Introduction
Sindbis virus-based gene transfer vectors have shown
remarkable antitumor efficacy and tumor-targeting capacity in
several animal models. After systemic delivery the vector is able
to target and destroy tumors without damaging normal tissues. It is
efficient in human xenograft tumors, metastatic models as well as
in spontaneously arising tumors in immunocompetent animals, making
this vector highly attractive for cancer gene therapy research.
Importantly, it has been reported that vectors carrying only marker
genes can induce efficient systemic tumor cell killing (1–4).
In addition, Sindbis virus has been shown to have oncolytic
potential (5,6). The Sindbis virus AR339 strain that
is non-pathogenic to humans was studied by Unno et al
(7) as an oncolytic virus for the
treatment of cervical and ovarian cancer with promising
results.
Sindbis virus is a member of the alphavirus family.
Alphaviruses are enveloped positive strand RNA viruses which
typically have a natural life cycle that is comprised of
invertebrate (hematophagous insect vectors) and vertebrate hosts
(8). The Sindbis virus is the
most cosmopolitan of all known alphaviruses, and several of its
strains have been isolated throughout the Old World. Birds are
primary vertebrate hosts for the Sindbis virus, and infection is
transmitted by mosquitos (8).
Several laboratory strains of this virus exist, and they have not
been reported to cause any human disease (8). The wild-type Sindbis is also usually
considered to be non-pathogenic in humans (8), except some strains in Northern
Europe and South Africa that cause rash, fever and polyarthritis
(8–12). One important characteristic of
alphaviruses is that they have a highly plastic genome which helps
them to maintain their complex life cycle and adapt to
environmental changes that can affect their host species (8). These viruses have the ability for
rapid evolution of the receptor binding domains of their envelope
glycoproteins, which allows modification of their tropism via
continuous passaging (13–16).
Genetic flexibility is a general characteristic of RNA-viruses
(17). In the case of Sindbis
virus, template switching by viral RNA-polymerase during negative
strand RNA synthesis seems to be a major mechanism of evolution
(18).
Several previous studies have suggested that when
one of the most commonly used commercially available helpers for
Sindbis, DH-BB (19), is used for
vector production, propagation-competent viruses are generated at a
relatively high frequency (20–22). Lu and Silver (21) demonstrated that after
co-electroporation of DH-BB-gfp helper and SinRepLacZ replicon
RNAs, the resulting vectors spread locally and produced clusters of
cells expressing both marker genes. Furthermore, when a highly
sensitive serial passage assay was used, in which the detection of
propagation-competent viruses was based on the appearance of a
cytopathic effect (CPE), the frequency of propagation-competent
virus formation with the DH-BB helper was reported to be as high as
5×10−2 (20) or
1×10−3 (22).
In our previous study, we demonstrated that Sindbis
vectors produced with a chimeric split helper system (23) can spread locally in BHK-21, BT4C
and 9L cell cultures without producing a CPE (20). The split helper system was
originally developed to prevent the formation of
propagation-competent recombinant viruses during vector production.
In the split helper system, the helper RNA containing the
structural domain of the Sindbis virus genome is divided into two
molecules, one of which (CSin helper) encodes the capsid and the
other one (CrrvΔ3 helper) the envelope genes. However, a
translational enhancer element located downstream of the AUG start
codon within the capsid sequence is also needed for efficient
expression of the envelope genes. In CrrvΔ3, this enhancer element
is replaced with an analogous enhancer of another alphavirus, Ross
River virus (23). According to
our observations, only the deleted RRV capsid and the Sindbis
envelope proteins encoding part of the split helper RNA (CrrvΔ3
helper) were needed for the generation of the propagating vector.
However, when both components of the split helper RNA were used in
vector production, the resulting titers were much higher than when
the replicon was supported only with the CrrvΔ3 component of the
split helper system (20).
Previous studies have shown that when the Sindbis
virus replicon is co-transfected with a helper RNA containing RNA
packaging signal, these two RNAs will complement each other and
produce infective particles with a bi-partite genome that are able
to produce plaques in cultures of permissive cells. Furthermore,
co-infection with a wild-type Sindbis virus and the virus with the
segmented genome lead to suppression of the wild-type virus
(24). However, all of the helper
RNA constructs we have used in our studies (single DH-BB helper RNA
and the split helper RNAs CSin and CrrvΔ3) lack the RNA packaging
signal but nonetheless unspecific packaging of low amounts of these
RNAs do occur (20,21,23).
The first evidence of the recombination between
Sindbis virus RNAs was published by Geigenmüller-Gnirke et
al (24). Weiss and
Schlesinger (25) sequenced the
junction region of several recombinants that emerged after
co-transfection of different parental Sindbis RNAs. Sequencing of
the crosses revealed that recombination may produce sequence
rearrangements, deletions or insertions derived from parental RNAs
or of cellular origin. According to their studies, sequence
homology-independent recombination events between Sindbis virus
replicon and helper RNAs occur frequently, although only the
recombinants with crosses that preserve the nsP genes as well as
the structural protein genes are viable and can be isolated.
Interferons (IFNs) are cytokines that are induced by
viral or bacterial infections. Type I IFNs (IFN-α/β) are produced
in direct response to viral infection by infected cells. In
surrounding cells, they trigger a signaling cascade leading to
expression of IFN-inducible genes (26). Consequently, the cells develop an
antiviral state, whereby the replication of the virus is inhibited
via several IFN-inducible pathways, e.g. dsRNA-dependent protein
kinase R (PKR), 2′-5′ oligoadenylate synthetases (OAS) leading to
activation of RNase L and myxovirus resistance (Mx) proteins.
Moreover, IFNs modulate the functions of various cell types,
leading to more effective and better directed immune responses
(27). However, viruses have
developed several strategies for inhibition of the host IFN
response. Usually these strategies cannot completely eliminate the
effects of the IFNs, since cells have multiple parallel pathways
for IFN induction and transcriptional initiation of IFN-induced
genes, which in turn encode proteins or enzymes that are able to
suppress viral replication by several unrelated means. This
reflects the crucial importance of the IFN response to the host
immune defense (27).
The aim of this study was to investigate the
features of propagation-competent vectors emerging upon Sindbis
virus vector production and to study whether recombination between
the replicon and helper RNAs could occur, possibly leading to
wild-type reversion of the vector. Furthermore, we tested human
osteosarcoma and rhabdomyosarcoma cell lines as targets for a
Sindbis vector and investigated the role of IFN response as a
limiting factor for vector propagation.
Materials and methods
Cell lines
BHK-21 baby hamster kidney cells (ATCC CCL-10), BT4C
rat glioma cells (28), TE-671
human rhabdomyosarcoma cells (ATCC CCL-136) and three human
osteosarcoma cell lines MG-63 (ATCC CRL-1427), U-2-OS (ATCC HTB-96)
and Saos-2 (HTB-85) were all grown in Dulbecco’s modified Eagle’s
medium (DMEM) with 10% fetal calf serum (FCS) and 50 μg/ml
gentamycin at 37°C and 5% CO2.
Production of Sindbis virus vectors
A TKGFP fusion gene containing Sindbis virus
replicon construct pSin-TKGFP (22) was used in this study for vector
production. The backbone of the pSin-TKGFP construct is pSinRep5
(Invitrogen Life Technologies, Carlsbad, CA, USA). We used two
different helper systems: the single component DH-BB helper plasmid
(Invitrogen Life Technologies) and the split helper system plasmids
DH-BBCSin (CSin helper) and DH-BBCrrvΔ3E1E2 (CrrvΔ3 helper) as
described previously (23). The
first split helper component contains Sindbis virus capsid gene and
the second component is a chimera of an extensively deleted Ross
River virus capsid gene and Sindbis virus envelope encoding
sequences (PE2, 6K and E1).
