Introduction
Inflammation in the brain, characterized by the
activation of microglia, has been closely associated with the
pathogenesis of Parkinson’s disease (PD), as well as several other
neurodegenerative disorders, including Alzheimer’s disease
(1,2). Microglia, the resident immune cells
in the brain, play a role in immune surveillance and host defense
under normal conditions (3).
However, in response to injury, infection, or inflammation,
microglia become readily activated and secrete a variety of
proinflammatory factors, including cytokines such as tumor necrosis
factor-α (TNF-α) and interleukin-1β (IL-1β), as well as the free
radicals, nitric oxide (NO) and reactive oxygen species (ROS). The
accumulation of these proinflammatory factors is considered to
contribute to the degeneration of dopaminergic neurons (4–8).
As the midbrain region that encompasses the substantia nigra is
particularly rich in microglia (6), the activation of microglia and the
release of proinflammatory factors may be a crucial component of
the degenerative process of dopaminergic neurons in PD. Hence, the
identification of compounds that inhibit microglial activation and
the release of proinflammatory factors is highly desirable in the
search for therapeutic agents for inflammation-mediated
neurodegenerative diseases, including PD.
In the investigation of more potent
anti-inflammatory agents, natural compounds have received much
attention in recent years (9,10).
Biochanin A, an O-methylated isoflavone, is a natural
organic compound and is classified as a phytoestrogen, due to its
structural similarity to estrogen and its estrogen-like activities.
It can be found predominantly in legume plants, such as chickpeas
and red clover. A number of studies have demonstrated that
biochanin A exerts beneficial effects on human health, including
the prevention of cancers, heart disease, menopausal symptoms and
osteoporosis (11,12,13). In our previous study, we found
that biochanin A inhibited the lipopolysaccharide (LPS)-induced
activation of microglia and the production of TNF-α, NO and
superoxide in mesencephalic neuron-glia cultures and
microglia-enriched cultures (11). However, the mechanisms underlying
the anti-inflammatory effects of biochanin A are not yet fully
understood.
In the present study, using murine BV2 microglial
cell cultures, we further investigated the potential
anti-inflammatory effects of biochanin A, as well as the possible
mechanisms involved.
Materials and methods
Materials
All reagents used for cell culture were purchased
from Gibco (Carlsbad, CA, USA). Biochanin A, LPS and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Griess
reagent and TRIzol RNA extraction reagent were provided by the
Beyotime Institute of Biotechnology (Haimen, China). RT-PCR
reagents were purchased from Thermo Scientific (Waltham, MA, USA).
Primary antibody specific for inducible NO synthase (iNOS; 2982s)
was obtained from Cell Signaling Technology (Danvers, MA, USA).
Primary antibodies against extracellular signal-regulated kinase
(ERK; BS6426), phosphorylated (p)-ERK (AP0484), c-Jun NH2-terminal
kinase (JNK; BS6706), p-JNK (BS4763), p38 (BS3566) and p-p38
(BS4635) were obtained from Bioworld Technology (St. Louis Park,
MN, USA). A secondary antibody for goat anti-rabbit immunoglobulin
(IgG) horseradish peroxidase (HRP) was acquired from Zhongshan
Biotechnology Corp. (Beijing, China). The TNF-α and IL-1β ELISA
kits were obtained from BD Biosciences (San Jose, CA, USA). Other
chemicals and reagents used in this study were of analytical
grade.
Cell culture and MTT assay
The BV-2 microglial cell line was obtained from
Shanghai Fuxiang Biological Corp. (Shanghai, China). The cells were
seeded in 96-well culture plates at 1×105 cells/well.
The cell cultures were maintained at 37°C in a humidified
atmosphere of 5% CO2 and 95% air in Dulbecco’s modified
Eagle’s medium/nutrient F12 (DMEM/F12) containing 10% fetal bovine
serum (FBS) and antibiotics (100 U/ml penicillin and 100
μg/ml streptomycin). For cell viability assay, the cells
were incubated with serum-free medium for 24 h, followed by
treatment with various concentrations of LPS or biochanin A.
Following incubation for 36 h, 100 μl of MTT (0.5 mg/ml
final concentration) were added and incubation was continued for a
further 4 h. The formazan crystals in each well were dissolved in
dimethyl sulfoxide (DMSO), and the absorbance was measured at 490
nm using an enzyme-linked immunosorbent assay (ELISA) microplate
reader. The absorbance of the untreated control group was also
measured in order to calculate relative cell viability.
Measurement of proinflammatory cytokine
production
The production of proinflammatory cytokines was
determined using an ELISA kit. BV2 cells were treated with various
concentrations of biochanin A and stimulated with LPS for 36 h. The
culture medium was collected and the concentrations of TNF-α and
IL-1β were measured according to the manufacturer’s instructions
with quantikine mouse TNF-α and IL-1β immunoassay.
Determination of NO production
The BV2 cells were pretreated with various
concentrations of biochanin A (1.25, 2.5, 5 μM) for 30 min
and then treated with LPS (10 μg/ml) for an additional 36 h.
The production of NO was measured by Griess reaction as previously
described (14). Briefly, after
36 h of treatment with LPS (10 μg/ml) and various
concentrations of biochanin A, 50 μl of culture supernatant
from each sample were mixed with an equal volume of Griess reagent
[1% sulfanilamide/0.1% N-(1-naphthyl)-ethylenediamine
dihydrochloride/2.5% phosphoric acid]. Following incubation for 15
min, the absorbance values were read at 540 nm using an ELISA
microplate reader. The nitrite content was calculated compared with
that of standard concentrations of sodium nitrite dissolved in
DMEM.
