Introduction
IgA nephropathy is the most common type of primary
glomerulonephritis (1). Renal
biopsy is required for definite diagnosis of primary
glomerulonephritis and IgA nephropathy accounts for 40% of cases
detected using this approach in East Asia and 20% in Europe. In
Japan, 47% of cases of primary glomerulonephritis are diagnosed as
IgA nephropathy. In addition, 20–30% of patients with IgA
nephropathy progress to end-stage renal disease (ESRD) within 20
years after onset (2,3) and require hemodialysis or renal
transplantation.
The pathology of IgA nephropathy is not fully
understood, but involves marked mesangial IgA deposits that are
often associated with complement component deposits. Furthermore,
recurrence of mesangial IgA deposits is seen in approximately 50%
of IgA nephropathy patients undergoing renal transplantation
(4), while these deposits
disappear in most patients without IgA nephropathy after renal
transplantation from a patient with IgA nephropathy (5,6).
In addition, patients with IgA nephropathy often develop gross
hematuria following upper respiratory infection, and tonsil
stimulation by an ultra short wave may cause deteriorated urinary
findings at 3 h after the mechanical stimulation (7,8).
These findings suggest that extrarenal factors and humoral factors
are involved in IgA nephropathy.
Since the pathophysiology of IgA nephropathy is
unclear, most current treatments aim to reduce immune reactions and
inflammation in glomeruli and tubulointerstitium, which result in
renal fibrosis. Corticosteroid-induced immune regulation delays
progression to ESRD (9), but is
only recommended for patients with increased pathological activity
due to possible adverse reactions (10). The Oxford classification of IgA
nephropathy was proposed in July 2009 as standard criteria for
evaluation of the pathological activity of IgA nephropathy. The
pathological findings are related to the clinical features
(11–13). Therefore, histological evaluation
in patients with IgA nephropathy is required. However, multiple
renal biopsies performed for pathological evaluation may cause
severe tissue damage and complications. Thus, it would be of value
to find a clinically useful biomarker that is correlated with
pathological findings in IgA nephropathy.
Biomarkers for various diseases have recently been
detected by mass spectrometry (14). Mass spectrometry is generally
limited for quantitative analysis, but a semiquantitative
assessment can be achieved using the ProteinChip surface-enhanced
laser desorption ionization (SELDI) system (15). In this study, we used this system
to explore the serum biomarkers which correlate with the
pathological activity evaluated histologically in accordance with
the Oxford classification in patients with IgA nephropathy.
Materials and methods
Clinical characteristics in patients with
IgA nephropathy
The first group of subjects (group 1) consisted of
25 patients with IgA nephropathy confirmed by renal biopsy from
2006 to 2007 and 14 healthy controls without renal dysfunction used
as controls. To verify the utility of serum biomarkers identified
in group 1, serum biomarker candidates were determined by enzyme
linked-immunosorbent assay (ELISA) in serum from 32 patients with
IgA nephropathy (group 2) diagnosed by renal biopsy from 2008 to
2011 (Table I). The backgrounds
of the patients and controls were the same, except for urinary
protein excretion and age (Table
I). Patients with eGFR <30 ml/min, patients who did not
provide appropriate serum at the time of diagnosis, and those with
<10 glomeruli in a renal biopsy specimen were excluded in each
group. The study was approved by the Ethics Committee of Kagoshima
University Hospital and Nanpuh Hospital. Written informed consent
was obtained from all subjects.
 | Table ICharacteristics of patients with IgA
nephropathy and healthy controls. |
Table I
Characteristics of patients with IgA
nephropathy and healthy controls.
