Introduction
Electrical stimulation plays an important role in
the cure of disease and relief of pain. It has been reported that
patients with spinal cord injury (SCI) have many factors that are
associated with pressure ulcer formation, including paralysis, loss
of sensation, poor nutrition, anemia, and skin maceration
associated with incontinence (1).
Chronic pain in SCI is disabling and resistant to common
pharmacologic approaches. It has been demonstrated that electrical
and magnetic neural stimulation techniques are potential tools for
use in the management of patients with this condition (2,3). The
stimulation of leg and gluteal muscles during functional electrical
stimulation cycling increases ankle excursion in individuals with
spinal cord injury (4).
However, it has been shown that electrical injuries
induce progressive tissue loss (5).
Electrical injuries are devastating and are challenging to treat
due to the complexity of the tissue damage and physiological impact
(6). When the injury occurs at a
high voltage, significant damage occurs to blood vessels via a
number of mechanisms; the rupture of a major vessel is a rare but
life-threatening sequela of electrical injury (7). Following high-voltage electrical
injury, the endothelial and smooth muscle functions of the brachial
artery are significantly decreased (8). In addition, electrical injury is
reportedly a major cause of morbidity (9).
It has previously been shown that the effects of
electrical injury include changes in the levels of type III
collagen expressed in cardiac tissue (10); however, little is known about the
changes in fatty acid-binding protein 1 (FABP1) and gastrin
receptor (gastrin R) expression in testes that have undergone
electrical injury.
The current study used immunohistochemistry to
evaluate the expression of FABP1 and gastrin R in the testes of
electrically injured rats, in order to determine whether their
expression at the protein level is altered by electrical
injury.
Materials and methods
Animal care
A total of 24 Sprague-Dawley rats, weighing 180–200
g, were provided by Sun Yat-Sen University (Guangzhou, China). All
animals were given free access to standard pellet chow and water
prior to the experiments. All procedures described in this study
were approved by the Ethics Committee of Sun Yat-Sen University
(Guangzhou, China).
Animal treatment and study design
The 24 Sprague-Dawley rats were divided into three
groups, namely a control group, a fatal electrocution group and an
electrical injury group (n=8 per group). Sixteen of the rats were
deeply anesthetized with sodium pentobarbital. A TMB 1000VA control
transformer was used to provide electrical current via an anode and
cathode (Zhejiang 001 Group Co., Ltd., Quzhou, Zhejiang, China).
With the anode connected to the left foreleg and the cathode to the
right hindleg, the rats were electrocuted (220 V, 50 Hz) for 60
sec. The 8 rats that died were defined as the fatal electrocution
group, and the others were defined as the electrical injury group.
The rats in the electrical injury group were sacrificed by cervical
dislocation. In the control group, 8 rats were sacrificed by
cervical dislocation without any prior electrical stimulus.
Following sacrifice, the rat testes were rapidly excised and
perfused with 10% neutral-buffered formalin solution (10).
Histopathological examinations
Specimens of the testes from each group were removed
for histopathological examination. The testicular tissues were
fixed in phosphate-buffered 4% formalin (pH 7.4) for 24 h and then
embedded in paraffin. Sections were cut into 4-µm slices, and
stained with hematoxylin and eosin. The slides were coded and
semiquantitative analysis of the sections was performed by a
pathologist who was blinded to the treatment that the rats had
received. The pathological changes in these testicular tissues were
then evaluated (11,12).
Tissue sections and
immunohistochemical staining
All rat testes were immersed in 10% neutral-buffered
formalin solution for 24 h, prior to being embedded in paraffin and
sectioned with a microtome into 4-µm sections. All rat testes were
investigated by immunohistochemistry to determine the difference
between the electrical injury and control groups or the fatal
electrocution and control groups with respect to FABP1 and gastrin
R expression. The immunohistochemical analyses were performed as
described previously (13–15). The primary antibodies were mouse
monoclonal anti-FABP1 [sc-271591; L-FABP (F-9); Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA] and anti-gastrin R
(BA2104; Wuhan Boster Biological Technology, Ltd., Wuhan, China),
which were used at a dilution of 1:400.
The total integrated optical density (IOD), a
parameter representing the expression levels of FABP1 and gastrin R
in the testicular tissue, was determined using a Bx41 cast-grid
microscope with DP10 camera (Olympus, Tokyo, Japan) together with
an image-analysis program (MetaMorph offline, version 4.65;
Molecular Devices, Sunnyvale, CA, USA). Under a magnification of
×200, five images were examined in each immunostained section and
the average IOD was calculated (15–17).
