Introduction
In adult humans, 40–50% of peripheral blood T cells
have memory phenotypes (1), which
can threaten graft survival (2,3). It
is hypothesized that continuous exposure to bacterial and viral
pathogens, blood transfusion or pregnancy may develop alloreactive
memory (TM) cells. Due to donor-reactive T cells, the
rejection of the majority of second organ transplantations is more
intense than the first transplantation (4–6).
Human and animal studies have indicated that alloreactive
TM cells are an important part of the barrier, and
understanding the underlying mechanisms may aid the inhibition of
the rejection response and induce transplant tolerance.
To date, animal models of retransplantation have
been studied less compared with animal models of transplantation,
since the majority of the recipient mice fail to survive two
surgeries, particularly in solid organ retransplantation with blood
vessel anastomosis. In the present study, through the improvement
of microscopic surgery skills, a consecutive skin-heart
retransplant mouse model was established. The model was based on
the rationale that the previous alloskin was a potent immunogen
that vigorously induced alloantigen-specific TM cells,
which thereafter ‘at the second exposure’ promptly and vigorously
launched an alloresponse upon the cardiac allograft following blood
reperfusion (7). We hypothesized
that this retransplantation mouse model may yield preclinical
results that more closely predict clinical outcomes.
It is well documented that the activation and
secretion of cytokines and chemokines regulate the recruitment of
inflammatory cells and lead to the upregulation in the expression
of cell-adhesion molecules (8).
Previous studies on experimental and clinical transplants have
demonstrated that chemokines play a critical role in the activation
of innate immunity (9),
ischemia-reperfusion injury (10)
and the induction of adaptive immune responses (11). Increased expression levels of C-X-C
motif chemokine ligand (CXCL) 9 and CXCL10 in a rat transplantation
model have been shown to be accompanied by T-cell recruitment
(12). In addition, alloreactive
TM cells have been previously demonstrated to contribute
to increased expression and secretion of RANTES, as well as the
migration of TM and other inflammatory cells into the
graft. With regard to previous observations, the present study
investigated the effect of CXCL9 and CXCL10 in a mouse
retransplantation model. As a result of previous allograft
rejection, a retransplantation model is more complicated and
exhibits a higher risk compared with primary transplantation.
Materials and methods
Mice
Female adult BALB/c and C57BL/6 mice (weight, 18–20
g; age, 8–10 weeks) were purchased from the Shanghai Laboratory
Animal Center (Shanghai, China), and used as donors and recipients,
respectively. All the mice were maintained under specific
pathogen-free conditions and the experimental procedures were
conducted in compliance with the Institutional Animal Care and Use
guidelines. The study was approved by the ethics committee of the
First Affiliated Hospital of Xiamen University, Xiamen, China.
Groups
In the experimental group (n=6), cardiac
transplantation was performed six weeks following skin grafting. In
the control group (n=6), cardiac transplantation was performed
without skin grafting. Untreated mice (n=6; C57BL/6) served as
blank controls.
Skin grafting
Full-thickness skin grafts were obtained from the
lateral thoracic skin of the BALB/c mice and were cut into squares
or rectangles of 1–1.5 cm2. Donor skin was engrafted
onto the lumbar regions of the C57BL/6 mice and was assessed
daily.
Heterotopic cardiac transplantation
Cardiac transplantation was performed six weeks
following skin grafting. Standard methods of murine heterotopic
intraneck (anastomosis of the vessels of the neck using a nonsuture
cuff technique) cardiac transplantation from BALB/c donors to
C57BL/6 recipients were performed as described previously (13). Donor hearts were assessed by daily
palpation following surgery. Cessation of a palpable heartbeat was
defined as graft rejection.
Histological examination
Cardiac grafts were harvested at day three following
cardiac transplantation and preserved with formalin.
Paraffin-embedded transventricular tissue sections (5 μm) were
stained with hematoxylin and eosin. A rejection score was assigned
by examining the extent of leukocytic infiltration and the
anatomical destruction of the myocytes, according to the
International Society for Heart & Lung Transplantation (ISHLT)
criteria (14,15).
Enzyme-linked immunosorbent assay
(ELISA)
Serum levels of CXCL9, CXCL10, interferon (IFN)-γ,
interleukin (IL)-2, IL-10 and tumor growth factor (TGF)-β in the
recipient mice were determined using ELISA kits (R&D Systems,
Minneapolis, MN, USA). A standard curve was generated using known
quantities of the purified recombinant murine cytokines.