| Characteristics | Healthy controls
(n=14) | Patient group 1
(n=25) | Patient group 2
(n=32) | P-valuea |
|---|
|
|---|
| C vs. G1 | C vs. G2 | G1 vs. G2 |
|---|
| Gender (M/F) | 5/9 | 11/14 | 16/16 | 0.614 | 0.371 | 0.652 |
| Age | 34.4±6.9 | 34.1±13.2 | 28.8±10.8 | 0.792 | 0.035 | 0.110 |
| UPE (g/g Cre) | ND | 0.80±0.72 | 0.49±0.55 | ND | ND | 0.081 |
| Serum Cre
(mg/dl) | 0.76±0.17 | 0.83±0.27 | 0.74±0.15 | 0.682 | 0.830 | 0.505 |
| eGFR (ml/min) | 81.9±15.8 | 82.0±26.2 | 95.8±29.1 | 0.942 | 0.133 | 0.085 |
| Serum IgA
(mg/dl) | 261.1±68.5 | 327.8±115.4 | 330.3±150.2 | 0.083 | 0.126 | 0.676 |
| Serum C3 (mg/dl) | 93.8±11.9 | 103.3±17.2 | 99.2±15.3 | 0.098 | 0.252 | 0.426 |
| Serum C4 (mg/dl) | 21.2±4.0 | 25.4±7.4 | 24.2±5.7 | 0.119 | 0.108 | 0.635 |
Pathological evaluation
Renal tissues obtained by needle biopsy under
ultrasound guidance were evaluated by light microscopy,
immunofluorescence microscopy and electron microscopy. Subjects
diagnosed with nephritis without IgA nephropathy, such as nephritis
related to hepatic dysfunction, lupus nephritis, and
poststreptococcal glomerulonephritis, based on clinical course and
pathological characteristics were excluded from the study. Subjects
with dominant IgA deposits found in the mesangial region by
immunofluorescence microscopy and with mesangial proliferative
glomerulonephritis confirmed by light microscopy were diagnosed
with IgA nephropathy.
Paraffin-embedded specimens were stained with
hematoxylin and eosin, periodic acid-Schiff reaction, Masson’s
trichrome, and periodic acid silver-methenamine, and were evaluated
according to the Oxford classification of IgA nephropathy (12,13).
Glomerular lesions were evaluated as follows; for
mesangial proliferation, all glomeruli without global sclerosis
were classified as normal, mild, moderate and severe based on
<4, 4–5, 6–7 and ≥8 mesangial cells/mesangial area,
respectively. The mesangial hypercellularity score was calculated
as the mean number of glomeruli for scoring mesangial proliferation
(normal, 0; mild, 1; moderate, 2; severe, 3). Active glomerular
lesions were defined as glomeruli with a cellular crescent,
fibrocellular crescent, or endocapillary proliferation. The
severity of glomerular lesions was defined as the ratio of active
glomerular lesions relative to the total number of glomeruli.
Chronic lesions of glomeruli were defined based on the number of
glomeruli with global sclerosis, segmental sclerosis, or a fibrous
crescent. The severity of chronic lesions was defined as the ratio
of chronic lesions relative to the total number of glomeruli.
ProteinChip SELDI system
A comprehensive protein analysis using the
ProteinChip SELDI system was performed as previously described
(16,17) under the following conditions, in
which several protein signals can be obtained in a stable manner.
Native serum was applied to a hydrophobic cation-exchange chip
(CM10, Bio-Rad Laboratories, Hercules, CA, USA). Sinapinic acid was
used as the matrix and 50 mM sodium acetate (pH 4.5) as the
binding/washing buffer. Protein signal intensities from 2,000 to
11,000 m/z were compared between the patient group 1 and control
groups.
Binding/washing buffers of pH 5.0–11.0 were prepared
in increments of pH 1.0 for use in experiments to predict the
isoelectric point of the target protein. These buffers were sodium
acetate (pH 5.0), phosphate (pH 6.0 and 7.0), Tris-HCl (pH 8.0 and
9.0), and sodium carbonate (pH 10.0 and 11.0). Each was used to
wash 7 spots on the CM10 chip and then the spectrum was obtained by
time-of-flight mass spectrometry. The pH at which the peak
disappeared was estimated as the approximate pI value. Peptides
were predicted from the pI value and the molecular weight based on
a published database (Tagldent: http://web.expasy.org/tagident/).