Measurement of intracellular ROS by
2′,7′-dichlorofluorescein diacetate (DCFH-DA) assay
The intracellular formation of ROS was assessed
using the non-fluorescent probe, DCFH-DA. DCFH-DA passively
diffuses into cells and is deacetylated by esterases to form
non-fluorescent DCFH. DCFH reacts with ROS to form the fluorescent
product, DCF, which is trapped inside the cells. The BV2 cells were
seeded at a density of 1×105 cells/well in a 96-plate
and pre-treated with various concentrations of biochanin A for 30
min, then treated with LPS (10 μg/ml) for an additional 36
h. The culture medium was first removed and the cells were washed
with PBS 3 times. DCFH-DA, diluted to a final concentration of 10
μM with DMEM/F12, was added to the culture medium and
incubated at 37°C for 20 min in the dark. After washing the cells 3
times with serum-free medium, the cells were visualized using an
inverted fluorescence microscope [Olympus Opticals, Tokyo, Japan;
excitation (Ex)/emission (Em), 352/461 nm]. Six continual fields in
each group were used for quantitative analysis. By using the
Image-Pro Plus 6.0 analysis system, the fluorescence intensity in
each group was measured to indicate the production of ROS.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
The BV2 cells (16×105 cells/well in
6-plate) were treated with LPS in the presence or absence of
biochanin A, and total RNA was extracted using TRIzol reagent
according to the manufacturer’s instructions. The yield and purity
of the RNA preparations were examined spectrophotometrically at 260
nm and 280 nm, respectively. An equal amount of RNA (2 μg)
was used for each cDNA synthesis reaction using a reverse
transcription system. The following primers derived from the
published cDNA sequences were used for the PCR amplifications:
IL-1β forward, 5′-CTC CAT GAG CTT TGT ACA AGG-3′ and reverse,
5′-TGC TGA TGT ACC AGT TGG GG-3′; iNOS forward, 5′-CCC TTC CGA AGT
TTC TGG CAG CAG C-3′ and reverse, 5′-GGC TGT CAG AGC CTC GTG GCT
TTG G-3′; TNF-α forward, 5′-TTC TGT CTA CTG AAC TTC GGG GTG ATC GGT
CC-3′ and reverse, 5′-GTA TGA GAT AGC AAA TCG GCT GAC GGT GTG
GG-3′; GAPDH forward, 5′-GGT GAA GGT CGG TGT GAA CG-3′ and reverse,
5′-TTG GCT CCA CCC TTC AAG TG-3′. The products were inspected
visually on a 1% precast agarose gel with ethidium bromide staining
and the bands were quantified by densitometry. Ratios were
calculated for the IL-1β, iNOS and TNF-α signals with the control
signals from GAPDH. The averages from these ratios were
presented.
Western blot analysis
Following treatment, the BV2 cells were rinsed in
ice-cold PBS and lysed in RIPA buffer (Invitrogen, Amersham, UK).
The protein concentration was determined by bicinchoninic acid
(BCA) assay (Wuhan Boster Biological Technology, Ltd., Wuhan,
China). The extracted protein samples were separated by 12%
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred
onto polyvinylidene difluoride (PVDF) membranes (Millipore Corp.,
Billerica, MA, USA). The membranes were incubated with blocking
solution (5% skim milk) to block non-specific protein binding,
followed by incubation with the following primary antibodies at 4°C
overnight: β-actin, p-JNK, JNK, p-ERK, ERK, p-p38, p38, iNOS. The
membranes were incubated with a horseradish peroxidase
(HRP)-conjugated secondary antibody at room temperature for 2 h,
and then specific protein bands were detected using the
ECL-chemiluminescence kit (Thermo Scientific) according to the
manufacturer’s instructions. The immunoblot bands were visualized
using a Bioshine ChemiQ 4600 mini Chemiluminescence imaging system
(Bioshine, Shanghai, China) and protein expression was quantified
using ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
The data are expressed as the means ± SD (n=3).
Statistical significance was assessed by one-way ANOVA, Duncan’s
multiple range tests were used to determine significant differences
between groups. A value of p<0.05 was considered to indicate a
statistically significant difference.
Results
Cytotoxic effects of biochanin A and LPS
on BV2 microglial cells
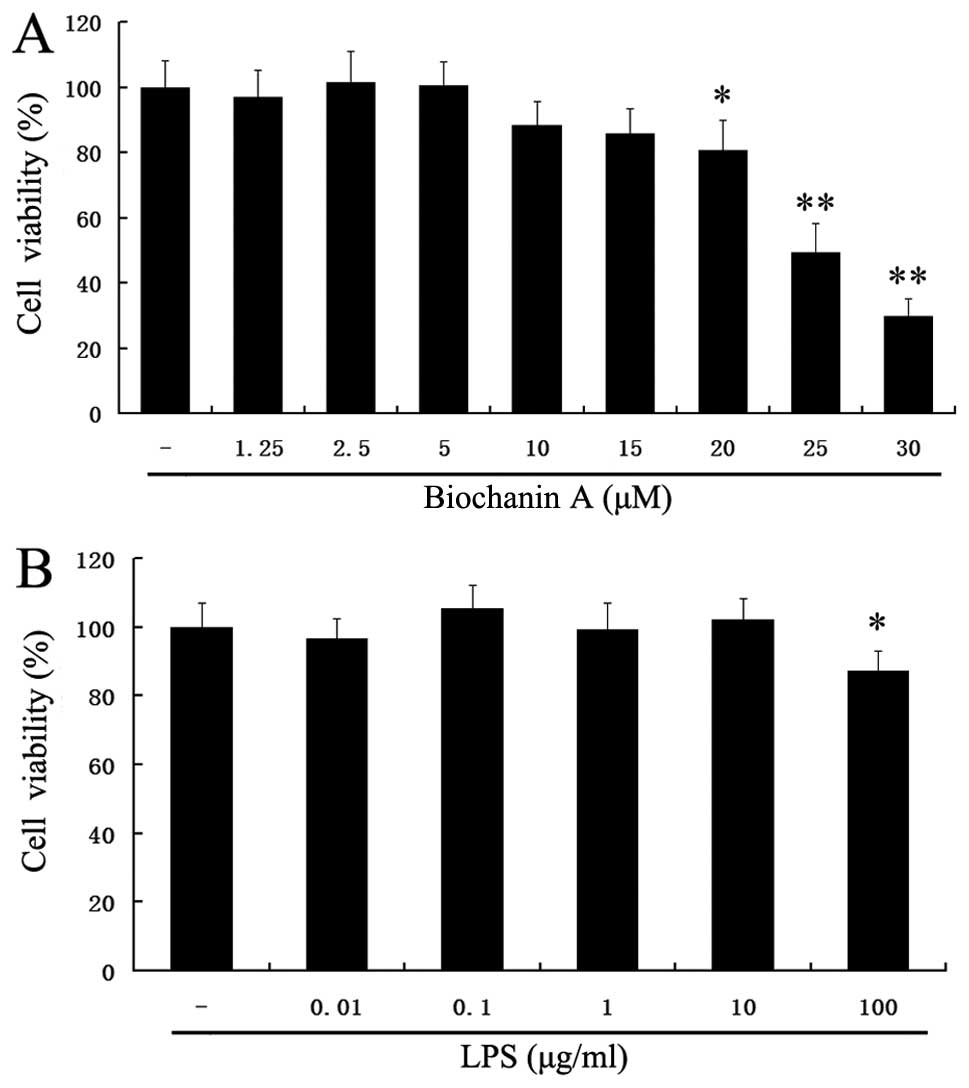
To exclude the cytotoxicity caused by biochanin A
treatment, the BV2 microglial cells were treated with biochanin A
at concentrations of 1.25–30 μM for 36 h. The viability of
the BV2 microglial cells was measured by MTT assay. At the examined
concentrations of 1.25–5 μM for 36 h, biochanin A did not
affect cell viability (Fig. 1A).
