Introduction
Fluoride is an essential trace element for human
beings and animals. Exposure to fluoride takes the form of food,
drinking water and burning coal (1–5).
Appropriate amount of fluoride is beneficial to maintain bone
strength and to protect against dental decay (6,7).
However, long-term excessive intake of fluoride has deleterious
effects on many organs and tissues including teeth, bone, liver,
kidney and brain (8–18).
Results of previous studies have shown that fluoride
has a higher and special affinity for calcium in bone tissue, which
could result in osteofluorosis (19,20). It has been reported that
osteofluorosis may increase the severity of osteoarthritis (OA)
characterized by progressive degeneration of articular cartilage
(21,22). Chondrocyte apoptosis may be
involved in the onset and development of osteoarthritic cartilage
degeneration (23). Previously,
chondrocytes were found to be responsible for the synthesis of
cartilage extracellular matrix (ECM) (24,25), while ECM is considered crucial to
the survival of chondrocytes (26,27). Subsequently, there is a close
correlation between chondrocyte apoptosis and the synthesis of ECM.
Additionally, fluoride may induce OA by promoting chondrocytes
apoptosis and disrupting the synthesis of ECM in cartilage;
however, the molecular mechanism involved remains to be
determined.
Hypoxia-inducible factor 1α (HIF-1α) is important in
the maintenance of the survival of chondrocytes. HIF-1α may inhibit
the apoptosis of chondrocytes and regulate the synthesis of ECM
(28–30). This synthesis may be mediated by
transactivation of sex determining region Y box gene 9 (Sox9), a
key transcription factor for chondrocyte-specific genes such as
collagen II (Col II) which encode the Col II protein (31,32). Thus, we hypothesized that sodium
fluoride (NaF) would induce chondrocytes apoptosis through the
downregulation of HIF-1α and cause matrix disruption through the
downregulation of HIF-1α via the Sox9 pathway.
The purpose of this study was to demonstrate the
molecular mechanism of NaF on apoptosis in primary cultured rat
chondrocytes.
Materials and methods
Culture of primary rat chondrocytes
Chondrocytes were obtained from arthrodial cartilage
of neonatal Wistar rats (Department of Laboratory Animal Science,
The First Affiliated Hospital of Harbin Medical University, China).
Cartilage was washed three times and minced in PBS with 100 IU/ml
penicillin and 100 μg/ml streptomycin (Sigma, St. Louis, MO, USA).
After digestion in 2.5 mg/ml trypsin for 40 min at 37°C, the cells
were digested with 0.05 mg/ml collagenase type II (Gibco-BRL,
Carlsbad, CA, USA) in DMEM/high glucose medium for 16 h at 37°C
with 5% CO2 and passed through a 70-mm cell strainer to
prepare a single-cell suspension. Approximately 1.5×105
cells/ml were seeded in 25 cm2 culture flask containing
DMEM/high glucose medium supplemented with 10% FBS, 100 IU/ml
penicillin, and 100 μg/ml streptomycin and cultured at 37°C with 5%
CO2 and 2% O2. After two days, cells adhering
to the plate were passaged. The third or fourth generation was used
in subsequent experiments.
Identification of primary rat chondrocyte
culture
Cells were grown for 1 day on glass coverslips
coated with polylysine in DMEM/high glucose medium with 10% FBS in
6-well plates. The cells were fixed in cold 4% paraformaldehyde for
15 min at room temperature and made permeable with ice-cold 0.2%
Triton X-100 in PBS for 8 min. Subsequent to blocking for 20 min
with goat serum, primary antibody to Col II (Abcam, Cambridge, MA,
USA) was applied at 1:50 overnight at 4°C. The cells were incubated
for 1 h in secondary anti-rabbit antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Diaminobenzidine (DAB)
was then used to detect positive staining (Zhongshan Biotechnology
Co., Ltd., Beijing, China).
In order to observe the normal morphology of primary
cultured rat chondrocytes, cells untreated with NaF were dyed with
hematoxylin and eosin (H&E). Immunohistochemistry was used to
identify the primary cultured rat chondrocytes by Col II
protein.
Analysis of cell viability
Cell viability was evaluated by MTT assay. A total
of 3×103 cells/well were plated in 96-well plates, and
subsequent to overnight incubation, the cells were exposed to NaF
for 24–72 h and washed with PBS. Fresh complete growth medium (1
ml) supplemented with 20 μl of MTT dye (0.5 mg/ml) was added to
each well, and the mixture was incubated for 4 h at 37°C.
Immediately after incubation, the cells were thoroughly washed with
PBS. Dimethyl sulfoxide (DMSO) (0.5 ml/well) was added to stop the
MTT reaction and to dissolve the formazan crystals. After agitation
for 10 min at room temperature, the optical density in each well
was detected at 490 nm with an ELISA reader (Bio-Rad, Hercules, CA,
USA). Untreated chondrocytes were considered as 100% viable cells.
The results were calculated using the formula: (OD treated well -
OD blank)/(OD untreated well - OD blank) ×100%.
Flow cytometric analysis of cell
apoptosis
Cells (1×105 cells/well) were seeded in
6-well plates and cultured overnight. Followin an increase in the
concentration of NaF treatment, the cells were digested with 2.5
mg/ml trypsin and washed twice with PBS. The cells were suspended
with 300 μl binding buffer, 2 μl Annexin V were added and the
mixture was carefully agitated. Then, 5 μl propidium iodine (PI)
(both from Baosai, Biotechnology, Beijing, China) was added to the
mixture. Following incubation for 5–15 min at room temperature, the
ratio of apoptosis was detected using a FACSCalibur flow cytometer
(Becton-Dickinson, San Jose, CA, USA).
Scanning electron microscopy
Cells were digested from the flask, centrifuged at
1500 rpm for 3 min to collect the cells, and fixed with 2.5%
formaldehyde for 24 h at 4°C. Subsequently, the samples were fixed
with 1% osmic acid for 1 h, dehydrated through a gradient ethanol
series, embedded with epoxy resin and cut into sections. The
sections were stained with uranyl acetate and lead citrate,
observed and images were captured with a 1,200 electron microscope
(Toshiba, Tokyo, Japan).
Cell cycle analysis
Cells (2×105 cells/well) were seeded in
6-well plates and starved in serum-free medium at 37°C. After 12-h
starvation, the cells were treated with NaF solution and complete
medium for 48 or 72 h. The cells were trypsinized and washed with
PBS. Subsequently, the cells were resuspended with 500 μl RNase A
(1 mg/ml) and PI (100 μg/ml) (Beyotime, Haimen, China) to stain the
cell DNA. After 20-min incubation at room temperature in the dark,
the DNA contents of the cells were analyzed using a FACSCalibur
flow cytometer and the data were analyzed by ModFitLT V2.0 software
(both from Becton-Dickinson).
