Introduction
Prostate cancer is the leading cause of
cancer-related mortality among males in economically developed
countries (1); indeed, in the
United States, it is only second to lung cancer as the most
frequently diagnosed type of cancer in males (2). Prostate cancer is considered
malignant as it is a mass of cells that can invade other parts of
the body. Currently, no therapies are curative after the cancer
invades beyond the gland, metastasizes to the bone and lymph nodes,
and becomes androgen-refratory (3). Most of the androgen-dependent stages
of prostate cancer respond well to androgen ablation therapy
(4). However, during hormonal
therapy, androgen-independent tumor cells eventually emerge,
leading to clinical relapse (5).
No effective therapy is available for such cases, and although
hormonal therapy is commonly used alone or in combination with
other therapies (6), it is
ultimately unsuccessful. A number of cancer types respond to
chemotherapy at the initiation of treatment; however, the ability
of cancer cells to become resistant to chemotherapeutic drugs
remains a significant impediment to successful chemotherapy
(7). Therefore, identifying novel
anticancer agents and strategies is important.
In general, natural or synthetic chemical agents are
employed in cancer chemoprevention to reverse, suppress or prevent
cancer progression (8). Flavonoid
compounds include a number of chemical subgroups, such as
flavonols, procyanidins or anthocyanidins, a broadly distributed
class of plant pigments (9).
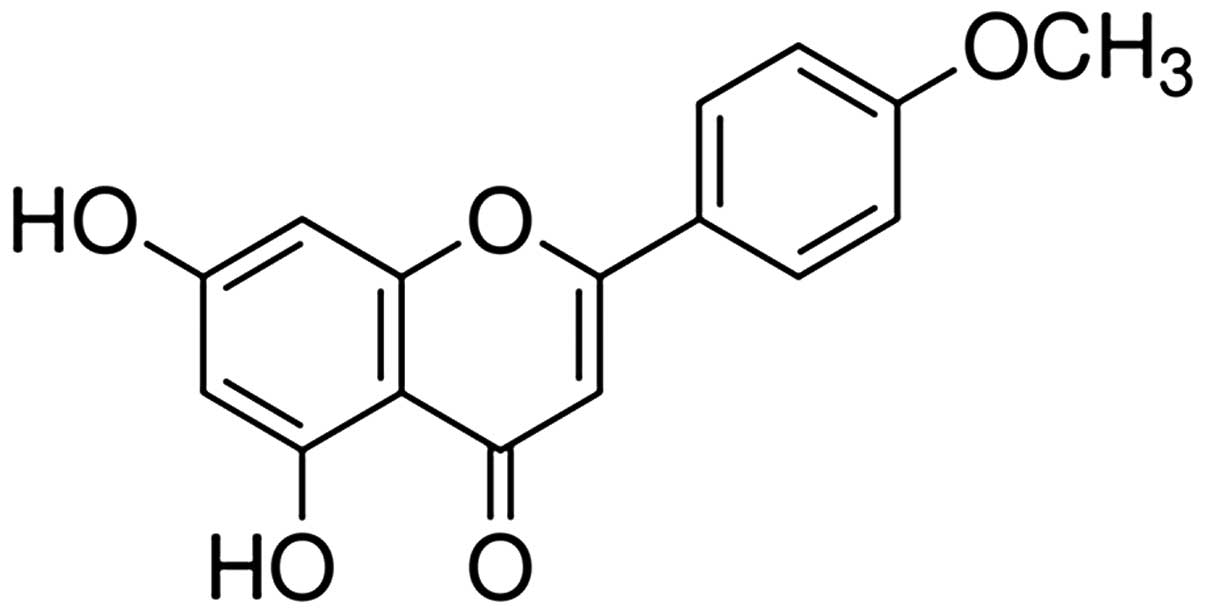
Acacetin (5,7-dihydroxy-4′-methoxyflavone) (Fig. 1) exerts antimutagenic (10), antiplasmodial (11,12), antiperoxidant (13), anti-inflammatory (14) and anticancer effects by
suppressing the invasion and migration of human cancer cells
(15,16). Acacetin has also been shown to
exert an antiproliferative effect by inducing apoptosis and
blocking cell cycle progression (8,17).
However, the mechanisms underlying the anticancer effects of
acacetin in human prostate tumors remain to be elucidated.
The nuclear factor (NF)-κB and phosphatidylinositol
3-kinase (PI3K)/Akt signaling pathways are major components of the
apoptotic machinery, and members of the NF-κB family play an
important role in the development and progression of several human
malignancies (18). The NF-κB
family is composed of five members, RelA, RelB, c-Rel, NF-κB1
(p105/p50) and NF-κB2 (p100/p52) (19,20). The activity of NF-κB is primarily
regulated by interactions with inhibitory IκBα proteins. Under
normal conditions, the typical NF-κB dimers (p50/p65) are bound to
inhibitory IκBα proteins, which sequester inactive NF-κB complexes
in the cytoplasm. The degradation of IκBα proteins is initiated
through phosphorylation by the IκB kinase (IKK) complex, which
consists of two catalytically active kinases, IKKα and IKKβ, and
the regulatory subunit, IKKγ. Phosphorylated IκBα is targeted for
ubiquitination and proteasomal degradation, which releases the
bound NF-κB dimers. The nuclear localization signals of the NF-κB
protein are exposed to allow nuclear translocation and
transcriptional activation, ultimately inducing the expression of a
number of target genes involved in cell growth, differentiation,
inflammatory responses and the regulation of apoptosis (21). NF-κB has been implicated in
oncogenesis (22). The
constitutive activation of NF-κB has been reported, not only in
androgen-independent prostate cancer cell lines, but also in
prostate cancer tissues (23,24), suggesting a pivotal role of NF-κB
in the progression of prostate cancer.
The PI3K/Akt signaling pathway is a potent survival
pathway that mediates resistance to the apoptotic effects of
chemotherapeutic drugs and radiation therapy in a variety of cancer
types (25). The activation of
membrane kinases, such as epidermal growth factor receptor (EGF-R)
and insulin-like growth factor receptor (IGF-R) by external growth
factors initiates the activation of PI3K and related intracellular
pathways (26). Once activated,
Akt, a major downstream target of PI3K, transduces signals from
growth factors and oncogenes to downstream targets that control
essential tumor-associated cellular processes, including cell
growth, cell cycle progression, survival, migration, tissue
invasion and angiogenesis (27).
In addition, Akt directly regulates NF-κB activation through the
phosphorylation of p65 by IKK (28,29). Therefore, Akt may also exert some
of its pro-survival effects by interacting with other pathways or
by exerting effects on nutrient uptake and metabolism.
In this study, we report the in vitro and
in vivo anticancer activity of acacetin in prostate cancer.
This study broadens the potential medicinal applications of
acacetin, a natural compound that may serve as a novel therapeutic
agent for human prostate cancer.
Materials and methods
Chemicals, drugs and antibodies
Acacetin was purchased from Sigma-Aldrich (St.
Louis, MO, USA), dissolved in dimethyl sulfoxide (DMSO) and stored
at −20°C. RPMI-1640 medium, penicillin-streptomycin, trypsin-EDTA
and fetal bovine serum (FBS) were purchased from HyClone
Laboratories Inc. (Logan, UT, USA).
