Introduction
The therapeutic indication of asymptomatic carotid
artery stenosis is based on the degree of stenosis, which
statistically correlates with the risk of stroke (1,2).
According to current concepts, the predominant pathological
substrate of cerebral ischemic events results from the embolization
of unstable or thrombogenic atherosclerotic plaque. Until now,
reliable clinical criteria differentiating between stable and risk
prone carotid plaque do not exist.
In addition to extensive image analysis, several
biomarkers have been examined, aiming to find predictive factors
that indicate an elevated risk of plaque instability and
consecutive embolization. Some prominent morphological features,
such as vascularization, the amount of necrotic core, thin fibrous
cap, intraplaque hemorrhage or increased intima-media thickness
(IMT) are associated with plaque vulnerability (3).
However, the cellular events leading to plaque
rupture are not yet fully understood. To further characterize the
biological activity of plaque and the areas adjacent to plaque, in
this study, we assessed the expression and localization of proteins
with a potential role in the pathogenesis of atherosclerosis in
correlation with qualitatively defined plaque components. In
contrast to several other previously described plaque models
focusing on plaque area only (4),
our study introduces a new model by additionally investigating
surrounding border zones neighboring different plaque areas. Plaque
from 20 patients that had undergone carotid endarterectomy (CEA)
were histologically mapped and stained by immunohistochemistry for
marker proteins associated with matrix remodeling, such as
matrix-metalloproteinase-9 (MMP-9), glycophorin A (GYPA),
osteoprotegerin (OPG), vascular cell adhesion molecule-1 (VCAM-1),
endothelin-1 (ET-1) and vascular endothelial growth factor (VEGF),
as well as for markers associated with inflammation, such as tumor
necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β),
interleukin-1β (IL-1β), C-reactive protein (CRP), pentraxin-3
(PTX-3), nuclear factor-κB (NF-κB) and CD68. Vascular smooth muscle
cell actin (VSMA) was used for the identification of vascular
smooth muscle cells (VSMCs).
Emerging evidence indicates that the increased
expression of MMP-9 is involved in plaque destabilization (5). MMP-9 belongs to a family of enzymes
that promotes the degradation of extracellular matrix components,
such as elastin, proteoglycans and laminin and is associated with
vascular remodeling and atherogenesis. MMP-9 is produced by
macrophages, macrophage foam cells and VSMCs and has been shown to
stimulate the production of TNF-α and IL-1, which in turn promotes
inflammation (6).
GYPA, a protein specific to erythrocytes, and OPG
are considered markers for structural damage. GYPA has been
reported to indicate intraplaque hemorrhage and to be associated
with necrotic cores and macrophage infiltration in coronary
atheromas (7). OPG is a
glycoprotein that acts as a receptor for the ‘receptor activator of
NF-κB’ ligand. Plasma OPG levels are elevated in patients with
vascular disease and the OPG concentration in carotid plaque is
associated with the prevalence of artery calcium and plaque
instability (8,9).
VCAM-1 plays a role in initiating atherosclerotic
cascades by mediating the firm adhesion between macrophages and
endothelial cells (10). Its
expression in activated endothelial cells is induced by TGF-β
(11), IL-1 (12) and by cholesterol accumulation
(13).
VEGF is a key player in angiogenesis and
neovascularization as well as in vascular permeability or
thrombogenicity (14). By the
initiation of neovascularization, VEGF also plays a role in
intraplaque microvessel formation and restenosis (15).
ET-1 is induced in endothelial cells by
biomechanical stimuli, hypoxemia, hormones or cytokines. It
stimulates the contraction and growth of smooth muscle cells
(16).
TNF-α and IL-1β are pro-inflammatory cytokines that
are released from activated macrophages. PTX-3 is a soluble
receptor of the pattern recognition type, labeling cellular debris
and pathogens for phagocytosis by macrophages. Accordingly, it is
found in macrophage foam cells which play a key role in
atherosclerosis, as well as in circulating blood. Elevated plasma
levels of PTX-3 have recently been shown to be associated with
plaque vulnerability (17).
Elevated plasma levels of CRP induce pro-inflammatory changes by
the activation of peripheral leukocytes with the ensuing secretion
of plaque-destabilizing mediators, such as MMP-9 (18).
The scavenger receptor, CD68, that interacts with
oxidized low-density lipoprotein (oxLDL) amongst others, is a
heavily glycosylated transmemberane protein that is predominantly
expressed in monocytes and macrophages. Upon interaction with
oxLDL, macrophages transform into foam cells. The presence of a
high CD68-positive macrophage count has been demonstrated to be
associated with plaque destabilization (19,20).