Quantitative polymerase chain reaction
(qPCR)
Reverse transcription (RT) was performed with 1 μg
total RNA, that had been isolated from the grafts, using TRIzol
reagent (Invitrogen Life Technologies, Gaithersburg, MD, USA). PCR
analysis of the RT product (2 μl) was performed using a real-time
PCR System (Applied Biosystems, Inc., Warrington, UK) with SYBR
Green I fluorescence and β-actin as the reference. The primer
sequences used for qPCR were as follows: β-actin forward, 5′-CAT
CCG TAA AGA CCT CTA TGC CAA C-3′ and reverse, 5′-ATG GAG CCA CCG
ATC CAC A-3′; IFN-γ forward, 5′-CGG CAC AGT CAT TGA AAG CCT A-3′
and reverse, 5′-GTT GCT GAT GGC CTG ATT GTC-3′; IL-2 forward,
5′-GGA GCA GCT GTT GAT GGA CCT AC-3′ and reverse, 5′-AAT CCA GAA
CAT GCC GCA GAG-3′; IL-10 forward, 5′-GAC CAG CTG GAC AAC ATA CTG
CTA A-3′ and reverse, 5′-GAT AAG GCT TGG CAA CCC AAG TAA-3′; TGF-β
forward, 5′-TGA CGT CAC TGG AGT TGT ACG G-3′ and reverse, 5′-GGT
TCA TGT CAT GGA TGG TGC-3′; CXCL9 forward, 5′-TGT GGA GTT CGA GGA
ACC CT-3′ and reverse, 5′-TGC CTT GGC TGG TGC TG-3′; and CXCL10
forward, 5′-AGA ACG GTG CGC TGC AC-3′ and reverse, 5′-CCT ATG GCC
CTG GGT CTC A-3′.
Statistical analysis
All the results are presented as the mean ± standard
deviation. The Kaplan-Meier method was used to plot the allograft
survival curve, while the log-rank test was performed to determine
differences in survival data. Data comparisons were analyzed with
