Introduction
Coagulation, the formation of a clot, is an
essential process in the maintenance of homeostasis under normal
conditions and during traumatic events. Numerous agents are used to
initiate clotting and prevent uncontrolled bleeding. However, only
a few agents, with limited efficacy, are used in clinical practice
to protect formed clots against premature lysis (1,2). The
crosslinked polymer, fibrin, is dissolved into fibrin degradation
products by plasmin. Thus, preventing the activation of plasmin is
a potential target for antihemorrhage therapy. Plasmin, the primary
fibrinolytic protease responsible for fibrin solvation, is formed
by proteolytic cleavage of the zymogen plasminogen by tissue
plasminogen activator (tPA) or urokinase plasminogen activator
(uPA) (3–6). Vascular endothelial cells synthesize
tPA, and tPA-mediated plasminogen activation is accelerated in the
presence of fibrin (5,6).
An appropriate level of plasminogen activation is
maintained through the competing activity of plasminogen activators
and plasminogen activator inhibitors (PAIs). The most relevant PAI
in clot formation is PAI-1 (7),
which is the quickest acting and most physiologically specific
inhibitor of tPA (5,8). The three conformational states of PAI-1
are latent (inactive), active and reactive-center-cleaved
(plasminogen-activator complexed) (9). The active form of PAI-1 is rapidly
converted (half-life, 1–2 h) into the latent form at physiological
temperatures and pH (6,7,10). This
relatively short half-life makes it difficult to use PAI-1
therapeutically as a clot protector. However, a previous study
described a novel, genetically-engineered, antifibrinolytic PAI-1
with a significantly longer half-life (>700 h) compared with the
wild-type (11).
The aim of the present study was to compare the
antifibrinolytic activity of this very long half-life PAI-1 (VLHL
PAI-1) with 6-aminocaproic acid (also known as ε-aminocaproic acid
or Amicar) in the presence of tPA by establishing dose-response
curves and determining the IC50 (half maximal inhibitory
concentration) using thromboelastography (TEG).
Materials and methods
Clot-protecting activity assay
A clot-protecting activity assay was performed using
a TEG® 5000 Thrombelastograph® (Haemonetics Corporation, Braintree,
MA, USA). The thrombelastograph measured coagulation with a pin
attached to a torsion wire suspended in a cup holding a plasma
sample. The TEG® was calibrated with plasma controls Level I
(reading of TEG parameters within: R, 0–4 min; Angle, 75–87°; MA,
42–63 mm; K, 0–2 min) and II (reading of TEG parameters within: R,
1–5 min; Angle, 60–80°; MA, 26–41 mm; K, 0–6 min) (Haemoscope
Corporation, Neils, IL, USA). Reference plasma (Coagulation
Specialty Assayed Reference Plasma; Helena Laboratories
Corporation, Beaumont, TX, USA) was reconstituted in 1 ml distilled
water and mixed with 20 µl kaolin (Haemonetics Corporation). In
trials where tPA was utilized, 20 µl tPA (2 mg/ml; Molecular
Innovations, Novi, MI, USA) was added directly to the plasma. Next,
320 µl plasma/kaolin solution was added to the TEG® cup containing
20 µl calcium chloride (0.2 M).
Trials
Each trial consisted of two samples running
simultaneously, containing the same plasma/kaolin solution. The
control TEG® cup received 10 µl distilled water and the
experimental TEG® cup received the appropriate combination of VLHL
PAI-1, 6-aminocaproic acid (Sigma-Aldrich, St. Louis, MI, USA) and
distilled water. The 6-aminocaproic acid was reconstituted with
distilled water. Expression and purification of VLHL PAI-1 was
conducted as described in a previous study (6). Serial dilutions of VLHL PAI-1 were
performed with saline (0.15 M) and each trial ran for 45 min at
37°C.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism (GraphPad Software, Inc., La Jolla, CA, USA), and differences
between the groups were analyzed by two-way analysis of variance.
