Introduction
Mast cell (MC) functions have classically been
associated with allergic responses (1), with previous studies indicating that
these cells contribute to other common diseases, such as
atherosclerosis, aortic aneurysm and cancer (2–4).
Liu et al (5) found that
MCs also contribute to obesity and diabetes. Mechanism studies have
demonstrated that MCs contribute to white adipose tissue (WAT) and
muscle angiogenesis. Aside from supplying WAT with nutrients,
microvessels also provide a path for leukocyte infiltration
followed by adipokine release (6,7).
Reduced angiogenesis should limit nutrient supply, thereby
impairing cell viability (8,9),
and the inhibition of angiogenesis blocks adipose tissue
development in mice.
Angiogenesis is a regulated balance between
stimulatory and inhibitory factors. This process is regulated by
several pro-angiogenic factors, such as vascular endothelial growth
factor (VEGF), angiopoietin-1 (Ang-1), fibroblast growth factor-2
[(FGF-2), also known as basic fibroblast growth factor (bFGF)], as
well as anti-angiogenic factors, such as thrombospondin-1,
angiostatin and Ang-2. Among various angiogenic factors,
angiogenesis is mainly regulated by the interplay between VEGF and
angiopoietins (10,11). A number of studies have shown that
VEGF and angiopoietins are associated with diabetes in angiogenesis
(12,13). However, angiogenesis in diabetes,
which is associated with the dysregulation of neovascularization,
has also been recognized. The molecular defects underlying these
angiogenic abnormalities have generated much interest but remain
elusive.
Microvascular endothelial cells are commonly used to
study the mechanisms of diabetic complications, since they play an
essential role in the abnormal angiogenesis process of several
diseases, including diabetes mellitus. In the present study, we
established a co-culture system to investigate the correlation
between angiogenesis and various growth factors in
vitro.
Materials and methods
Reagents
All cell culture reagents, alamarBlue®
cell viability reagent, TRIzol reagent, the SuperScript™ III
First-Strand Synthesis System for reverse transcription (RT)-PCR
and Platinum SYBR-Green qPCR SuperMix were purchased from
Invitrogen Life Technologies, Inc. (Grand Island, NY). Primary
antibodies against VEGF, fms-like tyrosine kinase-1 (Flt-1), fetal
liver kinase-1 (Flk-1), β-actin and secondary antibodies against
either rabbit or mouse IgG were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA). SuperSignal West Pico
Chemiluminescent Substrate was obtained from Pierce Biotechnology,
Inc. (Rockford, IL). All other reagents were obtained from
Sigma-Aldrich Chemical Co. (St. Louis, MO).
Cell culture of myocardial microvascular
endothelial cells (MMVECs)
Wistar male rats [8–9 weeks of age; Shanghai
Laboratory Animal Center (SLAC), Shanghai Institutes for Biological
Science, Shanghai, China] were used for primary MMVEC isolation.
The Animal Care Committee of Shanghai Jiaotong University approved
the animal protocols. MMVECs were isolated as previously described
(14). Briefly, the rats were
anesthetized with sodium pentobarbital (60 mg/kg). After
thoracotomy, the hearts were rapidly removed and washed in
phosphate-buffered saline (PBS). The atria, visible connective
tissue, valvular tissue, the right ventricle, and the epicardial
and endocardial surfaces of the left ventricle were carefully
removed, and the remaining myocardial tissue was cut into sections
(1 mm3). Myocardial tissues were then seeded on culture
plates pre-coated with rat-tail tendon gelatin and incubated at
37°C in a humidified atmosphere of 5% CO2/95% air. After
a 40-min attachment period, the tissues were cultured in DMEM (25
mmol/l D-glucose) supplemented with 20% fetal bovine serum (FBS),
50 U/ml heparin, 100 U/ml penicillin and 100 g/ml streptomycin. The
tissue pieces were discarded and the medium was changed after
approximately 70 h. MMVECs were identified by their typical
‘cobblestone’ appearance with positive CD31 and CD34
immunostaining. Only MMVECs at second passage were used for
experiments; cells were allowed to grow to 80–90% confluence
followed by serum-starvation for 24 h before the experiments
commenced.
Collection and isolation of MCs
MCs were isolated as previously described, with
minor modifications to the procedure (15). Briefly, MCs from the peritoneal
cavities of male Wistar rats (14–16 weeks of age; SLAC, Shanghai
Institutes for Biological Science) were collected by lavage with 15
ml of RPMI-1640 (1% FBS). After a 1-h attachment period in the
incubator, non-adherent cells, mainly MCs, were separated by using
percoll density gradient centrifugation at 2,500 rpm for 15 min at
4°C. Cells that remained at the percoll interface were aspirated
and re-suspended in PBS. MCs were washed in PBS twice and cultured
in RPMI-1640, supplemented with 10% FBS, 25 mM HEPES, 100 U/ml
penicillin and 100 mg/ml streptomycin. MCs isolated by this
procedure exceeded 90% purity based on staining with 0.05%
toluidine blue O.
Preparation of MC granules (MCGs)
The standard incubation was conducted in 200 μl of
RPMI-1640 containing 5.0×106 cell/ml MCs. After the MCs
were pre-incubated at 37°C for 15 min, compound 48/80 (5 μg/ml), a
non-cytotoxic MC-specific stimulator, was added to stimulate the
MCs for 1 min. The MCs were centrifuged at 800 × g for 5 min and
the supernatant containing the material released from the
stimulated MCs was stored at -80°C for further experiments
(16). The tryptase and chymase
activity of the MCGs was measured before each experiment, using the
same method as previously described (17). In principle, tryptase and chymase
activities were quantified using the chromogenic substrates,
Nα-benzoyl-DL-arginine-p-nitroanilide hydrochloride
(BAPNA) and N-succinyl-L-phenylalanine-p-nitroanilide
(SAAPP), respectively. Tryptase activity was determined by its
ability to cleave a synthetic substrate, BAPNA (2 mmol/l) in
Tris-HCl (0.1 mol/l; pH 8.0) and glycerol (1 mol/l) at 410 nm,
while chymase activity was determined spectrophotometrically by the
rate of hydrolysis of SAAPP (0.7 mmol/l) in NaCl (1.5 mol/l) and
Tris (0.3 mol/l; pH 8.0) at 405 nm. Protease activity was expressed
in milliunits/millilitre (mU/ml), in which 1 unit of enzyme
activity was defined as the 1 μmol of degraded substrate/min at
25°C. The tryptase activity of the MCGs used in the present study
was 9.46 mU/ml, and the chymase activity was 3.57 mU/ml.
