Introduction
It is generally accepted that precocious puberty
(PP) refers to signs of sexual development occurring in girls
younger than 8 years of age or boys younger than 9 years of age, or
the appearance of menstruation in girls before the age of 10 years.
The major manifestations of PP include a growth spurt, together
with maturity of the reproduction organs and sexual characteristics
that are significantly advanced compared with those of other
children of the same age. In girls, idiopathic central precocious
puberty (ICPP) accounts for >80% of the cases of central
precocious puberty (CPP).
The clinical diagnosis of this condition and the
evaluation of therapeutic effects are mainly conducted through
physical examination, and the assessment of bone age and growth
rate. Currently, owing to the high accuracy of provocation testing
with hypothalamic gonadotropin releasing hormone (GnRH), it
continues to be the gold standard in the diagnosis of CPP with
active suppression of gonadal axis post-treatment. However,
provocation testing with GnRH is not yet widely used due to it
being expensive, time-consuming and causing an uncomfortable
sensation similar to that of repeated hemospasia/pricking.
The pathogenesis of ICPP is currently unclear.
Previous studies have confirmed that the kisspeptin/G
protein-coupled receptor 54 (GPR54) signaling pathway initiates the
onset of puberty (1,2), which is probably closely associated
with the occurrence and development of ICPP. The early diagnosis of
PP and evaluation of therapeutic effects is very important;
however, to the best of our knowledge, no studies concerning the
kisspeptin levels of normal children, girls with premature
thelarche (PT) and girls with ICPP ante-and-post treatment have
been reported.
The purpose of present study was to explore the role
of kisspeptin in the initiation of pubertal growth and its
significance in the diagnosis of ICPP in girls and the evaluation
of the effects of ICPP therapy. The kisspeptin levels of girls with
ICPP ante- and post-treatment, girls with PT, and normal girls of
the same age were compared with the aim of elucidating the
correlation of the kisspeptin signaling mechanism with suppression
of the gonadal axis in girls with ICPP at onset and post-treatment.
This may facilitate the development of new guidelines for the early
diagnosis of ICPP in females and the evaluation of therapeutic
effects.
Materials and methods
Subjects
A case-control study design was adopted. Informed
consent was obtained from the subjects and their guardians and
approval was provided by ethics committee of the Children's
Hospital of Jiangxi (Nanchang, China).
ICPP group
A total of 24 girls diagnosed with ICPP in this
hospital from June 2012 to January 2013 formed the ICPP group. The
diagnosis standard of ICPP was in accordance with the diagnosis and
treatment guidelines of central (true) precocious puberty as
established by the Subspecialty Group of Endocrinology, Hereditary
and Metabolic Diseases of the Society of Pediatrics, Chinese
Medical Association in 2007 (3).
Normal control group
The normal control group comprised girls who
accepted a physical health examination at the above period in this
hospital. The inclusion criteria were: i) no manifestation of the
development of sexual characteristic; ii) height and weight between
the mean ±2 standard deviations (SD) of those of children with the
same gender and of the same age; iii) age matched as closely as
possible with the ICPP group (±1 year).
PT group
The PT group comprised girls diagnosed with PT in
this hospital during the aforementioned time period. The inclusion
criteria were: i) only premature thelarche, without occurrence of
other secondary sex characteristics, and no coloring of the areola
of the breast; ii) followed up for 1 year, showing a
non-progressive self-limited course; iii) height and weight between
mean ± 2SD of those of children with the same gender and of the
same age; iv) age matched as closely as possible with the ICPP
group (±1 year).
Research methods
Growth and development evaluation
Height, weight, body mass index (BMI) and Tanner
staging for girls were determined by clinical specialists to
evaluate the puberty developmental level. Bone age determination
via the Tanner-Whitehouse (TW2) method (4) and B ultrasonic examination of the
ovaries and uterus were conducted.
Detection of baseline levels of luteinizing
hormone (LH), follicle-stimulating hormone (FSH) and estradiol
(E2)
A chemiluminescence method was adopted to measure
the levels of LH, FSH and E2 using kits obtained from
Siemens Healthcare Diagnostics Inc., (Tarrytown, NY, USA).