The data were presented as the mean ± standard deviation, and
P<0.05 was considered to indicate a statistically significant
difference. Graph visualization was performed using Origin 8
software (OriginLab, Northampton, MA, USA).
Results
Clotting parameters for the reference
and tPA-treated plasma
Selected clotting parameters for the control and
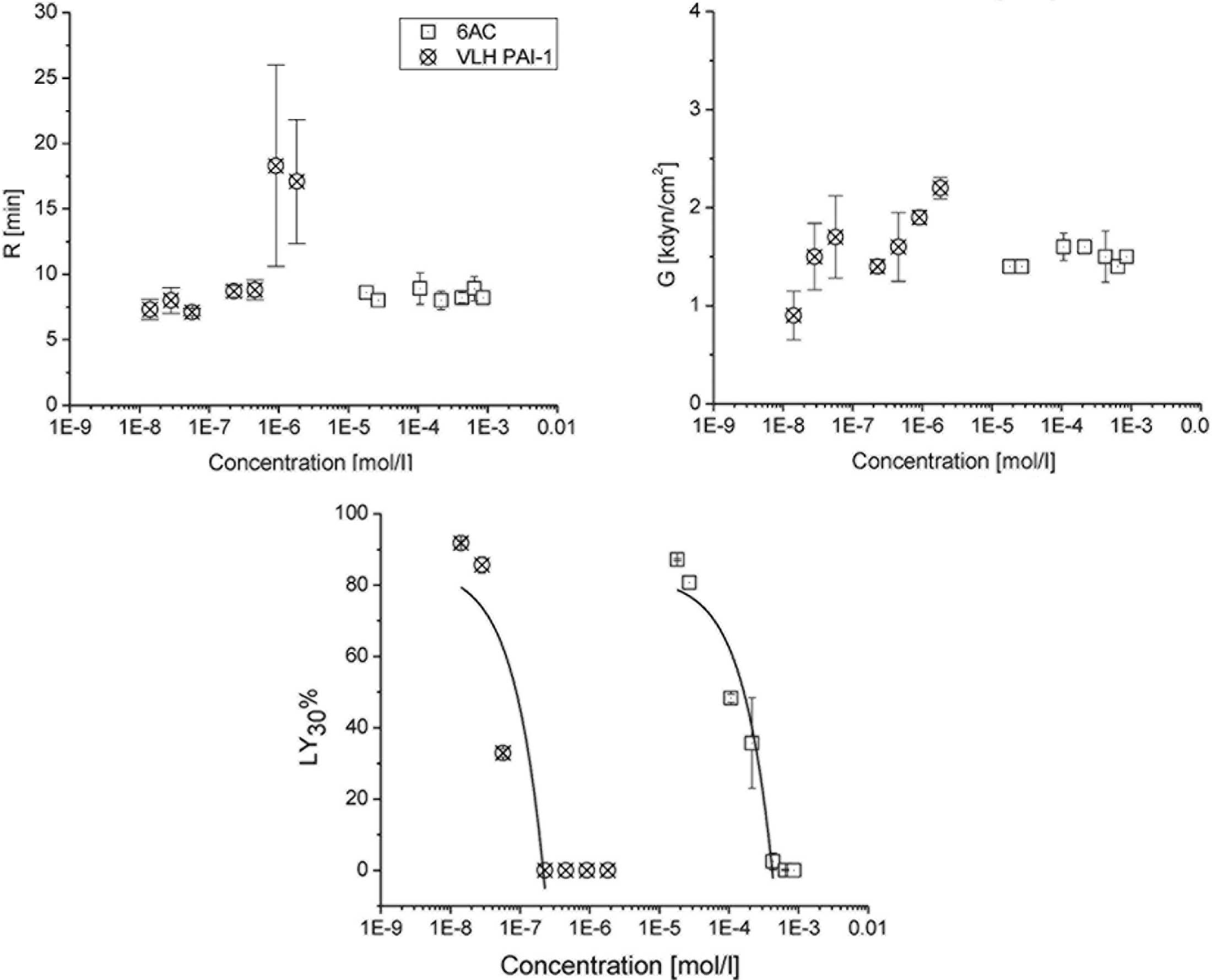
various doses of 6-aminocaproic acid and VLHL PAI-1 are shown in
Table I and Figs. 1 and 2. Control samples consisted of reference
and tPA-treated plasma, with tPA included to mimic clot lysis.