3-(4,5-Dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
and DMSO were obtained from Sigma-Aldrich. Antibodies against Bax,
Bcl-2, β-actin, p53, Akt, phospho-Akt (Ser473), phospho-glycogen
synthase kinase (GSK)-3β (Ser9), IκBα, phospho-IκBα (Ser32),
phospho-NF-κB p65 (Ser536), X-linked inhibitor of apoptosis protein
(XIAP), cyclooxygenase (COX)-2 and goat anti-rabbit horseradish
peroxidase (HRP) were purchased from Cell Signaling Technology
(Beverly, MA, USA). Cell lysis buffer and
4′,6-diamidino-2-phenylindole (DAPI) were purchased from Invitrogen
Life Technologies (Carlsbad, CA, USA). The DeadEnd™ fluorometric
terminal deoxyribonucleotide transferase-mediated dUTP nick
end-labeling (TUNEL) assay kit was purchased from Promega (Madison,
WI, USA).
Cell lines and culture
The human prostate carcinoma cell line, DU145, was
purchased from the Korean Cell Line Bank (Seoul, Korea), and
maintained in RPMI-1640 medium supplemented with 10% FBS and
penicillin-EDTA under standard culture conditions, at 37°C with 95%
humidified air and 5% CO2. The culture medium was
renewed every two to three days. For acacetin treatment, DU145
cells were seeded at a density of ~3×104
cells/cm2 in a 175-cm2 flask and allowed to
adhere overnight.
Cell viability assay
The anticancer effects of acacetin were assessed by
MTT assay. DU145 cells were seeded in a 96-well plate at a density
of 2×104/ml and a volume of 200 μl/well. After 24 h of
incubation, the cells were treated with 6.25, 12.5, 25, 50 or 100
μM acacetin for either 24 or 48 h in triplicate. Following
treatment, the medium was discarded, followed by the addition of 40
μl of a 5 mg/ml MTT solution and incubation for a further 2 h. The
medium was then aspirated and the formazan product generated by
viable cells was solubilized with the addition of 100 μl of DMSO.
The absorbance of the solutions at 595 nm was determined using a
microplate reader (Bio-Rad, Hercules, CA, USA). The percentage of
viable cells was estimated in comparison to the untreated control
cells.
Nuclear staining
To quantify acacetin-induced apoptotic cell death,
the DU145 cells were treated with either 12.5 or 25 μM acacetin for
24 h. Following treatment, the cells were fixed with 4%
paraformaldehyde containing 0.1% Triton X-100 and stained with DAPI
for 30 min at room temperature. The cells were washed twice with
PBS and examined under a fluorescence microscope (IX71; Olympus
Co., Tokyo, Japan).
Western blot analysis
Cells were grown in culture flasks under the same
conditions described above and treated with 12.5 or 25 μM acacetin
for 24 h. Cells were washed briefly with cold PBS and treated with
trypsin-EDTA. Cell pellets were obtained by centrifugation, lysed
in lysis buffer (Invitrogen Life Technologies) and centrifuged at
15,000 rpm for 5 min at 4°C to obtain whole-cell lysates. Protein
concentration was determined using the Bradford protein assay
(Bio-Rad), and the samples were stored at −80°C in small aliquots.
Protein extracts (50 μg) were resolved by sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and
electrotransferred pmto nitrocellulose membranes (Amersham
Biosciences, Uppsala, Sweden). The membranes were incubated at room
temperature for 1 h in a 5% non-fat milk powder solution in
Tris-buffered saline (TBS) to block non-specific reactivity. Each
membrane was incubated overnight with appropriate primary
antibodies at 4°C and washed with a TBS with Tween-20 (TBS-T)
solution. Subsequently, the membranes were incubated with secondary
HRP-conjugated goat anti-rabbit IgG for 2 h. After washing the
membrane three times for 10 min in TBS-T, bands were detected using
ECL western blotting detection reagents (Pierce, Rockford, IL, USA)
according to the manufacturer’s instructions. β-actin was used as a
loading control.
Annexin V apoptosis assay
The Annexin V/propidium iodide (PI) assay was
performed following the manufacturer’s instructions
(Becton-Dickinson, San Jose, CA, USA). Briefly, the DU145 cells
were treated with or without 12.5 or 25 μM acacetin for 24 h,
washed with cold PBS, and incubated with Annexin V and PI in
binding buffer at room temperature for 15 min in the dark. Samples
were analyzed using a FACSCalibur™ flow cytometer
(Becton-Dickinson). All analyses were performed in triplicate.
Animals and in vivo xenograft tumor
model
Five-week-old male BALB/c nude mice (nu/nu) were
purchased from Orient Bio Inc. (Gyeonggi-do, Korea). Experiments on
animals were performed in accordance with the Guidelines for the
Care and Use of Animals of the Kongju National University Animals
Care Committee (Chungcheongnam-do, Korea). Mice were maintained
under a 12-h light/dark cycle, and housed under controlled
temperature (23±3°C) and humidity (40±10%) conditions. Mice were
allowed access to laboratory pelleted food and water ad
libitum.
DU145 cells were injected subcutaneously
(1×107/0.2-ml medium/animal) with a 27-gauge needle into
the right flank. When the tumors were palpable, mice were randomly
assigned into three groups of five mice in each. Acacetin was
orally administered three times per week at a dose of 25 or 50
mg/kg body weight, while the vehicle-treated mice were orally
administered distilled water. The weight and tumor size were
monitored twice per week. The tumor sizes were measured using
vernier calipers and calculated using the following equation: size
(mm3) = 0.5 × length (mm) × width2. Mice were
sacrificed 48 days after treatment. The liver and kidneys from each
mouse were excised for histopathological examination, and the
tumors were also excised to measure tumor wet weight. A portion of
the tumor was embedded in paraffin and used for TUNEL assay.
TUNEL assay
Apoptotic cell death was observed using a Promega
DeadEnd™ Colorimetric TUNEL system kit according to the
manufacturer’s instructions. Briefly, tumor tissues were fixed in
10% formalin overnight and embedded in paraffin. These blocks were
cut into 5-μm-thick slices. The sections were deparaffinized and
hydrated by sequential immersion in xylene and graded alcohol
solutions. The tumor sections were visualized using
3′-diaminobenzidine tetrahydrochloride (DAB) solution. The sections
were stained with methyl green, treated with a mounting reagent and
observed under a microscope (x200).
Histological examination
The excised livers and kidneys were immediately
fixed in 10% neutral-buffered formalin and, after embedding in
paraffin, cut into 5-μm-thick sections. Following hematoxylin and
eosin (H&E) staining, the sections were examined under a light
microscope (x200).
Statistical analysis
The results are expressed as the means ± standard
deviation (SD). Differences between the mean values for the groups
were assessed by a one-way analysis of variance (ANOVA) and
Dunnett’s t-tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Induction of DU145 cell death by
acacetin
The antiproliferative effects of acacetin on DU145
prostate cancer cells were determined by MTT assay. The cells were
treated with 0, 6.25, 12.5, 25, 50 or 100 μM acacetin for 24 or 48
h. As shown in Fig. 2A, acacetin
inhibited DU145 cell proliferation in a time-dependent manner.