Linearized plasmids were used as templates for in
vitro RNA synthesis with SP6 RNA polymerase (Amersham
Biosciences China). The reaction was performed in 40 μl volume
containing 5 μl 10X SP6 buffer (Amersham), 2.5 μg template DNA, 5
μl 50 mM DTT (Promega, Madison, WI, USA), 5 μl volume of 5 μM rNTP
mixture (100 mM ATP, CTP, UTP and GTP), 5 μl 10 mM CAP
[m7G(5′)ppp(5′)G] and 1.5 μl RNase inhibitor (all from
Roche). After a 2-h incubation at 37°C, 50 μl 8 M LiCl and 60 μl
sterile H2O were added, and RNA was precipitated o/n at
−20°C. RNA was pelleted and washed with 70% EtOH, air dried and
resuspended in sterile H2O (Aquasteril; Baxter). The RNA
preparations were stored at −70°C before use.
Ten or forty micrograms of each RNA species
(Fig. 1A) was electroporated in
equal amounts to logarithmically growing BHK-21 cells. Supernatants
containing the vectors were collected 24 h later, purified using a
0.2-μm cellulose acetate filter (Whatman GmbH, Germany) and stored
in aliquots at −70°C.
Vector titers were determined essentially as
described previously (22).
Logarithmically growing BHK-21 cells were split onto 12-well plates
(2×105 cells/well) and incubated at 37°C for 4 h
allowing the cells to attach. Cells were transduced in duplicate
with serial dilutions of vector supernatants prepared in
Opti-MEM® I with GlutaMAX™ I medium (Gibco, GB) for 1 h
at 37°C. Then 1.5 ml growth medium was added onto each well and the
incubation was continued for 6 h. The cells were harvested and
fixed with 4% paraformaldehyde, and the proportion of
GFP-expressing cells was determined using flow cytometry
(FACSCalibur, Becton-Dickinson). Titers were calculated based on
the proportion of transgene-positive viable cells, the cell number
at the time of transduction and the dilution factor of the vector
supernatant.
Serial passage assay
Virus preparations were tested for
propagation-competent viruses by a method based on observing the
CPE after serial passaging in BHK-21 cells (Fig. 1B). Transductions were carried out
in triplicates on 12-well plates in a 300-μl volume of Opti-MEM I
with GlutaMAX I medium for 1 h, followed by addition of 700 μl
growth medium. For transductions (1st passage), seven 5-fold serial
dilutions of Sindbis-TKGFP split helper or Sindbis-TKGFP DH-BB
vectors were used. The highest MOIs used in this assay were 0.04
with Sindbis-TKGFP split helper and 0.05 with Sindbis-TKGFP DH-BB
vectors. After 2 days of incubation at 37°C, 500 μl supernatant
from each well was passaged onto fresh BHK-21 cells (2nd passage)
growing on 12-well plates (2×105 cells/well) in a 0.5-ml
volume of growth medium. These cells were incubated for 2 days and
the supernatants were passaged again onto fresh BHK-21 cells (3rd
passage), as described above. The frequency of
propagation-competent virus in each preparation was calculated
based on the virus titer and the last dilution yielding complete
CPE (more than 90% of cells dead based on microscopy) at least in
two of the three parallel wells. To study the kinetics of the
vector spreading, we observed the CPE also on plates from previous
passages and then fixed the cells with 4% paraformaldehyde for
fluorescence microscopy. Clusters of TKGFP-expressing cells were
counted in each well.
Plaque titration assay
For plaque titration, BHK-21 cells were split on
24-well plates (5×104 cells/well) and incubated o/n at
37°C. On the following day, the cells were transduced with serial
dilutions of the vector (6 parallel wells for each dilution) in a
200-μl transduction volume for 1 h followed by overlaying the cells
with 0.4% SeaPlaque agarose in growth medium (0.5 ml/well). Plates
were incubated at 37°C for 48 h. The cells were fixed and stained
o/n at 37°C using solution containing 5% formalin, 1% EtOH and
crystal violet (2 g/l) in PBS. The agarose was then removed by
inverting and knocking the plates on the desk. Plaques were counted
and titers were determined. For comparison, plaque titers were also
determined for a Sindbis-TKGFP DH-BB vector preparation containing
2×107 TU/ml (titer based on the flow cytometry
titration).
Fluorescence microscopy for detection of
localized non-cytopathic propagation
MG-63, Saos-2, U-2-OS, TE-671 and BHK-21 cells were
split on 12-well plates (5×104 cells/well). On the
following day, cells were transduced with Sindbis-TKGFP split
helper vector (MOI 0.7) (medium: Opti-MEM I with GlutaMAX I) in a
0.3-ml transduction volume for 1 h and then 1.5 ml growth medium
was added onto each well. Cells were incubated for 48 h at 37°C
followed by fixation with 4% paraformaldehyde and inspected by
fluorescence microscopy. Clusters of TKGFP-expressing cells
indicating local spreading of the vector were photographed (bright
field and fluorescence images).
PCR and cloning of PCR products for
sequencing of recombination sites
The potential recombination sites were amplified
with PCR using primers described in Table I and Fig. 2. Sindbis-TKGFP DH-BB and split
helper vectors and the Sindbis split helper CPE+
supernatant were used to infect BHK-21 cells followed by overlaying
with agarose (see above). Single plaques were isolated at the 48 h
time-point and used to infect logarithmically growing BHK-21 cells
on 10-cm plates. After a 24-h incubation, total cellular RNA was
extracted with TRIzol according to the manufacturer’s instructions.
RNAs were stored at −70°C until cDNA synthesis (1.5 μg RNA for each
reaction) with 0.25 μg random hexamer primers, 40 units M-MuLV
reverse transcriptase (both from Promega) and 20 units RiboLock
ribonuclease inhibitor (Fermentas). RNA was incubated with random
hexamer primers in a 11-μl volume at 70°C for 5 min, chilled on ice
and mixed with other reagents in total reaction volume of 21 μl,
followed by incubations at 25°C for 10 min, at 37°C for 60 min and
at 70°C for 10 min (termination of the reaction). The cDNAs were
stored at −20°C. The cDNAs were used as templates for PCR reactions
with 1 unit DynaZyme DNA polymerase, 0.5 μl 10 mM dNTP (both from
Finnzymes), 10 pmol of both primers and 2.5 μl template cDNA. PCR
conditions consisted of: 96°C for 30 sec, 60°C (for all primer
pairs) or 63°C (additional optimized reactions for primer pairs 1 +
7 and 9 + 2) for 1 min, and 72°C for 1 min, for 30 cycles. The PCR
products were then fractionated with agarose gel electrophoresis
(0.9% agarose in TAE buffer with EtBr) and each product was
extracted using Qiagen MinElute Gel extraction kit according to the
manufacturer’s instructions. The fragments were then cloned into
pCR-XL-TOPO plasmid using the TOPO® XL PCR Cloning kit
(Invitrogen Life Technologies), according to the manufacturer’s
instructions, transformed into competent DH5α E. coli cells
and plated on LB agar plates with kanamycin (50 μg/ml). After o/n
incubation at 37°C, single colonies were selected for further
analysis. Plasmids were purified using the QIAprep Spin Miniprep
kit (Qiagen) according to the microcentrifuge protocol for plasmid
DNA. The inserts were sequenced using M13 R and M13 F (−20) primers
included in the TOPO XL PCR Cloning kit.