Crude fractionation, demineralization and
concentration of serum samples
Crude fractionation of serum samples was performed
using a weak cation-exchange absorbent column (HiTrap CM FF 5 ml
column, GE Healthcare Bio-Sciences KK, Tokyo, Japan) to remove
proteins that inhibit reactions between biomarker candidate
proteins and specific antibodies. Serum containing biomarker
candidates was diluted 5 times with phosphate buffer (pH 7.5) and
applied to the column. Proteins and peptides bound to the column
were then eluted using 0.5 M NaCl in phosphate buffer. The eluent
was desalted and concentrated using an ultrafiltration column
(Vivaspin 500 Polyethersulfone 5,000 MWCO, Sartorius Stedim
Biotech, Goettingen, Germany) and an ultrafilter (5,000 MWCO).
Protein levels were determined by Bradford’s method (Quick Start
Protein Assay, Bio-Rad Laboratories).
Immunoprecipitation
A combination of 50% protein A (Protein A Sepharose
CL-4B, GE Healthcare) suspension and 1:5 diluted rabbit anti-human
C4a polyclonal antibody (#A206, Complement Technology, Inc., Tyler,
TX, USA) was incubated with shaking overnight at 4ºC. The rabbit
anti-human C4a antibody-bound protein A and serum samples
containing biomarker candidate proteins were mixed, incubated, and
then centrifuged to separate supernatant from precipitates of the
C4a-anti C4a antibody-protein A complex. Normal rabbit IgG
(sc-2027, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was
used as a control antibody. The supernatant was divided into two
parts; one was diluted with sodium acetate buffer (pH 4.5) and used
for ProteinChip SELDI system, while the other was incubated with
sample buffer with β-mercaptoethanol at 95ºC and then used in
tricine-SDS-PAGE. The collected precipitates were washed with PBS
several times and incubated with sample buffer with
β-mercaptoethanol to release C4a including C4a desArg from the
C4a-anti C4a antibody-protein A complex. The resulting mixture was
centrifuged to remove protein A and the remaining supernatant was
used for tricine-SDS-PAGE.
Tricine-SDS-PAGE and western blot
analysis
Tricine-SDS-PAGE was performed as previously
described (18), using 0.1 M
Tris/0.0225 M HCl and 0.1 M Tris/0.1M Tricine/0.1% SDS as the anode
and cathode buffers, respectively. For the western blotting, the
rabbit anti-human C4a antibody (#A206, Complement Technology, Inc.)
was used as the primary antibody and goat anti-rabbit IgG-HRP
(sc-2004, Santa Cruz Biotechnology, Inc.) as the secondary antibody
for visualization of the target proteins with a chemiluminescent
reagent (ECL Plus, GE Healthcare UK Ltd., Buckinghamshire, UK).
Purified C4a desArg using the ProteinChip
SELDI system
Purified C4a desArg (#A107, Complement Technology,
Inc.) was prepared as solutions of 1 and 5 μg/ml. These solutions
were diluted 10 times with sodium acetate buffer (pH 4.5) and
diluted solutions (100 μl) were applied to the ProteinChip SELDI
system with the CM10 chip. C4a desArg was also added to serum from
healthy controls to give a concentration of 50 μg/ml. The mixture
was diluted 10 times with PBS and 10 times with sodium acetate
buffer (pH 4.5) and then diluted solutions (100 μl) were applied to
the ProteinChip SELDI system.
Determination of serum levels of
complement C4a/C4a desArg
The level of serum C4a including C4a desArg was
determined using an ELISA kit (Human C4a ELISA kit, BD Biosciences,
Franklin Lakes, NJ, USA).
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). Differences in laboratory data and signal intensities
obtained by SELDI were evaluated by Mann-Whitney U test. Regression
analysis was performed to examine the correlation of continuous
variables. A P-value <0.05 was considered to indicate a
statistically significant difference. Analyses were performed with
StatView 4.5 software (Abacus Concepts, Berkeley, CA, USA) or
ProteinChip Software, version 3.2.1 (Bio-Rad Laboratories).