Patients and methods
Patients
Carotid artery plaque obtained from patients who had
undergone CEA was randomly selected from the vascular tissue bank
of the Department of Vascular Surgery/National Center for Tumor
Diseases (NCT), University of Heidelberg, Heidelberg, Germany. The
study population included 20 specimens, 10 from asymptomatic and 10
from symptomatic patients. Indications for CEA were high-grade
internal carotid artery stenosis (as determined by ultrasound) for
asymptomatic patients and transient ischemic attack (TIA),
amaurosis fugax (AF) or ischemic stroke for symptomatic patients.
Neurological events had occurred within 6 months prior to surgery.
Clinical data, including medication, blood work and risk factors
for atherosclerosis were recorded for all patients (Table I). Patients with atrial
fibrillation were excluded. A neurologist examined all symptomatic
patients according to clinical standards and the study followed
ethical guidelines.
 | Table IPatient characteristics and clinical
data. |
Table I
Patient characteristics and clinical
data.
| Patients |
|---|
|
|
|---|
| Items | Asymptomatic | Symptomatic |
|---|
| Characteristics |
| Gender |
| Male | 7 | 10 |
| Female | 3 | |
| Mean age
(years) | 68.6±9.9 | 73.7±8.7 |
| Mean BMI
(kg/m2) | 26.2±3.7 | 25.9±3.8 |
| Mean degree of
stenosis (%) | 81.5±8.2 | 81.5±14.2 |
| Risk factors |
| Arterial
hypertension | 9 | 10 |
| Diabetes
mellitus | 2 | 1 |
| Nicotin | 1 | 2 |
| Hyperlipidemia | 6 | 8 |
| Medication |
| Acetylsalicylic
acid | 10 | 9 |
| Statins | 6 | 9 |
| Oral
antidiabetics/insulin | 2 | 1 |
|
Anti-hypertonics | 9 | 9 |
Tissue processing
After dissection of the internal carotid artery from
the bifurcation, the intact specimen was harvested by a vascular
surgeon (routine eversion technique) (Fig. 1). The fresh carotid eversion
specimen was rinsed with saline to remove surface blood, and
defined proximal sections were divided into 3 tissue segments
(rings of approximately 3 mm thickness): 2 segments were
formalin-fixed and embedded in paraffin according to standard
procedures for conventional histological analysis and
immunohistochemistry. A third segment was shock-frozen and stored
in a freezer at −80°C.
Histological graduation
For the assessment of morphological features, the
American Heart Association (AHA) classification was adapted for
advanced carotid atherosclerotic lesions (21).
Plaque characterization and definition of
morphological zones
Based on the morphologial examination, we defined 3
different zones within the atherosclerotic lesions (Fig. 2A): plaque (zone 1), border zone
(zone 2) and normal vessel wall (zone 3, control). The plaque zone
was divided in 3 major subtypes: calcified (1a), lipid-rich (1b)
and mixed type (1c). Similarly, the border zone was classified as
bordering to calcified (2a), lipid-rich (2b) or mixed (2c) plaque.
The unaffected vessel wall of the same sample (zone 3) was used as
the control for comparison. To determine the dimension of the
different morphological areas, a grid was applied on each
transverse sliced sample (square measure of 9033.39 μm2
per grid unit) (Fig. 2B).
Analysis was performed using a microscope (CX40; Olympus, Tokyo,
Japan) at an original magnification of ×200 and a camera (QImaging,
Surrey, BC, Canada). Each grid unit was assigned to a plaque,
border or control zone. Corresponding protein expression, as
detected by immunohistochemistry, was semi-quantitatively scored by
2 independent investigators unaware of the clinical history of the
patient samples (for score evaluations see following section). The
plaque zone (zone 1) was characterized by necrotic areas, fibrous
tissue, large amounts of calcium and cholesterol. Areas with mixed
features demonstrated noticeable calcification and cholesterol-rich
areas and signs of surface disruption or fissures were observed
(Fig. 2G and H). The main
characteristics of zone 2 were large amounts of inflammatory cells,
neovascularization and deallocation of the intima and media, while
zone 3 represented intact vascular tissue with a clear separation
of the intima and media plus a significantly lower amount of
inflammatory cells (ratio 1:10 compared to zone 2).