 | Table IPCR primers. |
Table I
PCR primers.
| Number | Orientation | Binding site in
Sindbis virus genome | Sequence |
|---|
| 1 | F | 7097–7114,
nsP4 | 5′-CGT TAT CGC CAG
CAG AGT-3′ |
| 2 | R | 9750–9766, E2 | 5′-ATC ATC GCC ACG
GTA GC-3′ |
| 3 | F | 11018–11036,
E1 | 5′-GCA GTA TGT ATC
CGA CCG C-3′ |
| 4 | R | 1167–1188,
nsP1 | 5′-TGT TCC TGT TAG
TCC TAC CGT T-3′ |
| 5 | F | 684–701, nsP1 | 5′-CGT AAC ATC GGA
CTT TGC-3′ |
| 6 | F | 796–818, nsP1 | 5′-CGA CAC TTT ATC
CAG AAC ACA GA-3′ |
| 7 | R | 8005–8022, C | 5′-TGA CAT CTC CGT
CCT CGT-3′ |
| 8 | F | 7786–7800, C | 5′-CAG CCG TCA GTG
CCC-3′ |
| 9 | F | 8140–8156, C | 5′-AGT TCG CAC AGT
TGC CA-3′ |
Determination of transduction efficiency
and type I IFN response
TE-671, BT4C, U-2-OS, MG-63 and Saos-2 cells were
split onto 6-well plates (1.5×105 cells/well) and
incubated at 37°C o/n. On the following day, the cells were
transduced with Sindbis-TKGFP split helper vector (titer
2.3×106 TU/ml) (MOI 0.4) in Opti-MEM I with GlutaMAX I
medium (or medium only for controls) in a 1-ml transduction volume
for 4 h, and then 2 ml growth medium was added to each well. The
cells were harvested for flow cytometry (FACSCalibur,
Becton-Dickinson) and for western blotting 24, 48 and 72 h after
transduction. Flow cytometry samples were fixed with 4%
paraformaldehyde, and the proportion of GFP-expressing cells was
determined.
For western blotting, cell pellets were stored at
−70°C. Proteins were extracted and MxA western blot analyses were
performed as previously described (29). Briefly, 30 μg of the protein
samples were denatured and run on 12.5% SDS-PAGE gel using a
Laemmli buffer system. As a positive control, we used A549 cells
incubated with medium containing 10 U/ml human recombinant
interferon-α A/D for 48 h before sampling. A549 cells have been
previously shown to express MxA as a response to type I IFNs
(30). An equal amount of protein
from the positive control samples was loaded onto each gel.
Proteins were transferred onto Invitrolon™ PVDF membranes (45-μm
pore size; Invitrogen Life Technologies), and membranes were
incubated in blocking solution o/n at 4°C. Then membranes were
incubated with primary rabbit anti-MxA antibody (31) using a 1:2,000 dilution for 1 h at
RT, washed 3 times and then incubated with secondary peroxidase
(HRP)-conjugated donkey anti-rabbit IgG antibody (Amersham
Biosciences, Little Chalfont, UK) using a 1:200,000 dilution for 1
h at RT. Membranes were washed 4 times before chemiluminescence
detection with the ECL™ Plus Western Blotting Detection System
(Amersham Biosciences), according to the manufacturer’s
instructions. MxA western blotting could not be used for BT4C rat
glioma cells because of the differences between human and rat Mx
proteins. The type I interferon response in these cells was studied
by blocking vector spreading with IFN-α (as described below).
Blocking the vector propagation with
IFN-α
BHK-21 or BT4C cells were split on 24-well plates
(5×104 cells/well). The plates were incubated at 37°C
for 4 h (BHK-21) or o/n (BT4C) to allow the cells to attach. Cells
were transduced with Sindbis-TKGFP Split helper vector in Opti-MEM
I with GlutaMAX I in a 0.3-ml transduction volume for 1 h using
MOIs 0, 0.005, 0.05 and 0.5 for BHK-21 or MOIs 0, 0.003, 0.03 and
0.3 for BT4C cells. Then 300 μl growth medium containing IFN-α
(IFN-α-A/D, human recombinant expressed in E. coli;
Sigma-Aldrich, St. Louis, MO, USA) at different concentrations was
added. Final concentrations in the wells were 1,000, 100, 10, 1 or
0 U/ml IFN-α for BHK-21 cells and 1,000, 100 or 0 U/ml IFN-α for
BT4C cells. The cells were incubated at 37°C for 48 h and fixed
with 4% paraformaldehyde for fluorescence microscopy or harvested
for flow cytometry. Vector spreading was analyzed with fluorescence
microscopy (bright field and fluorescence photographs). Flow
cytometry samples were fixed with 4% paraformaldehyde and analyzed
to determine the proportion of TKGFP transgene-expressing
cells.
Results
Detection of propagation-competent
virus
Serial passage assays for the Sindbis-TKGFP DH-BB
vector revealed a constant formation of propagation-competent
viruses that produced complete CPE (more than 90% of cells appeared
dead by microscopy). The assay was carried out twice and both
analyses revealed that in a large proportion of the wells
displaying CPE during the third passage, the cells no longer
expressed GFP. In the first serial passage assay, GFP expression
was found in only 22% of the wells presenting CPE during the third
passage, whereas GFP was expressed in 33% of the corresponding
wells during the first passage. In the second assay, the respective
proportions of wells with GFP expression were 0% (3rd passage) and
100% (1st passage). These results suggest that in the context of
the propagation-competent vector, the presence of the transgene was
unstable and the variants lacking the relatively large TKGFP
transgene had a positive selection advantage over those with
preserved transgene expression capacity.
Serial passage assays for Sindbis-TKGFP split helper
vector showed only occasional CPE that was considerably milder
(approximately 50% of cells were dead) than that observed with the
Sindbis-TKGFP DH-BB vector. Thus, because this was suggestive of
formation of the propagation-competent virus, the supernatant was
collected from a well presenting with CPE for further studies
(Sindbis split helper CPE+ supernatant). The appearance
of CPE due to the presence of propagation-competent viruses or
virus-like particles in the cell cultures infected with Sindbis
split helper vectors was too rare an event for reliable detection
of the frequency of this phenomenon with the serial passage assay.
In both instances when the assay was performed, one or two wells
out of three were CPE-positive with the second dilution (MOI 0.008
or 0.007) but the wells infected with the 5-fold more concentrated
first dilution remained all CPE-negative. However, local
propagation of TKGFP transgene-carrying vector without associated
CPE (indicated by clusters of viable green fluorescent cells) was
noted in the serial passage assays far more frequently. Sometimes,
the TKGFP-expressing cell clusters were detected also after two
passages but in most cases, the clusters disappeared when the
supernatant was passaged twice (Table II).
 | Table IILoss of TKGFP transgene expression
during continued passaging of Sindbis-TKGFP vectors produced with
the split helper RNAs. |
Table II
Loss of TKGFP transgene expression
during continued passaging of Sindbis-TKGFP vectors produced with
the split helper RNAs.
| Serial passage
assay no. | Proportion of wells
containing clusters of transgene-expressing cells | Proportion of wells
containing only single transgene-expressing cells | Proportion of wells
negative for transgene-expression |
|---|
|
|
|
|---|
| 1st passage | 3rd passage | 1st passage | 3rd passage | 1st passage | 3rd passage |
|---|
| 1 | 9/21 (42.9%) | 3/21 (14.3%) | 0/21 (0%) | 1/21 (4.8%) | 12/21 (57.1%) | 17/21 (81.0%) |
| 2 | 7/21 (33.3%) | 2/21 (9.5%) | 5/21 (23.8%) | 0/21 (0%) | 9/21 (42.9%) | 19/21 (90.5%) |
Flow cytometry and plaque titers
Flow cytometry titer of the Sindbis-TKGFP DH-BB
vector, that was produced according to the standard procedure, was
2×107 TU/ml and the plaque titer was 4×103
pfu/ml. The supernatant collected from cells infected with the
Sindbis split helper CPE+ supernatant (see above) was
also titrated by using both flow cytometry and the plaque titration
assay. Flow cytometry titer could not be determined as no GFP
expression was detected in the virus-infected cells. However, the
plaque titration assay from the supernatant revealed titers as high
as 5.8×106 pfu/ml. Most plaques were pinpoint-sized but
also large plaques were present; their sizes close to the average
size of the plaques observed with the Sindbis-TKGFP DH-BB vector.