MMVEC-MC co-culture
MMVECs (1×105 cells/well) and MCGs
obtained from 1×105 activated MCs were co-cultured in
DMEM with low (5.56 mmol/l) and high glucose levels (25 mmol/l).
The inhibitors of tryptase [N-tosyl-L-lysine chloromethyl ketone
(TLCK)] and chymase [N-tosyl-L-phenylalanyl chloromethyl ketone
(TPCK)] were used in further experiments when MMVECs were grown to
85–90% confluence before being quiesced in DMEM with 1% FBS for 24
h and treated with MCGs.
Cell proliferation assay
The cell proliferation rate was assessed by cell
counting using alamarBlue® cell viability reagent.
Second passage MMVECs were seeded onto 96-well cell culture plates
at an initial density of 5×103 cells/well (100 μl) in
DMEM with 5% FBS in the presence or absence of MCGs. Following
overnight incubation, 10 μl of alamarBlue® cell solution
were added and further incubated for 2 h. Cell growth rate was
quantified by the difference in absorbance at 570 and 600 nm using
a microplate reader (Tecan Group Ltd., Männedorf, Switzerland), and
the absorbance reading was repeated for 5 consecutive days to
generate a cell growth curve.
Cell migration assay
Cell migration was evaluated using a transwell
chamber assay (Corning Inc., Corning, NY). In brief, second passage
MMVECs were trypsinized, resuspended in 200 μl serum-free DMEM
(4×104 cells/well) and were then placed onto the upper
chamber, while another 800 μl of DMEM with a different glucose
concentration and 2% FBS were placed in the lower compartment of
the transwell chamber. MCGs, TLCK or TPCK were also added to
examine their effects on cell migration activity, as described
above. Following incubation for 12 h at 37°C, allowing time for the
cells to migrate from the upper to the lower chamber, cells that
migrated to the lower side of the transwell inserts were washed,
fixed with methanol for 10 min and then stained with crystal violet
dye for cell counting. Cell migration ability was quantified by an
average of cells in 5 random microscopic fields (x200
magnification) at the lower side of the transwell insert.
Cell scratch wound healing assay
Wound healing assay was performed as described
previously (18). Once second
passage MMVECs reached approximately 90% confluence in 24-well
plates, a wound was made with a 200 μl pipette tip. The cells were
washed with PBS and further incubated in DMEM with a different
glucose concentration and 1% FBS in the presence or absence of
MCGs. The effects of TLCK and TPCK on MCGs were also examined.
After 24 h of incubation, cultured cells were photographed and cell
migration activity was quantified with ImageJ software (version
1.37) by measuring the area of the cells that moved beyond a
reference line.
Capillary-like tube formation assay on
Matrigel
Matrigel (BD Biosciences, Bedford, MA) was used for
the capillary-like tube formation assay. Briefly, 100 μl/well of
this matrix solution were added to a 24-well culture plate and 30
min were allowed for gelation at 37°C. MMVECs obtained as described
above, which had been resuspended in DMEM with a different glucose
concentration and 1% FBS, were added to the top of the Matrigel
(5×104 cells/well) and incubated for 18 h at 37°C. Tube
formation was carefully observed either in the presence or absence
of MCGs, and the effects of TLCK and TPCK were also examined. Once
cell culture images were captured, tube structures were quantified
by counting all branches in 3 random fields from each well
(19). Each experiment was
repeated 3 times.
Real-time RT-PCR
Total RNA was extracted from MMVECs using TRIzol
Reagent according to the manufacturer’s instructions. RNA (2 μg)
was then reverse-transcribed into cDNA using the SuperScript™ III
First-strand Synthesis System for RT-PCR and was further amplified
by SYBR-Green RT-PCR amplification with specific oligonucleotides
as follows: VEGF sense, 5′-GCA CTG GAC CCT GGC TTT AC-3′ and
antisense primer, 5′-CTG CAG GAA GCT CAT CTC TC-3′; Flk-1 sense,
5′-ACA GTT CCC AGA GTG GTT GG-3′ and antisense primer, 5′-GTC ACT
GAC AGA GGC GAT GA-3′; Flt-1 sense, 5′-CCA CTT CTG TCT TGC CAC
ACA-3′ and antisense primer, 5′-CCA ACC AAT TAA GAC CTT CTG-3′; and
β-actin sense, 5′-CAC CCG CGA GTA CAA CCT TC-3′ and antisense
primer, 5′-CCC ATA CCC ACC ATC ACA CC-3′. The SYBR-Green RT-PCR
amplification was carried out in a 25 μl reaction volume that
contained 12.5 μl 2X Platinum SYBR-Green qPCR SuperMix, 200 nM each
of the forward and reverse primer, and 2 μl of diluted cDNA using
the iCycler iQ™ Real-Time PCR Detection System (Bio-Rad, Richmond,
CA). The thermal profile for SYBR-Green real-time RT-PCR was
conducted for 40 cycles under the following conditions:
denaturation at 94°C for 30 sec, annealing at optimal temperature
for each primer pair for 30 sec, and extension at 72°C for 45 sec.
Final extension was at 72°C for 10 min. A single amplification
product was confirmed by running a melting curve for all PCRs. Each
sample was assayed in triplicate.
Western blot analysis for VEGF, Flt-1 and
Flk-1
MMVECs were lysed in lysis buffer, and the protein
content was measured using the BCA assay (both from Beyotime,
China). Equal amounts of protein were loaded and separated onto an
8 to 12% Tris-glycine gel and transferred onto PVDF membranes.
Primary antibodies against VEGF (1:500 dilution), Flt-1 (1:500
dilution), Flk-1 (1:500 dilution) and β-actin (1:500 dilution) were
added to the membranes, incubated overnight at 4°C, followed by
reaction with appropriate secondary antibodies (1:4,000 dilution,
goat anti-rabbit IgG and goat anti-mouse IgG-HRP linked; Cell
Signaling Technology) for 2 h. ECL reagent was then used to
determine the immunoreaction, which was measured by densitometry on
X-ray film, using GIS software (Bio-Tanon, Shanghai, China). All
western blot analysis experiments were repeated at least 3 times
with different cell preparations.
Statistical analysis
Data are presented as the means ± SD. Statistical
significance was assessed by one-way ANOVA followed by post hoc
analysis using the Student-Newman-Keuls test. A P-value <0.05
was considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS 16.0 statistical
software (SPSS Inc., Chicago, IL).