Provocation testing with GnRH
Gonadorelin was administered intravenously according
to a dosage level of 2.5 µg/kg (maximum dose, 100 g). Serum LH and
FSH levels were detected at 0, 30, 60 and 90 min after the
injection using a chemiluminescence-based method, as described
above. In this test, a peak stimulated LH level of >3.3–5.0 U/l
is considered to indicate a diagnosis of CPP, and a ratio of LH/FSH
>0.6 may be diagnosed as CPP.
Exclusion of ICPP due to organic causes
For girls considered to have ICPP, blood, urine and
feces routine tests were conducted. Tests of hepatic, renal and
thyroid gland function were also conducted, and the levels of
adrenocorticotropic hormone, growth hormone and
17α-hydroxyprogesterone were detected. Abdominal B ultrasound
scanning of the liver, gallbladder, spleen, pancreas, kidneys and
adrenal gland together with magnetic resonance imaging of the
hypothalamus and hypophysis were conducted to exclude ICPP due to
organic causes.
Detection of the plasma kisspeptin level by
ELISA
A 1-ml specimen of blood was put into an EDTA
anticoagulant tube containing 0.6TI aprotinin (Amresco; Shanghai
Haoran Bio Technologies Co., Ltd., Shanghai, China), immediately
mixed gently, allowing aprotinin to contact the blood sufficiently
to reduce the degradation of kisspeptin by protease, and then
conserved at 4°C. The blood specimen was centrifuged for 15 min at
l,600 × g, 4°C and the supernatant was extracted and conserved at
−80°C for detection. All of the above procedures were completed
within 1 h. The plasma specimen was then loaded onto a
C18-Sep-Column (Bejing H&E Technology Co., Ltd., Beijing,
China), and the eluent was evaporated and dried using a SIM FD-5
vacuum freeze dryer (SIM International Group Co., Ltd., Shanghai,
China), then reconstituted. The kisspeptin level was detected by
ELISA according to directions of the kisspeptin test kit (Phoenix
Pharmaceuticals, Inc., Burlingame, CA, USA).
ICPP treatment and evaluation of therapeutic
effect
i) Treatment. The first dosage of triptorelin was
administered according to a dosage level of 80–100 µg/kg (maximum
dose, 3.75 mg). Subsequently, one injection was given every 4
weeks. For those patients whose weight was >30 kg, 3.75 mg
triptorelin was injected intramuscularly every 4 weeks, and the
maintenance dosage was adjusted according to the suppression of
gonadal axis function (including sexual characteristics, sex
hormone levels and bone age).
ii) Therapeutic effect evaluation. Height and the
status of sexual characteristics development were determined every
3–6 months during the course of treatment. The GnRH provocation
test was readministered at 3–6 months after the first dose. Serum
E2 level measurements, B ultrasound scanning of the
ovaries and uterus, and bone age evaluation were conducted after 3
months of treatment. Plasma kisspeptin levels were monitored after
6 months of treatment (3).
Statistical analysis
Statistical analyses were performed using SPSS for
Windows, version 18.0 (SPSS, Inc., Chicago, IL, USA). All test
results are presented as the mean ± standard deviation. Students
t-test was used for pairwise comparison of measurement data.
Differences of multiple sample rates were compared by analysis of
variance. The correlation of kisspeptin levels in different groups
was analyzed by Pearson correlation analysis. Height is expressed
as SDS, which was calculated as follows: SDS = (measured value -
mean)/SD. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of clinical parameters and
growth among the different groups
A total of 24 girls with ICPP treatment, 21 girls
with PT and 25 normal girls as the control were enrolled in this
study. The average age at the first diagnosis was 7.53±0.71 years
in the ICPP group, 7.62±1.42 years in the PT group and 7.27±1.51
years in the control group. The healthy control group comprised
undeveloped prepubertal girls, and they had the lowest BMI
(15.02±1.93 kg/m2). The BMI for girls with ICPP prior to
treatment was the highest (17.09±3.05 kg/m2), which
indicated that the BMI increased along with pubertal development.
Compared with the PT and control groups, the girls in the ICPP
group had significantly advanced bone age. The ratio of bone
age/actual age was 1.57±0.64 (F=114.1, P<0.001). The SDS of
height suggested clearly accelerated growth in the ICPP group
(1.05±0.78; F=135, P<0.001). The E2 level and volume
of the ovaries and uterus in girls of the ICPP group were also
markedly higher than those of other two groups, with the difference
being statistically significant (P<0.001; Table I).
 | Table I.Comparison of clinical parameters
among four experimental groups (mean ± standard deviation). |
Table I.