Large plaques produced a titer of 4.2×105 pfu/ml. These
results indicate that propagation-competent vectors that no longer
carried the TKGFP transgene had a significant selection advantage
over those containing the functional TKGFP expression casette.
PCR and sequencing of the recombination
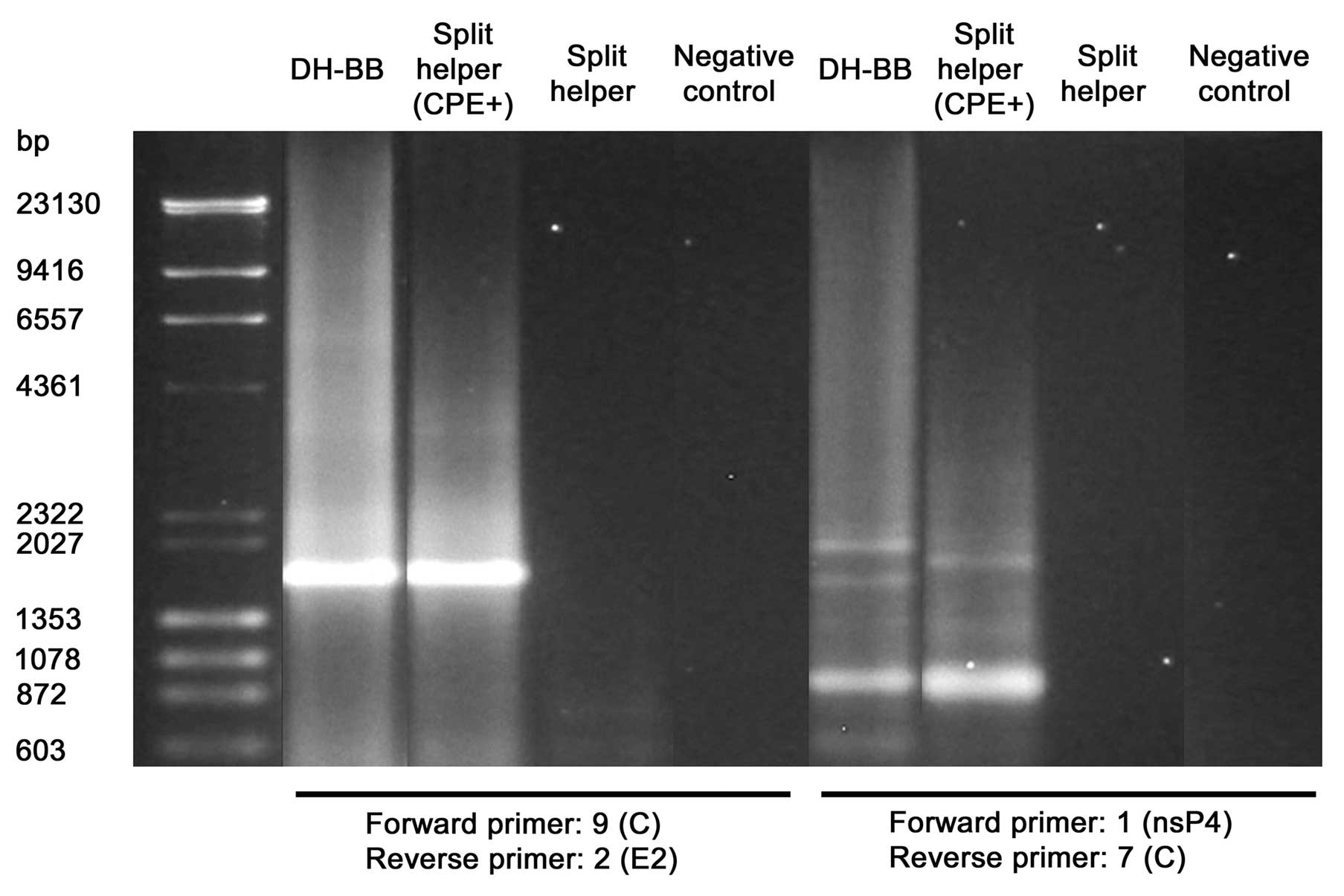
sites
Using Sindbis DH-BB vectors and Sindbis split helper
CPE+ supernatant, the selection of propagation-competent
vectors which were no longer capable of transgene expression during
serial passaging suggested that recombination could play a role in
acquisition of propagation competence. To detect possible
recombinants, cells were infected with the Sindbis split helper
CPE+ supernatant and vectors produced with DH-BB or
split helper RNAs and overlaid with agarose. All vectors gave rise
to plaques, although with standard produced Sindbis split helper
only pinpoint-sized plaques were present (Fig. 3) Single plaques were isolated and
used to infect fresh cells for RNA extraction and rtPCR. A series
of different PCR primers annealing to Sindbis virus genome were
designed (Table I and Fig. 2). In each primer pair, forward and
reverse primers were located in distinct vector RNAs (replicon and
helper RNAs or in the two components of the split helper RNA) in
such a way that possible recombination would lead to amplification
of the recombination site. PCR amplification with any single primer
pair gave rise to multiple PCR products of variable sizes. After
agarose gel electrophoresis, the different PCR products were
extracted from the gel and cloned for subsequent sequencing of the
recombination sites. The PCR products that, based on their size,
were suggestive of wild-type reversion of the virus due to
recombination of replicon and helper RNAs or both components of the
split helper, were chosen to be sequenced. We could verify
restoration of the wild-type junction between the structural and
non-structural open reading frames in both the Sindbis DH-BB and
Sindbis split helper CPE+ samples. Furthermore,
restoration of the complete wild-type structural open reading frame
was verified in the case of Sindbis split helper CPE+.
The results are summarized in Figs.