Reference and tPA-treated plasma exhibited similar average control
values, with the exception of a lower clot strength (G), maximum
amplitude (MA) and rate of clot lysis after 30 min
(LY30) in the tPA-treated plasma due to tPA-induced clot
lysis, which is consistent with previous studies (12–16). The
MA was lower, as reference plasma lacks platelets that strengthen
the blood clot (3,17–19).
There are no normal values of TEG parameters for reference plasma
in the literature. However, normal blood samples are considered to
have a reaction time (R) of 2–8 min, a 2–4-min period between the
reaction onset and the formation of a 20-mm clot, an MA of 51–69 mm
and a LY30 value of 0–8% (12).
 | Table I.Selected thrombelastography parameters
for the control-, VLHL PAI-1- and 6AC-treated reference plasma. |
Table I.
Selected thrombelastography parameters
for the control-, VLHL PAI-1- and 6AC-treated reference plasma.
| Reagent (µg) | R (min) | K (min) | A (°) | MA (mm) | LY30
(%) | G
(kdyn/cm2) |
|---|
| Control |
|
|
|
|
|
|
|
Reference |
8.57±0.45 |
2.04±0.62 |
63.7±5.6 |
26.2±2.2 |
0.0±0.0 |
1.77±0.19 |
|
tPA-treated |
8.09±0.71 |
2.30±0.55 |
63.9±4.7 |
19.3±2.5 |
87.5±7.5 |
1.21±0.19 |
| VLHL PAI-1 |
|
|
|
|
|
|
| 25.0 |
18.27±4.75 |
5.80±1.80 |
35.6±7.4 |
30.2±1.5 |
0.0±0.0 |
2.17±0.11 |
| 12.5 |
23.75±0.70 |
8.90±5.37 |
32.1±13.2 |
26.9±0.6 |
0.0±0.0 |
1.85±0.07 |
|
6.25 |
9.10±0.45 |
2.67±1.55 |
62.0±2.8 |
26.4±0.6 |
0.0±0.0 |
1.82±0.35 |
|
3.12 |
8.70±0.28 |
6.05±4.87 |
65.0±13.1 |
21.1±1.2 |
0.0±0.0 |
1.35±0.07 |
|
0.78 |
7.05±0.77 |
2.30±1.55 |
63.3±7.1 |
24.8±4.5 |
32.9±1.3 |
1.70±0.42 |
|
0.39 |
8.00±0.98 |
1.55±0.49 |
71.2±2.4 |
22.9±2.1 |
85.0±2.3 |
1.50±0.14 |
|
0.19 |
7.25±0.77 | N/A |
62.4±4.8 |
15.6±3.6 |
91.8±0.5 |
0.90±0.28 |
| 6AC |
|
|
|
|
|
|
|
36.0 |
8.20±0.00 |
2.10±0.42 |
63.3±8.4 |
22.8±0.2 |
0.0±0.0 |
1.50±0.00 |
|
27.0 |
8.85±0.91 |
5.10±2.68 |
54.3±2.9 |
21.1±1.4 |
0.1±0.1 |
1.35±0.07 |
|
18.0 |
8.20±0.40 |
2.10±0.30 |
67.0±2.8 |
23.0±2.7 |
2.4±2.3 |
1.50±0.26 |
|
9.0 |
8.00±0.70 |
1.60±0.28 |
68.3±3.6 |
24.0±0.6 |
35.7±12.7 |
1.55±0.07 |
|
4.5 |
8.85±1.20 |
1.65±0.07 |
67.3±1.6 |
24.2±1.1 |
48.3±1.1 |
1.60±0.14 |
|
1.12 |
8.00±0.00 |
1.80±0.00 |
70.2±0.0 |
21.8±0.0 |
80.7±0.0 |
1.40±0.00 |
|
0.75 |
8.55±0.07 |
2.25±0.07 |
62.9±2.4 |
21.4±0.6 |
87.1±0.3 |
1.35±0.07 |
6-aminocaproic acid treatment
decreases the incidence rate of clot lysis after 30 min
Doses of 6-aminocaproic acid that were ≥18 µg
generated LY30 values of ≤5%, indicating that the formed
fibrin clot was almost entirely intact. Serial dilutions of ≤9 µg
yielded significantly different (P<0.01) LY30 values,
ranging between 26.7 and 87.4%, as compared with the controls
(Fig. 1). The IC50 of
6-aminocaproic acid was 6.5 µg or 1.6×10−4 mol/l when
expressed as a concentration. No dosage of 6-aminocaproic acid