Immunohistological analysis
Staining procedures and
antibodies
For the immunohistochemical detection of protein
expression, serial 4-μm-thick sections were prepared from each
paraffin-embedded tissue specimen throughout the plaque at the
level of the highest degree of stenosis. After deparaffination and
rehydration, the samples were pre-treated according to individually
optimized protocols (detailed protocols and antibody dilutions are
available upon request). The following unconjugated primary
antibodies were used for detection: monoclonal mouse anti-MMP-9 was
obtained from Calbiochem (Merck, Darmstadt, Germany); polyclonal
rabbit anti-GYPA, rabbit anti-IL1β, rabbit anti-NF-κB, rabbit
anti-TGF-β, monoclonal mouse anti-VCAM, polyclonal rabbit anti-VEGF
and polyclonal rabbit anti-OPG were obtained from Santa Cruz
(Heidelberg, Germany); rabbit monoclonal anti-OPG was purchased
from Epigenomics - Biomol (Hamburg, Germany), and monoclonal mouse
anti-VSMA was from Sigma (Taufkirchen, Germany); monoclonal mouse
anti-CD68 was from USBiological - Biomol (Hamburg, Germany),
polyclonal sheep anti-CRP was from Biotrend (Koeln, Germany),
polyclonal rabbit anti-ET-1 was from Chemicon (Nuernberg, Germany),
polyclonal rabbit anti-TNF-α was from Genzyme (Neu-Isenburg,
Germany), and polyclonal rabbit anti-PTX-3 was from Alexis
Biochemicals (Loerrach, Germany). The slides were washed 2×5 min in
Tris-buffer and exposed to a biotinylated antibody [MultiLink, HK
340–9K (5%); BioGenex, San Ramon, CA, USA] for 20 min. After
washing again for 2×5 min in Tris-buffer, the samples were
incubated with peroxidase (horseradish peroxidase conjugate) for 20
min, washed again for 2×5 min in Tris-buffer and stained with Fast
Red (Zytomed Systems, Berlin, Germany). Colour development was
stopped by the addition of water and the sections were finally
counterstained with hematoxylin (Dako REAL, Dako North America,
Inc., Capinteria, CA, USA) 1:3 in distilled aqua. For the negative
controls, the application of the primary antibody was omitted.
Masson-Goldner trichrome staining was used according to standard
procedures for the visualization of different connective tissue
components.
Expression score
The level of antigen expression was determined
semi-quantitatively by a score composed of intensity and quantity.
Data for the intensity ran from 0, no staining; >1, faint
positive staining and 2, moderate positive staining to 3, strong
positive staining. Data for the quantity ran from 0, no staining;
1, <10%; 2, 10–50%; 3, 51–80% and 4, >80% of structures
positively stained within the field of view. The score was built by
the multiplication of both results (22,23). To avoid observation bias, we first
investigated the different zones in all the samples, not
discriminating between symptomatic or asymptomatic patients. During
a second revision, we focused on the zonal distribution to
differentiate between symptomatic and asymptomatic patients.
Statistical analysis
Statistical analysis was performed using SPSS
software version 16.0 (SPSS Inc., Chicago, IL, USA). For a
comparison of zonal protein scores between border zones and control
zones within the same sample, quantitative non-parametric
continuous variables were expressed as medians and compared by
using the Wilcoxon matched-pairs signed rank test. The Mann-Whitney
U test was applied to compare the expression score of each marker
derived from different border zones (adjacent to calcified,
lipid-rich or mixed plaque) between asymptomatic and symptomatic
patients; a value of p<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
As shown in Table
I, the mean age of the patients was 68.6±9.9 years for the
asymptomatic and 73.7±8.7 years for the symptomatic patients.
Neurological events of the symptomatic patients had occurred within
the last 6 months with a history of recent occurrence of cerebral
symptoms [7 patients with non-disabling ischemic stroke (Rankin
1–3), 2 with TIA and 1 with AF]. Nine asymptomatic and 10
symptomatic patients had hypertension, 6 asymptomatic and 8
symptomatic were afflicted with hypercholesterolemia. In total, 3
patients were diabetic and 3 had a history of >20 ‘pack years’
of nicotine abuse. The mean body mass index was 26.2±3.7
kg/m2 for the asymptomatic and 25.9±3.8 kg/m2
for the symptomatic patients.
Morphological plaque features according
to AHA classification
The assessment of morphological plaque features was
carried out as previously described in the study by Stary et
al (21). As visualized by
trichrome staining, all specimens showed severe lesions with
calcification, plaque rupture, intra-plaque hemorrhage or fissures
equivalent to type V and VI lesions, according the AHA criteria.
Four out of the 10 asymptomatic but only 1 out of the 10
symptomatic patients had type V lesions. This shows a tendency
toward the presence of type VI lesions in symptomatic patients,
without statistical significance, however.
Spatial resolution and differentiation of
zones with distinctive morphological features
All types of plaque zones and border zones, namely
calcified, lipid-rich and mixed type, were observed in the
atherosclerotic lesions from the symptomatic and asymptomatic
patients. The plaque and border zone accounted for approximately
72% (±1.3) of the vessel wall area, whereas the control zone
accounted for 28% (±1.3) of the total cross sectional area. Zone 1a
(calcified plaque) ranged between 0 and 29%, zone 1b (lipid-rich
plaque) ranged between 0 and 40% and zone 1c (mixed plaque) ranged
between 25 and 93% of the plaque area. The same spatial
distribution was observed in the border zones. No difference in
zonal expansion was observed between the symptomatic or
asymptomatic patients.