Comparison of clinical parameters
among four experimental groups (mean ± standard deviation).
| Group | N | Age (years) | Height SDS | Body weight (kg) | BMI
(kg/m2) | Bone age (years) | BA/CA | L-OV |
|---|
| Before ICPP
treatment | 24 | 7.53±0.71 | 1.05±0.78 | 31.55±7.70 | 17.09±3.15 | 8.59±0.98 | 1.57±0.64 | 2.55±1.06 |
| After ICPP
treatment | 24 | 8.67±1.01 | 1.19±0.48 | 33.83±4.35 | 17.39±1.61 | 9.12±0.84 | 1.62±0.49 | 2.22±1.12 |
| PT | 21 | 7.62±1.42 | 0.24±0.67 | 26.95±5.04 | 16.09±1.91 | 7.88±1.04 | 0.97±0.94 | 1.90±0.59 |
| Control | 25 | 7.27±1.51 | -0.41±0.52 | 23.20±5.81 | 15.02±1.92 | 7.79±0.62 | 1.02±0.79 | 1.51±0.94 |
| F-value |
|
| 135 |
| 2.87 |
| 114.1 | 206 |
| P-value |
|
| <0.001 |
| 0.044 |
| <0.001 | <0.001 |
|
|
| R-OV | UV | B-LH (IU/l) | P-LH (IU/l) | B-FSH (IU/l) | P-FSH (IU/l) | P-LH/P-FSH | E2
(pg/ml) |
|
| Before ICPP
treatment | 2.56±1.12 | 2.98±0.85 | 2.24±5.74 | 13.23±10.33 | 1.86±4.95 | 13.08±5.54 | 0.51±0.43 | 24.15±11.50 |
| After ICPP
treatment | 2.27±0.91 | 2.62±1.81 | 1.03±2.81 | 0.78±0.47 | 2.07±3.17 | 1.81±1.00 | 0.03±0.70 | 17.38±9.36 |
| PT | 1.86±0.63 | 2.14±1.15 | 1.65±2.46 | 1.37±2.94 | 5.25±5.90 | 1.74±1.83 | 0.12±0.48 | 18.81±10.58 |
| Control | 1.45±0.79 | 1.93±1.12 | 0.50±0.72 |
| 2.15±3.85 |
|
| 8.51±2.92 |
| F-value | 258 | 137 |
|
|
|
| 156 | 92.5 |
| P-value | <0.001 | <0.001 |
|
|
|
| <0.001 | 0.012 |
Comparison of plasma kisspeptin levels
among different groups
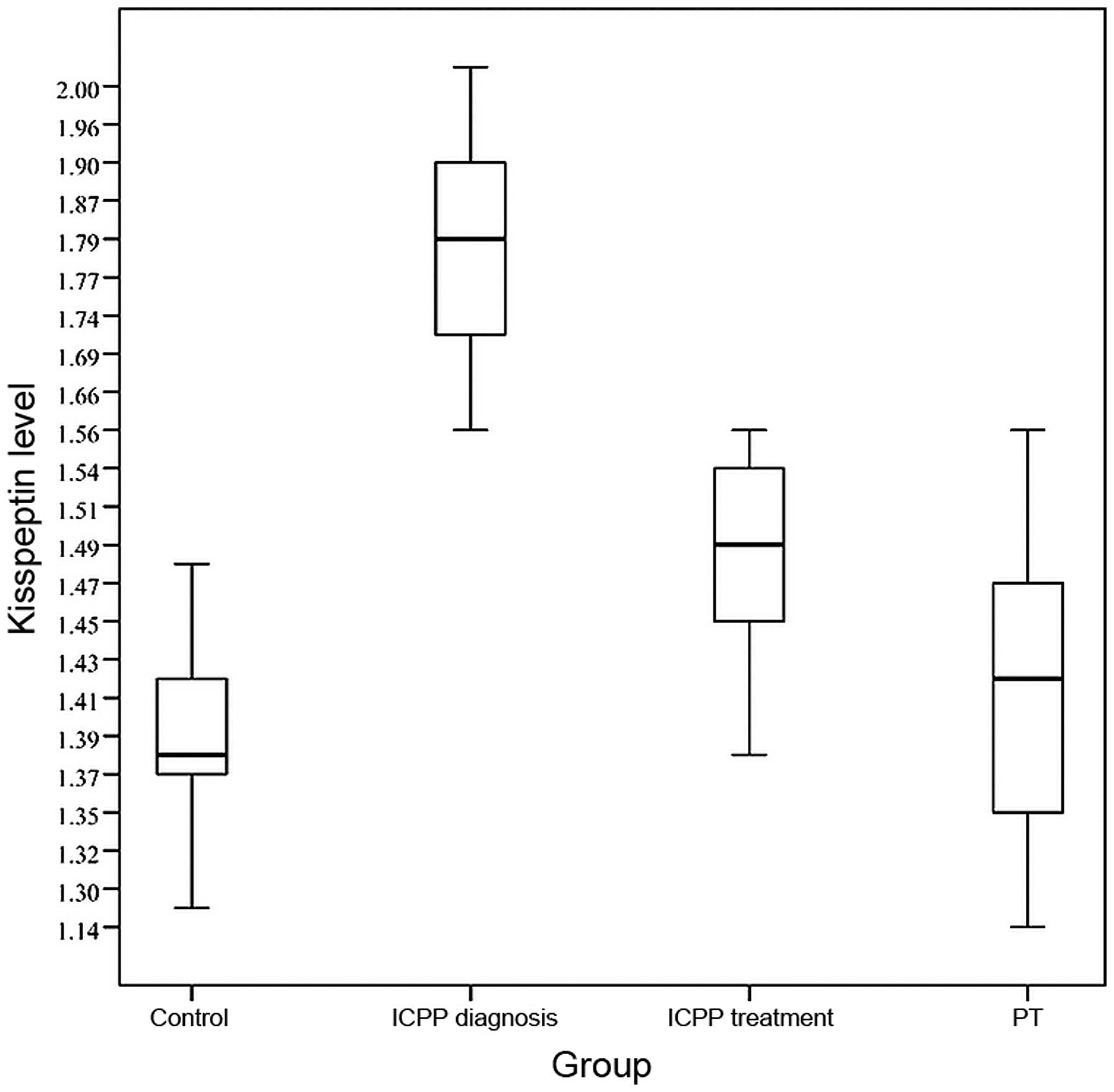
The average plasma kisspeptin level of the ICPP