Treatment with 12.5, 25, 50 and 100 μM acacetin for 24 h or 6.25,
12.5, 25, 50 and 100 μM for 48 h resulted in a significant decrease
in cell viability compared witht the control group (P<0.05).
Cell mortality increased by 50% upon treatment with 25 μM acacetin
for 48 h. These results suggest that acacetin induces cell death
and inhibits cell proliferation.
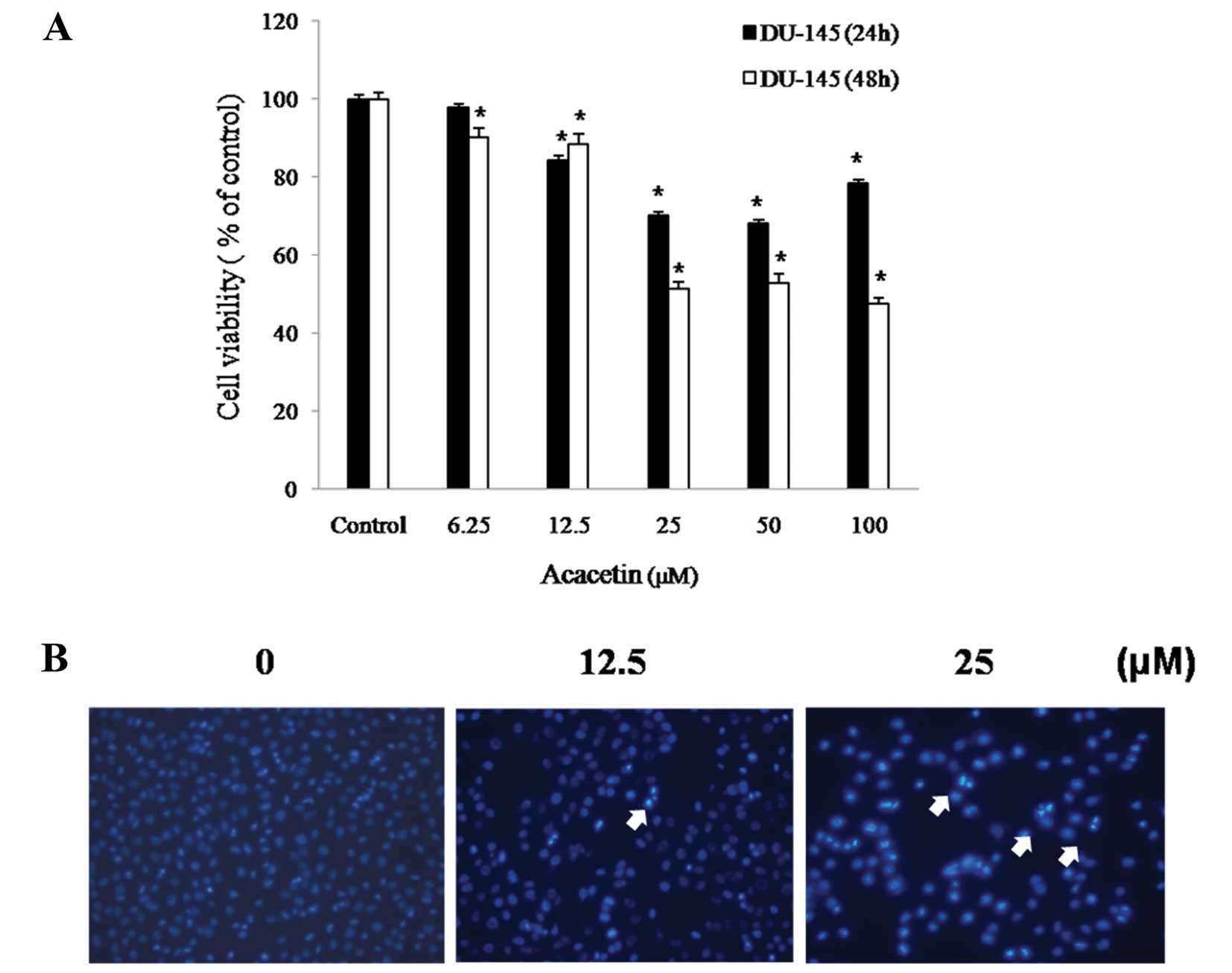 | Figure 2Effects of acacetin on cell viability
and apoptosis. (A) DU145 cells were treated with acacetin (0, 6.25,
12.5, 25, 50 and 100 μM) for 24 or 48 h, and cell viability was
determined by 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium
bromide (MTT) assay. The results are shown as the means ± standard
deviation (SD) of two independent experiments performed in
triplicate. Significance was determined by a Dunnett’s t-test with
*P<0.05 considered to indicate a statistically
significant difference compared with untreated control cells. (B)
DU145 cells were treated with acacetin (0, 12.5 and 25 μM) for 24
h, and apoptotic bodies stained with 4′,6-diamidino-2-phenylindole
(DAPI) (n=4, means ± SD from triplicate separated experiments). The
arrows indicate chromatin condensation in DU145 cells. Cleaved
nuclei were examined under a fluorescence microscope (x200). |
Induction of DU145 apoptosis by
acacetin
DNA damage was observed in DU145 cells, as indicated
by morphological changes in the nuclei. The presence of chromatin
condensation in the acacetin-treated cells was detected using a
fluorescence microscope (x200). DAPI forms fluorescent complexes
with double-I banded DNA, and stained nuclei show bright
fluorescence under a DAPI filter. The cells were treated with 0,
12.5 or 25 μM acacetin for 24 h. As shown in Fig. 2B, characteristic apoptotic
features were observed in the DU145 cells treated with acacetin,
including chromatin condensation, convoluted nuclei with
cavitations, nuclear fragmentation, and apoptotic bodies. Chromatin
condensation and the formation of apoptotic bodies, which are
characteristics of apoptosis, were not observed in the untreated
cells. These results suggest that acacetin induces the condensation
and formation of apoptotic bodies.