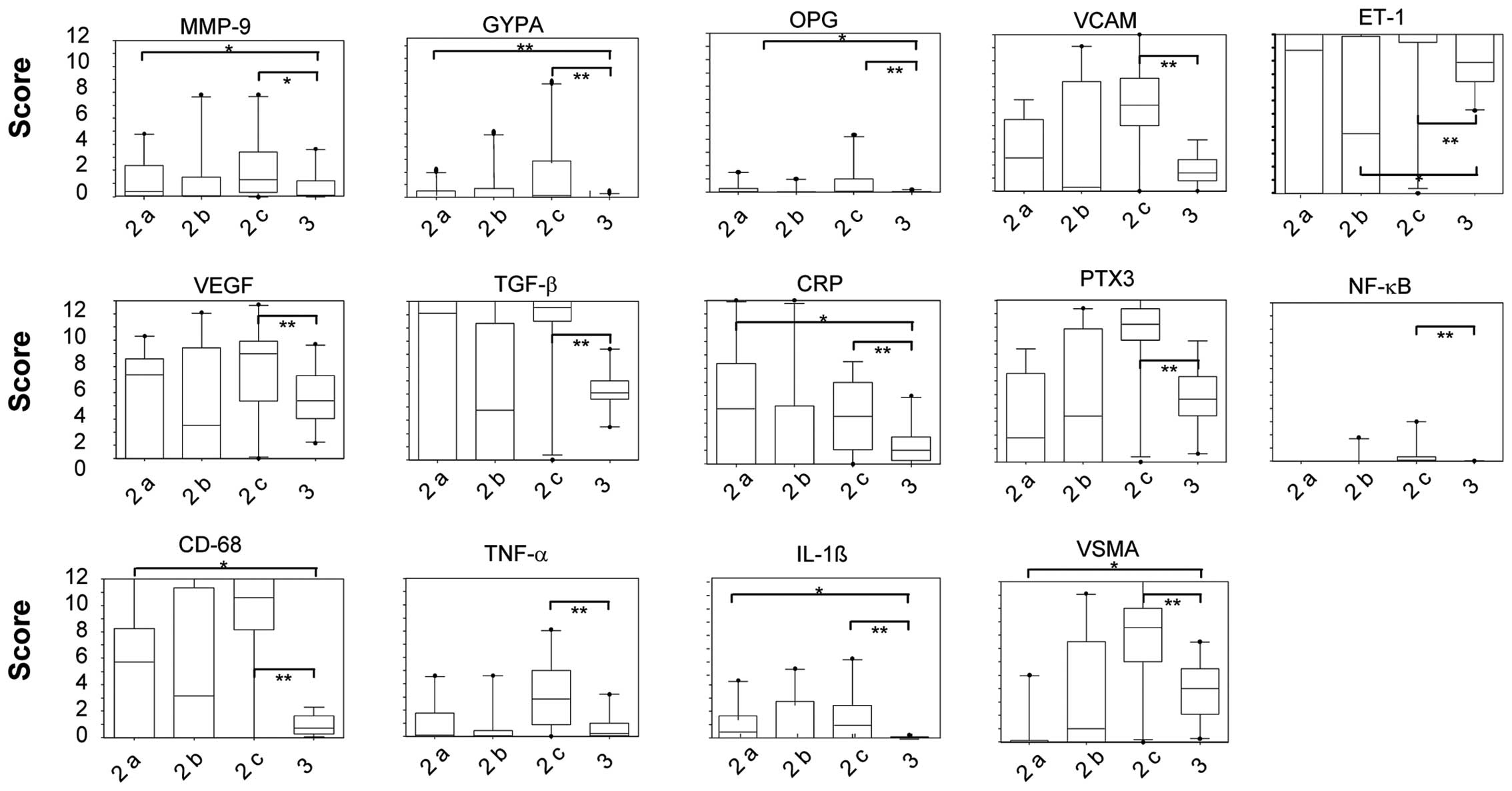
Zonal distribution of protein expression
according to immunohistochemical analysis
In general, all proteins tested were found to be
predominantly expressed in the border zones (zone 2), touching or
surrounding different plaque types. Except for VSMA, indicating the
presence of smooth muscle cells in the vascular media, little
protein expression was observed in the control zones (zone 3),
whereas almost no protein expression was present within the plaque
zones (zone 1). Statistical analysis revealed significantly
elevated expression scores of all tested proteins in the border
zones compared to control zones. Within the border zones, those
touching mixed plaque (zone 2c) presented significantly higher
protein expression scores than the border zones touching pure
calcified (zone 2a) or pure lipid-rich (zone 2b) plaque (Fig. 3). As indicated by the high VSMA
expression, the highest expression of the majority of proteins
tested (except for CRP) overlapped with the presence of a high
density of smooth muscle cells in zone 2c.
Furthermore, inflammatory markers, such as CD68,
NF-κB, IL-1β, TNF-α and PTX-3, representing the presence of
activated macrophages, displayed significantly higher expression
scores in zone 2c compared to the control zone (Fig. 2G, H and Fig. 3). This indicates that macrophage
infiltration is more prominent in the border zones adjacent to
mixed plaque than in other border zones.