Statistical analysis
Results are expressed as mean ± standard error of
the mean. The significance of differences in total IOD values was
tested by Kruskal-Wallis analysis. P<0.05 was considered to
indicate a statistically significant difference. All analyses were
performed using SPSS software, version 12.0 (SPSS Inc., Chicago,
IL, USA).
Results
Histological examination
Routine histological examination revealed little
morphological change in the rat testes from each group (not
shown).
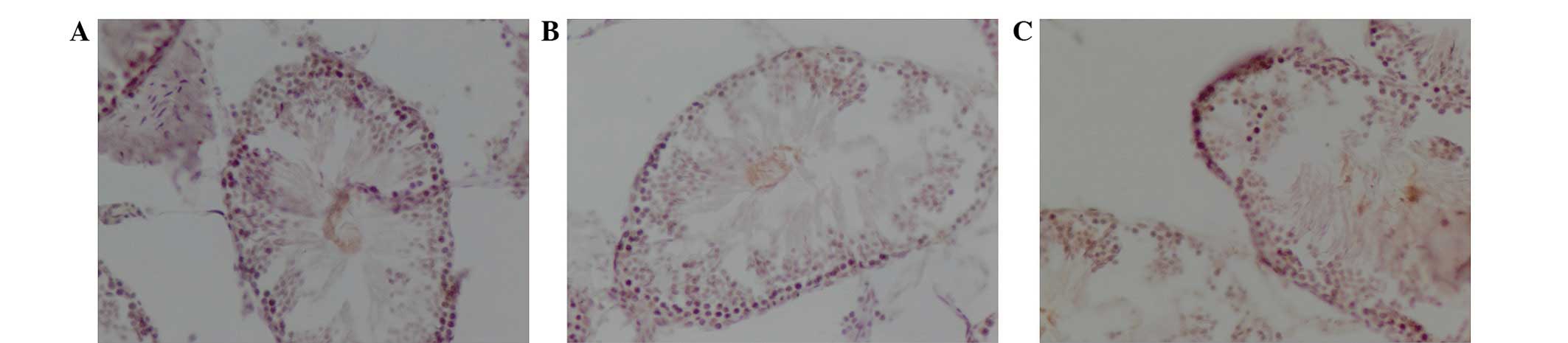
Expression of FABP1 protein
The distribution of FABP1 in the rat testes of the
control (Fig. 1A), fatal
electrocution (Fig. 1B) and
electrical injury (Fig. 1C) groups
is displayed in Fig. 1. The total
IOD for FABP1 in the testes of animals subjected to electrical
injury (0.0134±0.00056) was significantly higher than that of the
testes in the control group (0.0069±0.00035; P<0.05; Table I). The expression level of FABP1 in
the control group was the lowest among the three groups; however,
no significant difference in FABP1 expression was found between the
fatal electrocution (0.0077±0.00041) and control groups.
 | Table I.IOD of FABP1 and gastrin receptor in
immunohistochemically stained rat testes. |
Table I.
IOD of FABP1 and gastrin receptor in
immunohistochemically stained rat testes.
| Groups | FABP1 | Gastrin receptor |
|---|
| Control |
0.0069±0.00035 |
0.0066±0.00048 |
| Fatal
electrocution |
0.0077±0.00041 |
0.0075±0.00052 |
| Electrical
injury |
0.0134±0.00056a |
0.0126±0.00033a |
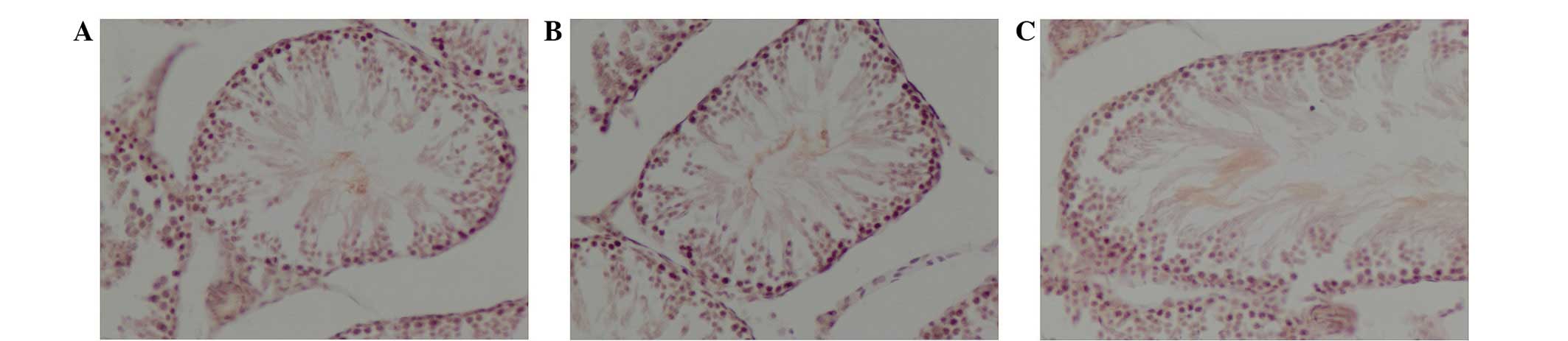
Expression of gastrin R protein
The distribution of gastrin R protein in the rat
testes of the control (Fig. 2A),
fatal electrocution (Fig. 2B) and
electrical injury (Fig. 2C) groups
is displayed in Fig. 2. The total
IOD of gastrin R in the testes of animals subjected to electrical
injury (0.0126±0.00033) was significantly higher than that of
control testes (0.0066±0.00048; P<0.05; Table I). The expression level of gastrin R
in the control group was the lowest among the three groups;
however, no significant difference in gastrin R expression was
found between the fatal electrocution (0.0075±0.00052) and control
groups.