4 and 5 and Table III. In addition, some of the
other PCR products were sequenced. These represented either
unspecific binding of the primers or replicon-helper recombinants
with some type of deletion extending over the junction area between
the non-structural and structural open reading frames. The
alternative deletions that were found represented nucleotides 7355
to 9626, 7292 to 9735, 830 to 8442, 817 to 9553 and 869 to 9567 in
the wild-type Sindbis virus genome. Notably, some of the Sindbis
split helper CPE+ plaques did not contain sequences
indicating wild-type reversion of the virus. Moreover, some of the
plaques arising from infection by the standard produced Sindbis
split helper vector completely failed to give rise to PCR products
with the used primers.
 | Table IIIWild-type reversion according to the
sequence of the PCR products. |
Table III
Wild-type reversion according to the
sequence of the PCR products.
| Primers | |
|---|
|
| |
|---|
| Vector | Forward | Reverse | Recombination |
|---|
| Sindbis DH-BB | 1 (nsP1) | 7 (C) | Restoration of
wild-type junction between the non-structural and structural open
reading frames due to recombination of replicon and DH-BB
helper |
| Sindbis split
helper CPE+ | 1 (nsP1) | 7 (C) | Restoration of
wild-type junction between the non-structural and structural open
reading frames due to recombination of replicon and CSin
helper |
| Sindbis split
helper CPE+ | 9 (C) | 2 (E2) | Restoration of
wild-type structural open reading frame sequence due to
recombination of split helper components |
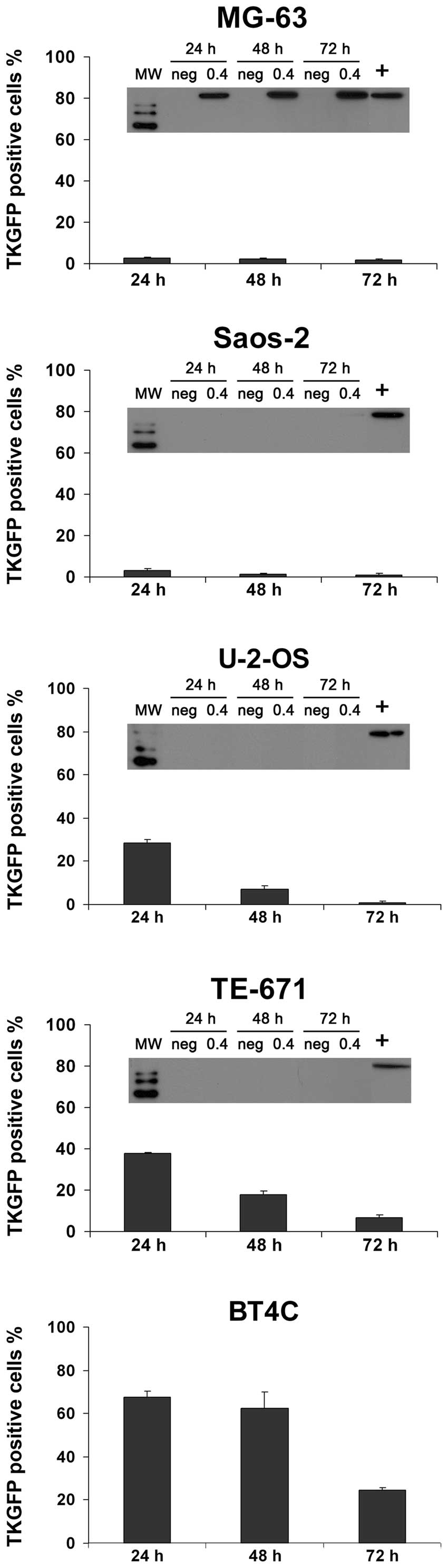
Local non-cytopathic spreading in human
osteosarcoma and rhabdomyosarcoma cells
Transduction of cells with the Sindbis-TKGFP split
helper vector revealed that 2 out of the 5 cell lines studied
(MG-63 and Saos-2) were relatively resistant to both transduction
and propagation of the Sindbis virus vector (Fig. 6). Some localized propagation was
noted in the human osteosarcoma cell line U-2-OS, whereas in TE-671
human rhabdomyosarcoma cells, local spreading of the vector was
frequently detected. In the BHK-21 cells, the Sindbis-TKGFP split
helper vector was able to spread throughout the cell culture
(Fig. 6, bottom panel).
Transduction efficiency
In all of the studied cell lines, the best
transduction efficiency was achieved at 24 h after transduction
with the Sindbis-TKGFP split helper vector, followed by a decline
in the proportion of transgene-expressing cells (Fig. 7). We previously demonstrated that
the Sindbis-TKGFP split helper vector spreads locally in BT4C rat
glioma cells (20). The average
size of the propagation foci in the BT4C cells was comparable to
that in the TE-671 cells, and these two cell lines also
demonstrated the highest numbers of transgene-positive cells at the
24 h time-point of all cell lines tested. The gene expression
declined slower in these two cell lines that were displaying
frequent local spreading of the vector, compared to the other cell
lines.
Effect of type I IFN response to vector
spreading
Induction of type I IFN response after transduction
with the Sindbis-TKGFP split helper vector was studied with MxA
expression analysis by western blotting (Fig. 7). IFN-α/β-induced MxA protein
accumulation is commonly used as a biomarker for the induction of a
type I IFN response (31). Only
one of the cell lines studied, the human osteosarcoma cell line
MG-63, exhibited a strong IFN response against this vector
(Fig. 7) and no vector spreading
was demonstrated in these cells (Fig.
6). In the other cell lines tested (Saos-2, U-2-OS and TE-671)
no evidence was detected for any type I IFN response, according to
the results of the MxA western blot analyses (Fig. 7).
To confirm the negative impact of a type I IFN
response on vector spreading, we transduced BHK-21 and BT4C cells
with Sindbis-TKGFP split helper vectors for 1 h and added
IFN-α-containing growth medium to the cells. The presence of IFN-α
in the growth medium effectively inhibited vector spreading in the
BHK-21 cells, which themselves produce little or no IFN (32) (Fig.
8). An inhibitory effect of IFN-α on vector spreading was also
detected in the BT4C cells (Fig.
9). This was demonstrated with both fluorescence microscopy
(Figs. 8A and 9A) and flow cytometry (Figs. 8B and 9B).
Discussion
Propagation by two different
mechanisms
According to the present study, it seems that
Sindbis vectors may acquire propagation competence by two different
mechanisms: (i) recombination leading to wild-type reversion and
(ii) co-infection with vector shells containing unspecifically
packaged helper RNA leading to local non-cytopathic spreading.
Serial passage assay based on observation of CPE is
a sensitive method for specific detection of propagation-competent
viruses generated upon Sindbis vector production due to
recombination and wild-type reversion of viral RNA. However, in the
case of the Sindbis split helper vector, clusters of marker
gene-expressing cells were frequently found without associated CPE
suggesting that this type of assay was not sufficiently sensitive
for detection of local non-cytopathic spreading.
Recombination leading to wild-type
reversion
The recombination sites were characterized using a
PCR-based method, followed by sequencing of the PCR products. The
results showed that one primer pair analysis of virus isolated from
a single plaque with any of the primer pairs used often gives rise
to multiple PCR products that represent different recombination
sites. This may be related to the high frequency of recombination
events, which enables rapid evolution of propagation-competent
vectors. Recombinations were found to lead to restored wild-type
sequences. On the other hand, many of the detected recombination
sites disrupted both the structural and non-structural open reading
frames. These recombinants may play a role as defective interfering
RNAs, i.e. helper dependent deletion mutants of the parental virus
genome which are capable of interfering with the virus replication
(33).
According to the present study, TK-GFP transgene
expression was often lost upon passaging of the
propagation-competent vectors. Propagation-competent viruses that
did not carry the functional TK-GFP transgene expression cassette
had a selection advantage over those expressing the transgene. This
observation is in line with the results of Weiss and Schlesinger
(25) with the recombinant
genomes that emerged due to recombination events between Sindbis
replicon and helper RNAs. The recombinant genomes larger than the
wild-type Sindbis genome were unstable and finally evolved to the
same size as the wild-type genome. Wild-type Sindbis virus has a
genomic RNA of 11.7 kb (8). In
the case that recombinations occur leading to the formation of a
propagation-competent virus genome from the replicon and DH-BB
helper or one (CrrvΔ3) or both components of the split helper RNA,
the resulting recombinant virus genome can be markedly larger than
the wild-type Sindbis genome. The Sindbis-TKGFP replicon RNA (10.28
kb) and the DH-BB helper RNA (4.8 kb) have together a size of ~15.1
kb. The total size of Sindbis-TKGFP replicon RNA together with both
split helper components CSin (2.05 kb) and CrrvΔ3 (4.75 kb) is
~17.1 kb. In such cases, a strong selection pressure towards a
smaller wild-type virus-like genome size exists.