Western blot analysis
Cells were collected and total cell lysates were
prepared using lysis buffer (Beyotime). Protein concentration was
measured using the Bradford method with bovine serum albumin as the
standard. Equal amounts (20–40 mg) of whole cell lysates were
loaded into 8–12% SDS-polyacrylamide gels for electrophoresis at 80
V constant voltage and transferred to PVDF membranes (Millipore,
Bedford, MA, USA) at 200 mA constant current for 3 h. The membrane
was incubated in blocking buffer (5% skim milk in TBS-T) at room
temperature for 2 h. The blocked membrane was then incubated with
anti-HIF-1α (1:500), anti-Sox9 (1:500) (Abcam, Cambridge, UK),
anti-Col II (1:250), anti-Bax (1:500), anti-Bcl-2 (1:500),
anti-caspase-9 (1:500), anti-caspase-3 (1:500), (Santa Cruz
Biotechnology, Inc.) and anti-caspase-12 (Biovision Inc., Mountain
View, CA, USA) primary antibody at 4°C overnight and washed with
TBS-T three times every 10 min, followed by incubation with a
secondary antibody (1:1,000) (Santa Cruz Biotechnology, Inc.) at
room temperature for 1 h. After washing in TBS-T, the
immunoreactive bands were visualized using enhanced
chemiluminescence kits (Pierce Biotechnology Inc., Rockford, IL,
USA). Blots were stained with anti-β-actin or -GAPDH antibody
(Santa Cruz Biotechnology, Inc.) as an internal control for the
amounts of target proteins.
Statistical analysis
Experiments were carried out three times. Data were
presented as means ± SD. Differences among means were analyzed
using the two-sided Student’s t-test or one-way ANOVA. P<0.05
was considered statistically significant.
Results
Morphology and identification of primary
cultured rat chondrocytes
In order to observe the normal morphology of primary
cultured rat chondrocytes, cells untreated with NaF were dyed with
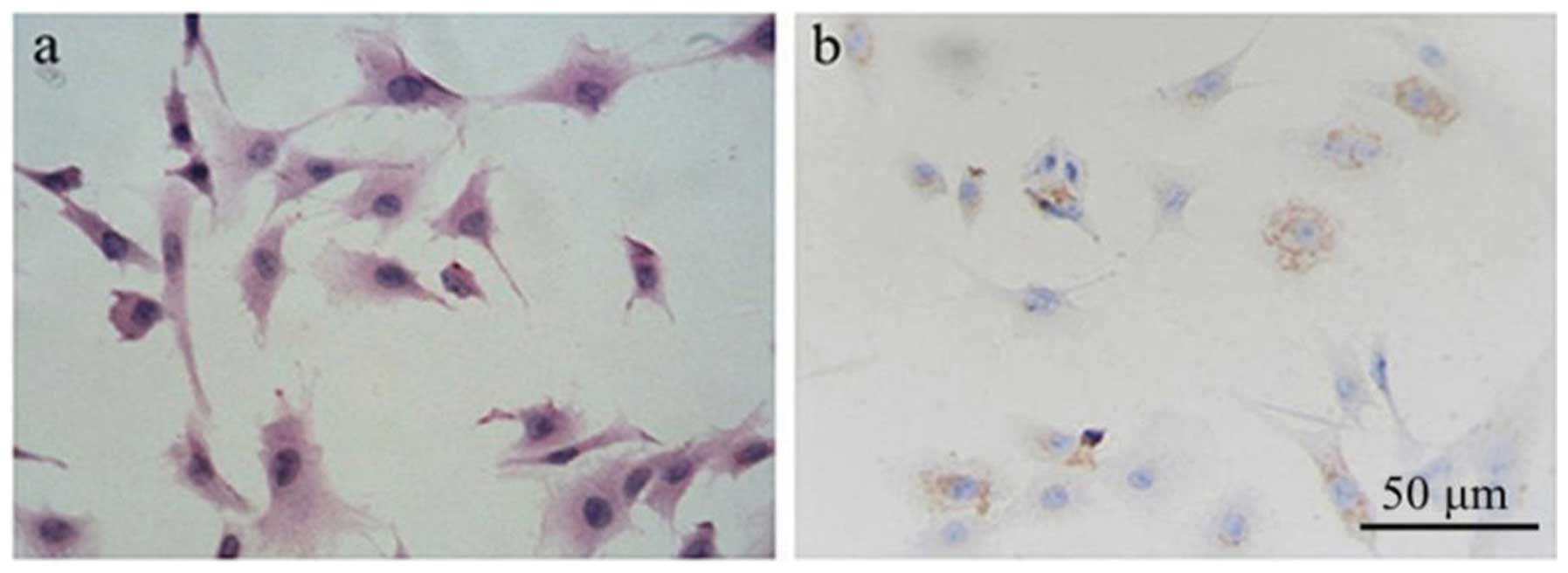
H&E. As is shown in Fig. 1a,
the primary chondrocytes are polygonal, with a number of
protrusions, and connect to each other. Some secretory granules are
also evident in the cytoplasm.
Col II is the chondrocyte-specific protein.
Immunohistochemistry was used to identify rat chondrocytes by Col
II protein (Fig. 1b). The results
showed that the primary cultured chondrocytes expressed Col II
protein in the cytoplasm, which is consistent with the
characteristics of chondrocytes.