However, treatment with concentrations >5 μM of biochanin
A decreased cell viability. Thus, we selected the dose of 1.25–5
μM of biochanin A for further experiments.
Furthermore, as we used LPS to induce microglial
inflammatory responses, it was necessary to ascertain the non-toxic
concentration of LPS in BV2 cells. Treatment with LPS (0.01–10
μg/ml) did not affect the cell viability (p>0.05)
(Fig. 1B). In addition, our
preliminary experimental results suggested that 10 μg/ml LPS
markedly induced proinflammatory cytokine production (data not
shown). Therefore, we selected the dose of 10 μg/ml of LPS
for further experiments.
Effects of biochanin A on LPS-induced
morphological changes in BV2 cells
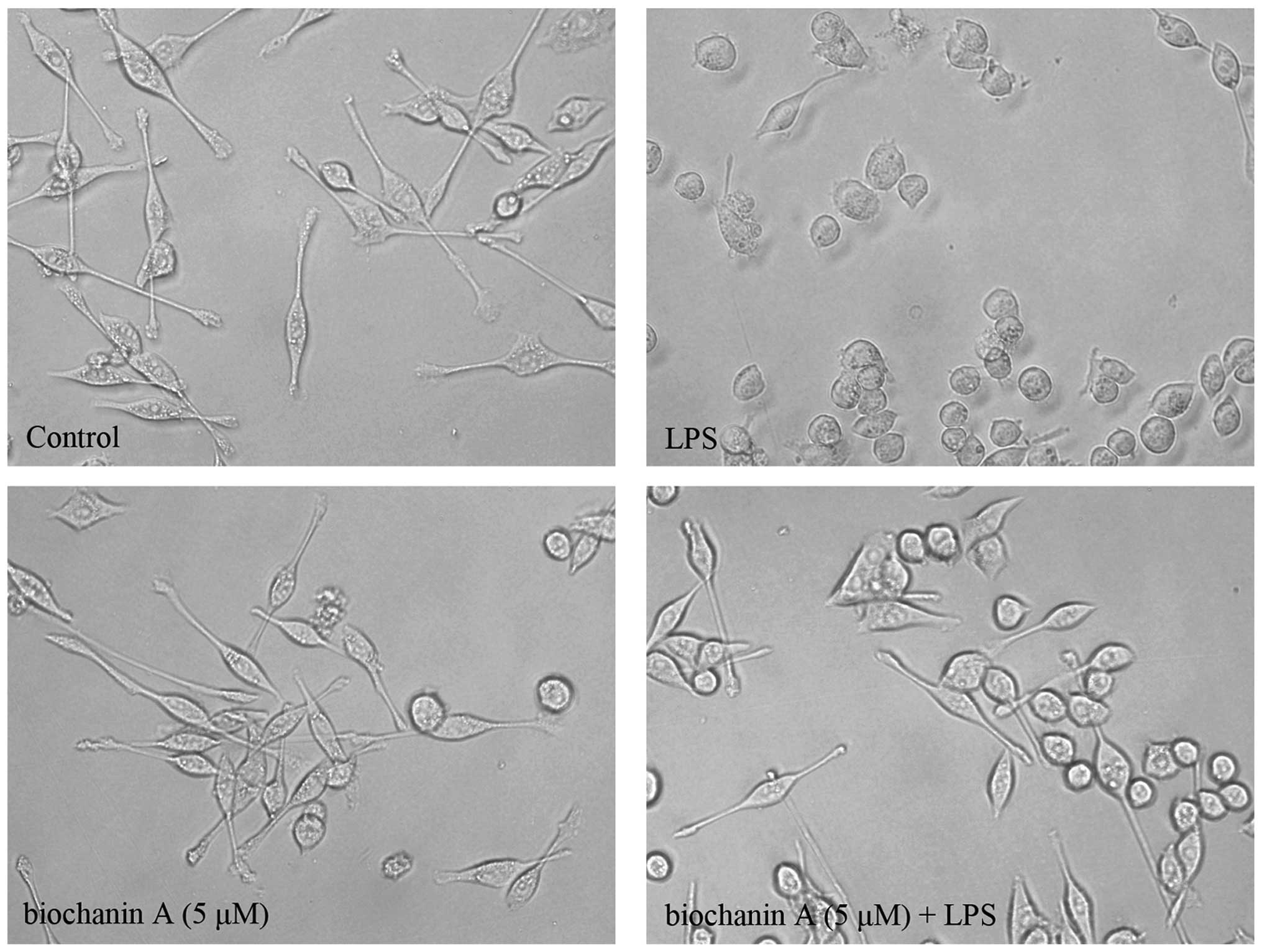
We also observed the morphological changes occurring
in microglial activation following treatment with LPS. As shown in
Fig. 2, the microglia exhibited
the typical ramified morphology of resting microglia in the control
group. Following treatment with LPS (10 μg/ml; 36 h), the
microglia became activated with a greatly enlarged cell body and
the characteristic shapes of activated microglia. However,
pretreatment with biochanin A (5 μM) significantly
attenuated the LPS-induced microglial activation.