Results
Exploration of biomarker candidates in
patients with IgA nephropathy
Serum spectra were obtained from 25 patients with
IgA nephropathy and 14 healthy controls using the ProteinChip SELDI
system (Fig. 1). A total of 558
signal clusters were detected from 2,000 to 11,000 m/z, and 93 of
these signals differed significantly between the patients and
controls (Table II). Simple
regression analysis was performed between the intensity of these 93
signals and the severity of glomerular lesion evaluated
histologically, with the goal of finding biomarker candidate
proteins correlated with the pathological activity of IgA
nephropathy. In this process, 3 signals (8592, 8757, 8806 m/z) were
identified as potential biomarkers (Table II, Fig. 2A-C). A protein at 8592 m/z was
also increased in the patients with IgA nephropathy compared to the
controls, with a mean intensity >5 in the patients (Table II).
 | Table IIRepresentative discriminatory signals
and mean values for patients with IgA nephropathy (group 1) and
healthy controls. |
Table II
Representative discriminatory signals
and mean values for patients with IgA nephropathy (group 1) and
healthy controls.
| Number | m/z | Patient group 1
(n=25) | Healthy controls
(n=14) | P-value |
|---|
| 1 | 3191 | 12.68±4.68 | 21.32±5.11 | <0.0001 |
| 2 | 8592 | 7.85±8.38 | 2.89±2.58 | 0.0008 |
| 3 | 4465 | 9.67±3.20 | 5.66±3.00 | 0.0009 |
| 4 | 6005 | 4.08±1.53 | 5.53±1.22 | 0.0012 |
| 5 | 4611 | 3.60±3.86 | 1.37±1.07 | 0.0013 |
| 6 | 6149 | 1.63±0.85 | 2.46±0.78 | 0.0013 |
| 7 | 8924 | 23.96±9.31 | 13.22±8.25 | 0.0013 |
| 8 | 4641 | 15.60±3.74 | 12.20±2.09 | 0.0014 |
| ⋮ | ⋮ | ⋮ | ⋮ | ⋮ |
| 27 | 8757 | 2.24±1.00 | 1.39±0.74 | 0.0084 |
| : | : | : | : | : |
| 38 | 8806 | 3.18±1.16 | 2.34±0.80 | 0.0118 |
| ⋮ | ⋮ | ⋮ | ⋮ | ⋮ |
| 93 | 10256 | 4.61±2.52 | 7.29±4.03 | <0.050 |
Identification of a biomarker candidate
protein (8592 m/z)
The protein signal at 8592 m/z was not detected
using the ProteinChip SELDI system (CM10 chip) in serum diluted
with buffer at pH 10.0, which suggested that the isoelectric point
of the protein at 8592 m/z was between pH 9.0–10.0. Based on the
Tagldent database of isoelectric point and molecular weight, the
signal at 8592 m/z was expected an inactivated peptide of C4a
anaphylatoxin (C4a desArg; 8590 Da, pI=9.6).
The protein signal at 8592 m/z detected in serum of
patients with IgA nephropathy (Fig.
3A) was not eliminated by immunoprecipitation using the control
antibody (Fig. 3B), but
disappeared with immunoprecipitation using anti-C4a antibody
(Fig. 3C). Western blot analysis
confirmed the presence of C4a or C4a desArg by the
immunoprecipitation assay (Fig.
3D). Purified C4a desArg diluted 10 times with sodium acetate
buffer (pH 4.5) also gave a signal at 8592 m/z using the
ProteinChip SELDI system (CM10 chip) and the signal intensity was
dependent on the concentration (Fig.
4B and C). Similarly, a signal at 8592 m/z appeared when
purified C4a desArg was added to serum from healthy controls
(Fig. 4E), at the same position
as the protein signal in serum from patients with IgA nephropathy
(Fig. 4A). These results show
that the peak at 8592 m/z was due to C4a desArg.
In addition, the levels of C4a, including C4a
desArg, determined by ELISA were strongly correlated with the
signal intensity at 8592 m/z (r=0.86, P<0.001, Fig. 4F). Thus, our results suggest that
most C4a evaluated by ELISA in patients with IgA nephropathy is in
the form of inactivated peptide C4a desArg.
Relationship between pathological data
and serum C4a/C4a desArg in patient group 1
The serum levels of C4a (mainly C4a desArg) in group
1 determined by ELISA were significantly higher in patients with
IgA nephropathy compared to healthy controls (1564.5±1129.0 vs.