Fluoride inhibits cell viability and
causes changes in cell morphology in chondrocytes
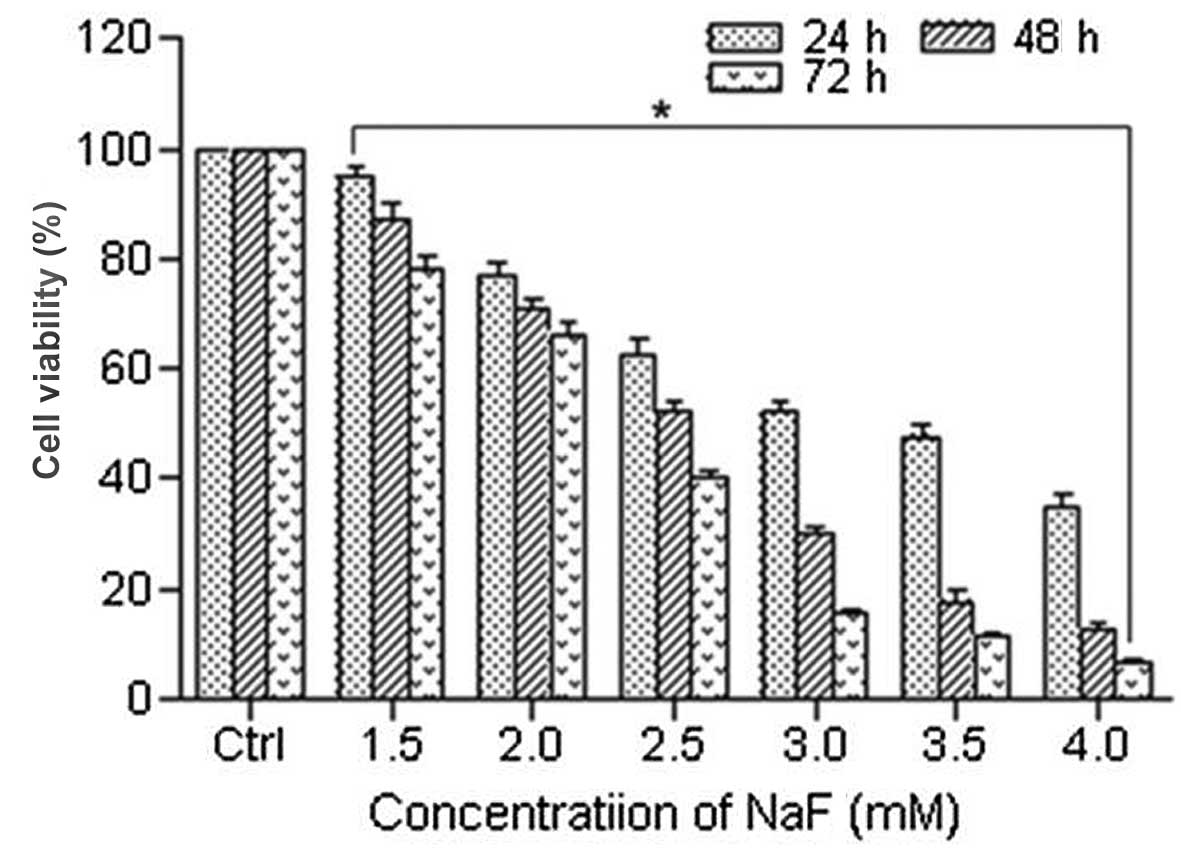
To evaluate the effect of NaF on chondrocytes, MTT
assay was used to measure cell viability. Cells were treated with
NaF at concentrations of 0, 1.5, 2.0, 2.5, 3.0, 3.5 and 4.0 mM for
24, 48 and 72 h. The viability of chondrocyte-exposed gradient
doses of NaF (0, 1.5, 2.0, 2.5, 3.0, 3.5 and 4.0 mM) for 24, 48 and
72 h was (100±0, 94.90±1.92, 77.10±2.55, 62.36±3.16, 52.21±2.10,
47.81±2.17 and 34.72±2.86%); (100.00±0, 87.27±2.97, 70.95±1.94,
52.18±1.90, 30.11±1.41, 17.70±2.06 and 12.47±1.37%); (100.00±0,
77.92±2.52, 66.39±2.36, 40.22±1.41, 15.35±1.06, 11.51±0.82 and
6.70±0.52%) respectively. The cell viability of the NaF groups was
significantly decreased compared with that of the control groups
(Fig. 2). NaF significantly
inhibited the viability of chondrocytes in a time- and
dose-dependent manner.
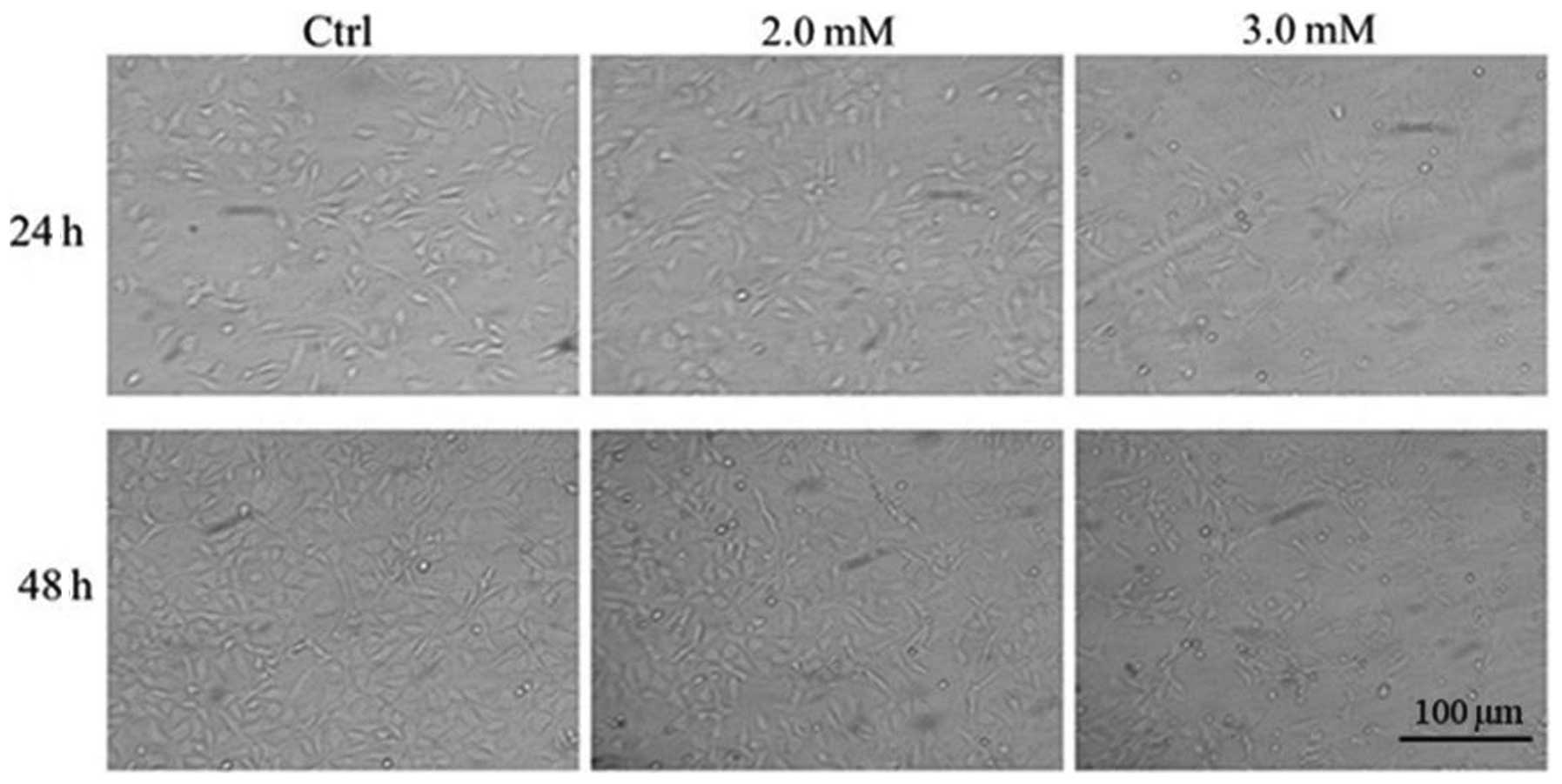
Morphological changes of chondrocytes following
treatment with NaF at 2.0 and 3.0 mM for 24 and 48 h are shown in
Fig. 3. Compared with
NaF-untreated chondrocytes, chondrocytes treated with NaF (2.0 and
3.0 mM) became smaller, spindle-shaped and floated. These
morphology changes demonstrated cell damage following NaF
treatment.