group at the time of diagnosis (1.80±0.13 ng/ml) was higher than
those of the PT group (1.41±0.10 ng/ml) and the control group
(1.39±0.13 ng/ml), with the differences being statistically
significant (F=109.7, P<0.001; Fig.
1). When pubertal development in the ICPP group was completely
inhibited after 6 months of treatment, plasma kisspeptin levels
(1.49±0.15 ng/ml) were clearly decreased compared with those prior
to treatment (1.80±0.13 ng/ml), and the difference was
statistically significant (t=10.80, P<0.001; Fig. 1). The difference in kisspeptin levels
between the PT group and the control group was not statistically
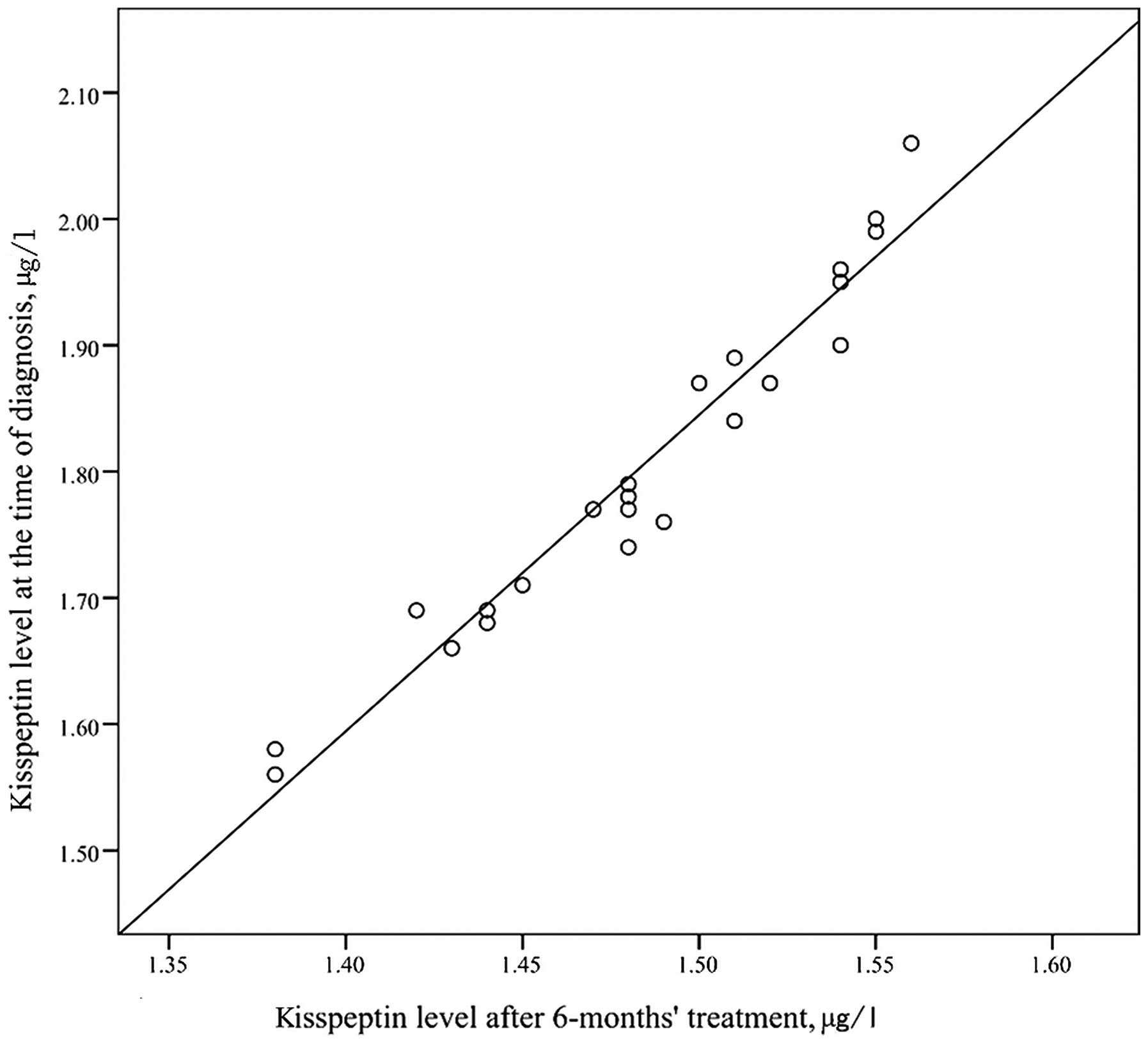
significant (t=10.97, P=0.095). The kisspeptin level at the time of
ICPP diagnosis and the kisspeptin level after treatment were
positively correlated in a statistically significant manner
(r=0.974, P<0.001; Fig. 2).
Discussion
The pubertal development of humans is a complex
process associated with hormone secretion, which is regulated by
the hypothalamic-pituitary-gonadal (HPG) axis. The activation of
the HPG axis is under integrated regulation by genetics, body
energy status and environmental and other factors (5). Activation of GnRH neurons is the key to
triggering the initiation of puberty. A previous study has shown
that at peri-puberty, very high Kiss-1 and GPR54 mRNA levels are
detectable in the rat hypothalamus, and Kiss-1 gene expression
levels are increased in the anteroventral periventricular nuclei