the Student’s unpaired t-test. SPSS 13.0 (SPSS Inc., Chicago, IL,
USA) and GraphPad Prism 5 (San Diego, CA, USA) were used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
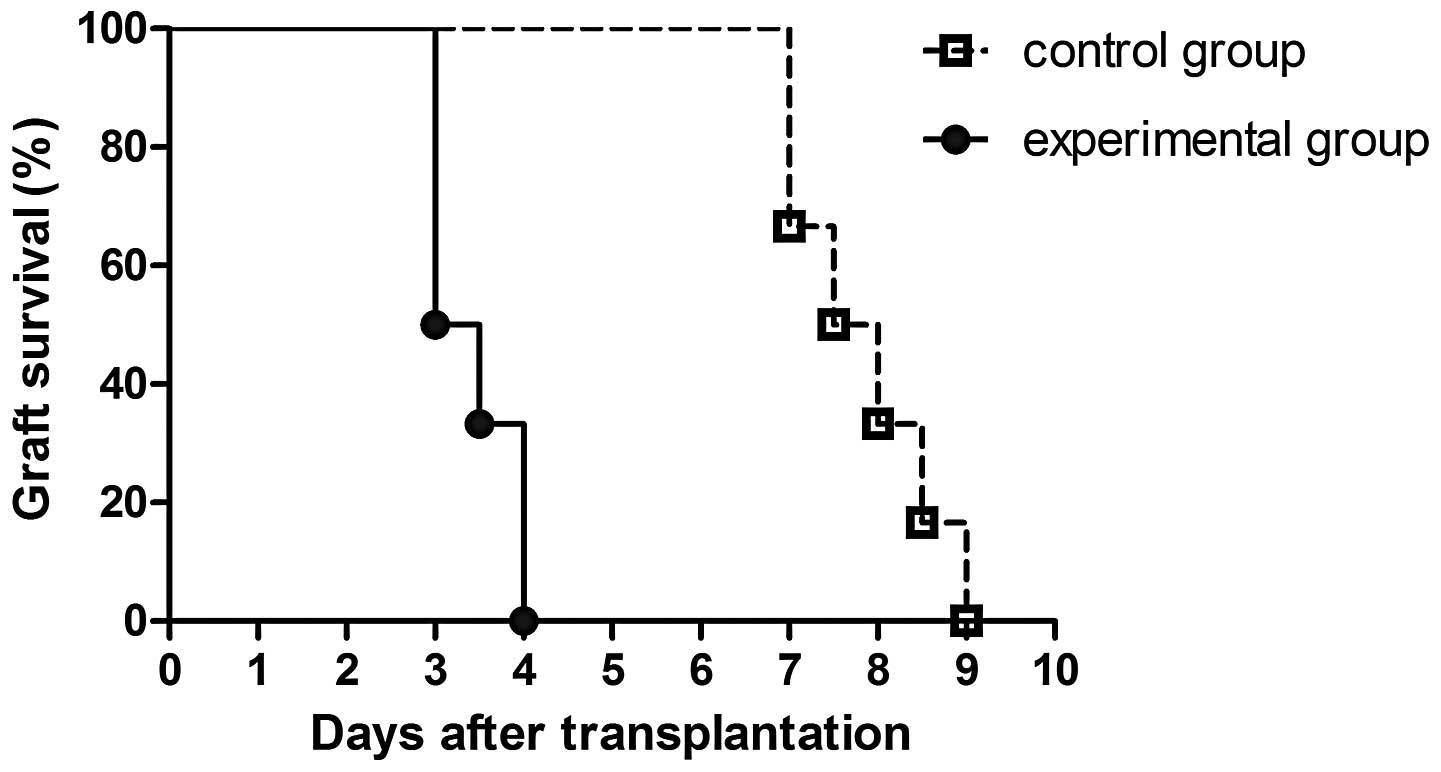
Survival times of the cardiac
allografts
In the experimental group, the median survival time
(MST) of the cardiac allografts was significantly shorter compared
with the controls (P<0.001). As shown in Fig. 1, the MST was 7.75 days in the
experimental group and 3.25 days in the control group.
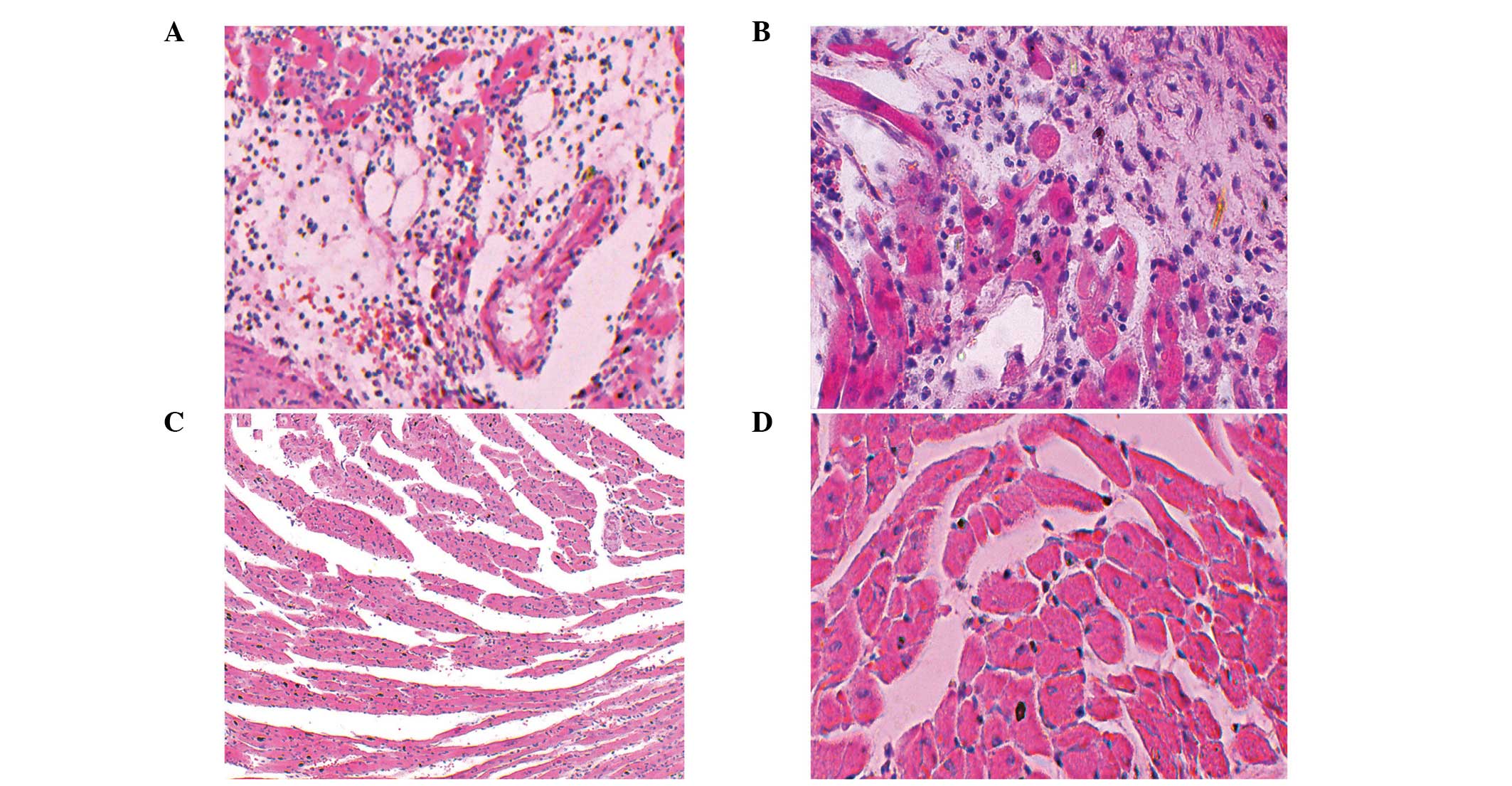
Histological evaluation
Compared with the control group, the destruction of
cardiac allografts in the experimental group was more intense. The
cardiac allografts were harvested from the transplanted mice on day
three following transplantation for histological examination. As
shown in Fig. 2, the experimental
group was assigned an ISHLT grade of 4 (Fig. 2), while the control group was
assigned an ISHLT grade of 1B-2 (Fig.
2), based on the extent of inflammatory cell infiltration and
the destruction of the cardiac allografts in the two groups.
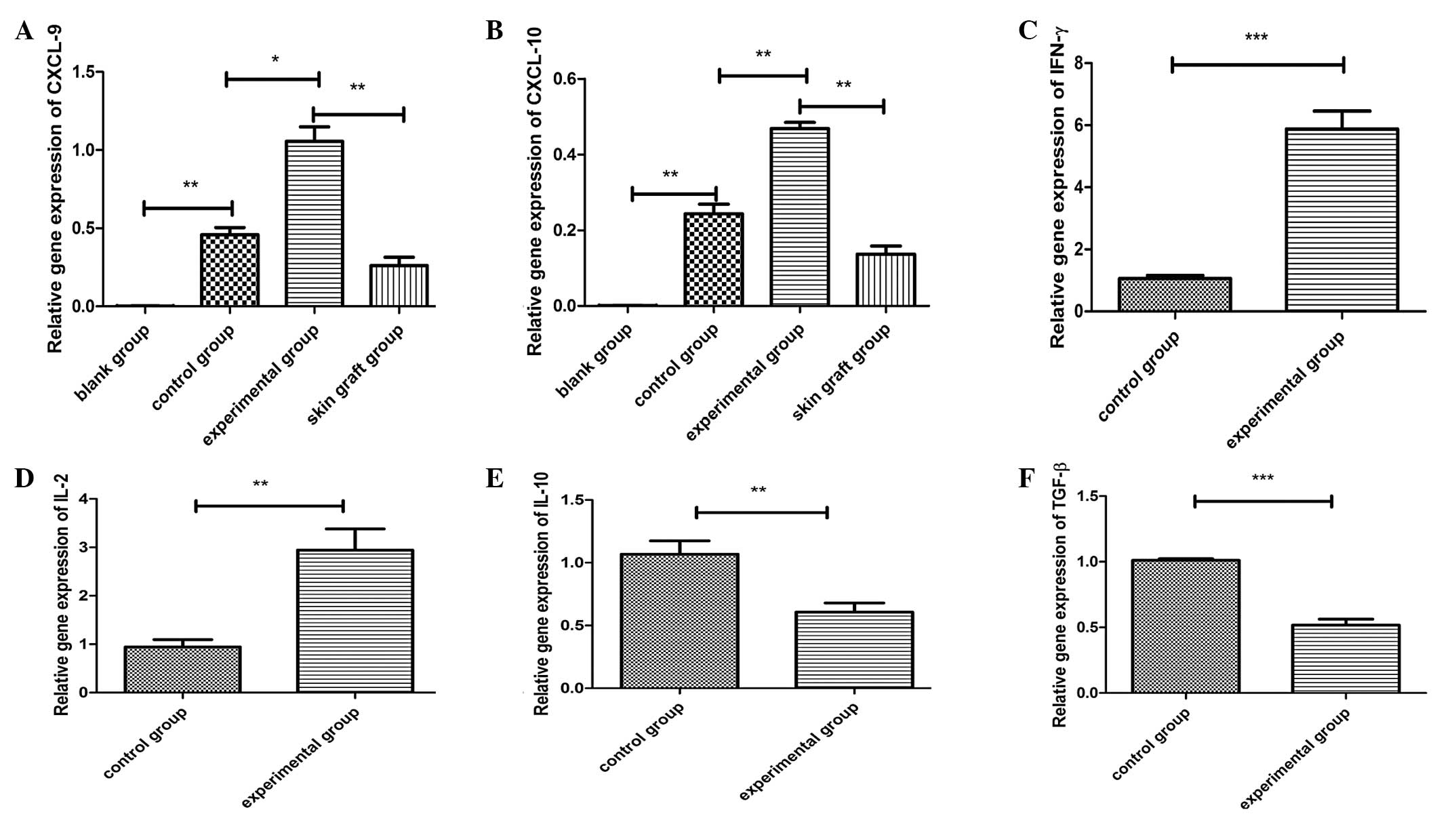
CXCL9 and CXCL10 gene expression and key
effector molecules in the cardiac grafts
RNA was isolated from the cardiac grafts on day
three following the transplantation in recipient mice, and the cDNA
products were synthesized and analyzed by qPCR. As shown in