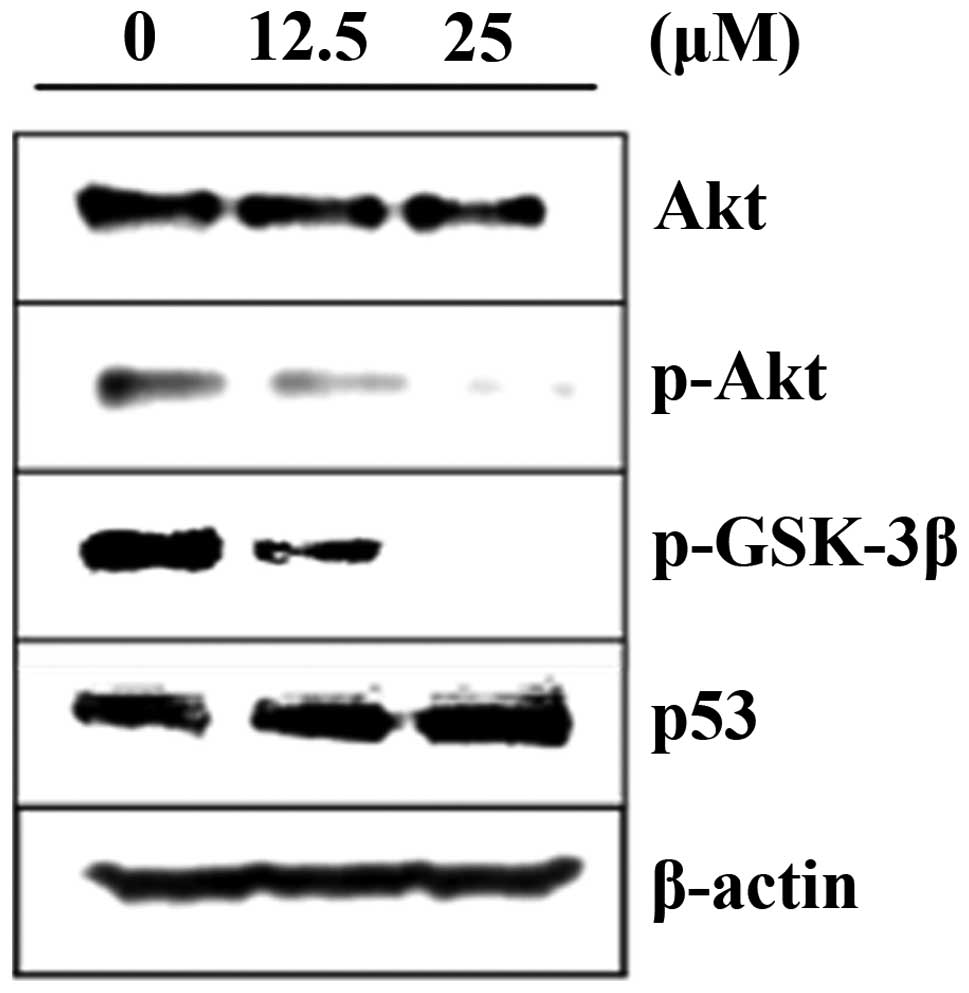
Inhibitory effects of acacetin on Akt
kinase activation
We examined the effects of acacetin on the Akt cell
survival pathway. The DU145 cells were treated with acacetin for 24
h, cell lysates prepared and the phosphorylation of Akt at Ser473
was determined by western blot analysis. The results revealed that
the treatment of DU145 cells with acacetin for 24 h decreased the
phosphorylation of Akt at Ser473 in a concentration-dependent
manner, although the total level of Akt remained unchanged
(Fig. 3). The expression of
downstream effectors of Akt, such as p-GSK-3β and p53, was also
evaluated. The total protein concentration of p-GSK-3β was
decreased, and that of p53 was increased in the acacetin-treated
DU145 cells. These results indicate that acacetin induces apoptosis
through the inhibition of Akt activation.
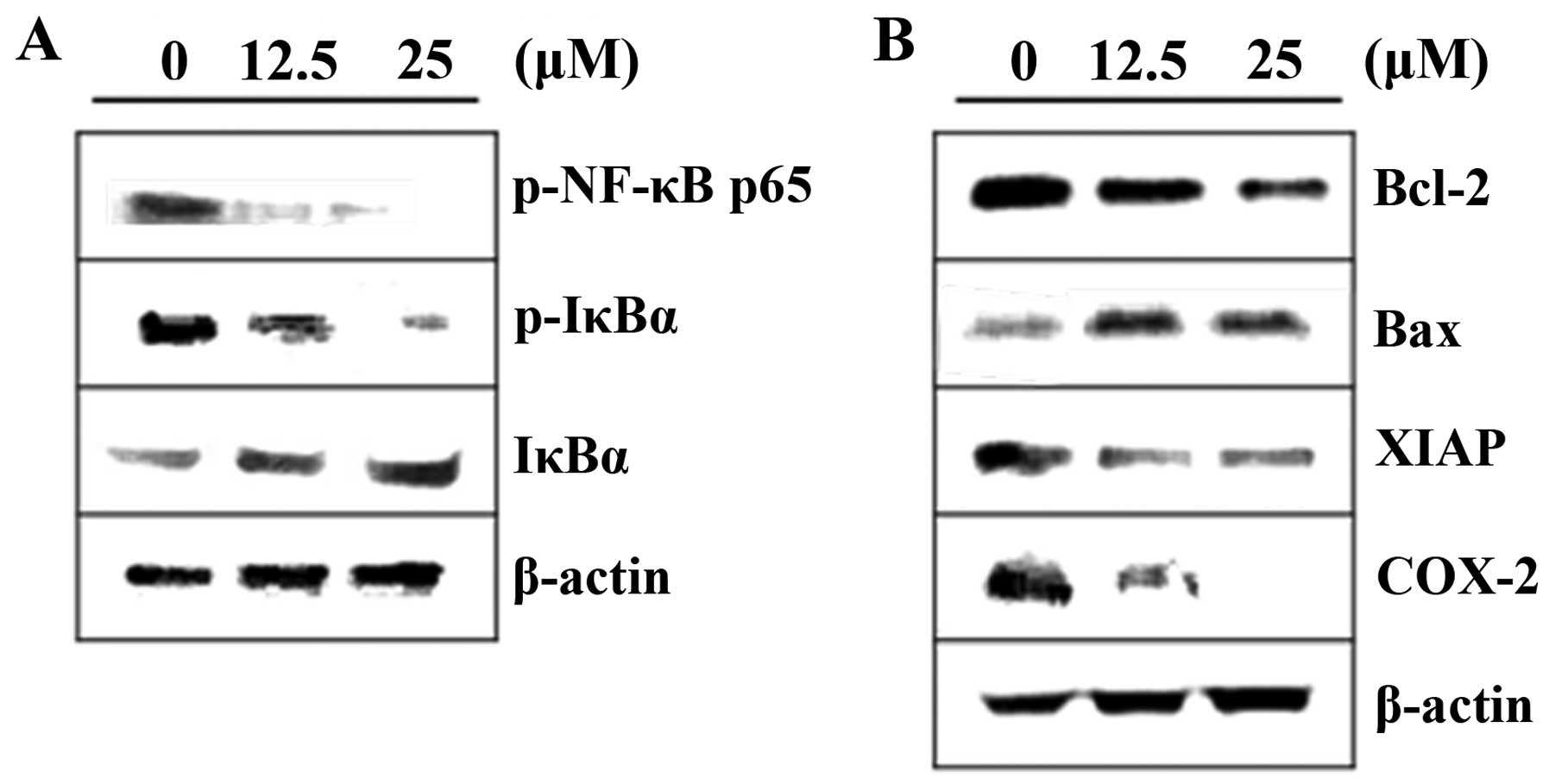
Inhibitory effects of acacetin on NF-κB
activation
To confirm that NF-κB is involved in
acacetin-induced apoptosis, we examined the expression levels of
NF-κB and NF-κB-regulated genes in acacetin-treated cells. As shown
in Fig. 4A, our results indicated
that acacetin treatment (0, 12.5 and 25 μM) markedly reduced the
phosphorylation of IκB, and NF-κB activity in DU145 cells. NF-κB
downstream effectors such as, XIAP, COX-2, Bax and Bcl-2 are key
mediators of apoptotic death and cell cycle arrest. Therefore, we
examined the effects of acacetin on the protein levels of Bax,
Bcl-2, COX-2 and XIAP by western blot analysis. Acacetin treatment
markedly increased the levels of Bax. In addition, decreased levels
of XIAP, COX-2 and Bcl-2 were detected in the acacetin-treated
cells (Fig. 4B). These results
suggest that the inhibition of NF-κB activation may be the
mechanism underlying acacetin-induced apoptosis in DU145 cells.