708.2±622.1 ng/ml, P=0.002). Correlations of the severity of
glomerular lesion with the serum levels of C4a/C4a desArg and other
laboratory data (urinary protein excretion, eGFR, IgA) were also
examined in group 1. The severity was positively correlated with
the serum levels of C4a/C4a desArg (r=0.62, P<0.001) and urinary
protein excretion (r=0.55, P=0.005). In addition, the mesangial
hypercellularity score, which is related to a decrease in renal
function (12), was positively
correlated with the serum levels of C4a/C4a desArg (r=0.54,
P=0.005). By contrast, the severity of chronic glomerular lesions
was not correlated with the serum levels of C4a/C4a desArg.
Clinical significance of serum C4a/C4a
desArg in patient group 2
To confirm the results from patient group 1, the
clinical significance of the serum levels of C4a/C4a desArg were
evaluated in a second group of patients with IgA nephropathy (group
2). The C4a/C4a desArg levels determined by ELISA in this group
were also significantly higher than those in healthy controls
(Fig. 5, P<0.001). The serum
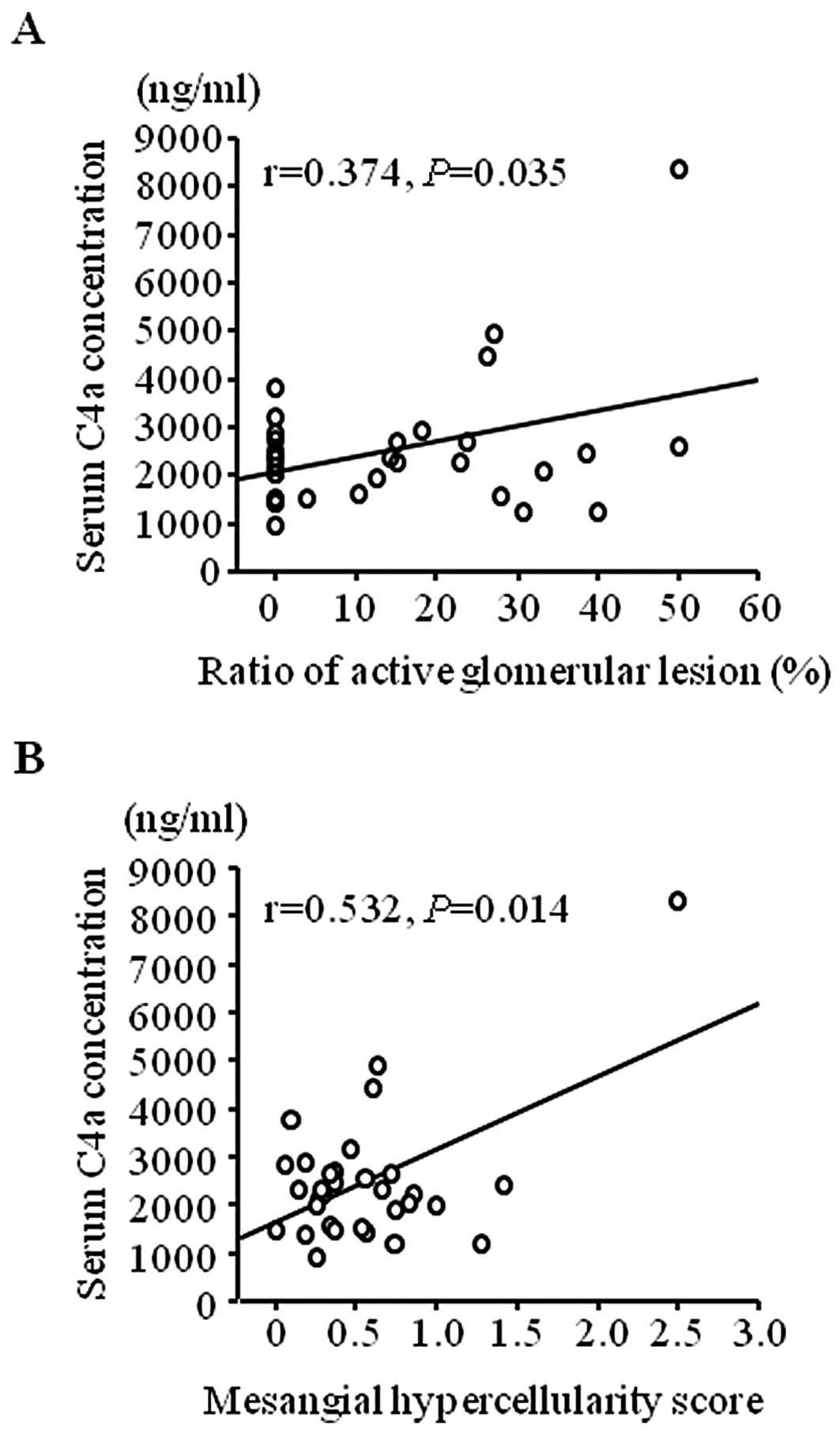
levels of C4a/C4a desArg also correlated with the severity of
glomerular lesion (Fig. 6A,
P=0.035) and were significantly correlated with mesangial
hypercellularity scores (Fig. 6B,
P=0.014) in group 2.