By contrast, the expression score of the
inflammatory protein, CRP, was highest in zone 2a, touching
calcified plaque, followed by border zones touching mixed plaque
(zone 2c), its epxression was still significantly higher in these
zones compare to the control zones. Of note, CRP was completely
absent from the border zone adjacent to lipid-rich plaque (zone
2b). Similar to CRP, the ET-1 expression score was significantly
reduced in zone 2b compared to the control and other border
zones.
Border zones touching calcified plaque (zone 2a)
revealed a statistically significant higher expression score of
GYPA, MMP-9, OPG, IL-1β, CRP and CD68 compared to the control zones
(Figs. 3 and 4). In addition to macrophage
infiltration, this points to greater intraplaque hemorrhage, matrix
degeneration and presumably ongoing calcification in these areas.
By contrast, the VSMA expression score was significantly reduced,
indicating a reduced number of VSMCs around calcified plaque.
Comparison of protein expression scores
between symptomatic and asymptomatic patients
The observation that the expression of inflammatory
markers and proteins involved in vascular remodeling was most
prominent in the border zones touching mixed plaque, suggested that
these plaque types may be more vulnerable. Accordingly, we were
interested in whether some of these protein scores differ
significantly between symptomatic and asymptomatic patients.
No difference in expression scores was observed for
any of the inflammatory marker proteins (CD68, IL-1β, NF-κB, CRP,
PTX-3, TNF-α and TGF-β) between the individual border zones of
symptomatic and asymptomatic patients (data not shown).
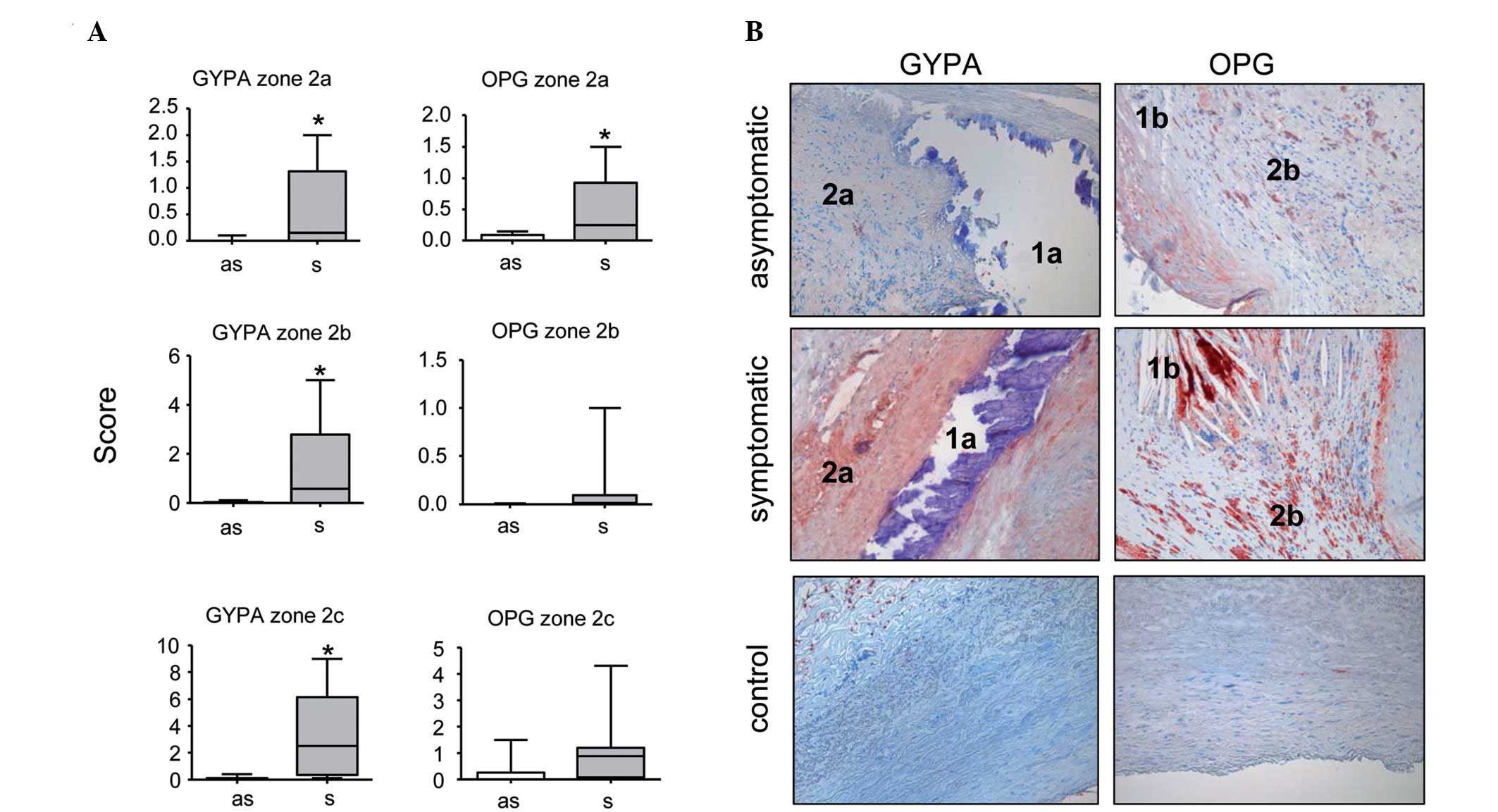
By contrast, the scores of some marker proteins
associated with vessel wall remodeling differed significantly
between the asymptomatic and symptomatic patients. The expression
scores of GYPA in the border zones around the calcified and mixed
plaque areas were slightly but statistically significantly higher
in the symptomatic compared to the asymptomatic patients (Fig. 5). The same tendency was observed
in the border zones around the lipid-rich plaque zones, without
reaching statistical significance, however. In addition, the
expression scores of OPG were significantly higher in all border
zones of symptomatic compared to asymptomatic patients, independent
of the adjacent plaque type (Fig.