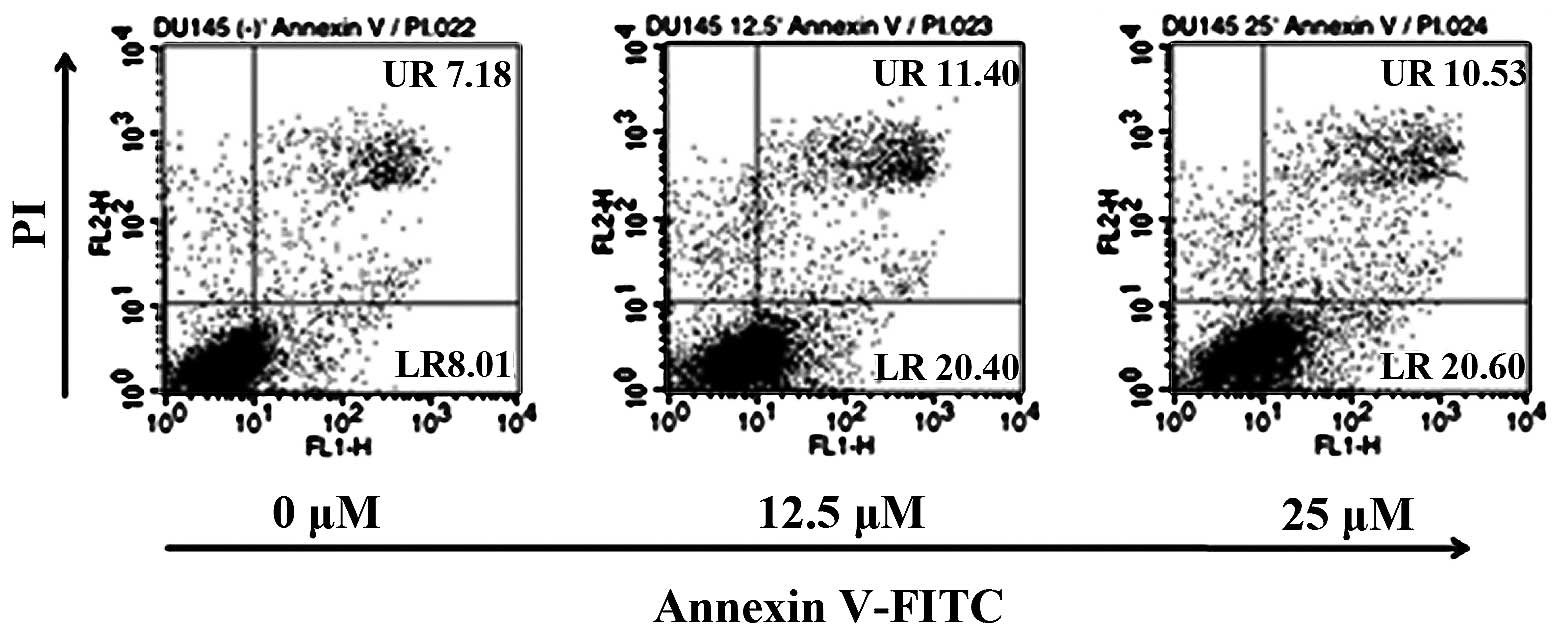
Induction of apoptosis in DU145 cells by
acacetin
To determine the effectd of acacetin treatment on
the induction of apoptosis in DU145 cells, we treated the cells
with various concentrations of acacetin and assessed the percentage
of apoptotic cells by Annexin V/PI double staining (Fig. 5). The cells in the lower right
(LR) quadrant of the histogram represent the number of early
apoptotic cells, while those in the upper right (UR) quadrant of
the histogram represent the cells in late apoptosis. Treatment of
the DU145 cells with acacetin for 24 h induced a marked,
dose-dependent induction of both the early and late stages of
apoptosis. Acacetin treatment increased the number of apoptotic
cells from 15.19% in the untreated cell group to 31.13% in the
group treated with 25 μM acacetin. These data suggest that the
induction of apoptosis is a key mechanism underlying the
acacetin-induced inhibition of DU145 cell viability (Fig. 2).
Inhibition of tumor growth by acacetin in
nude mice
Following the demonstration of the antitumor
potential of acacetin in prostate cancer cells in vitro, we
examined the in vivo effects of acacetin on prostate tumor
growth using a DU145 prostate cancer xenograft model. Mice were
assigned to three groups of five mice in each, and treated with
various doses of acacetin (0, 25 or 50 mg/kg). None of these doses
of acacetin had any detectable toxic effect, and there were no
statistically significant effects on body weight, behavior, or the
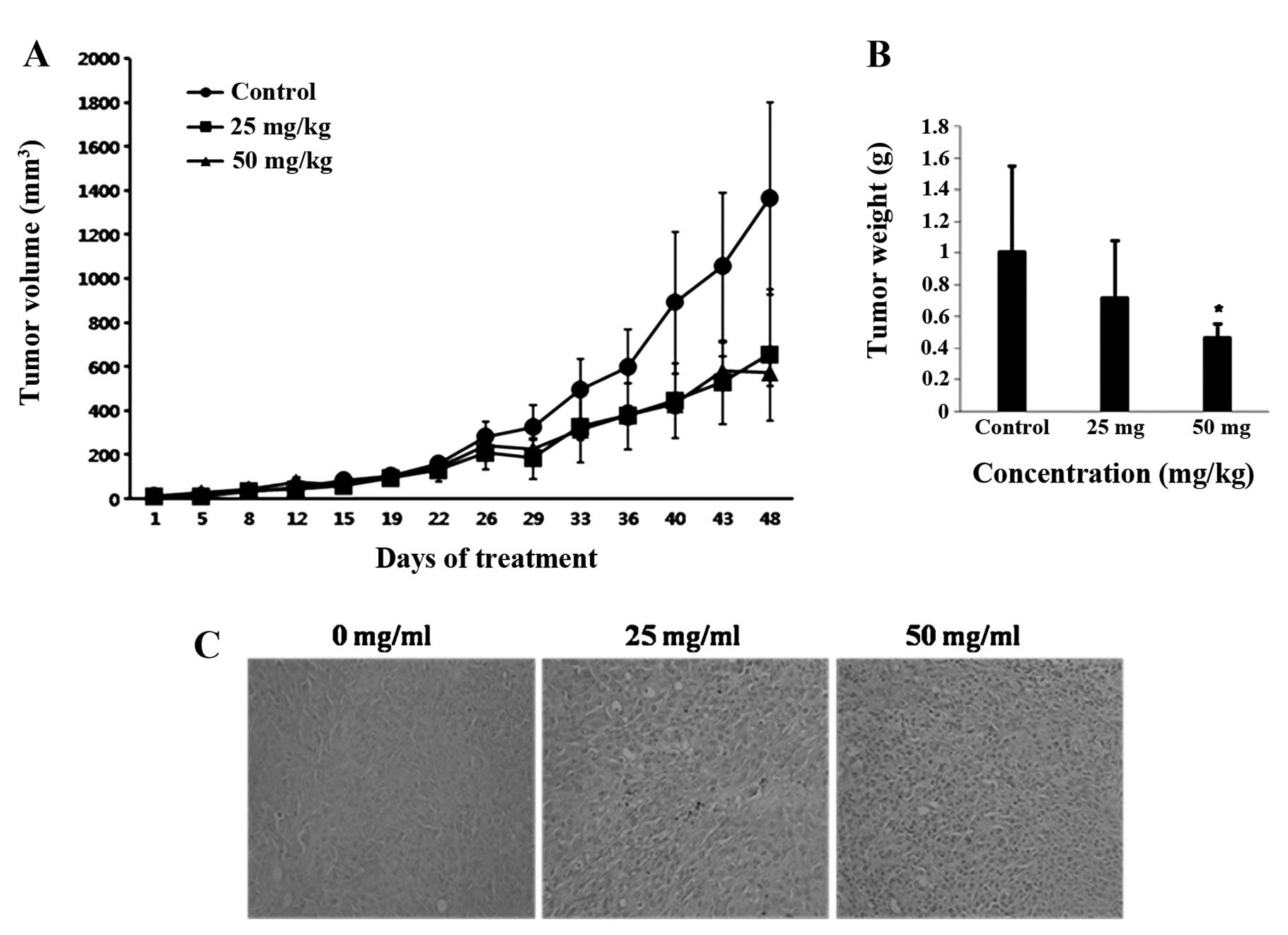
appearance of the mice (data not shown). As shown in Fig. 6A, tumor size was significantly
reduced in the mice treated with 25 or 50 mg/kg acacetin compared
with the control mice (P<0.05). On day 29 of acacetin treatment,