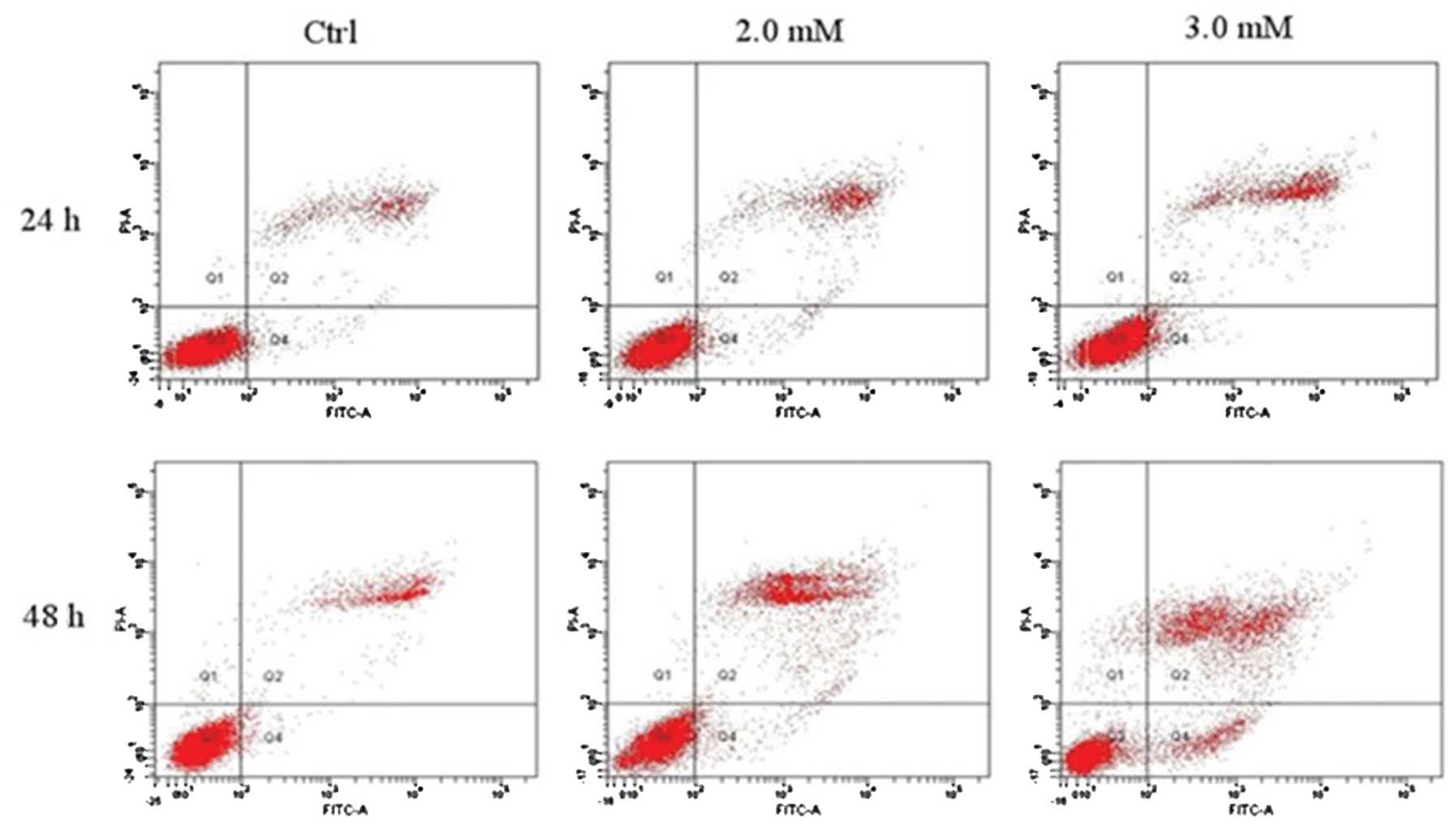
Analysis of cell apoptosis by Annexin
V/PI staining
The rate of early apoptotic, late apoptotic or dead
cells was analyzed with Annexin V/PI staining and flow cytometry.
The apoptotic rate of chondrocytes treated with doses of NaF (2.0
and 3.0 mM) for 24 and 48 h was (17.28±2.27 and 24.53±1.36%) and
(36.59±0.90 and 43.10±2.23%), respectively. The rates were
significantly higher than those of the control groups
(12.67±0.67%). As is shown in Fig.
4a and Table I, the rate of
apoptotic cells increased significantly with increasing
concentrations of NaF in a time- and dose-dependent manner.
 | Table IPercentage of quadrant distribution
(QD) in Annexin V/PI staining apoptosis assay. |
Table I
Percentage of quadrant distribution
(QD) in Annexin V/PI staining apoptosis assay.
| 24 h | 48 h |
|---|
|
|
|
|---|
| Variables | Control | 2.0 NaF | 3.0 NaF | Control | 2.0 NaF | 3.0 NaF |
|---|
| Necrosis | 0.20±0.17 | 0.25±0.26 | 0.13±0.06 | 0.50±0.17 | 0.55±0.38 | 1.87±0.25b |
| Early
apoptosis | 2.07±0.64 | 3.10±0.17 | 4.13±0.61a | 1.63±0.21 | 4.62±1.11b | 7.70±1.06b |
| Late apoptosis | 10.60±1.01 | 14.18±2.12 | 20.40±1.85a | 11.20±1.42 | 31.97±0.24b | 35.40±2.87b |
| Normal | 87.13±0.93 | 82.51±2.54 | 75.37±1.36a | 86.63±1.38 | 62.82±1.05b | 55.37±1.50b |
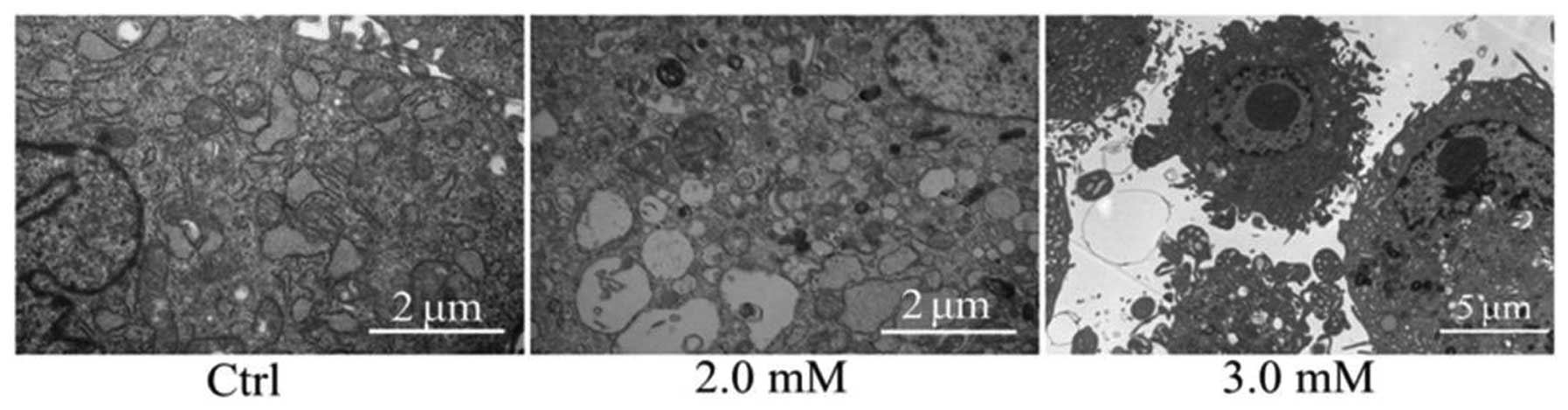
Ultrastructure observation
Chondrocytes were treated with NaF at 0, 2.0 and 3.0
mM for 48 h. The morphology of the control group was normal.