produced a delay in clot formation (Fig.
1). The maximum G achieved by 6-aminocaproic acid was 1.8
kdyn/cm2.
VLHL PAI-1 treatment decreases
incidence rate of clot lysis after 30 min
At doses of ≥3.125 µg VLHL PAI-1, tPA action was
completely inhibited, generating LY30 values of 0%.
Doses of ≤0.78 µg generated statistically significant differences
(P<0.01) in LY30 values, which ranged between 32.1
and 92.2%, as compared with the controls. The IC50 of
VLHL PAI-1 was 0.68 µg or 8.8×10−8 mol/l when expressed
as a concentration (Fig. 1). In
addition, VLHL PAI-1 doses of ≥12.5 µg produced a statistically
significant (P<0.01) increase in R, as compared with the
controls (Fig. 2). VLHL PAI-1
achieved a maximum G of 2.3 kdyn/cm2.
Discussion
There are three conformational states of PAI-1,
including latent (inactive), active and reactive-center-cleaved
(plasminogen-activator-complex) (9,10). The
active form of PAI-1 is rapidly converted (half-life, 1–2 h) into
the latent form at a physiological temperature and pH (7,9,10,20). The
reactive center loop of PAI-1, which contains the active site, is
connected to β-sheet C at the C-terminal and β-sheet A at the N
terminal (9). In the latent form,
the reactive center loop is inserted into β-sheet A (central
β-sheet), thereby eliminating the possibility of proteinase
interaction with the reactive peptide bond (P1-P1) of tPA or uPA,
and PAI-1 becomes inactive (7,9,10). Thus, only active PAI-1 can be used as
a clot protector. We previously developed a VLHL PAI-1 mutant that
remained active for >700 h, and despite the two cysteine
mutations, the VLHL PAI-1 structure was found to be almost
identical to wild-type PAI-1 (10,11,21,22). The
long half-life of VLHL PAI-1 has enabled this protein to become a
potential therapeutic agent for the mitigation of bleeding.
None of the current antifibrinolytics, including
aprotinin, 6-aminocaproic acid and tranexamic acid (TXA), are
without side effects, warranting the development of new hemostatic
agents (14). TXA has been shown to
have a statistical association with seizure induction, possibly due
to cerebral ischemia caused by a decrease in regional or global
cerebral blood flow (20). Aprotinin
functions via the same mechanism as PAI-1, while lysine analogs,
such as 6-aminocaproic acid and TXA, block the lysine-binding sites
on plasminogen, thereby preventing the activation of plasmin and
fibrin clot-degradation (19). The
USA Food and Drug Administration has removed aprotinin, a bovine
product, from the market due to statistical associations with renal
failure, cerebrovascular events and mortality, without
significantly stronger antifibrinolytic effects over other
medications within the class (23–25). As
a natural constituent of blood, VLHL PAI-1 may be free of side
effects in addition to being an efficient antifibrinolytic
agent.