When Sindbis vectors are produced with the split
helper packaging system, a two-step process is required for
generation of the full-length Sindbis genome (23). First, recombination between the
replicon and one of the split helper RNA components takes place,
followed by recombination with the second component. In case that
replicon-CSin helper recombinants are formed, the resulting
recombinant genome is dependent on simultaneous transmission of the
CrrvΔ3 helper RNA that is non-specifically packaged in the vector
particles. This was previously demonstrated when only the replicon
and CSin helper were used for vector production; infective vector
particles were not formed when the CrrvΔ3 helper was missing
(20). However, when the replicon
was supported with the CrrvΔ3 helper without the presence of the
CSin helper, low titers of infective and locally transmissive
vectors were generated (20) and
therefore we propose that replicon-CrrvΔ3 recombinants are not
dependent on co-transmission of the CSin helper RNA. Still, CSin
helper RNA that codes for Sindbis capsid protein could promote more
potent formation of vector particles and enhanced spreading.
Local non-cytopathic spreading
Local non-cytopathic spreading may be related to
recombinants that are strongly attenuated and almost non-viable due
to deletions or some other type of fatal changes in the
non-structural or structural open reading frames. Alternatively, it
could be attributable to non-specific co-packaging of helper RNAs
into replicon-containing vector particles that is too inefficient
to maintain a wild-type virus-like transmission cycle or
co-infection with replicon and helper RNAs or replicon-CSin
recombinants and CrrvΔ helper packaged into separate vector
particles. Our data suggest that recombination is not necessarily
required for vector propagation and plaque formation, as PCR
analysis of some of the Sindbis split helper plaques detected no
recombination sites.
If the two components of a bi-partite genome are
packaged into separate particles, high MOIs would be essential for
continued survival (24). The
observation that the spreading foci are often lost during the
continuous passage supports the hypothesis that the local spreading
of Sindbis-TKGFP split helper vectors could be mainly based on
co-infection with replicon and helper RNAs or replicon-CSin
recombinant and CrrvΔ3 helper preferentially packaged in separate
vector particles. A decrease in MOI after passage could be fatal to
co-infection-based vector spreading.
Transduction efficiency and local
spreading
BT4C, TE-671 and U-2-OS cells showed the highest
proportions of TKGFP transgene-expressing cells of the 5 cell lines
studied (Fig. 7). The local
spreading of Sindbis virus vectors produced with the split helper
system was detected in these 3 cell lines, while in the other 2
cell lines tested, we did not detect any spreading foci and the
gene transfer efficiencies were very low. The capacity of the
Sindbis-TKGFP split helper vector to spread locally in the target
cells appears to have a major impact on its ability to elicit an
effective gene transfer. The amount of primary positive cells after
transduction as well as subsequent vector spreading are both
affected by the repertoire of receptors expressed on the cell
surface. This usually differs between distinct cell types. However,
also the intracellular environment and host antiviral responses may
have a strong influence on the vector performance.
Utilization of propagation-competent
Sindbis vectors
The propagation-competent viruses that are present
in Sindbis DH-BB vector preparations could potentially function as
oncolytic vectors, selectively propagating in malignant cells and
leading to specific tumor cell death. In general, when
propagation-competent virus vectors are used for gene transfer,
vector propagation in tumor cells can eventually lead to widespread
expression of the transgene in tumors despite the low initial
transduction efficiency (34),
for example, after systemic delivery of relatively low doses of
vector compared to the number of cells in the tumor. Sindbis virus
vectors capable of tumor selective propagation, especially vectors
carrying transgenes that induce or enhance tumor-specific immune
responses [for example IL-12, that was found to enhance antitumor
effect of Sindbis vectors produced with the DH-BB helper (3)], could be promising agents for the
treatment of malignant tumors. However, due to the labile nature of
RNA virus genomes, propagative Sindbis vectors often evolve towards
the wild-type, expelling such transgenes which do not offer a
selection advantage compared to the wild-type genome. Clearly, in
the case of propagation-competent oncolytic alphavirus vectors,
safety issues should be carefully evaluated due to the unstable
nature of the virus genome, typical to RNA viruses in general.
Nevertheless, attenuated propagation-competent RNA viruses have
been widely used in humans throughout the world for vaccination
purposes. Almost complete eradication of polio using oral
poliovirus vaccine is a landmark of the successful use of
attenuated propagation-competent vaccine strains (35). However, live attenuated poliovirus
vaccine strains are capable of effective uncontrolled spreading to
unvaccinated subjects as well as capable of undergoing mutations
leading to restored neurovirulence (35).
In the production of propagation-incompetent Sindbis
virus vectors, it appears unlikely that splitting the helper RNA
into several parts would lead to complete elimination of the
contaminating propagation-competent viruses, due to the ease of
recombination between Sindbis viral RNAs. It has been demonstrated
that transfection of two non-overlapping and non-replicative RNA
precursors representing the two halves of the Sindbis virus genome,
one including the non-structural and the other the structural open
reading frame, undergo recombination leading to the formation of
full-length viral genome and production of infectious viruses
(36). At present, one successful
strategy to produce transmission incompetent alphavirus vectors is
to modify the vector envelope in a way that vectors need to be
pretreated before use to restore their infectivity (37). Additionally, to prevent formation
of propagation-competent Sindbis virus during vector production, a
similar approach as used for the construction of the SFV split
helper RNA could be utilized (38). This involves modification of the
capsid gene to abolish its self-cleaving activity. Thereby,
recombination events between the replicon and split helper
components cannot directly lead to wild-type reversion of the
virus. However, our observation that propagation-competent vectors
are formed also when only CrrvΔ3 RNA but not the Csin component of
the split helper RNA is used for packaging of the replicon suggests
that this modification may not totally abolish formation of
propagation-competent virus-like particles. Still, vectors capable
of subtle propagation due to co-infection with helper
RNA-containing vector particles in the absence of wild-type
reversion could serve as a tool for enhanced therapeutic gene
transfer.
Suppression of vector propagation by type
I IFN response
The 67-kDa laminin receptor is used by Sindbis for
virus entry. This receptor is highly conserved among different
species including invertebrates and vertebrates and is the most
important receptor for the Sindbis virus expressed in mammalian
cells (39). The 67-kDa laminin
receptor is frequently overexpressed in cancer cells, especially in
metastatic tumors (40–42), and the tumor-targeting properties
of Sindbis virus vectors have been suggested to result from
utilization of this receptor (2,3,43).
However, Unno et al (7)
studied Sindbis AR339 strain as an oncolytic virus and found that
normal keratinocytes also expressed high levels of the 67-kDa
laminin receptor but were resistant to AR339 strain infection and
subsequent induction of apoptosis whereas human ovarian and
cervical cancer cells were susceptible both to virus propagation
and to infection-induced apoptosis. Therefore, other mechanisms in
addition to overexpression of the 67-kDa laminin receptor are
probably involved in the tumor selectivity of Sindbis virus. One
possible mechanism of tumor selectivity is the sensitivity of
Sindbis virus to type I IFNs. Tumor cells frequently have defective
IFN pathways, because in addition to antiviral effects, the
IFN-mediated signaling cascade can lead to growth inhibitory and
apoptotic signals (44).
Additionally, it has been shown that dependence on defects in IFN
pathways in tumor cells can mediate tumor selectivity of an
oncolytic virus, for example in the case of oncolytic vesicular
stomatitis virus (45).