Effects of biochanin A on LPS-induced
TNF-α and IL-1β production and mRNA expression
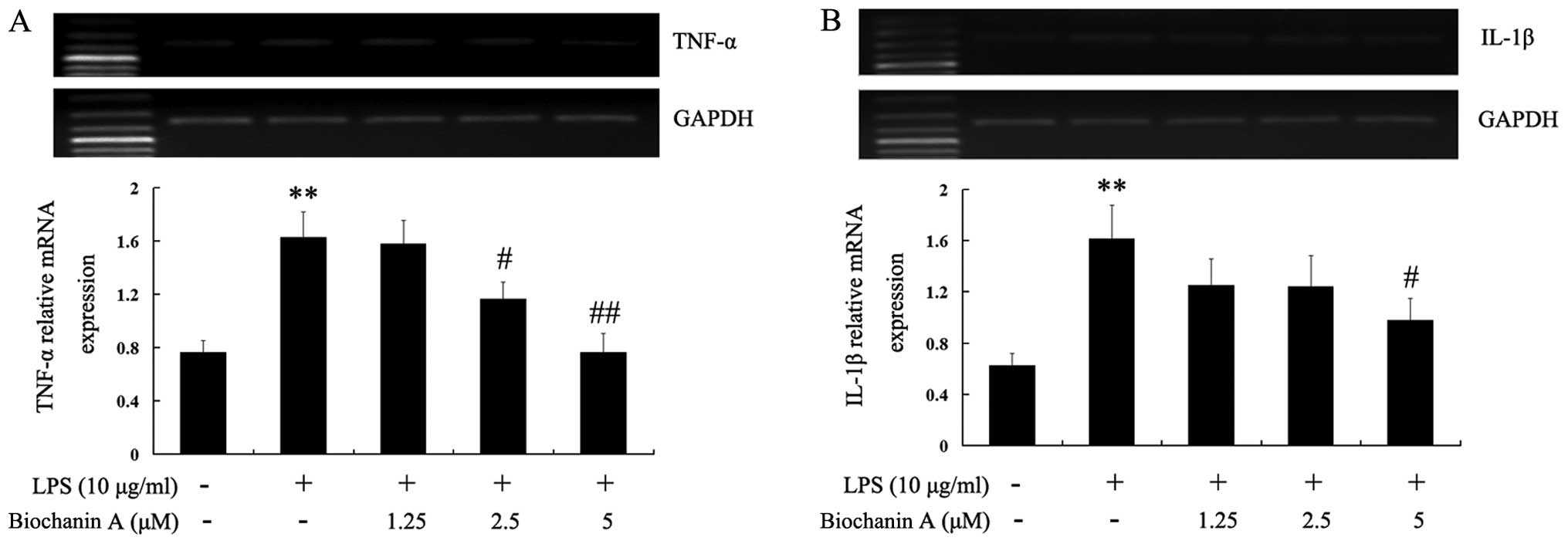
Activated microglia are known to be a major source
of various pro-inflammatory cytokines, such as TNF-α and IL-1β
(15). In this study, we examined
whether biochanin A reduces the generation of pro-inflammatory
cytokines induced by LPS stimulation. As shown in Table I, the TNF-α and IL-1β levels were
significantly increased in the culture medium of LPS-stimulated BV2
microglial cells. Pre-treatment with 1.25–5 μM biochanin A
significantly inhibited the production of TNF-α and IL-1β in a
dose-dependent manner. To elucidate the mechanisms responsible for
the inhibitory effects of biochanin A on TNF-α and IL-1β
production, we examined the cytokine mRNA expression levels by
RT-PCR. Consistent with the results obtained from cytokine
production, the LPS-induced mRNA levels of TNF-α and IL-1β were
reduced by biochanin A (Fig. 3),
suggesting that biochanin A negatively regulated the production of
TNF-α and IL-1β at the transcriptional level in the LPS-stimulated
microglial cells.
 | Table IEffects of biochanin A on
pro-inflammatory cytokine production in LPS-stimulated BV2
microglial cells. |
Table I
Effects of biochanin A on
pro-inflammatory cytokine production in LPS-stimulated BV2
microglial cells.