Fig. 3, statistically significant
differences with regard to cytokine gene expression were observed
among the groups (blank vs. control; control vs. experimental; skin
graft vs. experimental). As shown in Fig. 3, the relative gene expression
levels of IFN-γ and IL-2 were higher in the experimental group than
in the control group, whereas the relative gene expression levels
of IL-10 and TGF-β were lower in the experimental group compared
with the control group.
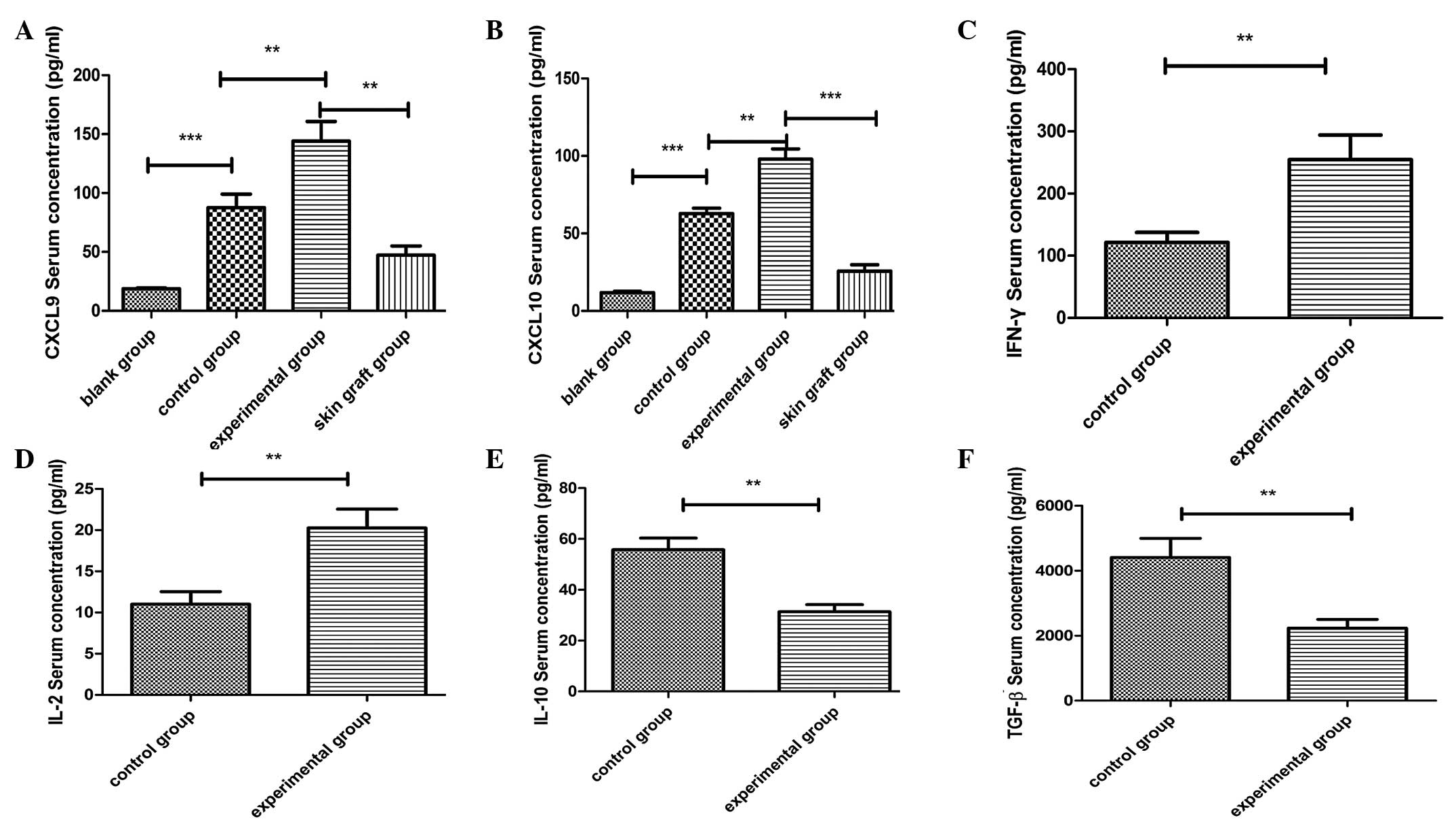
Effect of retransplantation on the
secretion of CXCL9, CXCL10 and key effector molecules in the
serum
ELISA was performed using sera obtained from the
recipient mice at day three following cardiac transplantation. As
shown in Fig. 4, statistically
significant differences were observed in the cytokine expression
levels between the groups (blank vs. control; control vs.
experimental; skin graft vs. experimental). Furthermore, the
expression levels of CXCL9 and CXCL10 in the serum were shown to
correlate with the development of acute rejection. In addition, in
the experimental group, the serum concentrations of IFN-γ and IL-2
were higher, while the concentrations of TGF-β and IL-10 were lower
when compared with the control group. These observations indicated
that the retransplantation model was more prone to the development
of acute rejection compared with the transplantation model.
Discussion
Acute rejection of allografts is a major