Using a number of in vitro, ex vivo and in
vivo models, previous studies have shown that VLHL PAI-1 is
able to reduce clot lysis and bleeding in animal experiments
(12–14,17).
However, the efficacy of this protein had not been compared with
other antifibrinolytics. The present study showed that VLHL PAI-1
is a more effective blood clot-protector than 6-aminocaproic acid.
Racanelli et al compared recombinant wild PAI-1 to
6-aminocaproic acid in a rabbit model and found that the molar
ED50 (effective dose for 50% of population base) was
25,000 times higher for 6-aminocaproic acid than for PAI-1. It was
also found that complete inhibition of blood loss was achieved with
PAI-1, but the highest dose of 6-aminocaproic acid did not
completely inhibit blood loss (26).
This outcome supports the conclusions of the present study.
However, the absence of platelets and associated platelet function
in reference plasma makes the clot weaker. This lack of
thrombocytes in the reference plasma may cause 6-aminocaproic acid
to exhibit an ED50 value ∼2,000 times higher than that
of VLHL PAI-1.
Notably, an increase in R was observed in ≥12.5 µg
VLHL PAI-1 (9.0×10−7 mol/l) cases. This phenomenon may
be explained by the interaction of thrombin with PAI-1 and the
simultaneous formation of cleaved PAI-1 and thrombin-PAI-1
complexes. The kinetics of this reaction are described by a suicide
substrate model with a branched reaction that ends in the
inhibitor/enzyme complex, the cleaved inhibitor and free enzyme.
Due to the branched pathway, it was proposed that 3 mol PAI-1 was
required to completely inhibit 1 mol thrombin (27). Since thrombin, a serine protease,
converts soluble fibrinogen into insoluble strands of fibrin
indirectly through factor XIII, inhibition of thrombin by excess
PAI-1 increases the time required for clot formation.
VLHL PAI-1 may also be implemented as a treatment
for PAI-1 deficiency. This condition is caused by a lack of PAI-1,
an abnormality in the PAI-1 molecule itself, or defects in the
secretory dynamics of PAI-1 in the blood (16,28–31).
PAI-1 deficiency, a clinically rare bleeding disorder, is
characterized by hyperfibrinolytic hemorrhage in the presence of
normal thrombus formation (7,9).
Patients experience multiple episodes of uncontrolled bleeding, and
in severe cases, require blood product transfusions (16,28–31). The
class of medications currently used to treat PAI-1 deficiency are
antifibrinolytics, which function by inhibiting the conversion of
plasminogen into plasmin (22).
There are three primary antifibrinolytics utilized in the clinical
treatment of the disease: Aprotinin, 6-aminocaproic acid and TXA,
which are not always effective and may exhibit a number of side
effects (26). Bleeding occurs due
to the unopposed conversion of plasminogen to plasmin by the action
of tPA and subsequent fibrinolytic activity. Infusion of VLHL
PAI-1, structurally homologous to wild-type PAI-1, may provide
prophylactic treatment for these patients and restore them to
normal health (12–14,28,30).
In conclusion, the inhibition of tPA by VLHL PAI-1
demonstrates an improved efficacy over 6-aminocaproic acid in
managing hemorrhagic events in the general patient population.
However, concentrations of >9.0×10−7 mol/l VLHL PAI-1
may delay the initiation and dynamics of clot formation. Therefore,
in therapy, VLHL PAI-1 should be used in concentrations
<9.0×10−7 mol/l.
Acknowledgements
This study was supported in part by grants from the
Frank Stranahan Endowed Chair and Childrens Miracle Network.