Microvillis, irregular karyotype and evenly distributed chromatin
were evident on the cell surface. Compared with the normal
structure of the control group, the microvillis of the cell surface
in the NaF groups were decreased, and the perinuclear cisterna was
widened. Chromatin was condensed into large clumps, surrounding the
nuclear membrane. Mitochondrias became swollen, degenerated and
pale. The endoplasmic reticulum appeared pale, and the apoptotic
cells appeared in NaF groups (Fig.
5).
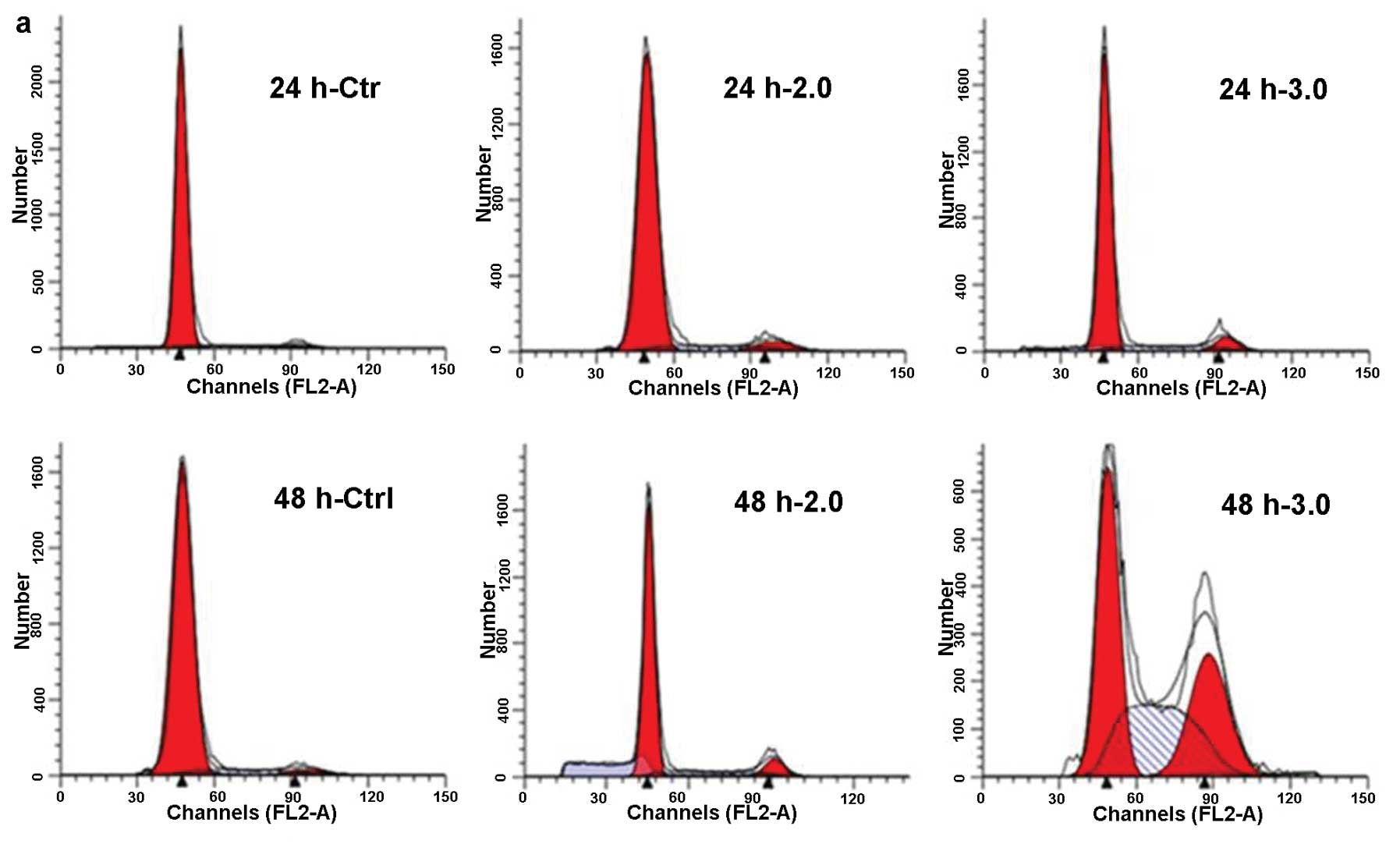
NaF induces cell cycle arrest in
chondrocytes
Cell cycle distribution of chondrocytes was analyzed
by flow cytometry, aiming to determine whether the inhibitory
effect was due to the cell cycle arrest. Chondrocytes were exposed
to NaF at 2.0 and 3.0 mM for 24 and 48 h. Chondrocytes exposed to
NaF showed G2 arrest by decreasing the fraction of G1 phase and
increasing the fraction of G2 phase, as compared with that of the
untreated cells (Fig. 6a and b).
These results revealed that NaF arrested chondrocyte proliferation
via cell cycle arrest at the G2 phase.