Results
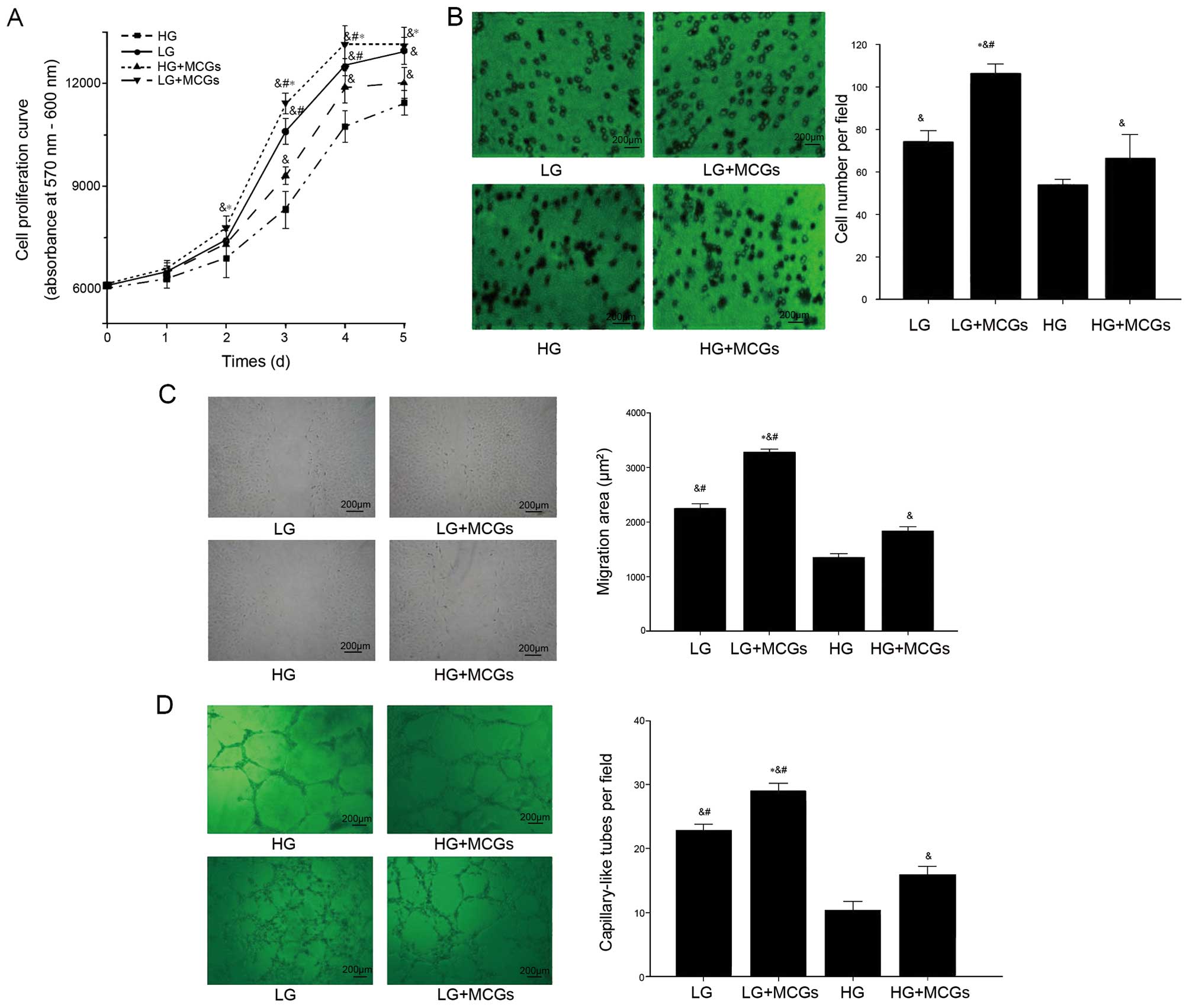
Impaired angiogenesis of MMVECs in high
glucose
MMVEC proliferation, migration and capillary-like
tube formation are important components of the process of
angiogenesis. The proliferation, migration and capillary-like tube
formation ability of the MMVECS co-cultured with MCGs increased
significantly in spite of high or low glucose. However, the MMVECs
cultured under low glucose conditions showed a higher proliferation
rate compared with those co-cultured with MCGs under hyperglycemic
conditions from day 3 to 5 (Fig.
1) (P<0.05). This phenomenon was also observed in both cell
scratch wound healing assays and the capillary-like tube formation
of MMVECs cultured under low glucose conditions compared to those
co-cultured with MCGs under hyperglycemic conditions (Fig. 1). The effect of MCGs on MMVECs was
significantly inhibited by the addition of TLCK and TPCK, resulting
in even lower migration ability and capillary-like tube formation
(P<0.05, respectively). There was no significant difference
observed between low and high glucose conditions when TLCK was
added to the MMVECs co-cultured with MCGs. When TPCK was added to
the MMVECs co-cultured with MCGs under low glucose conditions,
there was a significant increase in migration ability and
capillary-like tube formation compared to the MMVECs co-cultured
with MCGs under high glucose conditions (Fig. 2) (P<0.05).