5). No difference was observed for any of the remaining markers
tested (ET-1, MMP-9, VCAM-1, VEGF and VSMA; data not shown).
Discussion
It is well accepted that atherosclerotic lesions
represent complex heterogeneous structures composed of connective
tissue, calcification, inflammatory cells and lipids in proportions
differing from plaque to plaque (24–26). Increasing evidence suggests that
individual plaque morphology and plaque composition contribute to
the vulnerability and risk of rupture, in addition to the grade of
stenosis (27,28). Consequently, a histopathological
staging (AHA classification) was introduced for the classification
of atherosclerotic coronary lesions (21,29) that is likewise applied to carotid
lesions. Furthermore, a growing number of studies have reported the
analysis of single proteins expressed in carotid lesions to be used
as biomarkers for the evaluation of plaque rupture and stroke risk
at the early stages of disease. However, little progress has been
made to achieve this target. In addition, the contribution of these
proteins to plaque instability is controversially discussed in the
literature and the biological mechanisms leading to plaque rupture
are not yet completely understood (30).
To gain deeper insight into carotid plaque
composition of asymptomatic and symptomatic patients, in this
study, we introduced a new approach to analyze carotid lesions in
greater detail by morphologically subdividing the samples into
different zones of (cell-free) plaque, border zones touching the
plaque material and a control zone. Using this zoning system, we
scored the expression of 7 inflammatory marker proteins and 7
proteins associated with remodeling of the artery wall in
endarterectomized carotid samples from asymptomatic versus
symptomatic patients at punctum maximum of stenosis.
Our findings add to the body of research from
previous studies, which predominantly focused on differential
gene/protein expression in carotid lesions as a whole (27,31), or within selected areas, such as
calcification (32) or the plaque
shoulder without differentiating systematically between plaque
components. Moreover, the majority of studies on atherosclerotic
plaque heterogeneity and instability have been performed on
coronary artery lesions. Although it has been suggested that
carotid lesions follow a pattern of development and progression
similar to coronary arteries, and may encounter the same mechanisms
leading to rupture, this hypothesis is still controversially
discussed (25).
Our immunohistochemical analysis revealed a
significantly elevated protein expression of all markers
investigated in the border zone adjacent to plaque, whereas border
zones touching mixed plaque displayed the highest scores. Thus, the
area adjacent to a combination of both calcification and lipid
deposit showed the highest biological activity with respect to
inflammation and tissue remodeling, irrespective of the patient’s
symptomatic features.
Inflammation is considered a key mechanism in human
atherosclerotic plaque vulnerability and disruption (33). However, in our study, the grade of
inflammation and macrophage density, as assessed by the expression
scores of CD68, IL-1β, NF-κB, TNF-α, CRP, PTX-3 and TGF-β, did not
differ between the samples from asymptomatic and symptomatic
patients. In addition, the expression of markers indicating the
recruitment and adhesion of inflammatory cells (VCAM-1), matrix
degeneration (MMP-9) and neovascularization (VEGF) was similar in
both patient groups.
By contrast, GYPA and OPG expression, despite being
a rare event, was significantly more prominent in lesions from our
symptomatic patients. Increased levels of GYPA were found in border
zones around calcified and mixed plaque in patients who presented
with neurological events. The difference was likewise observed in
border zones around lipid-rich plaque without reaching statistical
significance. Although our results are based on a relatively small
sample size, this indicates that intraplaque haemorrhage may be
involved in plaque vulnerability. Our data is in line with findings
from coronary atheromas, where larger levels of GYPA were
associated with advanced plaque instability (7). Moreover, we previously demonstrated
that deposits of erythrocyte membrane-derived material (as assessed
by GYPA expression) was significantly more pronounced in the
coronary intima of patients with chronic renal disease compared to
non-renal patients (23).