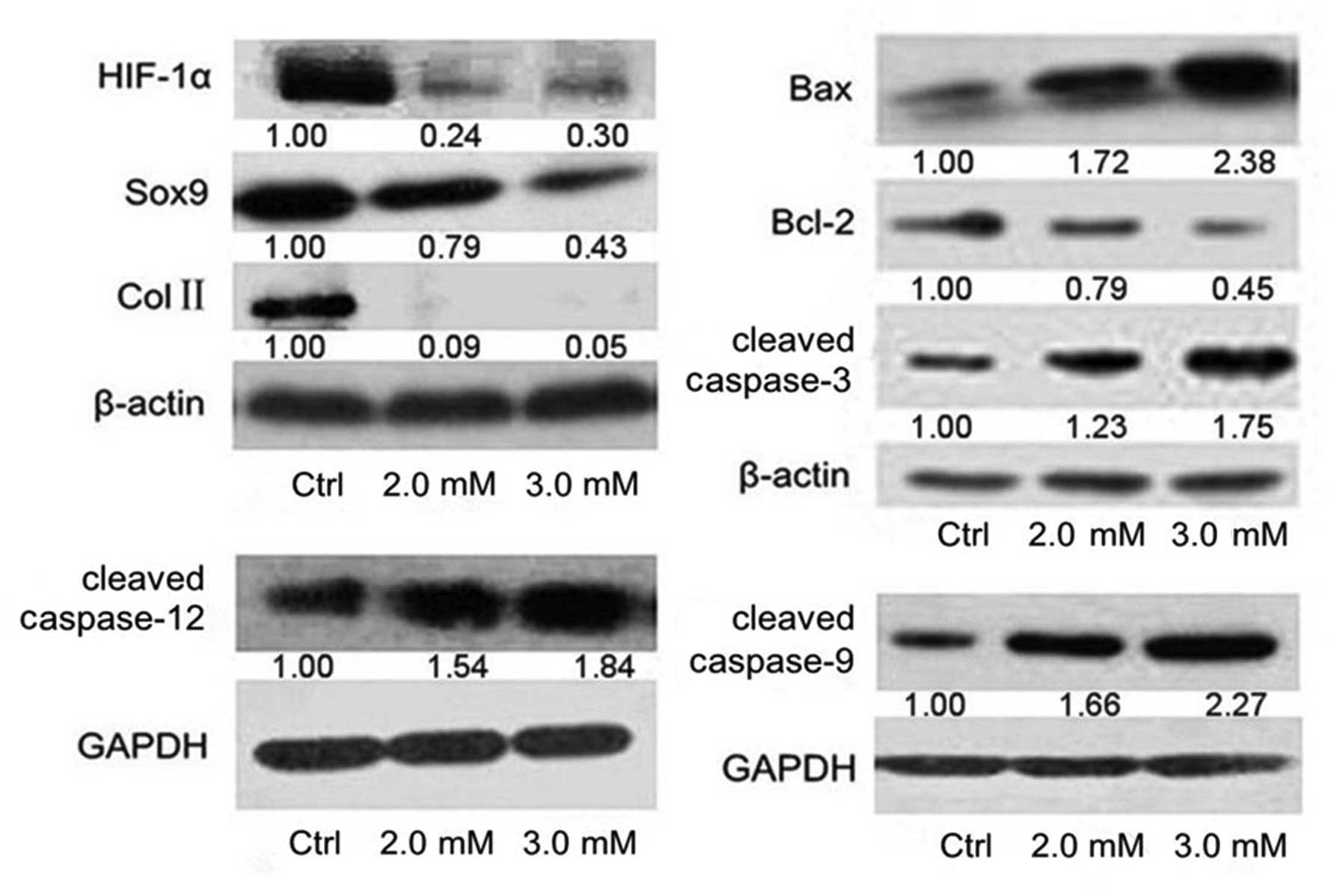
Mechanisms of apoptosis after NaF
treatment
To investigate the mechanisms of NaF causing
apoptosis, the expression of HIF-1α, Sox9 and Col II, Bcl-2, Bax,
caspase-9, −12 and −3 were assessed using western blotting
(Fig. 7). Downregulation of Bcl-2
and upregulation of Bax, cleavaged caspase-9, −12 and −3 indicated
that NaF induced apoptosis through the mitochondrial and
endoplasmic reticulum pathways. The results also showed that the
levels of HIF-1α, Sox9 and Col II protein of the NaF-treated groups
were lower than those of control groups. Thus, NaF may induce
apoptosis through the downregulation of HIF-1α and disrupt the
synthesis of ECM through the downregulation of HIF-1α via Sox9
pathway in primary cultured rat chondrocytes.
Discussion
OA, a degenerative joint disease, is the most common
form of arthritis. The mainly affected peripheral joints are knees,
hips and hands (33,34). The later clinic symptoms of OA
include joint space stenosis, joint pains and the dysfunction of
joint movement. Aging and mechanical overload have been considered
as risk factors of OA, while excessive fluoride could increase the
severity of OA (35,36). To the best of our knowledge, the
effects of NaF on the degeneration of chondrocytes in OA have yet
to be elucidated. In this study, NaF at concentrations of 1.5, 2.0,
2.5, 3.0, 3.5 and 4.0 mM was administered to primary cultured rat
chondrocytes. The effects of NaF on the proliferation, apoptosis
and synthesis of ECM in primary cultured rat chondrocytes were
observed.
Ren et al have shown that primary cultured
mouse osteoblasts treated with lower concentrations of NaF
(10−3-1 mM) resulted in higher cell activities compared
with control cells. However, the cell activities were significantly
decreased at higher concentrations of NaF (5–10 mM) (37). In this study, the MTT assay
indicated that NaF inhibited the cell activities of primary
cultured rat chondrocytes with increasing concentration of NaF
(1.5, 2.0, 2.5, 3.0, 3.5 and 4.0 mM) in a time- and dose-dependent
manner. However, the effects of NaF on primary cultured mouse
osteoblasts at lower concentrations of NaF (<1 mM) had no
effects on primary cultured rat chondrocytes. This result may be
due to cultivation conditions, incubation time and different cell
lines. Results of the present study also showed the cell cycle
distribution of chondrocytes in the subsequent experiments.
According to the result of the MTT assay, chondrocytes were treated
with NaF at concentrations of 2.0 and 3.0 mM in subsequent
experiments. Our data showed a reduced number of chondrocytes in G1
and S phase and an increased number of chondrocytes in G2 phase in
the NaF groups compared with the control groups. Additionally, the
number of G2 phase cells gradually increased with the increasing in
dose of NaF and treatment time. These results suggest that
excessive NaF inhibited the proliferation of chondrocytes by
inducing cell cycle arrest at G2 phase in a dose- and
time-dependent manner. The result is concordant with a study by
Wang et al (38). He and
Chen (18) demonstrated that the
number of rat oral mucosal cells and hepatocytes in G2/M phase was
lower in NaF groups than that in control groups, however, there
were no obvious changes in G0/G1 and S phase. The difference may be
due to NaF having a different effect on the cell cycle in different
types of cells.
Findings of previous studies have shown that
excessive NaF can trigger apoptosis in different types of cells
including osteoblasts, epithelial lung cells, sperm cells,
leukocytes and ameloblasts (38–42). In the present study, the rate of
apoptosis was assessed by Annexin V/PI staining and flow cytometric
analysis. The results showed the rate of apoptosis in NaF groups
was significantly increased compared with that of the control
groups, and that it gradually increased with the increasing NaF
concentrations and treatment time. This suggests that NaF induced
chondrocyte apoptosis in a dose- and time-dependent manner.
Furthermore, the ultrastructure of chondrocytes was detected by
transmission electron microscopy. The results revealed that the
control groups possessed integrated cell membrane, abundant
cytoplasm, evenly distributed chromatin, a large number of abnormal
mitochondrias and well-developed endoplasmic reticulum. However,
the NaF groups had various degenerative biological characteristics
including swelling of mitochondria, dilation of endoplasmic
reticulum, reduced electron dense material, chromatin condensation
and gathered chromatin at the nuclear periphery.