an important reduction in the tumor size was observed compared with
the control. This trend persisted over time and became more
pronounced at 40 days of acacetin treatment. On day 48, mice were
sacrificed and the tumors excised. Compared with the control,
acacetin treatment significantly reduced the mean tumor weight
(Fig. 6B). As shown in Table I, the groups treated with acacetin
showed significant reductions in tumor size on day 48; 52.00% for
the 25 mg/kg and 57.90% for the 50 mg/kg group (both P<0.05
compared with the control group, 0 mg/kg). Moreover, we assayed
tumor tissues with TUNEL so as to examine apoptotic cell death. As
shown in Fig. 6C, an increase in
the number of TUNEL-positive cells was observed in the mice treated
with acacetin compared with the control mice (P<0.05). These
findings confirmed that the treatment of mice with DU145 tumors
with acacetin significantly inhibited tumor growth by inducing the
apoptosis of the tumor cells.
 | Table IInhibitory effects of acacetin on
DU145 prostate tumor size. |
Table I
Inhibitory effects of acacetin on
DU145 prostate tumor size.
| Pre-treatment | Post-treatment | |
|---|
|
|
| |
|---|
| Acacetin dose | n | Size
(mm3) | n | Size
(mm3) | Inhibition
rateb (%) |
|---|
| 0 mg/kga | 5 | 12.79 | 5 | 1365.79 | |
| 25 mg/kg | 5 | 12.91 | 5 | 655.55 | 52.00 |
| 50 mg/kg | 5 | 13.86 | 5 | 575.03 | 57.90 |
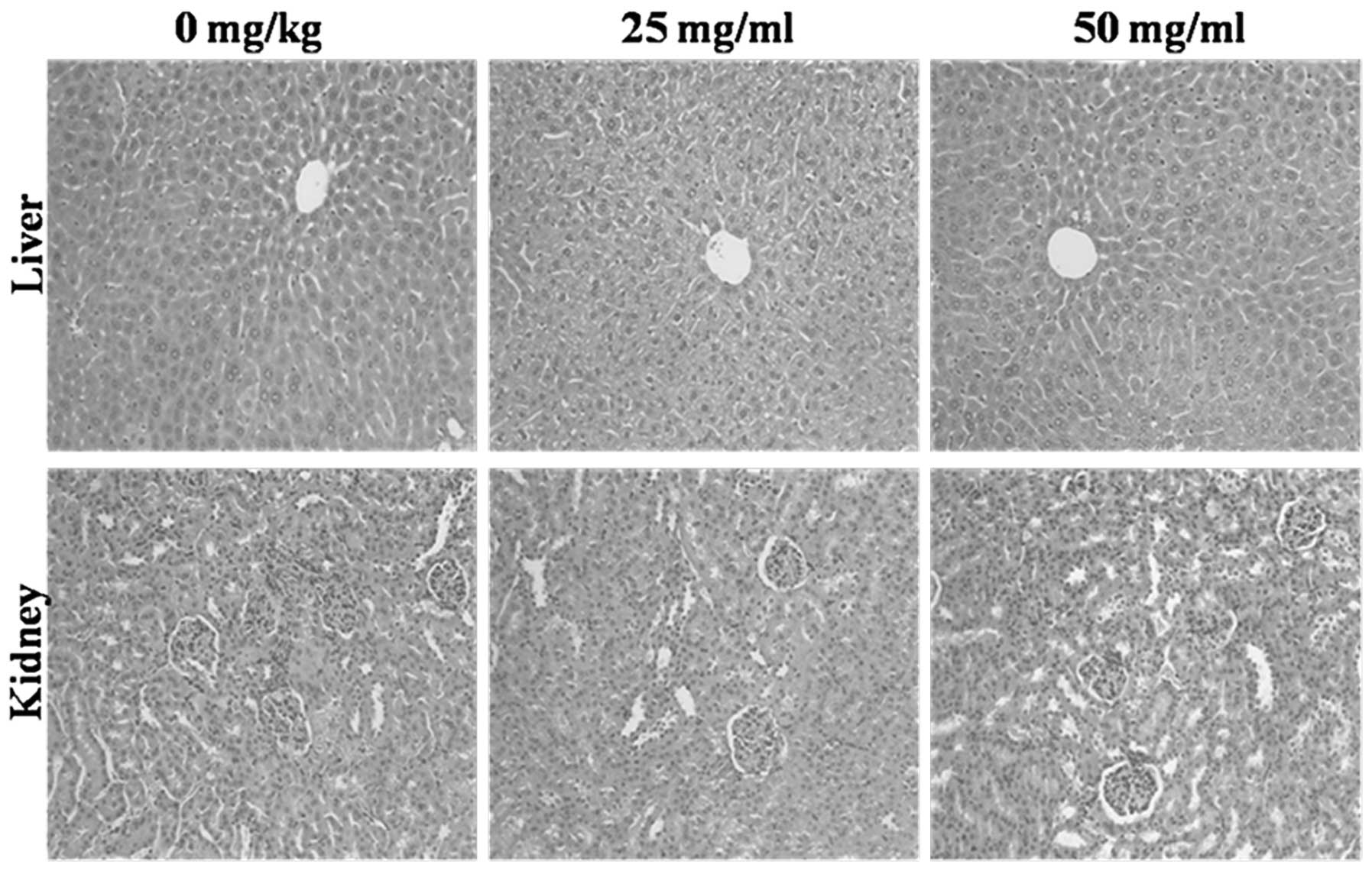
Toxicity evaluation of acacetin in liver
and kidney tissues
To ensure the safety of acacetin when administered
as a chemotherapeutic agent, the mice were sacrificed at the end of
the experiment, and liver and kidney tissues were removed and fixed
in formalin for histopathological evaluation by H&E staining
(Fig. 7). No pathological change
was observed in the acacetin-treated group compared with the
control group.