Discussion
FABP1, also known as liver fatty acid-binding
protein (L-FABP) has been demonstrated to be strongly associated
with several diseases. FABP1 is a highly conserved key factor in
lipid metabolism (18). The
structures, functions and expression of a whole family of FABPs
have been investigated extensively (19). Plasma and especially urinary levels
of intestinal (I)-FABP and L-FABP and urinary levels of ileal bile
acid binding protein (I-BABP) can improve the early diagnosis of
intestinal ischemia, and plasma I-BABP levels can assist in the
localization of ileal ischemia (20). It has been suggested that L-FABP may
have an important role in the prevention of age- or diet-induced
obesity (21). A previous study has
indicated that alterations in FABP1 may be associated with the
development of aspirin-exacerbated respiratory disease (22). The FABP1 gene is highly transcribed
in liver-derived cells, and regulated predominantly by
liver-enriched transcription factors HNF3β and C/EBPα (23). A role of L-FABP in the attenuation of
hepatic steatosis has also been demonstrated (24). SCP-2 expression has also been
indicated to play a significant role in high density
lipoprotein-mediated cholesterol efflux by regulating the size of
rapid versus slow cholesterol efflux pools and/or eliciting the
concomitant upregulation of L-FABP in cultured primary hepatocytes
(25).
FABP1 serves as a key regulator of hepatic lipid
metabolism, and FABP1 gene polymorphisms have been associated with
several metabolic traits (26). It
has also been shown that phosphorylation of Sar1b disrupts the
FABP1-containing four-membered 75-kDa protein complex in the
cytosol, enabling it to bind to the endoplasmic reticulum and
generate pre-chylomicron transport vesicle (27). FABP1 plays an important role in the
male reproductive system (28). In
the present study, FABP1 expression was found to be significantly
increased in electrically injured rat testes, indicating that
elevated FABP1 expression is associated with testicular injury.
Gastrin is a peptide hormone, which regulates
gastric acid secretion and exerts physiological actions such as the
regulation of sodium balance (29).
Gastrin has been suggested to be involved in blood pressure
regulation, possibly via the regulation of sodium and water
metabolism and/or the renin-angiotensin-aldosterone system
(29). Measurements of gastrin
levels are taken primarily for the diagnosis of gastrin-producing
tumors (gastrinomas), which cause Zollinger-Ellison syndrome.
Gastrin circulates as several bioactive peptides, and the peptide
pattern in gastrinoma patients often deviates from that in normal
individuals (30). Gastrin and its
precursors have been shown to promote mitogenesis and angiogenesis
in gastrointestinal tumors (31).
Gastrin-releasing peptide (GRP), which is an
unfolded protein response regulator that functions as a
Ca2+-binding molecular chaperone in the endoplasmic
reticulum, is a regulatory human peptide that induces the release
of gastrin and regulates gastric acid secretion and enteric motor
function (32). GRP is a member of
the bombesin-like peptide family. It has been reported that GRP
stimulates the proliferation and invasiveness of
androgen-independent prostate carcinoma (33). GRP mediates its action through the
membrane-bound receptor, GRP receptor (GRPR), which is
characterized by high-affinity binding for GRP and bombesin
(33). GRPR is an attractive target
for therapeutic and diagnostic applications, and is overexpressed
in prostate cancer (34). The
expression of GRPR is elevated in mucosa adjacent to head and neck
squamous cell carcinoma (HNSCC) compared with mucosa from
cancer-free controls, suggesting that elevated GRPR expression may
indicate presence of HNSCC (35).
