erosive reaction in
periodontitis (25). TNF-α is
produced by monocytes and macrophages in response to oral bacterial
components, such as LPS. Elevated levels of TNF-α may promote the
release of collagenase from human gingival fibroblasts (10), leading to cartilaginous collagen
destruction and bone resorption (25). It has been demonstrated that there
is no correlation between the level of TNF-α and representations of
chronic degenerative changes, such as gingival index, plaque index
or probing depth (26). Therefore,
it has been suggested that TNF-α may be a marker of inflammatory
activity. In the present results, the concentration of TNF-α showed
little difference among the 19 patients, which contrasted with the
fluctuations of the IL-6 or IL-8 levels. Furthermore, the TNF-α
expression did not correlate with the IL-6 or IL-8 levels. The
results suggest that ILs and TNF-α may be expressed through
different pathways in the pathophysiology of periodontitis.
In conclusion, the levels of three cytokines (TNF-α,
IL-6 and IL-8) in the human gingival tissues of 19 patients with
periodontitis were detected by ELISA. The mean levels of TNF-α,
IL-6 and IL-8 were 8.41±0.25, 34.01±1.09 and 20.70±0.31 pg/ml,
respectively. The expression of the two ILs (IL-6 and IL-8) was
revealed to be positively correlated (r=0.932, P=0.01). It was
suggested that the increased levels of these inflammatory cytokines
in periodontitis may have diagnostic and prognostic potentials for
the monitoring of the disease and the therapeutic decisions.
Acknowledgements
This study was supported by the Bio R&D program
of the National Research Foundation, Ministry of Education, Science
and Technology of Korea (grant no. 2009-0092562).
References
|
1
|
Van Dyke TE and Serhan CN: Resolution of
inflammation: a new paradigm for the pathogenesis of periodontal
diseases. J Dent Res. 82:82–90. 2003.PubMed/NCBI
|
|
2
|
Pozo P, Valenzuela MA, Melej C, et al:
Longitudinal analysis of metalloproteinases, tissue inhibitors of
metalloproteinases and clinical parameters in gingival crevicular
fluid from periodontitis-affected patients. J Periodontal Res.
40:199–207. 2005. View Article : Google Scholar
|
|
3
|
DeCarlo AA Jr, Windsor LJ, Bodden MK, et
al: Activation and novel processing of matrix metalloproteinases by
a thiol-proteinase from the oral anaerobe Porphyromonas
gingivalis. J Dent Res. 76:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fentoğlu Ö, Köroğlu BK, Hiçyılmaz H, et
al: Pro-inflammatory cytokine levels in association between
periodontal disease and hyperlipidaemia. J Clin Periodontol.
38:8–16. 2011.PubMed/NCBI
|
|
5
|
Vernal R, Dezerega A, Dutzan N, et al:
RANKL in human periapical granuloma: possible involvement in
periapical bone destruction. Oral Dis. 12:283–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cazalis J, Tanabe S, Gagnon G, Sorsa T and
Grenier D: Tetracyclines and chemically modified tetracycline-3
(CMT-3) modulate cytokine secretion by
lipopolysaccharide-stimulated whole blood. Inflammation.
32:130–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sorsa T, Tjäderhane L, Konttinen YT, et
al: Matrix metalloproteinases: contribution to pathogenesis,
diagnosis and treatment of periodontal inflammation. Ann Med.
38:306–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Birkedal-Hansen H: Role of cytokines and
inflammatory mediators in tissue destruction. J Periodontal Res.
28:500–510. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uitto VJ, Overall CM and McCulloch C:
Proteolytic host cell enzymes in gingival crevice fluid.
Periodontol 2000. 31:77–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okada H and Murakami S: Cytokine
expression in periodontal health and disease. Crit Rev Oral Biol
Med. 9:248–266. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirano T, Akira S, Taga T and Kishimoto T:
Biological and clinical aspects of interleukin 6. Immunol Today.
11:443–449. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishimi Y, Miyaura C, Jin CH, et al: IL-6
is produced by osteoblasts and induces bone resorption. J Immunol.
145:3297–3303. 1990.PubMed/NCBI
|
|
13
|
Baggiolini M, Walz A and Kunkel SL:
Neutrophil-activating peptide-1/interleukin 8, a novel cytokine
that activates neutrophils. J Clin Invest. 84:1045–1049. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bastos MF, Lima JA, Vieira PM, et al:
TNF-alpha and IL-4 levels in generalized aggressive periodontitis
subjects. Oral Dis. 15:82–87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dongari-Bagtzoglou AI and Ebersole JL:
Increased presence of interleukin-6 (IL-6) and IL-8 secreting
fibroblast subpopulations in adult periodontitis. J Periodontol.
69:899–910. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
César-Neto JB, Duarte PM, de Oliveira MC,
et al: Smoking modulates interleukin-6:interleukin-10 and
RANKL:osteoprotegerin ratios in the periodontal tissues. J
Periodontal Res. 42:184–191. 2007.PubMed/NCBI
|
|
17
|
Ribeiro FV, de Mendonça AC, Santos VR, et
al: Cytokines and bone-related factors in systemically healthy
patients with chronic periodontitis and patients with type 2
diabetes and chronic periodontitis. J Periodontol. 82:1187–1196.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Javed F, Al-Askar M and Al-Hezaimi K:
Cytokine profile in the gingival crevicular fluid of periodontitis
patients with and without type 2 diabetes: a literature review. J
Periodontol. 83:156–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim KA, Chung SB, Hawng EY, et al:
Correlation of expression and activity of matrix
metalloproteinase-9 and −2 in human gingival cells of periodontitis
patients. J Periodontal Implant Sci. 43:24–29. 2013.
|
|
20
|
Seymour GJ and Gemmell E: Cytokines in
periodontal disease: where to from here? Acta Odontol Scand.
59:167–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bartold PM and Haynes DR: Interleukin-6
production by human gingival fibroblasts. J Periodontal Res.
26:339–345. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reinhardt RA, Masada MP, Kaldahl WB, et
al: Gingival fluid IL-1 and IL-6 levels in refractory
periodontitis. J Clin Periodontol. 20:225–231. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Botero JE, Contreras A and Parra B:
Profiling of inflammatory cytokines produced by gingival
fibroblasts after human cytomegalovirus infection. Oral Microbiol
Immunol. 23:291–298. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chung RM, Grbíc JT and Lamster IB:
Interleukin-8 and beta-glucuronidase in gingival crevicular fluid.
J Clin Periodontol. 24:146–152. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boström L, Linder LE and Bergström J:
Clinical expression of TNF-alpha in smoking-associated periodontal
disease. J Clin Periodontol. 25:767–773. 1998.PubMed/NCBI
|
|
26
|
Rossomando EF, Kennedy JE and Hadjimichael
J: Tumour necrosis factor alpha in gingival crevicular fluid as a
possible indicator of periodontal disease in humans. Arch Oral
Biol. 35:431–434. 1990. View Article : Google Scholar : PubMed/NCBI
|