(6), which suggests that kisspeptin
may play an important role in the regulation of GnRH secretions and
may act directly on GnRH neurons (7).
Pielecka-Fortuna and Moenter (8) observed that the intraventricular
injection of kisspeptins into mice promoted the release of GnRH,
and a parallel increase in plasma LH occurred. Plant, Ramaswamy and
Dipietro (9) repeatedly administered
kisspeptin-10 to juvenile monkeys intravenously and GnRH secretion,
indicating sexual maturity, occurred prematurely. These findings
imply that the secretion of kisspeptin has a close association with
the initiation of puberty.
These data indicate the important role of kisspeptin
in pubertal development. However, the majority of studies have
focused on the role of kisspeptin in the activation of the HPG axis
and its mechanism. Studies concerning the clinical application of
kisspeptin are rare.
In the present study, the kisspeptin levels of girls
with ICPP were detected prior to treatment and following 6 months
of treatment, and were compared with those of girls with PT and
healthy controls. The results demonstrated that the kisspeptin
levels of girls in the ICPP group prior to treatment were
significantly higher than those of the other groups, which supports
the hypothesis that kisspeptin is an activating effector of the HPG
axis. Significant increases in Kiss-1 gene expression trigger the
pulsed release of GnRH, activate the HPG axis and initiate pubertal
development, thereby causing the occurrence of PP (10).