GRP acts as a neuropeptide through G protein-coupled receptors
involved in signal transmission in the central and peripheral
nervous systems (36). It has also
been proposed that GRPR is an alternative chemotactic receptor that
may be involved in the pathogenesis of inflammatory disorders
(36). In addition, gastrin plays an
important role in the male reproductive system (37,38). In
the current study, it was revealed that the expression level of
gastrin R following electrical injury was higher than that in the
control testes, indicating that elevated gastrin R levels are
associated with testicular injury.
In the present study, it was demonstrated that the
levels of expression of FABP1 and gastrin R in the testes were
altered in electrically injured rats. Following an electrical
injury, the expression levels of FABP1 and gastrin R were
significantly elevated in the rat testes, indicating an association
with testicular trauma in rats undergoing electrical injury.
In conclusion, the current findings indicate that
such alterations would be reflected in abnormal testis
function.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81260466) and
Dali University (no. KYBS201104).
References
|
1
|
Recio AC, Felter CE, Schneider AC and
McDonald JW: High-voltage electrical stimulation for the management
of stage III and IV pressure ulcers among adults with spinal cord
injury: Demonstration of its utility for recalcitrant wounds below
the level of injury. J Spinal Cord Med. 35:58–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moreno-Duarte I, Morse LR, Alam M, et al:
Targeted therapies using electrical and magnetic neural stimulation
for the treatment of chronic pain in spinal cord injury.
Neuroimage. 85:1003–1013. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gorgey AS, Harnish CR, Daniels JA, et al:
A report of anticipated benefits of functional electrical
stimulation after spinal cord injury. J Spinal Cord Med.
35:107–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fornusek C, Davis GM and Baek I:
Stimulation of shank muscles during functional electrical
stimulation cycling increases ankle excursion in individuals with
spinal cord injury. Arch Phys Med Rehabil. 93:1930–1936. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benlier E, Eskiocak S, Puyan FO, et al:
Effect of lidocaine on reducing injury in a rat electrical burn
model. Ann Plast Surg. 69:152–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shupp JW, Moffatt LT, Nguyen T, et al:
Examination of local and systemic in vivo responses to electrical
injury using an electrical burn delivery system. J Burn Care Res.
33:118–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toy J, Ball BJ and Tredget EE: Carotid
rupture following electrical injury: a report of two cases. J Burn
Care Res. 33:e160–e165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park KH, Park WJ, Kim MK, et al:
Alterations in arterial function after high-voltage electrical
injury. Crit Care. 16:R252012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mashreky SR, Hossain MJ, Rahman A, et al:
Epidemiology of electrical injury: Findings from a community based
national survey in Bangladesh. Injury. 43:113–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang QY, Chen YC and Liu SP: Connexin 43,
angiotensin II, endothelin 1, and type III collagen alterations in
heart of rats having undergone fatal electrocution. Am J Forensic
Med Pathol. 33:215–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Helin HO, Lundin ME, Laakso M, et al:
Virtual microscopy in prostate histopathology: Simultaneous viewing
of biopsies stained sequentially with hematoxylin and eosin and
alpha-methylacyl-coenzyme A racemase/p63 immunohistochemistry. J
Urol. 175:495–499. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Rossi A, Rocha LB and Rossi MA:
Application of fluorescence microscopy on hematoxylin and
eosin-stained sections of healthy and diseased teeth and supporting
structures. J Oral Pathol Med. 36:377–381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang QY, Li XF and Liu SP: E-cadherin and
5-HT alterations in the heart of rats having undergone
atropine-induced toxicity. Mol Med Rep. 5:700–704. 2012.PubMed/NCBI
|
|
14
|
Huang QY, Li XF and Liu SP: Elevated
enolase and caveolin-1 in the heart of rats following
dexamethasone-induced toxicity. Mol Med Rep. 5:1232–1236.
2012.PubMed/NCBI
|
|
15
|
Huang QY, Li XF and Liu SP: Connexin43 and
angiotensin II alterations in hearts of rats having undergone an
acute exposure to alcohol. Am J Forensic Med Pathol. 34:68–71.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong J, Yin H, Liu W, Wang P, Jiang Y and
Chen J: Congenital iodine deficiency and hypothyroidism impair LTP
and decrease C-fos and C-jun expression in rat hippocampus.
Neurotoxicology. 26:417–426. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Kuijk AW, Gerlag DM, Vos K, et al: A
prospective, randomised, placebo-controlled study to identify
biomarkers associated with active treatment in psoriatic arthritis:
Effects of adalimumab treatment on synovial tissue. Ann Rheum Dis.