| Groups | Pro-inflammatory
cytokine concentration
|
|---|
| TNF-α (pg/ml) | IL-1β (pg/ml) |
|---|
| Control | 107.82±18.14 | 54.12±9.413 |
| LPS (10
μg/ml) |
2269.75±62.38a |
213.12±15.64a |
| Biochanin A (1.25
μM) + LPS |
2037.37±66.56b | 203.65±14.13 |
| Biochanin A (2.5
μM) + LPS |
1776.62±47.71c |
170.80±13.34b |
| Biochanin A (5
μM) + LPS |
1577.67±50.84c |
147.09±14.36b |
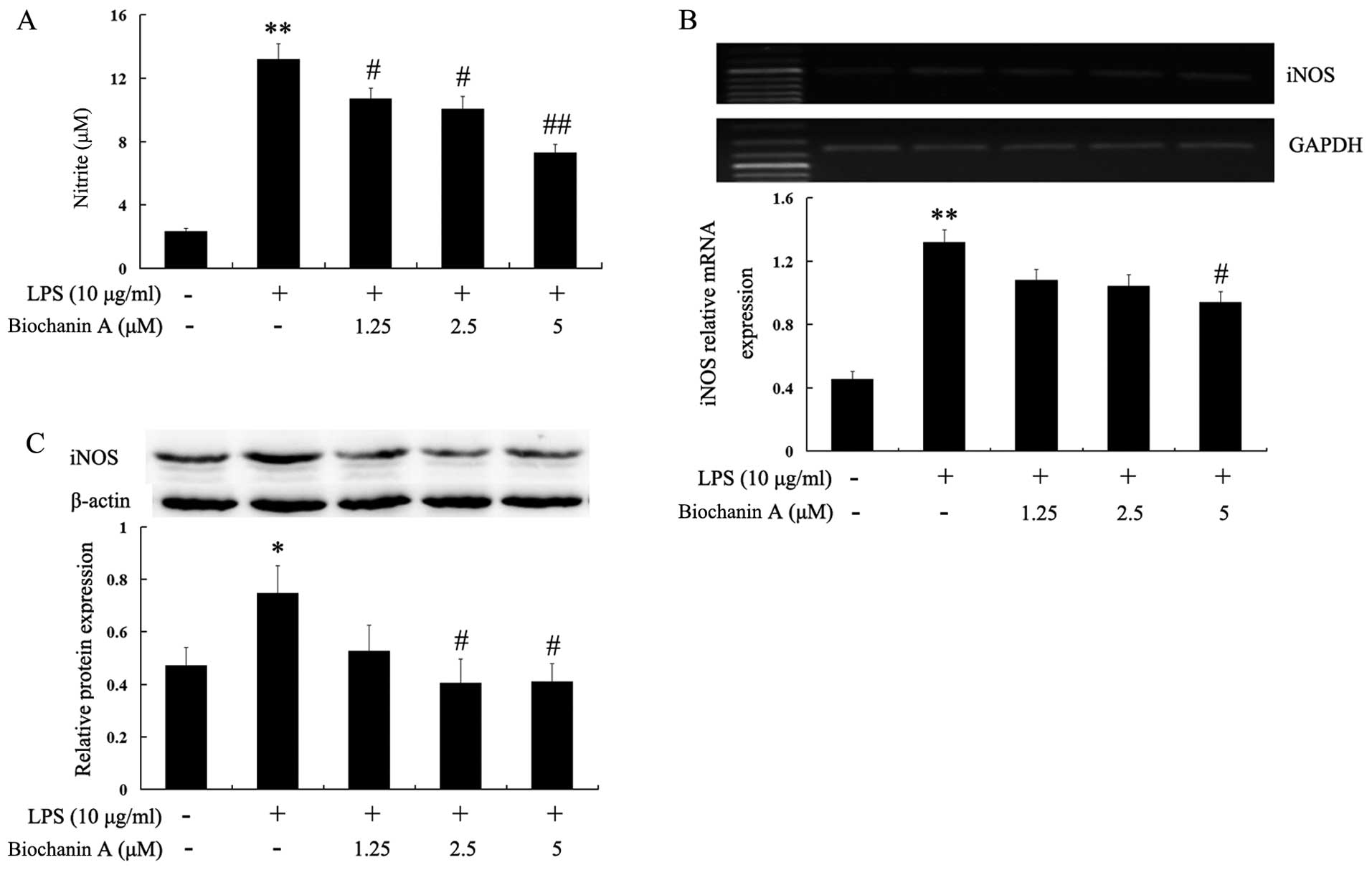
Effects of biochanin A on the production
of NO and the expression of iNOS
NO is generated from L-arginine by iNOS, and high
levels of NO in the brain have been associated with the progression
of various neurodegenerative diseases (16). In this study, to investigate the
effects of biochanin A on NO production in LPS-stimulated BV2
cells, the BV2 cells were pre-treated with biochanin A (1.25–5
μM) for 30 min and then stimulated with LPS (10
μg/ml) for another 36 h. The levels of nitrite in the
culture medium were determined by Griess assay. The accumulation of
nitrite in the culture supernatant was an indicator of the
production of NO. Biochanin A significantly decreased the
LPS-induced production of NO in the BV2 cells in a dose-dependent
manner (Fig. 4A). Subsequently,
to elucidate the mechanisms responsible for the inhibitory effects
of biochanin A on NO production, we determined the mRNA and protein
levels of iNOS by RT-PCR and western blot analysis, respectively.
As shown in Fig. 4B and C,
biochanin A effectively inhibited the mRNA and protein expression
of iNOS in the LPS-stimulated BV2 cells. These results indicated
that biochanin A inhibited NO production through the downregulation
of the mRNA and protein expresion of iNOS in LPS-stimulated BV2
microglial cells.