complication following transplantation and is characterized by
intragraft infiltration of activated mononuclear cells. However,
the mechanism of leukocyte recruitment has not been fully
established. In recent years, an increasing number of studies have
demonstrated that the activation and secretion of chemokines
regulate inflammatory cell recruitment and lead to the upregulation
of cell-adhesion molecule expression (8–11,16),
thus, revealing the critical role of chemokines in the development
of acute rejection (8). Chemokines
are a group of low-molecular weight (8–11 kDa) cytokines that
mediate cellular trafficking and can be divided into four families
(C, CC, CXC and CX3C) based on conserved cysteine residues in the
amino-terminal end of the molecule. CXCL9/MIG (a monokine induced
by IFN-γ) and CXCL10/IP-10 (IFN-γ-inducible protein 10) belong to
the CXC chemokine subgroup of the chemokine superfamily. CXCL9 and
CXCL10 bind to their shared receptor, CXCR3, which is expressed on
Th1 cells, a number of B cells and natural killer cells, and
participate in lymphocyte recruitment in virtually all stages
following transplantation. In human and animal studies, CXCL9 and
CXCL10 expression levels have been shown to be elevated in solid
organ rejection, including skin, heart, renal and lung (16–19).
In addition, neutralization of CXCL9 and CXCL10 has been shown to
prolong the allograft survival time by reducing the infiltration of
mononuclear cells and attenuating the acute rejection. However, the
role of CXCL9 and CXCL10 in retransplantation models remains
unknown. The model used in the present study is known to closely
mimic the clinical setting, as a quicker and more vigorous
alloresponse is induced compared with other models (20). In the present study, this
retransplantation model was used to determine whether the levels of
CXCL9 and/or CXCL10 increase post-transplantation. Previous
research using this model indicated that it is an effective method
to acquire TM cells in small mice following skin
grafting (21). In contrast to
naïve T cells, TM cells are programmed to activate
quickly, with a reduced requirement for costimulatory signals and a
lower threshold for activation (1). Alloreactive TM cells are
insensitive to conventional immunosuppressive treatments, including
rapamycin or tacrolimus, indicating that TM cells may
induce a faster and stronger immune response in terms of
proliferation and IFN-γ secretion on reexposure to the same
antigen. As shown in Figs. 3 and
4, gene expression and serum
concentration levels of CXCL9 and CXCL10 in the experimental group
were markedly higher compared with the control group. Similarly,
the infiltration of mononuclear cells was more intense in the
experimental group than in the control group following cardiac
allograft transplantation (Fig.