Finally, GYPA expression has recently been shown to correlate with
thin fibrous cap and the clinical risk of CEA in a study using a
CEA risk classification, which is independent of the symptomatic
events if the patients (34).
Based on their results, the authors recommended identifying
patients with intraplaque haemorrhage by improved imaging and by
performing CEA in those patients to avoid neurologic events.
Of note, we identified OPG expression scores to be
significantly elevated in all types of border zones in our group of
symptomatic patients compared to asymptomatic patients. The role of
OPG in vascular calcification and atherosclerosis is not yet clear.
Several in vitro studies and animal models have suggested
that OPG inhibits vascular calcification, whereas serum OPG levels
have been associated with an increased risk of cardiovascular
disease in clinical studies (35). OPG has been reported to be
expressed in the normal vascular wall by endothelial and VSMCs.
Moreover, increased OPG immunoreactivity and mRNA expression have
been localized to areas surrounding calcification in the medial
layer of Mönckeberg’s sclerosis and areas adjacent to calcified
neointimal lesions in carotid atherosclerotic arteries (36). Our data, demonstrating an
increased OPG expression around different types of plaque not
restricted to calcification, point to a more complex mechanism of
OPG activity in vascular remodelling that requires further
investigation. In particular, it would be of interest to elucidate
its role in plaque instability.
In conlcusion, in this study, we present evidence
that intraplaque hemorrhage and OPG expression in areas touching
mixed plaque that are composed of calcified and lipid-rich
components, may be associated with plaque instability and
subsequent neurological events in patients with carotid lesions
independent of AHA classification. This argues for the need of a
more detailed plaque analysis in non-symptomatic patients; for
example, by the development of advanced imaging procedures that may
aid in the identification of hemorrhage in these patients.
Acknowledgements
We thank Diana Lutz and Heike Ziebart for their
excellent technical assistance in performing immunohistochemical
staining. The assistance of Dr Dmitriy Dovzhanskiy in the
configuration of the Box-Whisker plots is greatly appreciated.
References
|
1
|
Beneficial effect of carotid
endarterectomy in symptomatic patients with high-grade carotid
stenosis. North American Symptomatic Carotid Endarterectomy Trial
Collaborators. N Engl J Med. 325:445–453. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Halliday AW, Thomas DJ and Mansfield AO:
The asymptomatic carotid surgery trial (ACST). Int Angiol.
14:18–20. 1995.
|
|
3
|
Golledge J: Carotid intervention in
asymptomatic patients. Stroke. 39:e172008. View Article : Google Scholar
|
|
4
|
Mauriello A, Sangiorgi GM, Virmani R, et
al: A pathobiologic link between risk factors profile and
morphological markers of carotid instability. Atherosclerosis.
208:572–580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galis ZS, Sukhova GK, Lark MW and Libby P:
Increased expression of matrix metalloproteinases and matrix
degrading activity in vulnerable regions of human atherosclerotic
plaques. J Clin Invest. 94:2493–2503. 1994. View Article : Google Scholar
|
|
6
|
Galis ZS and Khatri JJ: Matrix
metalloproteinases in vascular remodeling and atherogenesis: the
good, the bad, and the ugly. Circ Res. 90:251–262. 2002.PubMed/NCBI
|
|
7
|
Kolodgie FD, Gold HK, Burke AP, et al:
Intraplaque hemorrhage and progression of coronary atheroma. N Engl
J Med. 349:2316–2325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abedin M, Omland T, Ueland T, et al:
Relation of osteoprotegerin to coronary calcium and aortic plaque
(from the Dallas Heart Study). Am J Cardiol. 99:513–518. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Golledge J, McCann M, Mangan S, Lam A and
Karan M: Osteoprotegerin and osteopontin are expressed at high
concentrations within symptomatic carotid atherosclerosis. Stroke.
35:1636–1641. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adams DH and Shaw S: Leucocyte-endothelial
interactions and regulation of leucocyte migration. Lancet.
343:831–836. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramana KV, Bhatnagar A and Srivastava SK:
Inhibition of aldose reductase attenuates TNF-alpha-induced
expression of adhesion molecules in endothelial cells. FASEB J.
18:1209–1218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bevilacqua MP: Endothelial-leukocyte
adhesion molecules. Annu Rev Immunol. 11:767–804. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cybulsky MI and Gimbrone MA Jr:
Endothelial expression of a mononuclear leukocyte adhesion molecule
during atherogenesis. Science. 251:788–791. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stephan CC and Brock TA: Vascular
endothelial growth factor, a multifunctional polypeptide. P R
Health Sci J. 15:169–178. 1996.PubMed/NCBI
|
|
15
|
O’Brien ER, Garvin MR, Dev R, et al:
Angiogenesis in human coronary atherosclerotic plaques. Am J
Pathol. 145:883–894. 1994.
|
|
16
|
Schiffrin EL and Touyz RM: Vascular
biology of endothelin. J Cardiovasc Pharmacol. 32(Suppl 3): S2–S13.