Studies on the blood levels of kisspeptin in
children have particularly focused on comparisons between girls
with CPP and those with PT or normal undeveloped girls, and there
have been no comparative studies on the plasma kisspeptin levels of
girls with ICPP prior to and following treatment in China. In the
present study, the kisspeptin levels of girls with ICPP before
treatment and after 6 months of treatment were compared, and the
results revealed that the kisspeptin level of the ICPP group
decreased significantly during this treatment period. Based on this
result, combined with a significant reduction of the LH peak/FSH
peak ratio in the GnRH provocation test when checked after
treatment, it is considered that the HPG axis was suppressed by the
treatment and the expression of Kiss-1 gene fell to a level typical
of ante-pubertal development. As for comparison of the ICPP group
after treatment with the control group, the results of the two
groups were similar, and the kisspeptin level following ICPP
treatment was only slightly higher than that of the control.
However, the average age of the ICPP group post-treatment was
nearly 1.5 years greater than that of the control group. Further
research is required to assess whether kisspeptin levels could
decrease to the same level as those of the control group or lower
with continued treatment.
Demirbilek et al (11) detected the plasma kisspeptin levels
of 28 girls with ICPP prior to treatment and after 6 months of
treatment, and the results demonstrated that the kisspeptin level
was clearly decreased following the treatment, which is consistent
with the results of the present study. In addition, statistical
analysis showed that the plasma kisspeptin levels of girls with
ICPP prior to and following treatment were positively correlated,
all of which implies that the plasma level of kisspeptin could be
used an indicator to evaluate the therapeutic effects in the
treatment of precocious puberty.
Liu et al (12) detected the plasma kisspeptin levels
of 20 healthy girls at the Tanner I stage of development and 20
girls with ICPP at the Tanner II and III stages; the results showed
that the kisspeptin levels of girls with ICPP were clearly higher
than those of the healthy controls, and were positively correlated
with LH peak value and bone age. A study of kisspeptin levels in 30
girls with CPP and 30 undeveloped girls of the same age conducted
by Rhie et al (13) confirmed
this outcome. Ma et al (14)
detected the plasma kisspeptin levels of girls with ICPP, girls
with PT and normal girls at Tanner stages I-V. The results showed
that the plasma kisspeptin levels of girls with ICPP were
significantly increased compared with those of girls with PT.
Kisspeptin levels at the Tanner II stage of pubertal development
were significantly higher than those at other Tanner stages;
moreover, the kisspeptin level began to gradually decrease
following the Tanner II stage, which demonstrated that the
Kiss-1/GPR54 system has a switching effect on pubertal development.
Activation of the Kiss-1 gene is probably the key to triggering the
initiation of puberty, and Kiss-1 gene expression in the HPG axis
may be affected by regulatory feedback from sex hormones.
Kisspeptin levels gradually decline as pubertal development
progresses (15). In the present
study, the kisspeptin level of girls with ICPP prior to treatment
was significantly higher than that of the PT group, which implies
that pubertal development in the ICPP group has been initiated and
the Kiss-1 gene activated, and demonstrates that the measurement of
kisspeptin levels has some significance in the early identification
of ICPP and PT.
A study of 20 girls with normal development and 20
girls with PT by Akinci, Cetin and Ilhan (16) demonstrated that the plasma kisspeptin
level in the girls with PT was significantly higher than that of
the girls with normal development and was correlated with the
prolactin level. In the present study, the plasma kisspeptin level
of the PT group was similar to that of the control group; no
significantly statistical difference was identified. The age
difference between the two groups was 1.5 years, and the kisspeptin
levels of the two groups were not measured following PT treatment.
Therefore, it is necessary to expand the sample size in order to
investigate whether kisspeptin can be used in the auxiliary
diagnosis of PT.
In conclusion, plasma kisspeptin levels rise at the
initiation of pubertal development, and the kisspeptin levels of
girls with PP decline following treatment with GnRH, which
indicates that the detection of kisspeptin can contribute to the
early diagnosis of ICPP and may be used as an indicator of
therapeutic effects in the treatment of precocious puberty. The
results of the study support the viewpoint that the Kiss-1/GPR54
gene is the key to regulating the initiation of pubertal
development and that kisspeptin is closely associated with the
initiation and development of ICPP (17). Research into kisspeptin is
continuing, and the clinical use of kisspeptin for ICPP diagnosis
and the evaluation of the effectiveness of therapy remains to be
further explored.