Discussion
Three signals (8592, 8757, 8806 m/z) of potential
biomarkers related to the severity of glomerular lesions were
identified in serum proteomics of patients with IgA nephropathy.
The signal at 8592 m/z was confirmed to be due to C4a desArg, which
was also shown to be positively correlated with mesangial
hypercellularity scores in two groups of patients with IgA
nephropathy.
C4a is an anaphylatoxin and strong
inflammation-inducing agent derived from complement component C4.
C4a desArg, which has low anaphylatoxin activity, is formed from
C4a by fast cleavage of arginine by carboxypeptidase N in serum. It
is considered that the majority of C4a in serum is C4a desArg, and
we confirmed a strong correlation between C4a levels determined by
ELISA and the level of C4a desArg detected by SELDI. The complement
pathway is activated in patients with IgA nephropathy and, in this
study, C4a desArg levels were significantly higher in these
patients than in healthy controls. By contrast, there was no
significant difference in C4 levels between the patients and
healthy controls. These results suggest that the C4a desArg level
can serve as an index of complement activation in IgA
nephropathy.
Serum levels of C4a (mainly C4a desArg) determined
by ELISA were significantly correlated with pathologically
estimated mesangial hypercellularity scores and with the severity
of glomerular lesions in both groups of patients with IgA
nephropathy. The mesangial hypercellularity score is a measure of
mesangial cell proliferation and is likely to be a prognostic
factor for renal function and to indicate the severity of
glomerular lesions. In proposing the Oxford classification, the
working group of the International IgA Nephropathy Network and the
Renal Pathology Society suggested that the mesangial
hypercellularity score was the most reproducible pathological
finding among investigators (13). Therefore, our results suggest that
the serum level of complement C4a desArg may serve as a biomarker
to estimate the mesangial hypercellularity score, as a prognostic
factor, and as an index of the effect of immunoregulatory therapy
in patients with IgA nephropathy. A long-term follow-up study is
required to confirm these results.
Complement deposition is observed in IgA deposition
sites in patients with IgA nephropathy, which suggests that the
complement system may be involved in the pathology, and increased
plasma levels of the anaphylatoxins C3a and C4a have been reported
in these patients (19). However,
the mechanism and clinical significance of complement activation in
IgA nephropathy is unclear. Activation may occur through an
alternative pathway (20), but it
has recently been proposed that the lectin pathway is involved in
complement activation in IgA nephropathy (21,22). Furthermore, C4d deposition in
glomeruli and activation of the lectin pathway influence the
prognosis of IgA nephropathy and the severity of renal injury
(23,24). The current study also showed that
serum C4a desArg levels were correlated with mesangial
hypercellularity scores and the severity of glomerular lesions.
Complement components other than C4 remain to be investigated;
however, our study indicates that complement activation by C4 may
be important in IgA nephropathy.