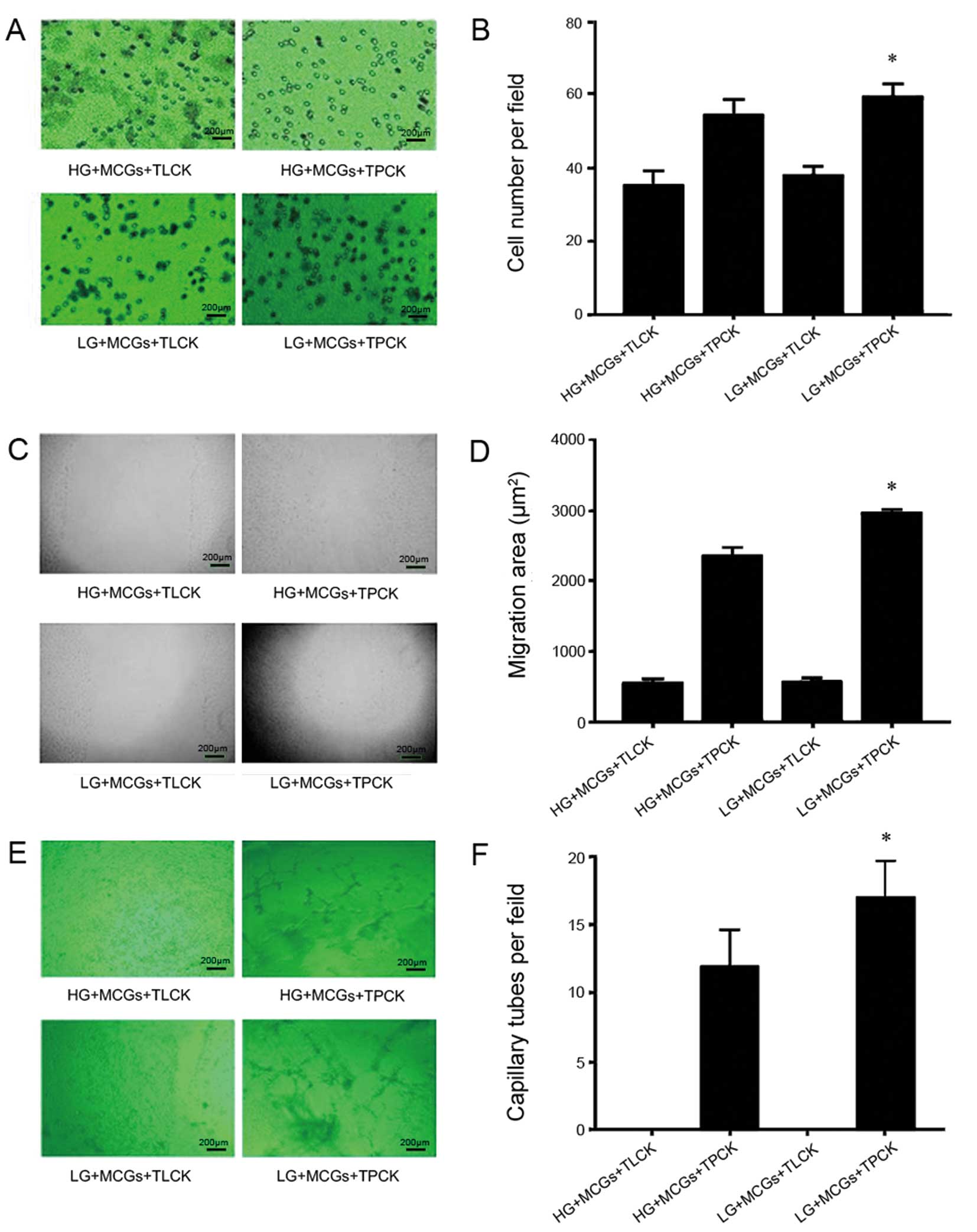 | Figure 2Effect of TLCK or TPCK on
proliferation, scratch wound healing and tube formation of
myocardial microvascular endothelial cells (MMVECs) cultured in
normal and high-glucose medium. Shown are representative (A, C and
E) images and (B, D and F) values obtained for cell number,
migration area and capillary tube formation, respectively in the
cells treated with TLCK or TPCK. (*P<0.05 vs. HG +
MCGs + TPCK). TLCK, N-tosyl-L-lysine chloromethyl ketone; TPCK,
N-tosyl-L-phenylalanyl chloromethyl ketone; HG, high glucose; LG,
low glucose; MCGs, mast cell granules. |
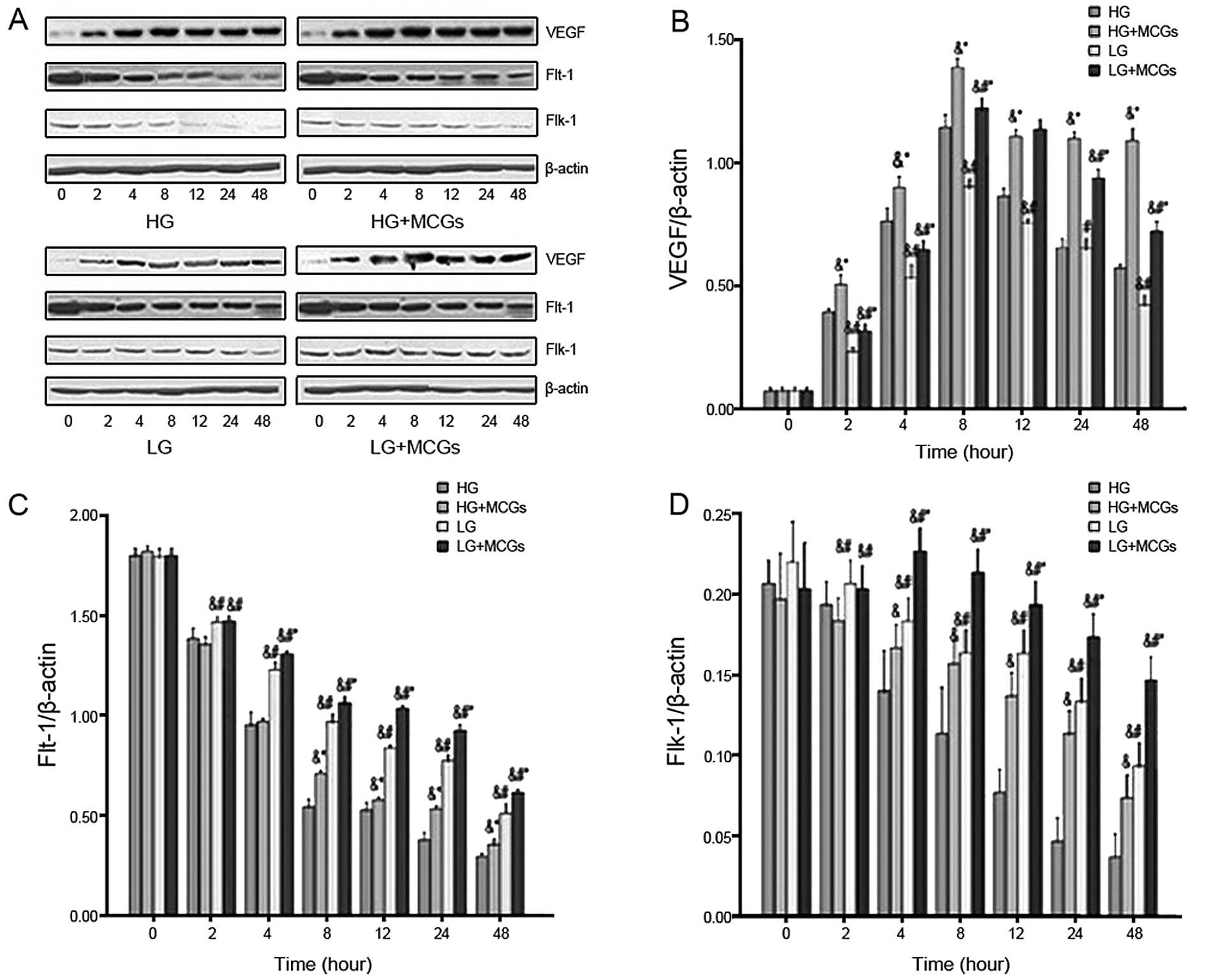
Analysis of the mRNA expression changes
by qRT-PCR
VEGF mRNA expression increased in a time-dependent
manner in the MMVECs co-cultured with MCGs within 2 h, with the
peak occurring after 4 h of culture in hyperglycemic medium and
after 8 h of culture in low-glucose medium (P<0.05). VEGF mRNA
expression in the MMVECs cultured under hyperglycemic conditions
was significantly higher compared to that in MMVECs cultured under
low glucose conditions, in spite of the MCGs, and this increase
continued for up to 24 h (Fig.