We used the IFN-induced protein MxA as a biomarker
for the type I IFN response. Mx proteins are highly conserved large
GTPases that have been found in all vertebrate species studied to
date (27). According to our
results, Sindbis virus vectors produced with the split helper
system could not induce a detectable type I IFN response in TE-671
and U-2-OS cells, which were also the cell lines most permissive
for transduction and local spreading of the vector. In contrast,
MG-63 cells are known to respond with a vigorous IFN production to
a variety of stimuli (46). In
these cells, the few TKGFP-expressing cells detected with
fluorescence microscopy usually appeared to be non-viable, and a
strong MxA response was detected after transduction with the
Sindbis virus vector. However, in Saos-2 cell cultures, the number
of transgene-expressing cells was very low at all time-points, but
no MxA protein accumulation was detected. This supports the
hypothesis that vector spreading is affected by multiple
independent factors in intracellular environment as well as the
type of receptors expressed in the target cells. Efficient local
spreading of the Sindbis-TKGFP split helper vector was demonstrated
also in BT4C rat glioma cells. However, the human MxA antibody
could not be used for detection of type I IFN response in rat cells
because of the differences between human and rat Mx proteins.
Therefore, we verified the negative impact of type I IFN response
to vector propagation in BT4C cells by adding IFN-α-containing
growth medium to the transduced cells.
In this study, we demonstrated that extensive
spreading of the Sindbis-TKGFP split helper vectors in BHK-21 cells
deficient of IFN synthesis (32)
were suppressed with administration of IFN-α to the cells. Double
stranded RNA produced during viral replication is a potent inducer
of type I IFNs in infected cells (27) and it has been proposed that the
wild-type Sindbis virus suppresses host type I IFN responses by
shutting down both host cell transcription and translation
(47). In addition, one of the
Sindbis non-structural proteins, nsP2, plays a role in the
suppression of IFN production (48). These strategies to circumvent the
negative impacts of IFNs help Sindbis virus to hide from the host
antiviral defense mechanisms. However, it has been demonstrated
that administration of IFN-α/β protects adult mice from fatal
Sindbis viral infection (49).
Therefore, the sensitivity for IFN could be a potentially important
mechanism for controlling the vector propagation.
In conclusion, Sindbis vector preparations produced
with the DH-BB helper RNA contain full-length Sindbis genomic RNA
comprising both non-structural and structural protein encoding
domains. The formation of propagation-competent virus genome via
recombination events was markedly reduced but not completely
prevented by using split helper RNA, in which the structural genes
are divided into two separate helper RNAs.
The non-pathogenic Sindbis AR339 strain (7) and Sindbis vectors produced using a
method allowing frequent formation of propagation-competent virus
were shown to effectively target and kill tumor cells after
systemic delivery in various animal models (3). Several studies indicate that
propagation of Sindbis virus is sensitive to type I IFNs. In our
study, we found that spreading of Sindbis split helper vectors was
effectively suppressed by a type I IFN response. Previous research
has shown that type I IFNs have a protective effect against Sindbis
virus infection in vivo (49). Therefore, propagation-competent
Sindbis vectors or oncolytic Sindbis viruses may be effectively
controlled by administration of type I IFNs to prevent potential
adverse effects.
Acknowledgements
This study was financially supported by the Finnish
Medical Foundation and the North-Savo Regional Fund of the Finnish
Cultural Foundation (grants to A.H.). We want to thank Dr Outi
Rautsi for her skillful technical assistance; Kati Pulkkinen and Dr
Anne Uimari for the practical advice concerning PCR; Docent Tero
Ahola for the valuable comments; and Professor Ilkka Julkunen
(Department of Viral Diseases and Immunology, National Public
Health Institute, Finland) for the MxA antibody used in the western
blot analyses.
References
|
1
|
Hurtado A, Tseng JC and Meruelo D: Gene
therapy that safely targets and kills tumor cells throughout the
body. Rejuvenation Res. 9:36–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tseng JC, Hurtado A, Yee H, Levin B,
Boivin C, Benet M, Blank SV, Pellicer A and Meruelo D: Using
sindbis viral vectors for specific detection and suppression of
advanced ovarian cancer in animal models. Cancer Res. 64:6684–6692.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tseng JC, Levin B, Hurtado A, Yee H, Perez
de Castro I, Jimenez M, Shamamian P, Jin R, Novick RP, Pellicer A
and Meruelo D: Systemic tumor targeting and killing by Sindbis
viral vectors. Nat Biotechnol. 22:70–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang J, Frolov I and Russell SJ: Gene
therapy for malignant glioma using Sindbis vectors expressing a
fusogenic membrane glycoprotein. J Gene Med. 6:1082–1091. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mettler NE, Clarke DH and Casals J: Virus
inoculation in mice bearing Ehrlich ascitic tumors: antigen
production and tumor regression. Infect Immun. 37:23–27.
1982.PubMed/NCBI
|
|
6
|
Wollmann G, Tattersall P and van den Pol
AN: Targeting human glioblastoma cells: comparison of nine viruses
with oncolytic potential. J Virol. 79:6005–6022. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Unno Y, Shino Y, Kondo F, Igarashi N, Wang
G, Shimura R, Yamaguchi T, Asano T, Saisho H, Sekiya S and
Shirasawa H: Oncolytic viral therapy for cervical and ovarian
cancer cells by Sindbis virus AR339 strain. Clin Cancer Res.
11:4553–4560. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strauss JH and Strauss EG: The
alphaviruses: gene expression, replication, and evolution.
Microbiol Rev. 58:491–562. 1994.PubMed/NCBI
|
|
9
|
Kurkela S, Manni T, Myllynen J, Vaheri A
and Vapalahti O: Clinical and laboratory manifestations of Sindbis
virus infection: prospective study, Finland, 2002–2003. J Infect
Dis. 191:1820–1829. 2005.PubMed/NCBI
|
|
10
|
Kurkela S, Manni T, Vaheri A and Vapalahti
O: Causative agent of Pogosta disease isolated from blood and skin
lesions. Emerg Infect Dis. 10:889–894. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Laine M, Luukkainen R and Toivanen A:
Sindbis viruses and other alphaviruses as cause of human arthritic
disease. J Intern Med. 256:457–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niklasson B, Espmark A, LeDuc JW, Gargan
TP, Ennis WA, Tesh RB and Main AJ Jr: Association of a Sindbis-like
virus with Ockelbo disease in Sweden. Am J Trop Med Hyg.
33:1212–1217. 1984.PubMed/NCBI
|
|
13
|
Greene IP, Wang E, Deardorff ER, Milleron
R, Domingo E and Weaver SC: Effect of alternating passage on
adaptation of sindbis virus to vertebrate and invertebrate cells. J
Virol. 79:14253–14260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kerr PJ, Weir RC and Dalgarno L: Ross
River virus variants selected during passage in chick embryo
fibroblasts: serological, genetic, and biological changes.
Virology. 193:446–449. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klimstra WB, Ryman KD and Johnston RE:
Adaptation of Sindbis virus to BHK cells selects for use of heparan
sulfate as an attachment receptor. J Virol. 72:7357–7366.
1998.PubMed/NCBI
|
|
16
|
Smit JM, Waarts BL, Kimata K, Klimstra WB,
Bittman R and Wilschut J: Adaptation of alphaviruses to heparan
sulfate: interaction of Sindbis and Semliki forest viruses with
liposomes containing lipid-conjugated heparin. J Virol.