Our results indicated a correlation between serum
levels of complement C4a (mainly C4a desArg) and histological
activity estimated by severity of glomerular lesions and mesangial
hypercellularity scores in both groups of patients with IgA
nephropathy. By contrast, serum levels of complement C4a or C4a
desArg were not related to the severity of chronic glomerular
lesions. Urinary protein excretion is used as a laboratory test of
the severity of renal injury and is strongly influenced by chronic
glomerular lesions, which are unlikely to be responsive to
anti-inflammatory therapy. By contrast, serum levels of complement
C4a and C4a desArg are not influenced by urinary protein excretion.
The independence of the serum level of C4a desArg with respect to
urinary protein excretion may also make it a useful index of the
effect of anti-inflammatory therapy in IgA nephropathy.
One limitation of identification of a
complement-related molecule, including an anaphylatoxin such as C4a
desArg, as a biomarker candidate is that complement may be
activated even after blood sample collection (25,26). Pfeifer et al showed that
C4a/C4a desArg levels increased over time in plasma samples from
patients with systemic lupus erythematosus stored at 37ºC without
addition of futhan (25). The
C4a/C4a desArg levels in plasma samples of healthy controls
collected in the same manner and stored for 60 min at 37ºC were
also higher than those measured immediately after collection
(26). Thus, the serum levels of
complement C4a/C4a desArg measured in the current study were likely
to be higher than those in blood in vivo. However, the
effects ex vivo can be minimized by shortening the time from
sample collection to centrifugation and freezing to eliminate
sample variability. The measured C4a desArg levels might reflect
complement activation ex vivo as well as that in
vivo, but these levels still showed a considerable correlation
with the severity of glomerular lesion and mesangial
hypercellularity scores in two separate groups of patients with IgA
nephropathy. Therefore, we conclude that the C4a/C4a desArg levels
in these patients are useful as a surrogate marker of disease
severity. Verification of these findings requires measurements in
samples with control of complement activation ex vivo. A
prospective study is also required to confirm the correlation
between serum C4a desArg levels and the pathological activity of
IgA nephropathy.
In conclusion, the serum level of C4a (mainly C4a
desArg) is significantly higher in patients with IgA nephropathy
compared to healthy controls and is significantly correlated with
the severity of glomerular lesions and mesangial hypercellularity
scores evaluated histologically using the Oxford classification of
IgA nephropathy. Thus, serum C4a desArg is a potential biomarker
for the severity of histological findings in patients with IgA
nephropathy.
References
|
1
|
Donadio JV and Grande JP: IgA nephropathy.
N Engl J Med. 347:738–748. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
D’Amico G: Influence of clinical and
histological features on actuarial renal survival in adult patients
with idiopathic IgA nephropathy, membranous nephropathy, and
membranoproliferative glomerulonephritis: survey of the recent
literature. Am J Kidney Dis. 20:315–323. 1992.
|
|
3
|
Alamartine E, Sabatier JC, Guerin C,
Berliet JM and Berthoux F: Prognostic factors in mesangial IgA
glomerulonephritis: an extensive study with univariate and
multivariate analyses. Am J Kidney Dis. 18:12–19. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Odum J, Peh CA, Clarkson AR, et al:
Recurrent mesangial IgA nephritis following renal transplantation.
Nephrol Dial Transplant. 9:309–312. 1994.PubMed/NCBI
|
|
5
|
Sanfilippo F, Croker BP and Bollinger RR:
Fate of four cadaveric donor renal allografts with mesangial IgA
deposits. Transplantation. 33:370–376. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koselj M, Rott T, Vizjak A and Kveder R:
IgA nephropathy as a donor-transmitted disease in renal transplant
recipients. Transplant Proc. 23:2643–2646. 1991.PubMed/NCBI
|
|
7
|
Shiraishi S, Tomoda K, Matsumoto A,
Kyomoto R and Yamashita T: Investigation of the local provocation
test to PPP and IgA nephritis. Acta Otolaryngol Suppl. 523:178–181.
1996.PubMed/NCBI
|
|
8
|
Yamabe H, Osawa H, Inuma H, et al:
Deterioration of urinary findings after tonsil stimulation in
patients with IgA nephropathy. Acta Otolaryngol Suppl. 523:169–171.