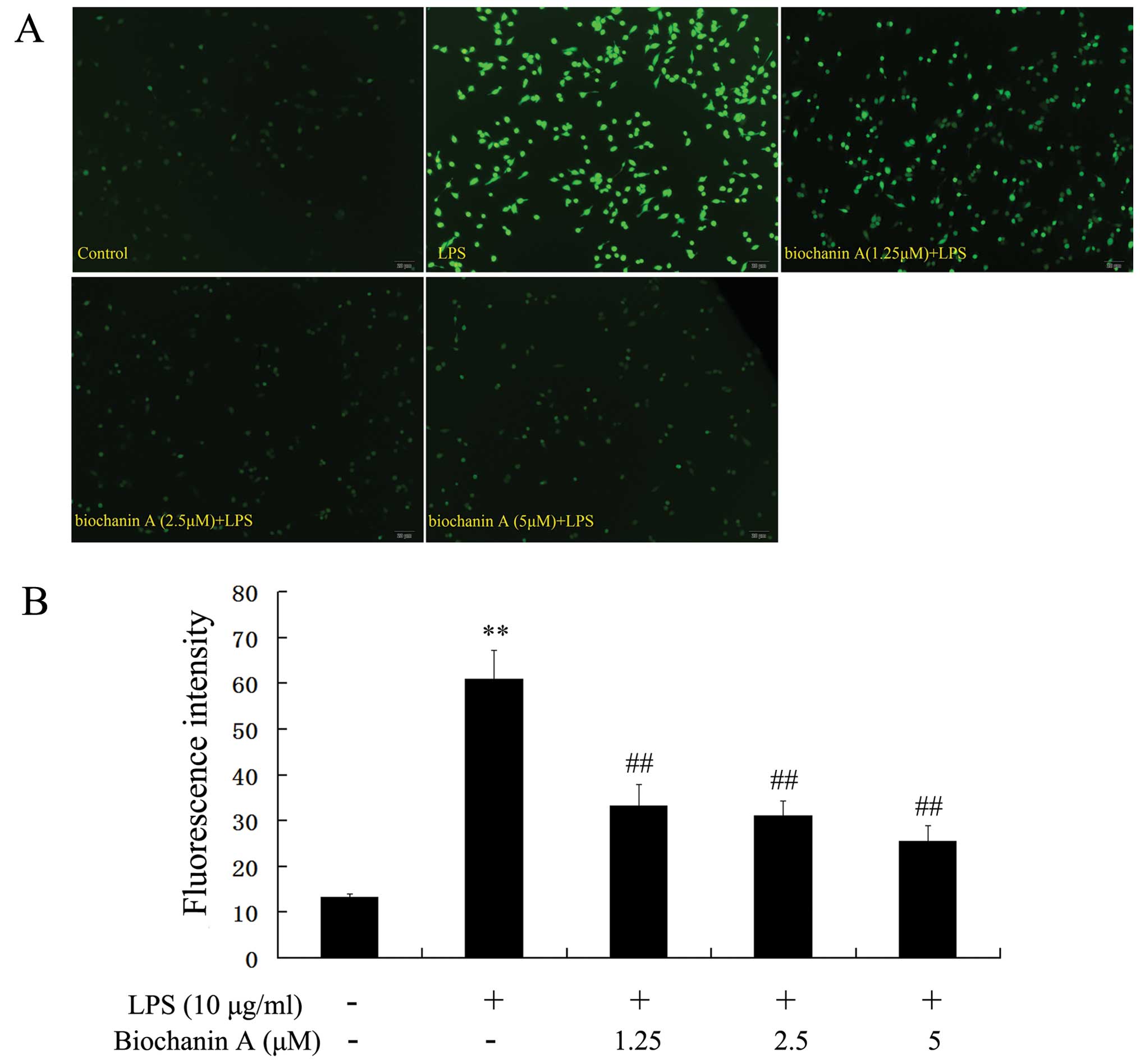
Effects of biochanin A on intracellular
ROS production
To determine whether the neuroprotective effects of
biochanin A are due to a reduction in ROS production, the
intracellular ROS production in BV2 cells was measured by DCFH-DA.
As shown in Fig. 5, following
treatment with LPS, the DCF fluorescence intensity was
significantly increased. However, pre-treatment with biochanin A
(1.25–5 μM) attenuated the LPS-induced fluorescence
intensity in the BV2 cells in a dose-dependent manner. These
results confirm that biochanin A exerts a marked inhibitory effect
on intracellular ROS production.
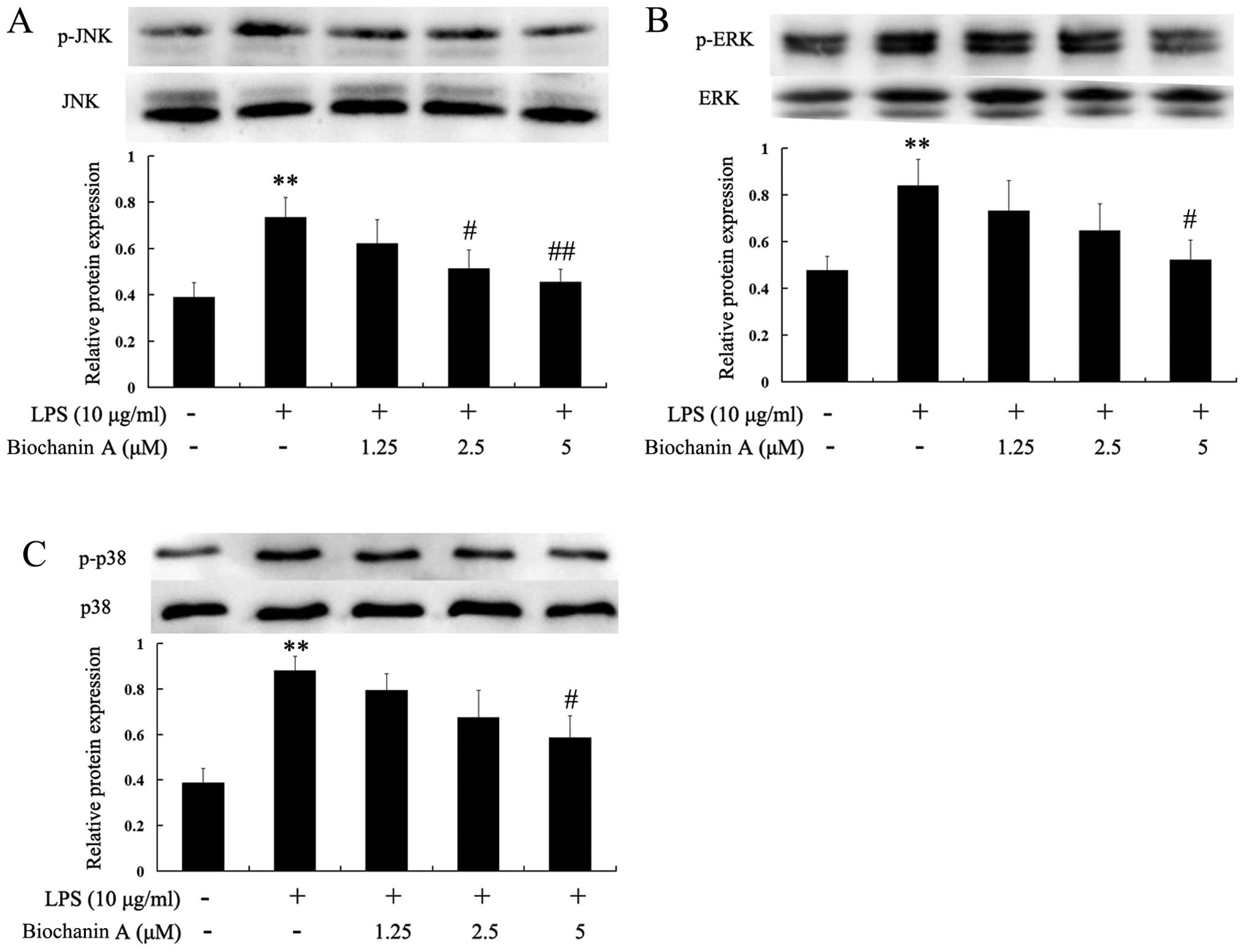
Effects of biochanin A on the
phosphorylation of JNK, ERK and p38
Mitogen-activated protein kinase (MAPK) signaling is
known to be a major regulator of pro-inflammatory cytokines in
LPS-stimulated microglia (17,18). In order to investigate whether
biochanin A inhibits the production of pro-inflammatory cytokines
through the MAPK signaling pathway, we examined the effects of
biochanin A on the LPS-induced phosphorylation of JNK, ERK and p38
in BV2 microglia by western blot analysis. As shown in Fig. 6, stimulation with LPS markedly
increased the phosphorylation of JNK, ERK and p38. However,
pre-treatment with biochanin A (5 μM) markedly inhibited the
phosphorylation of JNK, ERK and p38. These results suggest that the
phosphorylation of JNK, ERK and p38 is involved in the inhibitory
effects of biochanin A on LPS-induced proinflammatory cytokine
production in BV2 microglial cells.