3A). Flt-1 mRNA expression in the MMVECs decreased in a
time-dependent manner, with a peak present after 4 h of culture
under hyperglycemic conditions and after 8 h of culture under low
glucose conditions (P<0.05). Flt-1 mRNA expression in the MMVECs
co-cultured with MCGs was significantly higher than that in MMVECs
cultured in different concentrations of glucose. Flt-1 mRNA
expression in the MMVECs co-cultured with MCGs under hyperglycemic
conditions was significantly lower compared to that in the MMVECs
cultured under low glucose conditions and this tendency continued
for up to 48 h (Fig. 3B). Flk-1
mRNA expression in the MMVECs decreased in a time-dependent manner.
Flk-1 mRNA expression in the MMVECs co-cultured with MCGs was
significantly higher than that in MMVECs cultured in different
concentrations of glucose. Flk-1 mRNA expression in the MMVECs
co-cultured with MCGs under hyperglycemic conditions was
significantly lower compared to that in MMVECs cultured under low
glucose conditions and the tendency continued for up to 48 h
(Fig. 3C).
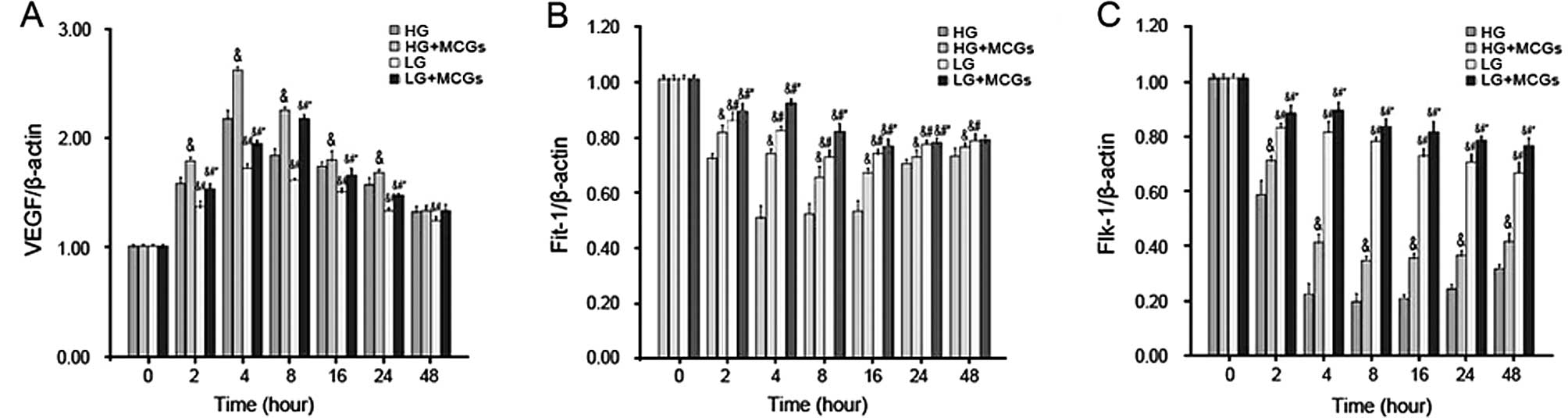 | Figure 3Vascular endothelial growth factor
(VEGF), fms-like tyrosine kinase-1 (Flt-1) and fetal liver kinase-1
(Flk-1) mRNA expression in myocardial microvascular endothelial
cells (MMVECs). (A) VEGF expression increased significantly in
MMVECs cultured with MCGs at 2 h, VEGF expression reached at its
peak point at 4 h in the HG group, and at 8 h in the LG group. Even
in the presence of MCGs, VEGF expression in the LG group was
significantly lower than that in the HG group (P<0.05). (B)
Flt-1 expression decreased significantly in the MMVECs at 2 h
(P<0.05), and reached at its low point at 4 h in the HG group,
and at 8 h in the LG group. (C) Flt-1 expression was significantly
different between the HG and LG group (P<0.05). Flt-1 expression
was significantly higher in the presence of MCGs. Flk-1 expression
decreased significantly in the HG group at 2 h, and reached its low
point at 8 h. Even in the presence of MCGs, Flk-1 expression in the
HG group was significantly lower than that in the LG group
(P<0.05). &P<0.05 vs. HG;
#P<0.05 vs. HG + MCGs; and *P<0.05 vs.