The ultrastructural changes suggest that NaF has
adverse effects on chondrocytes through the mitochondrial and
endoplasmic reticulum pathways. In order to demonstrate whether NaF
was actually capable of inducing apoptosis through the
mitochondrial and endoplasmic reticulum pathways in primary rat
chondrocytes, apoptotic markers, including Bax, Bcl-2, caspase-9,
−12 and −3 were detected by western blotting. In this study, the
level of Bcl-2 protein in the NaF groups was significantly lower
than that of the control groups, while the levels of Bax, cleavaged
caspase-9, −12 and −3 proteins were significantly higher than those
of the control groups. These data confirmed that NaF was capable of
inducing the apoptosis of chondrocytes through the mitochondrial
and endoplasmic reticulum pathways, although the mechanism of NaF
on apoptosis in chondrocytes remains to be clarified.
HIF-1α is known to play a key role in the
development and progression of articular cartilage degeneration in
OA. It can maintain the cartilage homeostasis by regulating the
downstream genes involved in the proliferation, apoptosis and
synthesis of ECM components in chondrocytes (28–30). This synthesis may, at least
partly, be mediated by the transactivation of Sox9, a key
transcription factor for cartilage-specific marker genes such as
Col II and aggrecan (31,32). Yudoh et al (43) reported that HIF-1α-deficient
chondrocytes showed significantly increased levels of apoptosis
compared with the control groups. This observation suggests that a
decreased expression of HIF-1α may have the ability to promote
chondrocyte apoptosis. In the present study, the level of HIF-1α
protein was significantly decreased with increasing concentrations
of NaF. In other words, NaF may induce cell apoptosis by inhibiting
the expression of HIF-1α protein in primary cultured rat
chondrocytes. Moreover, in order to investigate the possible
molecular mechanisms of NaF-induced matrix disruption in
chondrocytes, the expression of Sox9 and Col II proteins were
assessed in vitro. The level of Col II protein was
significantly decreased along with the decreasing Sox9 protein.
Thus, NaF may inhibit the synthesis of Col II protein through the
downregulation of HIF-1α via the Sox9 pathway in primary cultured
rat chondrocytes. It appears that there is a stong correlation
between the NaF-induced apoptosis and synthesis of ECM in
chondrocytes. Chondrocyte apoptosis may disrupt matrix synthesis.
Conversely, matrix disruption also affects the survival of
chondrocytes.
Taken together, results of the present study suggest
that NaF inhibits the synthesis of Col II protein through
downregulation of HIF-1α via Sox9 pathway and induces cell
apoptosis through the downregulation of HIF-1α expression in
primary cultured chondrocytes. The apoptotic pathway included
endoplasmic reticulum intrinsic and mitochondrial intrinsic
pathways. However, future studies should be conducted to clarify
the exact mechanisms involved.
Acknowledgements
We would like to thank Mr. Jing Li (Department of
Central Electron Microscope, Harbin Medical University), Mr. Bowen
Dong and Ms. You Zhou (Department Central Laboratory, The First
Affiliated Hospital of Harbin Medical University) for technically
supports. This study was supported by the Science and Technology
Fund of Heilongjiang (GC09C412-2) and the Research Fund for the
Doctoral Program of Higher Education of China (20070226016).
References
|
1
|
Malde MK, Scheidegger R, Julshamn K and
Bader HP: Substance flow analysis: a case study of fluoride
exposure through food and beverages in young children living in
Ethiopia. Environ Health Perspect. 119:579–584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pandey J and Pandey U: Fluoride
contamination and fluorosis in rural community in the vicinity of a
phosphate fertilizer factory in India. Bull Environ Contam Toxicol.
87:245–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hussain I, Arif M and Hussain J: Fluoride
contamination in drinking water in rural habitations of Central
Rajasthan, India. Environ Monit Assess. 184:5151–5158. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao YH, Sun F, Li CB, Shi JQ, Gu J, Xie
C, Guan ZZ and Yu YN: Effect of endemic fluoride poisoning caused
by coal burning on the oxidative stress in rat testis (In Chinese).
Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 33:357–361. 2011.PubMed/NCBI
|
|
5
|
Li HL, Yu YN, Chen Y and Huang L: Effect
of fluoride on oxidative stress and Mn-SOD expression in rats with
endemic fluorosis of coal burning (In Chinese). Zhonghua Bing Li
Xue Za Zhi. 41:627–630. 2012.PubMed/NCBI
|
|
6
|
Gutiérrez-Salinas J, Morales-González JA,
Madrigal-Santillán E, Esquivel-Soto J, Esquivel-Chirino C,
González-Rubio MG, Suástegui-Domínguez S and Valadez-Vega C:
Exposure to sodium fluoride produces signs of apoptosis in rat
leukocytes. Int J Mol Sci. 11:3610–3622. 2010.PubMed/NCBI
|
|
7
|
Ricomini Filho AP, Tenuta LM, Fernandes
FS, Calvo AF, Kusano SC and Cury JA: Fluoride concentration in the
top-selling Brazilian toothpastes purchased at different regions.
Braz Dent J. 23:45–48. 2012.PubMed/NCBI
|
|
8
|
Vieira AP, Hanocock R, Eggertsson H,
Everett ET and Grynpas MD: Tooth quality in dental fluorosis
genetic and environmental factors. Calcif Tissue Int. 76:17–25.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Denbesten P and Li W: Chronic fluoride
toxicity: dental fluorosis. Monogr Oral Sci. 22:81–96. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Whyte MP, Essmyer K, Gannon FH and Reinus
WR: Skeletal fluorosis and instant tea. Am J Med. 118:78–82. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bezerra de Menezes LM, Volpato MC, Rosalen
PL and Cury JA: Bone as a biomarker of acute fluoride toxicity.
Forensic Sci Int. 137:209–214. 2003.PubMed/NCBI
|
|
12
|
Wang AG, Xia T, Chu QL, Zhang M, Liu F,
Chen XM and Yang KD: Effects of fluoride on lipid peroxidation, DNA
damage and apoptosis in human embryo hepatocytes. Biomed Environ
Sci. 17:217–222. 2004.PubMed/NCBI
|
|
13
|
Shanthakumari D, Srinivasalu S and
Subramanian S: Effect of fluoride intoxication on lipidperoxidation
and antioxidant status in experimental rats. Toxicology.
204:219–228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santoyo-Sanchez MP, del Carmen
Silva-Lucero M, Arreola-Mendoza L and Barbier OC: Effects of acute
sodium fluoride exposure on kidney function, water homeostasis, and
renal handling of calcium and inorganic phosphate. Biol Trace Elem
Res. 152:367–372. 2013. View Article : Google Scholar
|
|
15
|
Basha PM and Madhusudhan N: Pre and post
natal exposure of fluoride induced oxidative macromolecular
alterations in developing central nervous system of rat and
amelioration by antioxidants. Neurochem Res. 35:1017–1028. 2010.