1996.PubMed/NCBI
|
|
9
|
Pozzi C, Andrulli S, Del Vecchio L, et al:
Corticosteroid effectiveness in IgA nephropathy: long term results
of a randomized, controlled trial. J Am Soc Nephrol. 15:157–163.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barratt J and Feehally J: Treatment of IgA
nephropathy. Kidney Int. 69:1934–1938. 2006. View Article : Google Scholar
|
|
11
|
Feehally J, Barratt J, Coppo R, Cook T and
Roberts I: International IgA Nephropathy Network: International IgA
nephropathy network clinico-pathological classification of IgA
nephropathy. Contrib Nephrol. 157:13–18. 2007.PubMed/NCBI
|
|
12
|
Working Group of the International IgA
Nephropathy Network and the Renal Pathology Society. Cattran DC,
Coppo R, Cook HT, et al: The Oxford classification of IgA
nephropathy: rationale, clinicopathological correlations, and
classification. Kidney Int. 76:534–545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Working Group of the International IgA
Nephropathy Network and the Renal Pathology Society. Roberts IS,
Cook HT, Troyanov S, et al: The Oxford classification of IgA
nephropathy: pathology definitions, correlations and
reproducibility. Kidney Int. 76:546–556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshida M, Hatano N, Nishiumi S, Irino Y,
Izumi Y, Takenawa T and Azuma T: Diagnosis of gastroenterological
diseases by metabolome analysis using gas chromatography-mass
spectrometry. J Gastroenterol. 47:9–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomosugi N, Kawabata H, Wakatabe R,
Higuchi M, Yamaya H, Umehara H and Ishikawa I: Detection of serum
hepcidin in renal failure and inflammation by using ProteinChip
System. Blood. 108:1381–1387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanmura S, Uto H, Kusumoto K, et al: Early
diagnostic potential for hepatocellular carcinoma using the SELDI
ProteinChip system. Hepatology. 45:948–956. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanmura S, Uto H, Sato Y, et al: The
complement component C3a fragment is a potential biomarker for
hepatitis C virus-related hepatocellular carcinoma. J
Gastroenterol. 45:459–467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schägger H: Tricine-SDS-PAGE. Nat Protoc.
1:16–22. 2006.
|
|
19
|
Abou-Ragheb HH, Williams AJ, Brown CB and
Milford-Ward A: Plasma levels of the anaphylatoxins C3a and C4a in
patients with IgA nephropathy/Henoch-Schönlein nephritis. Nephron.
62:22–26. 1992.
|
|
20
|
Wyatt RJ, Kanayama Y, Julian BA, Negoro N,
Sugimoto S, Hudson EC and Curd JG: Complement activation in IgA
nephropathy. Kidney Int. 31:1019–1023. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsuda M, Shikata K, Wada J, Sugimoto H,
Shikata Y, Kawasaki T and Makino H: Deposition of mannan binding
protein and mannan binding protein-mediated complement activation
in the glomeruli of patients with IgA nephropathy. Nephron.
80:408–413. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Endo M, Ohi H, Ohsawa I and Fujita T,
Matsushita M and Fujita T: Glomerular deposition of mannose-binding
lectin (MBL) indicates a novel mechanism of complement activation
in IgA nephropathy. Nephrol Dial Transplant. 13:1984–1990. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Espinosa M, Ortega R, Gomez-Carrasco JM,
Lopez-Rubio F, Lopez-Andreu M, Lopez-Oliva MO and Aljama P:
Mesangial C4d deposition: a new prognostic factor in IgA
nephropathy. Nephrol Dial Transplant. 24:886–891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roos A, Rastaldi MP, Calvaresi N, et al:
Glomerular activation of the lectin pathway of complement in IgA
nephropathy is associated with more severe renal disease. J Am Soc
Nephrol. 17:1724–1734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pfeifer PH, Kawahara MS and Hugli TE:
Possible mechanism for in vitro complement activation in blood and
plasma samples: futhan/EDTA controls in vitro complement
activation. Clin Chem. 45:1190–1199. 1999.PubMed/NCBI
|
|
26
|
Morgan E, Varro R, Sepulveda H, et al:
Cytometric bead array: a multiplexed assay platform with
applications in various areas of biology. Clin Immunol.
110:252–266. 2004. View Article : Google Scholar : PubMed/NCBI
|