LG. HG, high glucose; LG, low glucose; MCGs, mast cell
granules. |
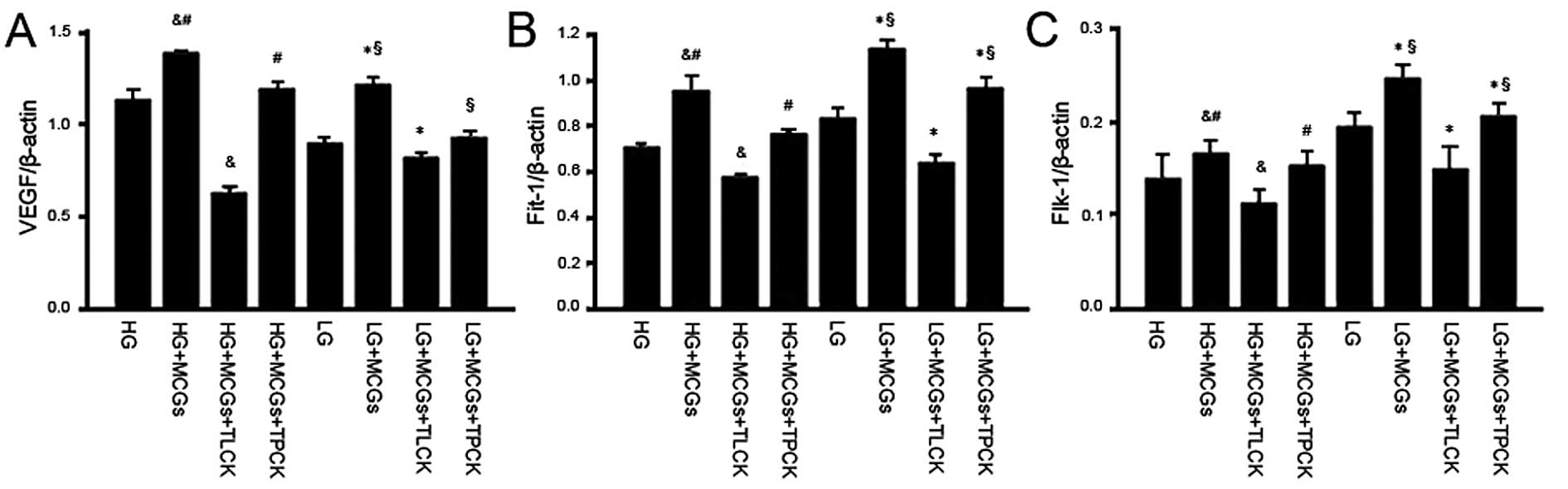
The addition of either TLCK or TPCK to the
co-culture system abolished the effects of MCGs on VEGF, Flt-1 and
Flk-1 mRNA expression in the MMVECs cultured in different
concentrations of glucose; the effects of TLCK however, were more
significant. However, the VEGF, Flt-1 and Flk-1 mRNA expression
levels in the MMVECs co-cultured with MCGs following the addition
of TLCK were much lower in the absence of MCGs. When the MMVECs
were co-cultured with MCGs under hyperglycemic conditions,
following the addition of TLCK, VEGF and Flt-1 expression
significantly decreased (Fig.
4).
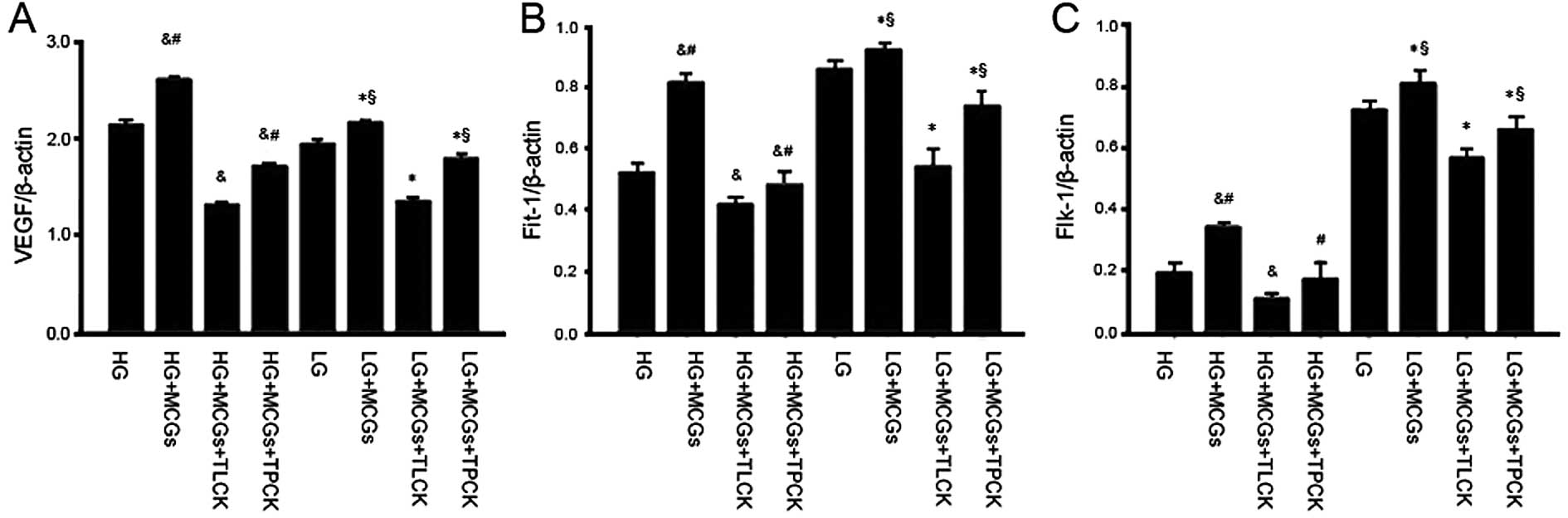 | Figure 4Vascular endothelial growth factor
(VEGF), fms-like tyrosine kinase-1 (Flt-1) and fetal liver kinase-1
(Flk-1) mRNA expression in myocardial microvascular endothelial
cells (MMVECs) cultured in the presence of TLCK or TPCK. The mRNA
expression of VEGF, Flt-1 and Flk-1 decreased significantly in the
presence of TLCK or TPCK, particularly in the presence of TLCK,
though the mRNA expression of VEGF in the LG group was not
significantly different from that in the HG group; however, the
expression of Flt-1 and FLK-1 in the LG group was significantly
higher than that in the HG group (P<0.05).
&P<0.05 vs. HG; #P<0.05 vs. HG +
MCGs + TLCK; *P<0.05 vs. LG; and §LG +
MCGs + TLCK). TLCK, N-tosyl-L-lysine chloromethyl ketone; TPCK,
N-tosyl-L-phenylalanyl chloromethyl ketone; HG, high glucose; LG,
low glucose; MCGs, mast cell granules. |
VEGF, Flt-1 and Flk-1 protein
expression
VEGF protein expression increased in the MMVECs
co-cultured with MCGs with the increase commencing at 2 h and
reaching a peak at 8 h (Fig. 5)
(P<0.05). Flt-1 protein expression decreased in a time-dependent
manner in the MMVECs. Flt-1 protein expression in the MMVECs
co-cultured with MCGs was significantly higher than that in the
MMVECs exposed to different concentrations of glucose. Flt-1
protein expression in the MMVECs cultured under hyperglycemic
conditions was significantly lower than that in MMVECs exposed to
low glucose levels (Fig. 5B).