References
|
1
|
Antun AG, Gleason S, Arellano M, et al:
Epsilon aminocaproic acid prevents bleeding in severely
thrombocytopenic patients with hematological malignancies. Cancer.
119:3784–3787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dhir A: Antifibrinolytics in cardiac
surgery. Ann Card Anaesth. 16:117–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jankun J and Skrzypczak-Jankun E:
Plasminogen activator inhibitor with very long half-life (VLHL
PAI-1) can reduce bleeding in PAI-1-deficient patients. Cardiovasc
Hematol Disord Drug Targets. 13:144–150. 2013.PubMed/NCBI
|
|
4
|
Mosesson MW: Fibrinogen and fibrin
structure and functions. J Thromb Haemost. 3:1894–1904. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rau JC, Beaulieu LM, Huntington JA and
Church FC: Serpins in thrombosis, hemostasis and fibrinolysis. J
Thromb Haemost. 5:(Suppl 1). 102–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zorio E, Gilabert-Estellés J, España F,
Ramón LA, Cosín R and Estellés A: Fibrinolysis: the key to new
pathogenetic mechanisms. Curr Med Chem. 15:923–929. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dellas C and Loskutoff DJ: Historical
analysis of PAI-1 from its discovery to its potential role in cell
motility and disease. Thromb Haemost. 93:631–640. 2005.PubMed/NCBI
|
|
8
|
Cesarman-Maus G and Hajjar KA: Molecular
mechanisms of fibrinolysis. Br J Haematol. 129:307–321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Egelund R, Schousboe SL, Sottrup-Jensen L,
Rodenburg KW and Andreasen PA: Type-1 plasminogen-activator
inhibitor - conformational differences between latent, active,
reactive-centre-cleaved and plasminogen-activator-complexed forms,
as probed by proteolytic susceptibility. Eur J Biochem.
248:775–785. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jankun J, Yang J, Zheng H, Han FQ,
Al-Senaidy A and Skrzypczak-Jankun E: Remarkable extension of PAI-1
half-life surprisingly brings no changes to its structure. Int J
Mol Med. 29:61–64. 2012.PubMed/NCBI
|
|
11
|
Chorostowska-Wynimko J, Swiercz R,
Skrzypczak-Jankun E, Wojtowicz A, Selman SH and Jankun J: A novel
form of the plasminogen activator inhibitor created by cysteine
mutations extends its half-life: relevance to cancer and
angiogenesis. Mol Cancer Ther. 2:19–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jankun J, Aleem AM, Selman SH, et al:
Highly stable plasminogen activator inhibitor type one (VLHL PAI-1)
protects fibrin clots from tissue plasminogen activator-mediated
fibrinolysis. Int J Mol Med. 20:683–687. 2007.PubMed/NCBI
|
|
13
|
Jankun J, Keck R, Selman SH and
Skrzypczak-Jankun E: Systemic or topical application of plasminogen
activator inhibitor with extended half-life (VLHL PAI-1) reduces
bleeding time and total blood loss. Int J Mol Med. 26:501–504.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jankun J, Selman SH, Keck RW,
Łysiak-Szydłowska W and Skrzypczak-Jankun E: Very long half-life
plasminogen activator inhibitor type 1 reduces bleeding in a mouse
model. BJU Int. 105:1469–1476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jankun J, Skotnicka M, Łysiak-Szydłowska
W, Al-Senaidy A and Skrzypczak-Jankun E: Diverse inhibition of
plasminogen activator inhibitor type 1 by theaflavins of black tea.
Int J Mol Med. 27:525–529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jankun J and Skrzypczak-Jankun E: Yin and
yang of the plasminogen activator inhibitor. Pol Arch Med Wewn.
119:410–417. 2009.PubMed/NCBI
|
|
17
|
Jankun J, Aleem AM, Struniawski R,
Lysiak-Szydłowska W, Selman SH and Skrzypczak-Jankun E: Accelerated
thrombus lysis in the blood of plasminogen activator inhibitor
deficient mice is inhibited by PAI-1 with a very long half-life.