Acknowledgements
The abstract was presented as a poster (P1-D3-224)
at the 53rd Annual Meeting of the European Society for Paediatric
Endocrinology (ESPE), Sep 18–20, 2014 in Dublin, Ireland.
References
|
1
|
Roa J, Navarro VM and Tena-Sempere M:
Kisspeptins in reproductive biology: Consensus knowledge and recent
developments. Biol Reprod. 85:650–660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sonigo C and Binart N: Overview of the
impact of kisspeptin on reproductive function. Ann Endocrinol
(Paris). 73:448–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Subspecialty Group of Endocrinology:
Hereditary and Metabolic Diseases, Society of Pediatrics, Chinese
Medical Association: The diagnosis and treatment guidelines of
central (true) precocious puberty. Zhonghua Er Ke Za Zhi.
45:426–427. 2007.[(In Chinese)]. PubMed/NCBI
|
|
4
|
Murray RO: Assessment of Skeletal Maturity
and Prediction of Adult Height (TW2 Method). Proc R Soc Med.
69:5421976.
|
|
5
|
Yang L, Tang K, Qi Y, et al: Potential
metabolic mechanism of girls' central precocious puberty: A network
analysis on urine metabonomics data. BMC Syst Biol. 6:(Suppl 3).
S192012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takumi K, Iijima N and Ozawa H:
Developmental changes in the expression of kisspeptin mRNA in rat
hypothalamus. J Mol Neurosci. 43:138–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gopurappilly R, Ogawa S and Parhar IS:
Functional significance of GnRH and kisspeptin and their cognate
receptors in teleost reproduction. Front Endocrinol (Lausanne).
4:242013.PubMed/NCBI
|
|
8
|
Pielecka-Fortuna J and Moenter SM:
Kisspeptin increases gamma-aminobutyric acidergic and glutamatergic
transmission directly to gonadotropin-releasing hormone neurons in
an estradiol-dependent manner. Endocrinology. 151:291–300. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Plant TM, Ramaswamy S and Dipietro MJ:
Repetitive activation of hypothalamic G protein-coupled receptor 54
with intravenous pulses of kisspeptin in the juvenile monkey
(Macaca mulatta) elicits a sustained train of
gonadotropin-releasing hormone discharges. Endocrinology.
147:1007–1013. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dedes I: Kisspeptins and the control of
gonadotrophin secretion. Syst Biol Reprod Med. 58:121–128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Demirbilek H, Gonc EN, Ozon A,
Alikasifoglu A and Kandemir N: Evaluation of serum kisspeptin
levels in girls in the diagnosis of central precocious puberty and
in the assessment of pubertal suppression. J Pediatr Endocrinol
Metab. 25:313–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu F, Zeng YE, Xia HB, et al: Change of
serum kisspeptins and its significance in girls with idiopathic
central precocious puberty. Shi Yong Er Ke Lin Chuang Za Zhi.
26:1558–1559. 2011.[(In Chinese)].
|
|
13
|
Rhie YJ, Lee KH, Eun SH, et al: Serum
kisspeptin levels in Korean girls with central precocious puberty.
J Korean Med Sci. 26:927–931. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma XY, Ni JH, Liu MZ, et al: Plasma
kisspeptin levels in normal female pubertal stages and in girls
with idiopathic central precocious puberty. Zhonghua Nei Fen Mi Dai
Xie Za Zhi. 27:36–39. 2011.[(In Chinese)].
|
|
15
|
Yang Y and Xiong XY: Study progress of
central precocious puberty and KISS-1/G protein-coupled receptor 54
gene. Shi Yong Er Ke Lin Chuang Za Zhi. 27:1548–1551. 2012.[(In
Chinese)].
|
|
16
|
Akinci A, Cetin D and Ilhan N: Plasma
kisspeptin levels in girls with premature thelarche. J Clin Res
Pediatr Endocrinol. 4:61–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rønnekleiv OK and Kelly MJ: Kisspeptin
excitation of GnRH neurons. Adv Exp Med Biol. 784:113–131. 2013.
View Article : Google Scholar : PubMed/NCBI
|