Flk-1 protein expression decreased in a time-dependent manner in
the MMVECs. The Flk-1 protein expression levels in the MMVECs
co-cultured with MCGs was significantly higher than that in MMVECs
exposed to different glucose concentrations. Flk-1 protein
expression in the MMVECs co-cultured with MCGs under hyperglycemic
conditions was significantly lower compared to that in MMVECs
exposed to low glucose and this tendency continued for up to 48 h
(Fig. 5C).
The addition of either TLCK or TPCK to the
co-culture system abolished the effects of MCGs on VEGF, Flt-1 and
Flk-1 protein expression in the MMVECs exposed to different
concentrations of glucose; the effects of TLCK though were more
significant. The protein expression of VEGF, Flt-1 and Flk-1
following the addition of TLCK to the MMVECs co-cultured with MCGs
was much lower than in the MMVECs cultured without MCGs. The
protein expression of Flt-1 and Flk-1 in the MMVECs co-cultured
with MCGs, after the addition of TLCK under low glucose conditions,
was significantly higher than that in the MMVECs cultured without
MCGs. However, it is noteworthy that this phenomenon was not
observed in the MMVECs cultured under hyperglycemic conditions
(Fig. 6).
Discussion
Angiogenesis is tightly regulated by pro- and
anti-angiogenic factors. In tumor models, MCs have been shown to
play a decisive role in inducing the angiogenic switch that
precedes malignant transformation. MCs are a rich source of several
potent angiogenic factors, including VEGF, bFGF, transforming
growth factor (TGF)-β, tumor necrosis factor (TNF)-α and
interleukin (IL)-8 (20,21). In our study, we found that a close
correlation exists between angiogenesis and various growth factors
in MMVECs co-cultued with MCGs. In our co-culture system, MCGs
increased the migration and lumen formation in MMVECs, as well as
the expression of VEGF, Flt-1 and Flk-1.
VEGF, as a major regulator of angiogenesis, binds
and activates 2 tyrosine kinase receptors, Flt-1 and Flk-1. These
receptors regulate physiological, as well as pathological
angiogenesis. Flk-1 has strong tyrosine kinase activity, and
transduces the major signals for angiogenesis. Flk-1 is a direct
signal transducer for pathological angiogenesis, including cancer
and diabetic retinopathy. Although the affinity of Flt-1 to VEGF is
more than 10-fold that of Flk-1 (22), Flk-1 plays an important role in
proliferation, migration and the survival process. Thus, Flk-1 and
its signaling appear to be critical targets for the suppression of
these diseases. It has been established that high glucose levels
are the direct cause of capillary vessel damage in diabetes
mellitus (23,24). Sasso et al (25) found that the expression of VEGF in
individuals with chronic coronary artery disease and diabetes
mellitus was significantly higher compared to individuals who did
not suffer from diabetes mellitus; however, in our study, even in
the presence of MCGs exposed to high glucose, this tendency still
existed. The expression of VEGF in the MMVECs exposed to high
glucose was significantly higher than that in the MMVECs exposed to
low glucose; the expression of Flt-1, Flk-1 and their downstream
signals was significantly suppressed. Chou et al found that
the expression of VEGF, Flt-1 and Flk-1 were significantly
decreased in heart tissues but were increased 2-fold in the retina
and kidney in diabetes mellitus patients (25). In the current study, we found that
capillary-like tube formation and the migration of MMVECs cultured
under low glucose conditions increased compared to MMVECs cultured
under high glucose conditions; however, Flt-1 and Flk-1 expression
decreased, particularly Flk-1 expression. The reason that
capillary-like tube formation and the migration of MMVECs cultured
under low glucose conditions increased significantly, compared to
the MMVECs cultured under high glucose conditions, even in the
presence of MCGs, may be the fact that the expression of VEGF
increased. However, the expression of the receptors decreased and
the expression of Ang-2 increased, all these factors likely
affecting the angiogenic ability of MMVECs in high glucose. These
results demonstrate that high glucose levels are an important
factor for the angiogenic ability of MMVECs in vitro. We
also found that the duration of culture had a pronounced effect on
MMVEC growth under high glucose conditions. Su found that the
number of MMVECs cultured in high glucose for 24–96 h increased in
the transcriptionally silent period and decreased in the DNA
synthesis period (26). Abe found
that the number of retina cells cultured in high glucose decreased
by 78% after 10 days (27). We
also found that the expression of VEGF and its receptors in MMVECs
significantly decreased as the culture duration increased.
Tryptase and chymase are the predominant proteases
present in MCGs; the amount of these proteases is 20–50% in MCs.
Tryptase directly added to dermal microvascular endothelial cells
caused a significant augmentation of capillary growth, which was
suppressed by specific tryptase inhibitors. Tryptase also directly
induced the cell proliferation of human dermal microvascular
endothelial cells (HDMECs) in a concentration-dependent manner
(28). MCs act at sites of new
vessel formation by secreting tryptase, which then functions as a
potent and previously unrecognized angiogenic factor (28). In our study, we found that a close
correlation existed between MMVECs and MCGs and angiogenesis. In
our co-culture system, MCGs increased migration and lumen formation
in MMVECs, but the application of TLCK or TPCK significantly
attenuated angiogenesis, in spite of the glucose concentration;
TLCK was particularly effective in this respect. We also found that
capillary-like tube formation and the migration of MMVECs
co-cultured with MCGs, when exposed to TLCK or TPCK, significantly
decreased. It seems that MCGs contain or secrete many of the
angiogenic and anti-angiogenic factors. When the main mediators of
angiogenesis, tryptase and chymase, were inhibited, anti-angiogenic
factors in MCGs, such as endothelin-1 (ET-1), had a significant
anti-angiogenic effect.
Liu et al found that MCs contribute to
obesity and diabetes. Mechanism studies’ have revealed that IL-6
and interferon (IFN)-γ in MCs contribute to WAT and muscle
angiogenesis (5). However, this
result was not so different from our findings. The reason may be
that: i) our study focused on MMVECs co-cultured with MCGs in
vitro; ii) diabetes mellitus is a chronic process, while cell
culture studies are necessarily conducted over a much shorter time
period; it may well be a completely different pathophysiological
process; and iii) the angiogenic process of MCs isolated from
animals is likely to be complex and perhaps differs from what
occurs in other cell types in vitro.
In conclusion, our results demonstrate unequivocally
that tryptase is the main angiogenic mediator in MCGs. High glucose
levels have a profound effect on angiogenesis; this effect may be
more pronounced than the effects of MCGs on angiogenesis.