Discussion
Biochanin A, an isoflavone compound, is found
predominantly in chickpeas and red clover. In our previous study,
we demonstrated that biochanin A inhibited the LPS-induced
activation of microglia and the production of TNF-α, NO and
superoxide in mesencephalic neuron-glia cultures and
microglia-enriched cultures (11). However, the mechanisms underlying
the anti-inflammatory effects of biochanin A are not yet fully
understood. Therefore, in this study, in order to elucidate the
molecular mechanisms responsible for the anti-inflammatory effects
of biochanin A, we further investigated the inhibitory effects of
biochanin A on the production of pro-inflammatory factors induced
by LPS in murine BV2 microglial cells. Our results demonstrated
that biochanin A significantly inhibited the LPS-induced microglial
activation and the release of TNF-α, IL-1β, NO and ROS. Biochanin A
also significantly attenuated the LPS-induced mRNA and protein
expression of TNF-α, IL-1β and iNOS. Furthermore, biochanin A
significantly inhibited the LPS-induced phosphorylation of JNK, ERK
and p38 in BV2 microglial cells.
Microglia are the resident immune cells of the
brain. In response to injury or infection, microglia become readily
activated and consequently release pro-inflammatory cytokines, free
radicals and eicosanoids. These factors are believed to contribute
to microglia-mediated neurodegeneration (2,19,20). Therefore, the inhibition of
microglial activation may prove to be an effective therapeutic
approach for alleviating the progression of neurodegenerative
diseases, such as PD and Alzheimer’s disease. In the present study,
our results indicated that biochanin A significantly inhibited the
LPS-induced microglial activation and the release of
pro-inflammatory factors.
Pro-inflammatory cytokines, such as TNF-α and IL-1β,
have been shown to induce neuronal cell damage; therefore, the
suppression of their production is important for the prevention of
neurodegenerative diseases. Microglia are the primary producers of
TNF-α in the brain and play a role in a number of pathological
conditions in the brain (21).
TNF-α overexpression has been implicated in the pathogenesis of
several human disorders of the central nervous system (CNS)
(22–25). IL-1β is a potent pro-inflammatory
cytokine that acts through IL-1 receptors found on numerous cell
types, including neurons and microglia. Moreover, the importance of
IL-1β as a mediator of neuroimmune interactions that participate
directly in neurodegeneration has been demonstrated (26). In this study, our results revealed
that the production of the pro-inflammatory cytokines, TNF-α and
IL-1β, was significantly inhibited by biochanin A in a
dose-dependent manner. Moreover, the LPS-stimulated mRNA expression
levels of TNF-α and IL-1β were also reduced by biochanin A,
suggesting that biochanin A suppressed the production of TNF-α and
IL-1β through the downregulation of their gene expression in the
LPS-stimulated microglial cells.
In mammalian cells, NO is synthesized from three
different isoforms of NOS: endothelial NOS (eNOS), neuronal NOS
(nNOS) and iNOS. Activated microglia are a major cellular source of
iNOS in the brain. The excessive release of NO by activated
microglia correlates with the progression of neuro-degenerative
disorders. The excessive accumulation of NO has long been known to
be toxic to neurons (8,27,28). The generation of NO has been
implicated in a variety of neuroinflammatory and neurodegenerative
disorders. In the brain, iNOS is the main contributor to NO
production following an inflammatory assault, compared with the
other isoforms of NOS (29,30). Accumulating evidence indicates
that the expression of iNOS during inflammation in the CNS plays an
important role in the neurodegeneration in PD (31). It has been reported that activated
microglia cause neuronal death with mechanisms involving the
expression of iNOS and the release of NO in mixed neuron-glia
co-cultures (19). This study
also demonstrated that LPS induced the activation of microglia,
which subsequently led to the upregulation of NO production and
iNOS expression. However, pre-treatment with biochanin A exerted a
significant inhibitory effect on the LPS-induced the production of
NO and attenuated the expression of iNOS in a dose-dependent
manner.
Previous studies have suggested that LPS increases
the levels of intracellular ROS, which serve as second messengers
to enhance the LPS-induced expression of genes encoding a variety
of pro-inflammatory factors (32,33). ROS, including superoxide anion,
hydroxyl radical, lipid hydroperoxides and their byproducts (e.g.,
hydrogen peroxide), may play an important role in the pathogenesis
of neurodegenerative diseases. ROS generated by activated microglia
are toxic to neurons by inducing lipid peroxidation, DNA
fragmentation and protein oxidation (34). Moreover, oxygen free radicals,
such as superoxide can react with NO to form much more deadly
intermediates, such as peroxynitrite (35). In fact, a previous study
identified peroxynitrite as a key mediator of neurotoxicity induced
by LPS-activated microglia (36).