2). Injury to the graft was predominantly due to the
graft-infiltrating T cells that produced IFN-γ, affecting the
function of the donor graft. CXCL9 and CXCL10 can traffic Th1
cells, and other inflammatory cells migrate throughout the
recipient’s blood stream to the graft and are activated to express
effector functions. Prior to heterotopic cardiac transplantation,
the serum concentration and gene expression levels of CXCL9 and
CXCL10 were higher in the transplantation group compared with the
blank control group; similarly, levels of other cytokines,
including IFN-γ and IL-2, were higher in the transplantation group
compared with the blank control group. Furthermore, the MST of the
cardiac allografts was significantly shorter in the experimental
group (3.25 days) compared with the control group (7.75 days).
Therefore, CXCL9 and CXCL10 may serve as possible biomarkers to
predict the development of acute rejection following
transplantation. The observations of the present study are
supported by the evidence that chemokines play a pivotal role in
directing immune cell differentiation, migration and proliferation
during acute rejection; therefore, their circulating levels in the
serum and plasma are strictly associated with the immune status of
the patient (22,23). Compared with biopsy samples,
circulating cytokine concentrations can be easily assessed from
serum collected from the peripheral blood of a transplant patient.
However, further studies are required to investigate additional
factors that influence the circulating levels of chemokines in
vivo and/or in vitro.
As stated previously, TM cells are not
sensitive to conventional immunosuppressive regimens (24). A number of studies have
demonstrated that the use of anti-CXCL9 and anti-CXCL10 antibodies
can prolong allograft survival time (25,26).
Thus, further research is required to determine whether anti-CXCL9
and/or anti-CXCL10 molecules may be an effective approach to
prevent TM cells and other inflammatory cells from
infiltrating into the grafts, alleviating acute retransplant
rejection.
Collectively, the results of the present study
indicate that compared with naïve T cells, TM cells
induce higher levels of CXCL9 and CXCL10, which in turn leads to
increased chemokine-mediated inflammatory cell trafficking into the
grafts, aggravating the development of acute rejection. The
differences in the levels of CXCL9 and CXCL10 between pre- and
post-transplant mice indicate that CXCL9 and CXCL10 may be used as
possible biomarkers to predict acute rejection. Future studies
should determine whether strategies of inhibiting CXCL9 and CXCL10,
combined with other immunosuppressive treatments, can alleviate
acute retransplant rejection.
Acknowledgements
The study was supported by a grant from the Key
Project of Program of Science and Technology of Fujian Province of
China (no. MKJ 2008–59).
References
|
1
|
Luo L, Li C, Wu W, Lu J, et al: Functional
analysis of alloreactive memory CD4+ T cells derived
from skin transplantation recipient and naïve CD4+ T
cells derived from untreated mice. J Surg Res. 176:649–656.
2012.PubMed/NCBI
|
|
2
|
McFarland RD, Douek DC, Koup RA and Picker
LJ: Identification of a human recent thymic emigrant phenotype.
Proc Natl Acad Sci USA. 97:4215–4220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Douek DC, McFarland RD, Keiser PH, et al:
Changes in thymic function with age and during the treatment of HIV
infection. Nature. 396:690–695. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Arendonk KJ, Garonzik Wang JM,
Deshpande NA, James NT, Smith JM, Montgomery RA, Colombani PM and
Segev DL: Practice patterns and outcomes in retransplantation among
pediatric kidney transplant recipients. Transplantation.
95:1360–1368. 2013.PubMed/NCBI
|
|
5
|
Johnson MR, Aaronson KD, Canter CE,
Kirklin JK, Mancini DM, Mehra MR, Radovancevic B, Taylor DO and
Webber SA: Heart retransplantation. Am J Transplant. 7:2075–2081.
2007. View Article : Google Scholar
|
|
6
|
Saito A, Novick RJ, Kiaii B, McKenzie FN,
Quantz M, Pflugfelder P, Fisher G and Chu MW: Early and late
outcomes after cardiac retransplantation. Can J Surg. 56:21–26.
2013. View Article : Google Scholar
|
|
7
|
Richters CD, Hoekstra MJ, et al:
Immunology of skin transplantation. Clin Dermatol. 23:338–342.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Game DS and Lechler RI: Pathways of
allorecognition: implications for transplantation tolerance.
Transpl Immunol. 10:101–108. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sánchez Lázaro IJ, Almenar Bonet L, Moro
López J, Sánchez Lacuesta E, Martínez-Dolz L, Agüero Ramón-Llín J,
et al: Influence of traditional cardiovascular risk factors in the
recipient on the development of cardiac allograft vasculopathy
after heart transplantation. Transplant Proc. 40:3056–3057.