Discussion
Compelling evidence suggests that the tumorigenic
growth of prostate cancer depends on the disruption of the normal
apoptotic process (30). Both the
incidence of prostate cancer and the associated mortality rate are
increasing steadily. Currently available chemotherapeutic agents
for the treatment of prostate cancer are associated with various
side-effects and the development of resistance. Therefore,
non-toxic and more selective pharmacotherapies that target prostate
cancer are required.
Over the past decade, a number of naturally
occurring dietary agents of reduced toxicity have been reported to
induce apoptosis and inhibit tumor growth. One of these, acacetin,
exhibits a number of biological effects, including anticancer
activity (8,15,16,17,31); however, its underlying mechanisms
of action remain unknown. In this study, we demonstrate that
acacetin induces the apoptosis of DU145 human prostate cancer cells
by inhibiting the activation of the Akt-NF-κB signaling pathway. We
found that acacetin suppressed constitutive NF-κB activation
through the inhibition of Akt in DU145 human prostate carcinoma
cells. In addition, acacetin altered the expression of signaling
effectors, including GSK-3β, IκB, Bcl-2 and Bax, as well as that of
inhibitors of apoptosis (IAP) family members, such as XIAP and
COX-2 (Figs. 3 and 4). Furthermore, we provide evidence that
acacetin can effectively inhibit the growth of prostate cancer
tumors without overt toxicity. These results provide mechanistic
insight into the in vivo and in vitro anticancer
effects of acacetin, which we suggest are mediated, at least in
part, by blocking the proliferative and anti-apoptotic effects of
NF-κB signaling through the reduction of the translocation of this
protein complex to the nucleus and inhibition of Akt
phosphorylation.
In order to evaluate the cytotoxicity of acacetin,
an MTT assay was performed to determine cell viability. Acacetin
induced a potent time- and a dose-dependent decrease in DU145 cell
number. Overall, the data suggest that acacetin inhibits the growth
of human prostate cancer cells. This result confirms an earlier
report on the antiproliferative effects of acacetin in the AGS
cancer cell line (31).
Apoptosis, otherwise known as programmed cell death,
is characterized by a number of well-defined features, such as
condensation and fragmentation of chromatin, inter-nucleosomal DNA
cleavage, caspase activation and the translocation of
phosphatidylserine from the inner to the outer leaflet of the
plasma membrane (32). The
induction of apoptosis is one of the most effective approaches in
cancer therapeutics. To determine whether the acacetin-induced cell
death in DU145 cells involved apoptosis, we performed DAPI staining
and flow cytometric analysis. As shown in Figs. 2 and 5, acacetin was a potent inhibitor of
cell viability and induced the rapid induction of apoptosis,
concurrent with chromatin condensation and the apoptotic appearance
of DU145 cells, in agreement with previous reports on human
prostate cancer cells (8,16). Further studies are required to
elucidate the mechanisms behind the reduced cell viability and the
induction of apoptosis in DU145 cells by acacetin.
The Akt signaling pathway is a critical component in
the regulation of cell growth, survival and apoptosis (33). The aberrant activation of the Akt
pathway and, hence, of these tumor-associated pathways, has been
reported in a number of human malignancies (34). Furthermore, Akt signaling
activates the NF-κB signaling pathway to promote the resistance of
cancer cells to apoptosis (35).
Therefore, the specific inhibition of the Akt pathway may be an
effective approach to prevent and treat malignancies. In the
present study, we demonstrated that Akt phosphorylation was
inhibited and the expression of p53 was increased by acacetin
treatment. Moreover, the acacetin-mediated suppression of Akt
phosphorylation in DU145 cells was coupled to the inhibition of
GSK-3β. These data suggest that the inhibition of the activation of
Akt is potentially one of the underlying mechanisms of
acacetin-induced apoptosis in DU145 cells.
NF-κB is a family of dimeric transcription factors
that regulate diverse biological processes, including immune
responses and cell growth and survival (36,37). In response to most activating
stimuli, NF-κB signaling occurs through the sequential activation
of IKK, the phosphorylation of IκBα at Ser32 and Ser36, leading to
IκBα degradation, and the translocation of NF-κB to the nucleus,
where it regulates the transcription of a series of genes,
including those that promote cell proliferation and survival
(38). In this study, we found
that acacetin was a potent inhibitor of NF-κB activation in DU145
cells. Acacetin inhibited NF-κB phosphorylation and IκB
phosphorylation and degradation, eventually leading to the
inhibition of NF-κB nuclear translocation. Acacetin also affected
the levels of NF-κB-regulated proteins involved in apoptosis (Bax),
anti-apoptosis (Bcl-2 and XIAP) and proliferation (COX-2), thereby
suppressing cell proliferation and inducing apoptosis in DU145
cells.
The in vivo anticancer efficacy of acacetin
was substantiated by our experiments on DU145 tumor-bearing nude
mice. Acacetin was administered three times per week at a dose of
25 or 50 mg/kg. As shown in Table
I, the mice administered with acacetin showed a marked
reduction in tumor size at day 48: 52.00% for the 25 mg/kg group
and 57.90% for the 50 mg/kg group compared with the control group.
This in vivo antitumor effect correlated with the increased
levels of apoptosis in the tumor cells of the acacetin-treated
group, as supported by the increased DNA fragmentation observed in
the TUNEL-positive cells. The in vivo results support our
in vitro studies and suggest that acacetin induces apoptotic
cell death in DU145 prostate tumor cells. Evaluation of the
potential toxic effects of acacetin on healthy tissues is an
important factor considered during the development of novel
anticancer drugs, aiming to prevent side-effects of the tested drug
on non-targeted cells, such as severe DNA damage (39). As shown in Fig. 7, none of the mice in the three
groups showed any significant clinical symptoms during the study
period. Therefore, acacetin showed no significant toxicity in mice
at the doses tested (<50 mg/kg).
In conclusion, we provide evidence that acacetin
significantly attenuates tumor progress, and that the antitumor
effects of this flavonoid are mediated by inhibiting the
phosphorylation of Akt and reducing NF-κB DNA binding. The present
study provides a molecular basis for the use of acacetin as a
cancer chemopreventive and chemotherapeutic agent. Overall, our
results indicate that flavonoid compounds, such as acacetin, may be
useful in the treatment of prostate cancer.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Siegal R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
3
|
Kar S, Palit S, Ball WB and Das PK:
Carnosic acid modulates Akt/IKK/NF-κB signaling by PP2A and induces
intrinsic and extrinsic pathway mediated apoptosis in human
prostate carcinoma PC-3 cells. Apoptosis. 17:735–747.