76:10128–10137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Domingo E and Holland JJ: RNA virus
mutations and fitness for survival. Annu Rev Microbiol. 51:151–178.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hajjou M, Hill KR, Subramaniam SV, Hu JY
and Raju R: Nonhomologous RNA-RNA recombination events at the 3′
nontranslated region of the Sindbis virus genome: hot spots and
utilization of nonviral sequences. J Virol. 70:5153–5164. 1996.
|
|
19
|
Bredenbeek PJ, Frolov I, Rice CM and
Schlesinger S: Sindbis virus expression vectors: packaging of RNA
replicons by using defective helper RNAs. J Virol. 67:6439–6446.
1993.PubMed/NCBI
|
|
20
|
Ketola A, Schlesinger S and Wahlfors J:
Properties of Sindbis virus vectors produced with a chimeric split
helper system. Int J Mol Med. 15:999–1003. 2005.PubMed/NCBI
|
|
21
|
Lu X and Silver J: Transmission of
replication-defective Sindbis helper vectors encoding capsid and
envelope proteins. J Virol Methods. 91:59–65. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wahlfors JJ, Zullo SA, Loimas S, Nelson DM
and Morgan RA: Evaluation of recombinant alphaviruses as vectors in
gene therapy. Gene Ther. 7:472–480. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frolov I, Frolova E and Schlesinger S:
Sindbis virus replicons and Sindbis virus: assembly of chimeras and
of particles deficient in virus RNA. J Virol 1997. 71:2819–2829.
1997.PubMed/NCBI
|
|
24
|
Geigenmüller-Gnirke U, Weiss B, Wright R
and Schlesinger S: Complementation between Sindbis viral RNAs
produces infectious particles with a bipartite genome. Proc Natl
Acad Sci USA. 88:3253–3257. 1991.PubMed/NCBI
|
|
25
|
Weiss BG and Schlesinger S: Recombination
between Sindbis virus RNAs. J Virol. 65:4017–4025. 1991.PubMed/NCBI
|
|
26
|
Katze MG, He Y and Gale M Jr: Viruses and
interferon: a fight for supremacy. Nat Rev Immunol. 2:675–687.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goodbourn S, Didcock L and Randall RE:
Interferons: cell signalling, immune modulation, antiviral response
and virus countermeasures. J Gen Virol. 81:2341–2364.
2000.PubMed/NCBI
|
|
28
|
Laerum OD, Rajewsky MF, Schachner M, et
al: Phenotypic properties of neoplastic cell lines developed from
fetal rat brain cells in culture after exposure to ethylnitrosourea
in vivo. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol.
89:273–295. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rautsi O, Lehmusvaara S, Salonen T,
Häkkinen K, Sillanpää M, Hakkarainen T, Heikkinen S, Vähäkangas E,
Ylä-Herttuala S, Hinkkanen A, Julkunen I, Wahlfors J and Pellinen
R: Type I interferon response against viral and non-viral gene
transfer in human tumor and primary cell lines. J Gene Med.
9:122–135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ronni T, Matikainen S, Sareneva T, Melén
K, Pirhonen J, Keskinen P and Julkunen I: Regulation of
IFN-alpha/beta, MxA, 2′,5′-oligoadenylate synthetase, and HLA gene
expression in influenza A-infected human lung epithelial cells. J
Immunol. 158:2363–2374. 1997.
|
|
31
|
Ronni T, Melén K, Malygin A and Julkunen
I: Control of IFN-inducible MxA gene expression in human cells. J
Immunol. 150:1715–1726. 1993.PubMed/NCBI
|
|
32
|
Schlesinger S and Dubensky TW: Alphavirus
vectors for gene expression and vaccines. Curr Opin Biotechnol.
10:434–439. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Monroe SS and Schlesinger S: Common and
distinct regions of defective-interfering RNAs of Sindbis virus. J
Virol. 49:865–872. 1984.PubMed/NCBI
|
|
34
|
Galanis E, Vile R and Russell SJ: Delivery
systems intended for in vivo gene therapy of cancer: targeting and
replication competent viral vectors. Crit Rev Oncol Hematol.
38:177–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kew OM, Wright PF, Agol VI, Delpeyroux F,
Shimizu H, Nathanson N and Pallansch MA: Circulating
vaccine-derived polioviruses: current state of knowledge. Bull
World Health Organ. 82:16–23. 2004.PubMed/NCBI
|
|
36
|
Raju R, Subramaniam SV and Hajjou M:
Genesis of Sindbis virus by in vivo recombination of nonreplicative
RNA precursors. J Virol. 69:7391–7401. 1995.PubMed/NCBI
|
|
37
|
Berglund P, Sjöberg M, Garoff H, Atkins
GJ, Sheahan BJ and Liljeström P: Semliki Forest virus expression
system: production of conditionally infectious recombinant
particles. Biotechnology. 11:916–920. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Smerdou C and Liljeström P: Two-helper RNA
system for production of recombinant Semliki forest virus
particles. J Virol. 73:1092–1098. 1999.PubMed/NCBI
|
|
39
|
Wang KS, Kuhn RJ, Strauss EG, Ou S and
Strauss JH: High-affinity laminin receptor is a receptor for
Sindbis virus in mammalian cells. J Virol. 66:4992–5001.
1992.PubMed/NCBI
|
|
40
|
Castronovo V: Laminin receptors and
laminin-binding proteins during tumor invasion and metastasis.
Invasion Metastasis. 13:1–30. 1993.PubMed/NCBI
|
|
41
|
Martignone S, Ménard S, Bufalino R,
Cascinelli N, Pellegrini R, Tagliabue E, Andreola S, Rilke F and
Colnaghi MI: Prognostic significance of the 67-kilodalton laminin
receptor expression in human breast carcinomas. J Natl Cancer Inst.
85:398–402. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sobel ME: Differential expression of the
67 kDa laminin receptor in cancer. Semin Cancer Biol. 4:311–317.
1993.PubMed/NCBI
|
|
43
|
Tseng JC, Zanzonico PB, Levin B, Finn R,
Larson SM and Meruelo D: Tumor-specific in vivo transfection with
HSV-1 thymidine kinase gene using a Sindbis viral vector as a basis
for prodrug ganciclovir activation and PET. J Nucl Med.
47:1136–1143. 2006.PubMed/NCBI
|
|
44
|
Everts B and van der Poel HG:
Replication-selective oncolytic viruses in the treatment of cancer.
Cancer Gene Ther. 12:141–161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stojdl DF, Lichty B, Knowles S, Marius R,
Atkins H, Sonenberg N and Bell JC: Exploiting tumor-specific
defects in the interferon pathway with a previously unknown
oncolytic virus. Nat Med. 6:821–825. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Billiau A, Edy VG, Heremans H, Van Damme
J, Desmyter J, Georgiades JA and De Somer P: Human interferon: mass
production in a newly established cell line, MG-63. Antimicrob
Agents Chemother. 12:11–15. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gorchakov R, Frolova E and Frolov I:
Inhibition of transcription and translation in Sindbis
virus-infected cells. J Virol. 79:9397–9409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Frolova EI, Fayzulin RZ, Cook SH, Griffin
DE, Rice CM and Frolov I: Roles of nonstructural protein nsP2 and
Alpha/Beta interferons in determining the outcome of Sindbis virus
infection. J Virol. 76:11254–11264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ryman KD, Klimstra WB, Nguyen KB, Biron CA
and Johnston RE: Alpha/beta interferon protects adult mice from
fatal Sindbis virus infection and is an important determinant of
cell and tissue tropism. J Virol. 74:3366–3378. 2000. View Article : Google Scholar : PubMed/NCBI
|