Pharmacol Rep. 61:673–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morimoto Y, Yoshioka A, Imai Y, Takahashi
Y, Minowa H and Kirita T: Haemostatic management of intraoral
bleeding in patients with congenital deficiency of alpha2-plasmin
inhibitor or plasminogen activator inhibitor-1. Haemophilia.
10:669–674. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schulman S: Pharmacologic tools to reduce
bleeding in surgery. Hematology Am Soc Hematol Educ Program.
2012:517–521. 2012.PubMed/NCBI
|
|
20
|
Makhija N, Sarupria A, Kumar Choudhary S,
Das S, Lakshmy R and Kiran U: Comparison of epsilon aminocaproic
acid and tranexamic acid in thoracic aortic surgery: clinical
efficacy and safety. J Cardiothorac Vasc Anesth. 27:1201–1207.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harrop SJ, Jankova L, Coles M, et al: The
crystal structure of plasminogen activator inhibitor 2 at 2.0 A
resolution: implications for serpin function. Structure. 7:43–54.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sharp AM, Stein PE, Pannu NS, et al: The
active conformation of plasminogen activator inhibitor 1, a target
for drugs to control fibrinolysis and cell adhesion. Structure.
7:111–118. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fergusson DA, Hébert PC, Mazer CD, et al:
BART Investigators: A comparison of aprotinin and lysine analogues
in high-risk cardiac surgery. N Engl J Med. 358:2319–2331. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Howell N, Senanayake E, Freemantle N and
Pagano D: Putting the record straight on aprotinin as safe and
effective: results from a mixed treatment meta-analysis of trials
of aprotinin. J Thorac Cardiovasc Surg. 145:234–240. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iribarren JL, Jimenez JJ, Hernández D, et
al: Postoperative bleeding in cardiac surgery: the role of
tranexamic acid in patients homozygous for the 5G polymorphism of
the plasminogen activator inhibitor-1 gene. Anesthesiology.
108:596–602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Racanelli AL, Diemer MJ, Dobies AC, Dubin
JR and Reilly TM: Comparison of recombinant plasminogen activator
inhibitor-1 and epsilon amino caproic acid in a hemorrhagic rabbit
model. Thromb Haemost. 67:692–696. 1992.PubMed/NCBI
|
|
27
|
van Meijer M, Smilde A, Tans G, Nesheim
ME, Pannekoek H and Horrevoets AJ: The suicide substrate reaction
between plasminogen activator inhibitor 1 and thrombin is regulated
by the cofactors vitronectin and heparin. Blood. 90:1874–1882.
1997.PubMed/NCBI
|
|
28
|
Fay WP, Parker AC, Condrey LR and Shapiro
AD: Human plasminogen activator inhibitor-1 (PAI-1) deficiency:
characterization of a large kindred with a null mutation in the
PAI-1 gene. Blood. 90:204–208. 1997.PubMed/NCBI
|
|
29
|
Jankun J and Skrzypczak-Jankun E: Bleeding
diathesis is associated with an A15T heterozygous mutation in exon
2 of the plasminogen activator inhibitor type 1. Exp Ther Med.
1:575–577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mehta R and Shapiro AD: Plasminogen
activator inhibitor type 1 deficiency. Haemophilia. 14:1255–1260.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schleef RR, Higgins DL, Pillemer E and
Levitt LJ: Bleeding diathesis due to decreased functional activity
of type 1 plasminogen activator inhibitor. J Clin Invest.
83:1747–1752. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Genét GF, Ostrowski SR, Sørensen AM and
Johansson PI: Detection of tPA-induced hyperfibrinolysis in whole
blood by RapidTEG, KaolinTEG, and functional fibrinogenTEG in
healthy individuals. Clin Appl Thromb Hemost. 18:638–644. 2012.
View Article : Google Scholar : PubMed/NCBI
|