68:1303–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao N, Qu X, Yan J, Huang Q, Yuan HY and
Ouyang DS: L-FABP T94A decreased fatty acid uptake and altered
hepatic triglyceride and cholesterol accumulation in Chang liver
cells stably transfected with L-FABP. Mol Cell Biochem.
345:207–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ono T and Odani S: Initial studies of the
cytoplasmic FABP superfamily. Proc Jpn Acad Ser B Phys Biol Sci.
86:220–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thuijls G, van Wijck K, Grootjans J, et
al: Early diagnosis of intestinal ischemia using urinary and plasma
fatty acid binding proteins. Ann Surg. 253:303–308. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Atshaves BP, Martin GG, Hostetler HA,
McIntosh AL, Kier AB and Schroeder F: Liver fatty acid-binding
protein and obesity. J Nutr Biochem. 21:1015–1032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim TH, Lee JY, Park JS, et al: Fatty acid
binding protein 1 is related with development of
aspirin-exacerbated respiratory disease. PLoS One. 6:e227112011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu YL, Peng XE, Wang D, Chen WN and Lin X:
Human liver fatty acid binding protein (hFABP1) gene is regulated
by liver-enriched transcription factors HNF3β and C/EBPα.
Biochimie. 94:384–392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Newberry EP, Kennedy SM, Xie Y, et al:
Decreased body weight and hepatic steatosis with altered fatty acid
ethanolamide metabolism in aged L-Fabp−/− mice. J Lipid
Res. 53:744–754. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Storey SM, Atshaves BP, McIntosh AL, et
al: Effect of sterol carrier protein-2 gene ablation on
HDL-mediated cholesterol efflux from cultured primary mouse
hepatocytes. Am J Physiol Gastrointest Liver Physiol.
299:G244–G254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng XE, Wu YL, Lu QQ, Hu ZJ and Lin X:
Two genetic variants in FABP1 and susceptibility to non-alcohol
fatty liver disease in a Chinese population. Gene. 500:54–58. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Siddiqi S and Mansbach CM II:
Phosphorylation of Sar1b protein releases liver fatty acid-binding
protein from multiprotein complex in intestinal cytosol enabling it
to bind to endoplasmic reticulum (ER) and bud the pre-chylomicron
transport vesicle. J Biol Chem. 287:10178–10188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu RZ, Li X and Godbout R: A novel fatty
acid-binding protein (FABP) gene resulting from tandem gene
duplication in mammals: Transcription in rat retina and testis.
Genomics. 92:436–445. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang X, Wang W, Ning B, et al: Basal and
postprandial serum levels of gastrin in normotensive and
hypertensive adults. Clin Exp Hypertens. 35:74–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rehfeld JF, Bardram L, Hilsted L, Poitras
P and Goetze JP: Pitfalls in diagnostic gastrin measurements. Clin
Chem. 58:831–836. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiao L, Kovac S, Chang M, Shulkes A,
Baldwin GS and Patel O: Induction of gastrin expression in
gastrointestinal cells by hypoxia or cobalt is independent of
hypoxia-inducible factor (HIF). Endocrinology. 153:3006–3016. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ni C, Zhao X, Sun T, Liu Y, Gu Q and Sun
B: Role of gastrin-releasing peptides in breast cancer metastasis.
Hum Pathol. 43:2342–2347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagasaki S, Nakamura Y, Maekawa T, et al:
Immunohistochemical analysis of gastrin-releasing peptide receptor
(GRPR) and possible regulation by estrogen receptor βcx in human
prostate carcinoma. Neoplasma. 59:224–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beer M, Montani M, Gerhardt J, et al:
Profiling gastrin-releasing peptide receptor in prostate tissues:
Clinical implications and molecular correlates. Prostate.
72:318–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Egloff AM, Liu X, Davis AL, et al:
Elevated gastrin-releasing peptide receptor mRNA expression in
buccal mucosa: Association with head and neck squamous cell
carcinoma. Head Neck. 35:270–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Czepielewski RS, Porto BN, Rizzo LB, et
al: Gastrin-releasing peptide receptor (GRPR) mediates chemotaxis
in neutrophils. Proc Natl Acad Sci USA. 109:547–552. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Levy R, Eustache F, Pilikian S, et al:
Effect of gastrin-releasing peptide on sperm functions. Mol Hum
Reprod. 2:867–872. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sakamoto H, Takanami K, Zuloaga DG, et al:
Androgen regulates the sexually dimorphic gastrin-releasing peptide
system in the lumbar spinal cord that mediates male sexual
function. Endocrinology. 150:3672–3679. 2009. View Article : Google Scholar : PubMed/NCBI
|