Acknowledgements
The present study was supported by an Institutional
Research Grant (to M.W.) and the Shanghai PuJiang Program [PJ
(2008) 00586 to Q.Z.].
References
|
1
|
Robbie-Ryan M and Brown M: The role of
mast cells in allergy and autoimmunity. Curr Opin Immunol.
14:728–733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun J, Sukhova GK, Wolters PJ, et al: Mast
cells promote atherosclerosis by releasing proinflammatory
cytokines. Nat Med. 13:719–724. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun J, Sukhova GK, Yang M, et al: Mast
cells modulate the pathogenesis of elastase-induced abdominal
aortic aneurysms in mice. J Clin Invest. 117:3359–3368. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coussens LM, Raymond WW, Bergers G, et al:
Inflammatory mast cells up-regulate angiogenesis during squamous
epithelial carcinogenesis. Genes Dev. 13:1382–1397. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Divoux A, Sun J, et al: Genetic
deficiency and pharmacological stabilization of mast cells reduce
diet-induced obesity and diabetes in mice. Nat Med. 15:940–945.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pang C, Gao Z, Yin J, Zhang J, Jia W and
Ye J: Macrophage infiltration into adipose tissue may promote
angiogenesis for adipose tissue remodeling in obesity. Am J Physiol
Endocrinol Metab. 295:E313–E322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kintscher U, Hartge M, Hess K, et al:
T-lymphocyte infiltration in visceral adipose tissue: a primary
event in adipose tissue inflammation and the development of
obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol.
28:1304–1310. 2008. View Article : Google Scholar
|
|
8
|
Rupnick MA, Panigrahy D, Zhang CY, et al:
Adipose tissue mass can be regulated through the vasculature. Proc
Natl Acad Sci USA. 99:10730–10735. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sato K, Tsuchihara K, Fujii S, et al:
Autophagy is activated in colorectal cancer cells and contributes
to the tolerance to nutrient deprivation. Cancer Res. 67:9677–9684.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holash J, Maisonpierre PC, Compton D, et
al: Vessel cooption, regression, and growth in tumors mediated by
angiopoietins and VEGF. Science. 284:1994–1998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holash J, Wiegand SJ and Yancopoulos GD:
New model of tumor angiogenesis: dynamic balance between vessel
regression and growth mediated by angiopoietins and VEGF. Oncogene.
18:5356–5362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zygalaki E, Kaklamanis L, Nikolaou NI, et
al: Expression profile of total VEGF, VEGF splice variants and VEGF
receptors in the myocardium and arterial vasculature of diabetic
and non-diabetic patients with coronary artery disease. Clin
Biochem. 41:82–87. 2008. View Article : Google Scholar
|
|
13
|
Chen JX and Stinnett A: Disruption of
Ang-1/Tie-2 signaling contributes to the impaired myocardial
vascular maturation and angiogenesis in type II diabetic mice.
Arterioscler Thromb Vasc Biol. 28:1606–1613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ning YX, Wang XH, Jin HM, Zhao FD and Yin
LH: Study on the culture of rat myocardium microvascular
endothelial cells and microarray analysis. Zhongguo Bing Li Sheng
Li Za Zhi. 21:2295–2300. 2005.(In Chinese).
|
|
15
|
Kokkonen JO and Kovanen PT: Low density
lipoprotein degradation by rat mast cells. Demonstration of
extracellular proteolysis caused by mast cell granules. J Biol
Chem. 260:14756–14763. 1985.PubMed/NCBI
|
|
16
|
Kokkonen JO and Kovanen PT: Accumulation
of low density lipoproteins in stimulated rat serosal mast cells
during recovery from degranulation. J Lipid Res. 30:1341–1348.
1989.PubMed/NCBI
|
|
17
|
He S, Peng Q and Walls AF: Potent
induction of a neutrophil and eosinophil-rich infiltrate in vivo by
human mast cell tryptase: selective enhancement of eosinophil
recruitment by histamine. J Immunol. 159:6216–6225. 1997.PubMed/NCBI
|
|
18
|
Ettenson DS and Gotlieb AI: Centrosomes,
microtubules, and microfilaments in the reendothelialization and
remodeling of double-sided in vitro wounds. Lab Invest. 66:722–733.
1992.PubMed/NCBI
|
|
19
|
Pollman MJ, Naumovski L and Gibbons GH:
Endothelial cell apoptosis in capillary network remodeling. J Cell
Physiol. 178:359–370. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qu Z, Liebler JM, Powers MR, et al: Mast
cells are a major source of basic fibroblast growth factor in
chronic inflammation and cutaneous hemangioma. Am J Pathol.
147:564–573. 1995.PubMed/NCBI
|
|
21
|
Ribatti D, Crivellato E, Candussio L, et
al: Mast cells and their secretory granules are angiogenic in the
chick embryo chorioallantoic membrane. Clin Exp Allergy.
31:602–608. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shibuya M: Structure and dual function of
vascular endothelial growth factor receptor-1 (Flt-1). Int J
Biochem Cell Biol. 33:409–420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klein R: Hyperglycemia and microvascular
and macrovascular disease in diabetes. Diabetes Care. 18:258–268.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chou E, Suzuma I, Way KJ, et al: Decreased
cardiac expression of vascular endothelial growth factor and its
receptors in insulin-resistant and diabetic states: a possible
explanation for impaired collateral formation in cardiac tissue.
Circulation. 105:373–379. 2002. View Article : Google Scholar
|
|
25
|
Sasso FC, Torella D, Carbonara O, et al:
Increased vascular endothelial growth factor expression but
impaired vascular endothelial growth factor receptor signaling in
the myocardium of type 2 diabetic patients with chronic coronary
heart disease. J Am Coll Cardiol. 46:827–834. 2005. View Article : Google Scholar
|
|
26
|
Su Y, Liu XM, Sun YM, et al: Endothelial
dysfunction in impaired fasting glycemia, impaired glucose
tolerance, and type 2 diabetes mellitus. Am J Cardiol. 102:497–478.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abe T, Yoshida M, Yoshioka Y, et al: Iris
pigment epithelial cell transplantation for degenerative retinal
diseases. Prog Retin Eye Res. 26:302–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blair RJ, Meng H, Marchese MJ, et al:
Human mast cells stimulate vascular tube formation. Tryptase is a
novel, potent angiogenic factor. J Clin Invest. 99:2691–2700. 1997.
View Article : Google Scholar : PubMed/NCBI
|