View Article : Google Scholar
|
|
16
|
Seraj B, Shahrabi M, Shadfar M, Ahmadi R,
Fallahzadeh M, Eslamlu HF and Kharazifard MJ: Effect of high water
fluoride concentration on the intellectual development of children
in makoo/iran. J Dent (Tehran). 9:221–229. 2012.PubMed/NCBI
|
|
17
|
Cicek E, Aydin G, Akdogan M and Okutan H:
Effects of chronic ingestion of sodium fluoride on myocardium in a
second generation of rats. Hum Exp Toxicol. 24:79–87. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He LF and Chen JG: DNA damage, apoptosis
and cell cycle changes induced by fluoride in rat oral mucosal
cells and hepatocytes. World J Gastroenterol. 12:1144–1148.
2006.PubMed/NCBI
|
|
19
|
Levy SM, Eichenberger-Gilmore J, Warren
JJ, Letuchy E, Broffitt B, Marshall TA, Burns T, Willing M, Janz K
and Torner JC: Associations of fluoride intake with children’s bone
measures at age 11. Community Dent Oral Epidemiol. 37:416–426.
2009.
|
|
20
|
Chachra D, Vieira AP and Grynpas MD:
Fluoride and mineralized tissues. Crit Rev Biomed Eng. 36:183–223.
2008. View Article : Google Scholar
|
|
21
|
Savas S, Cetin M, Akdoğan M and Heybeli N:
Endemic fluorosis in Turkish patients: relationship with knee
osteoarthritis. Rheumatol Int. 21:30–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishida T, Kubota S, Aoyama E and Takigawa
M: Impaired glycolytic metabolism causes chondrocyte
hypertrophy-like changes via promotion of phospho-Smad1/5/8
translocation into nucleus. Osteoarthritis Cartilage. 21:700–709.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goggs R, Carter SD, Schulze-Tanzil G,
Shakibaei M and Mobasheri A: Apoptosis and the loss of chondrocyte
survival signals contribute to articular cartilage degradation in
osteoarthritis. Vet J. 166:140–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blanco FJ, Guitian R, Vázquez-Martul E, de
Toro FJ and Galdo F: Osteoarthritis chondrocytes die by apoptosis.
A possible pathway for osteoarthritis pathology. Arthritis Rheum.
41:284–289. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hashimoto S, Ochs RL, Komiya S and Lotz M:
Linkage of chondrocyte apoptosis and cartilage degradation in human
osteoarthritis. Arthritis Rheum. 41:1632–1638. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peters HC, Otto TJ, Enders JT, Jin W, Moed
BR and Zhang Z: The protective role of the pericellular matrix in
chondrocyte apoptosis. Tissue Eng Part A. 17:2017–2024. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomas CM, Fuller CJ, Whittles CE and
Sharif M: Chondrocyte death by apoptosis is associated with
cartilage matrix degradation. Osteoarthritis Cartilage. 15:27–34.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Provot S and Schipani E: Fetal growth
plate: a developmental model of cellular adaptation to hypoxia. Ann
NY Acad Sci. 1117:26–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schipani E, Ryan HE, Didrickson S,
Kobayashi T, Knight M and Johnson RS: Hypoxia in cartilage:
HIF-1alpha is essential for chondrocyte growth arrest and survival.
Genes Dev. 15:2865–2876. 2001.PubMed/NCBI
|
|
30
|
Pfander D, Cramer T, Schipani E and
Johnson RS: HIF-1alpha controls extracellular matrix synthesis by
epiphyseal chondrocytes. J Cell Sci. 116:1819–1826. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kypriotou M, Fossard-Demoor M,
Chadjichristos C, Ghayor C, de Crombrugghe B, Pujol JP and Galéra
P: SOX9 exerts a bifunctional effect on type II collagen gene
(COL2A1) expression in chondrocytes depending on the
differentiation state. DNA Cell Biol. 22:119–129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Apichart V, Wong R, Rabie B and Lei S: The
effect of quercetin on expression of SOX9 and subsequent release of
type II collagen in spheno-occipital synchondroses of
organ-cultured mice. Angle Orthod. 82:247–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Andrianakos AA, Kontelis LK, Karamitsos
DG, Aslanidis SI, Georgountzos AI, Kaziolas GO, Pantelidou KV,
Vafiadou EV and Dantis PC: Prevalence of symptomatic knee, hand,
and hip osteoarthritis in Greece. The ESORDIG study. J Rheumatol.
33:2507–2513. 2006.PubMed/NCBI
|
|
34
|
Bennell K, Hinman RS, Wrigley TV, Creaby
MW and Hodges P: Exercise and osteoarthritis: cause and effects.
Compr Physiol. 1:1943–2008. 2011.
|
|
35
|
Zhang Y, Niu J, Kelly-Hayes M, Chaisson
CE, Aliabadi P and Felson DT: Prevalence of symptomatic hand
osteoarthritis and its impact on functional status among the
elderly: The Framingham Study. Am J Epidemiol. 156:1021–1027. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Horisberger M, Fortuna R, Valderrabano V
and Herzog W: Long-term repetitive mechanical loading of the knee
joint by in vivo muscle stimulation accelerates cartilage
degeneration and increases chondrocyte death in a rabbit model.
Clin Biomech (Bristol, Avon). 28:536–543. 2013. View Article : Google Scholar
|
|
37
|
Ren G, Ferreri M, Wang Z, Su Y, Han B and
Su J: Sodium fluoride affects proliferation and apoptosis through
insulin-like growth factor I receptor in primary cultured mouse
osteoblasts. Biol Trace Elem Res. 144:914–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Z, Yang X, Yang S, Ren G, Ferreri M,
Su Y, Chen L and Han B: Sodium fluoride suppress proliferation and
induce apoptosis through decreased insulin-like growth factor-I
expression and oxidative stress in primary cultured mouse
osteoblasts. Arch Toxicol. 85:1407–1417. 2011. View Article : Google Scholar
|
|
39
|
Thrane EV, Refsnes M, Thoresen GH, Låg M
and Schwarze PE: Fluoride-induced apoptosis in epithelial lung
cells involves activation of MAP kinases p38 and possibly JNK.
Toxicol Sci. 61:83–91. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun Z, Niu R, Wang B, Jiao Z and Wang J,
Zhang J, Wang S and Wang J: Fluoride-induced apoptosis and gene
expression profiling in mice sperm in vivo. Arch Toxicol.
85:1441–1452. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan Q, Zhang Y, Li W and Denbesten PK:
Micromolar fluoride alters ameloblast lineage cells in vitro. J
Dent Res. 86:336–340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qu WJ, Zhong DB, Wu PF, Wang JF and Han B:
Sodium fluoride modulates caprine osteoblast proliferation and
differentiation. J Bone Miner Metab. 26:328–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yudoh K, Nakamura H, Masuko-Hongo K, Kato
T and Nishioka K: Catabolic stress induces expression of
hypoxia-inducible factor (HIF)-1 alpha in articular chondrocytes:
involvement of HIF-1 alpha in the pathogenesis of osteoarthritis.
Arthritis Res Ther. 7:R904–R914. 2005. View
Article : Google Scholar : PubMed/NCBI
|