2012.PubMed/NCBI
|
|
4
|
Kim EJ, Lim SS, Park SY, Shin HK, Kim JS
and Park JH: Apoptosis of DU145 human prostate cancer cells induced
by dehydrocostus lactone isolated from the root of Saussurea
lappa. Food Chem Toxicol. 46:3651–3658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Linja MJ, Savinainen KJ, Saramäki OR,
Tammela TL, Vessella RL and Visakorpi T: Amplification and
overexpression of androgen receptor gene in hormone-refractory
prostate cancer. Cancer Res. 61:3550–3555. 2001.PubMed/NCBI
|
|
6
|
Lam JS, Leppert JT, Vemulapalli SN,
Shvarts O and Belldegrun AS: Secondary hormonal therapy for
advanced prostate cancer. J Urol. 175:27–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu LZ, Jing Y, Jiang LL, et al: Acacetin
inhibits VEGF expression, tumor angiogenesis and growth through
AKT/HIF-1α pathway. Biochem Biophys Res Commun. 413:299–305.
2011.PubMed/NCBI
|
|
8
|
Singh RP, Agrawal P, Yim D, Agarwal C and
Agarwal R: Acacetin inhibits cell growth and cell cycle
progression, and induces apoptosis in human prostate cancer cells:
structure-activity relationship with linarin and linarin acetate.
Carcinogenesis. 26:845–854. 2005. View Article : Google Scholar
|
|
9
|
Schuier M, Sies H, lllek B and Fischer H:
Cocoa-related flavonoids inhibit CFTR-mediated chloride transport
across T84 human colon epithelia. J Nutr. 135:2320–2325.
2005.PubMed/NCBI
|
|
10
|
Miyazawa M and Hisama M: Antimutagenic
activity of flavonoids from Chrysanthemum morifolium. Biosci
Biotechnol Biochem. 67:2091–2099. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martínez-Vázquez M, RamírezApan TO, Lastra
AL and Bye R: A comparative study of the analgesic and
anti-inflammatory activities of pectolinarin isolated from
Cirsium subcoriaceum and linarin isolated from Buddleia
cordata. Planta Med. 64:134–137. 1998.PubMed/NCBI
|
|
12
|
Kraft C, Jenett-Siems K, Siems K, et al:
In vitro antiplasmodial evaluation of medicinal plants from
Zimbabwe. Phytother Res. 17:123–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cholbi MR, Paya M and Alcaraz MJ:
Inhibitory effects of phenolic compounds on CC14-induced micresomal
lipid peroxidation. Experientia. 47:195–199. 1991. View Article : Google Scholar
|
|
14
|
Yin Y, Gong FY, Wu XX, et al:
Anti-inflammatory and immunosuppressive effect of flavones isolated
from Artemisia vestita. J Ethnopharmacol. 120:1–6. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fong Y, Shen KH, Chiang TA and Shih YW:
Acacetin inhibits TPA-induced MMP-2 and u-PA expressions of human
lung cancer cells through inactivating JNK signaling pathway and
reducing binding activities of NF-kappaB and AP-1. J Food Sci.
75:H30–H38. 2010. View Article : Google Scholar
|
|
16
|
Shen KH, Hung SH, Yin LT, et al: Acacetin,
a flavonoid, inhibits the invasion and migration of human prostate
cancer DU145 cells via inactivation of the p38 MAPK signaling
pathway. Mol Cell Biochem. 333:279–291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsu YL, Kuo PL and Lin CC: Acacetin
inhibits the proliferation of Hep G2 by blocking cell cycle
progression and inducing apoptosis. Biochem Pharmacol. 67:823–829.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park MH, Choi MS, Kwak DH, et al:
Anti-cancer effect of bee venom in prostate cancer cells through
activation of caspase pathway via inactivation of NF-κB. Prostate.
71:801–812. 2011.PubMed/NCBI
|
|
19
|
Xiao G, Harhaj EW and Sun SC:
NF-kappaB-inducing kinase regulates the processing of NF-kappaB2
p100. Mol Cell. 7:401–409. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Senftleben U, Cao Y, Xiao G, et al:
Activation by IKKalpha of a second, evolutionary conserved,
NF-kappa B signaling pathway. Science. 293:1495–1499. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu YQ, Hu XY, Lu T, et al: Retigeric acid
B exhibits antitumor activity through suppression of nuclear
factor-κB signaling in prostate cancer cells in vitro and in vivo.
PLoS One. 7:e380002012.PubMed/NCBI
|
|
22
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 107:241–246. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen CD and Sawyers CL: NF-kappa B
activates prostate-specific antigen expression and is upregulated
in androgen-independent prostate cancer. Mol Cell Biol.
22:2862–2870. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Palayoor ST, Youmell MY, Calderwood SK,
Coleman CN and Price BD: Constitutive activation of IkappaB kinase
alpha and NF-kappaB in prostate cancer cells is inhibited by
ibuprofen. Oncogene. 18:7389–7394. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boreddy SR, Pramanik KC and Srivastava SK:
Pancreatic tumor suppression by benzyl isothiocyanate is associated
with inhibition of PI3K/AKT/FOXO pathway. Clin Cancer Res.
17:1784–1795. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaur P, Shukla S and Gupta S: Plant
flavonoid apigenin inactivates Akt to trigger apoptosis in human
prostate cancer: an in vitro and in vivo study. Carcinogenesis.
29:2210–2217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sizemore N, Leung S and Stark GR:
Activation of phosphatidylinositol 3-kinase in response to
interleukin-1 leads to phosphorylation and activation of the
NF-kappaB p65/RelA subunit. Mol Cell Biol. 19:4798–4805.
1999.PubMed/NCBI
|
|
29
|
Meng F, Liu L, Chin PC and D’Mello SR: Akt
is a downstream target of NF-kappa B. J Biol Chem. 277:29674–29680.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bruckheimer EM and Kyprianou N: Apoptosis
in prostate carcinogenesis. A growth regulator and a therapeutic
target. Cell Tissue Res. 301:153–162. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pan MH, Lai CS, Hsu PC and Wang YJ:
Acacetin induces apoptosis in human gastric carcinoma cells
accompanied by activation of caspase cascades and production of
reactive oxygen species. J Agric Food Chem. 53:620–630. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Talib WH and Mahasneh AM:
Antiproliferative activity of plant extracts used against cancer in
traditional medicine. Sci Pharm. 78:33–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Franke TF, Yang SI, Chan TO, et al: The
protein kinase encoded by the Akt proto-oncogene is a target of the
PDGF-activated phosphatidylinositol 3-kinase. Cell. 81:727–736.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song L, Xiong H, Li J, et al: Sphingosine
kinase-1 enhances resistance to apoptosis through activation of
PI3K/Akt/NF-κB pathway in human non-small cell lung cancer. Clin
Cancer Res. 17:1839–1849. 2011.PubMed/NCBI
|
|
36
|
Ghosh S, May MJ and Kopp EB: NF-κB and Rel
proteins: evolutionarily conserved mediators of immune responses.
Annu Rev Immunol. 16:225–260. 1998.
|
|
37
|
Barkett M and Gilmore TD: Control of
apoptosis by Rel/NF-kappaB transcription factors. Oncogene.
69:6910–6924. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karin M and Lin A: NF-kappaB at the
crossroads of life and death. Nat Immunol. 3:221–227. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carneiro ML, Peixoto RC, Joanitti GA, et
al: Antitumor effect and toxicity of free rhodium (II) citrate and
rhodium (II) citrate-loaded maghemite nanoparticles in mice bearing
breast cancer. J Nanobiotechnology. 11:42013. View Article : Google Scholar : PubMed/NCBI
|