2008.PubMed/NCBI
|
|
10
|
Springer TA: Traffic signals on
endothelium for lymphocyte recirculation and leukocyte emigration.
Annu Rev Physiol. 57:827–872. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Flach R, Speidel N, Flohé S, Börgermann J,
Dresen IG, Erhard J and Schade FU: Analysis of intragraft cytokine
expression during early reperfusion after liver transplantation
using semi-quantitative RT-PCR. Cytokine. 10:445–451. 1998.
View Article : Google Scholar
|
|
12
|
Rosenblum JM, Shimoda N, Schenk AD, Zhang
H, Kish DD, Keslar K, Farber JM and Fairchild RL: CXC chemokine
ligand (CXCL) 9 and CXCL10 are antagonistic costimulation molecules
during the priming of alloreactive T cell effectors. J Immunol.
184:3450–3460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuura A, Abe T and Yasuura K:
Simplified mouse cervical heart transplantation using a cuff
technique. Transplantation. 51:896–898. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Billingham ME, Cary NR, Hammond ME,
Kemnitz J, Marboe C, McCallister HA, et al: A working formulation
for the standardization of nomenclature in the diagnosis of heart
and lung rejection: Heart Rejection Study Group. The International
Society for Heart Transplantation. J Heart Transplant. 9:587–593.
1990.
|
|
15
|
Winters GL, Marboe CC and Billingham ME:
The International Society for Heart and Lung Transplantation
grading system for heart transplant biopsy specimens: clarification
and commentary. J Heart Lung Transplant. 17:754–760.
1998.PubMed/NCBI
|
|
16
|
Koga S, Auerbach MB, Engeman TM, Novick
AC, Toma H and Fairchild RL: T cell infiltration into class II
MHC-disparate allografts and acute rejection is dependent on the
IFN-gamma-induced chemokine Mig. J Immunol. 163:4878–4885.
1999.PubMed/NCBI
|
|
17
|
Watarai Y, Koga S, Paolone DR, Engeman TM,
Tannenbaum C, Hamilton TA and Fairchild RL: Intraallograft
chemokine RNA and protein during rejection of MHC-matched/multiple
minor histocompatibility-disparate skin grafts. J Immunol.
164:6027–6033. 2000. View Article : Google Scholar
|
|
18
|
Miura M, Morita K, Kobayashi H, Hamilton
TA, Burdick MD, Strieter RM and Fairchild RL: Monokine induced by
IFN-gamma is a dominant factor directing T cells into murine
cardiac allografts during acute rejection. J Immunol.
167:3494–3504. 2001. View Article : Google Scholar
|
|
19
|
Hancock WW, Gao W, Csizmadia V, Faia KL,
Shemmeri N and Luster AD: Donor-derived IP-10 initiates development
of acute allograft rejection. J Exp Med. 193:975–980. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang H, Zhao Y, San Z, Liao C, Sha C, Xie
B, Chen J, Xia J, Wang Y and Qi Z: The recall alloresponse
following retransplantation is more intense compared with the T
cell memory-transfer model. Immunol Invest. 39:39–53. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou X, Shan Z, Liang H, et al: Role of
regulated upon activation normal T-cell expressed and secreted in a
model of retransplantation acute rejection mediated by alloreactive
memory CD4+ T cells. Transplant Proc. 45:546–551. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roedder S, Vitalone M, Khatri P and Sarwal
MM: Biomarkers in solid organ transplantation: establishing
personalized transplantation medicine. Genome Med. 3:372011.
View Article : Google Scholar
|
|
23
|
Romagnani P and Crescioli C: CXCL10: a
candidate biomarker in transplantation. Clin Chim Acta.
413:1364–1373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang H, Liao C, Qi Z, et al: Rapamycin or
tacrolimus alone fails to resist cardiac allograft accelerated
rejection mediated by alloreactive CD4(+) memory T cells in mice.
Transpl Immunol. 22:128–136. 2010.
|
|
25
|
Agostini C, Calabrese F, Rea F, et al:
Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells
infiltrating lung allografts and mediate chemotaxis of T cells at
sites of rejection. Am J Pathol. 158:1703–1711. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Belperio JA, Keane MP, Burdick MD, et al:
Role of CXCL9/CXCR3 chemokine biology during pathogenesis of acute
lung allograft rejection. J Immunol. 171:4844–4852. 2003.
View Article : Google Scholar : PubMed/NCBI
|