1998.
|
|
17
|
Soeki T, Niki T, Kusunose K, et al:
Elevated concentrations of pentraxin 3 are associated with coronary
plaque vulnerability. J Cardiol. 58:151–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bisoendial RJ, Birjmohun RS, Akdim F, et
al: C-reactive protein elicits white blood cell activation in
humans. Am J Med. 122:582 e1–e9. 2009.PubMed/NCBI
|
|
19
|
Zeibig S, Li Z, Wagner S, et al: Effect of
the oxLDL binding protein Fc-CD68 on plaque extension and
vulnerability in atherosclerosis. Circ Res. 108:695–703. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Artese L, Ucchino S, Piattelli A, et al:
Factors associated with apoptosis in symptomatic and asymptomatic
carotid atherosclerotic plaques. Int J Immunopathol Pharmacol.
18:645–653. 2005.PubMed/NCBI
|
|
21
|
Stary HC, Chandler AB, Dinsmore RE, et al:
A definition of advanced types of atherosclerotic lesions and a
histological classification of atherosclerosis. A report from the
Committee on Vascular Lesions of the Council on Arteriosclerosis,
American Heart Association. Arterioscler Thromb Vasc Biol.
15:1512–1531. 1995. View Article : Google Scholar
|
|
22
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German).
|
|
23
|
Gross ML, Meyer HP, Ziebart H, et al:
Calcification of coronary intima and media: immunohistochemistry,
backscatter imaging, and x-ray analysis in renal and nonrenal
patients. Clin J Am Soc Nephrol. 2:121–134. 2007. View Article : Google Scholar
|
|
24
|
Verstraete M: Coronary atherosclerosis and
thrombosis. Recenti Prog Med. 81:221–227. 1990.
|
|
25
|
Slevin M, Wang Q, Font MA, et al:
Atherothrombosis and plaque heterology: different location or a
unique disease? Pathobiology. 75:209–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aidinian G, Weiswasser JM, Arora S,
Abularrage CJ, Singh N and Sidawy AN: Carotid plaque morphologic
characteristics. Perspect Vasc Surg Endovasc Ther. 18:63–70. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Naghavi M, Libby P, Falk E, et al: From
vulnerable plaque to vulnerable patient: a call for new definitions
and risk assessment strategies: Part I. Circulation. 108:1664–1672.
2003. View Article : Google Scholar
|
|
28
|
Kher N and Marsh JD: Pathobiology of
atherosclerosis - a brief review. Semin Thromb Hemost. 30:665–672.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stary HC, Chandler AB, Dinsmore RE, et al:
A definition of advanced types of atherosclerotic lesions and a
histological classification of atherosclerosis. A report from the
Committee on Vascular Lesions of the Council on Arteriosclerosis,
American Heart Association. Circulation. 92:1355–1374. 1995.
View Article : Google Scholar
|
|
30
|
Koenig W and Khuseyinova N: Biomarkers of
atherosclerotic plaque instability and rupture. Arterioscler Thromb
Vasc Biol. 27:15–26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fagerberg B, Ryndel M, Kjelldahl J, et al:
Differences in lesion severity and cellular composition between in
vivo assessed upstream and downstream sides of human symptomatic
carotid atherosclerotic plaques. J Vasc Res. 47:221–230. 2010.
View Article : Google Scholar
|
|
32
|
Wahlgren CM, Zheng W, Shaalan W, Tang J
and Bassiouny HS: Human carotid plaque calcification and
vulnerability. Relationship between degree of plaque calcification,
fibrous cap inflammatory gene expression and symptomatology.
Cerebrovasc Dis. 27:193–200. 2009.
|
|
33
|
Libby P, Ridker PM and Hansson GK:
Inflammation in atherosclerosis: from pathophysiology to practice.
J Am Coll Cardiol. 54:2129–2138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hao H, Iihara K, Ishibashi-Ueda H, Saito F
and Hirota S: Correlation of thin fibrous cap possessing
adipophilin-positive macrophages and intraplaque hemorrhage with
high clinical risk for carotid endarterectomy. J Neurosurg.
114:1080–1087. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Van Campenhout A and Golledge J:
Osteoprotegerin, vascular calcification and atherosclerosis.
Atherosclerosis. 204:321–329. 2009.PubMed/NCBI
|
|
36
|
Schoppet M, Al-Fakhri N, Franke FE, et al:
Localization of osteoprotegerin, tumor necrosis factor-related
apoptosis-inducing ligand, and receptor activator of nuclear
factor-kappaB ligand in Mönckeberg’s sclerosis and atherosclerosis.
J Clin Endocrinol Metab. 89:4104–4112. 2004.
|