In this study, our results demonstrated that LPS increased
intracellular ROS production in BV2 cells. However, pre-treatment
with biochanin A attenuated the LPS-induced intracellular ROS
production in a dose-dependent manner.
The mammalian MAPK family consists of ERK, p38, and
JNK (37). Previous studies have
demonstrated that MAPKs are involved in the regulation of iNOS,
COX-2, TNF-α, IL-1β expression in microglia (38–42). In this study, we found that
biochanin A inhibited the LPS-induced phosphorylation of JNK, ERK
and p38. In addition, we found that biochanin A inhibited the gene
expression of iNOS, TNF-α and IL-1β in LPS-stimulated BV2
microglia, the mechanisms of which at least in part, may involve
the inhibition of MAPKs. Taken together, the results from the
present study suggest that the inhibition of the MAPK pathway may
be a molecular mechanism underlying the anti-inflammatory effects
of biochanin A in LPS-stimulated BV2 microglial cells.
In conclusion, to the best of our knowledge, in this
study, we demonstrate for the first time that biochanin A markedly
attenuates LPS-induced microglial activation and pro-inflammatory
responses in BV2 microglial cells. Biochanin A also inhibited the
production of IL-1β, TNF-α, NO and ROS, which may be mediated
through the inhibition of MAPK signaling pathways in BV2 cells.
These observations suggested that biochanin A may be considered as
a potential anti-inflammatory agent. However, further in
vivo investigations using animal models are required to confirm
the effectiveness of biochanin A.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (NSFC) fund (grant no. 31171650), the
National Basic Research Program of China (973 program, no.
2012CB525003) and the Natural Science Foundation of Anhui Province
Education Department (Grant no. KJ2014A115).
References
|
1
|
Liu B and Hong JS: Role of microglia in
inflammation-mediated neurodegenerative diseases: mechanisms and
strategies for therapeutic intervention. J Pharmacol Exp Ther.
304:1–7. 2003. View Article : Google Scholar
|
|
2
|
McGeer PL, Itagaki S, Boyes BE and McGeer
EG: Reactive microglia are positive for HLA-DR in the substantia
nigra of Parkinson’s and Alzheimer’s disease brains. Neurology.
38:1285–1291. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kreutzberg GW: Microglia: a sensor for
pathological events in the CNS. Trends Neurosci. 19:312–318. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao HM, Hong JS, Zhang W and Liu B:
Distinct role for microglia in rotenone-induced degeneration of
dopaminergic neurons. J Neurosci. 22:782–790. 2002.PubMed/NCBI
|
|
5
|
Gayle DA, Ling Z, Tong C, Landers T,
Lipton JW and Carvey PM: Lipopolysaccharide (LPS)-induced dopamine
cell loss in culture: roles of tumor necrosis factor-alpha,
interleukin-1beta, and nitric oxide. Brain Res Dev Brain Res.
133:27–35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu
B and Hong JS: Regional difference in susceptibility to
lipopolysaccharide-induced neurotoxicity in the rat brain: role of
microglia. J Neurosci. 20:6309–6316. 2000.PubMed/NCBI
|
|
7
|
Liu B, Du L and Hong JS: Naloxone protects
rat dopaminergic neurons against inflammatory damage through
inhibition of microglia activation and superoxide generation. J
Pharmacol Exp Ther. 293:607–617. 2000.PubMed/NCBI
|
|
8
|
Liu B, Gao HM, Wang JY, Jeohn GH, Cooper
CL and Hon JS: Role of nitric oxide in inflammation-mediated
neurodegeneration. Ann NY Acad Sci. 962:318–331. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jung WK, Ahn YW, Lee SH, Choi YH, Kim SK,
Yea SS, Choi I, Park SG, Seo SK, Lee SW and Choi IW: Ecklonia cava
ethanolic extracts inhibit lipopolysaccharide-induced
cyclooxygenase-2 and inducible nitric oxide synthase expression in
BV2 microglia via the MAP kinase and NF-kappaB pathways. Food Chem
Toxicol. 47:410–417. 2009. View Article : Google Scholar
|
|
10
|
Lei M, Wang JG, Xiao DM, Fan M, Wang DP,
Xiong JY, Chen Y, Ding Y and Liu SL: Resveratrol inhibits
interleukin 1β-mediated inducible nitric oxide synthase expression
in articular chondrocytes by activating SIRT1 and thereby
suppressing nuclear factor-κB activity. Eur J Pharmacol. 674:73–79.
2012. View Article : Google Scholar
|
|
11
|
Chen HQ, Jin ZY and Li GH: Biochanin A
protects dopaminergic neurons against lipopolysaccharide-induced
damage through inhibition of microglia activation and
proinflammatory factors generation. Neurosci Lett. 417:112–117.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Puthli A, Tiwari R and Mishra KP:
Biochanin A enhances the radiotoxicity in colon tumor cells in
vitro. J Environ Pathol Toxicol Oncol. 32:189–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su SJ, Yeh YT and Shyu HW: The preventive
effect of biochanin a on bone loss in ovariectomized rats:
involvement in regulation of growth and activity of osteoblasts and
osteoclasts. Evid Based Complement Alternat Med.
2013:5948572013.PubMed/NCBI
|
|
14
|
Green LC, Wagner DA, Glogowski J, Skipper
PL, Wishnok JS and Tannenbaum SR: Analysis of nitrate, nitrite, and
[15N]nitrate in biological fluids. Anal Biochem. 126:131–138. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruce-Keller AJ, Keeling JL, Keller JN,
Huang FF, Camondola S and Mattson MP: Antiinflammatory effects of
estrogen on microglial activation. Endocrinology. 141:3646–3656.
2000.PubMed/NCBI
|
|
16
|
Boje KM: Nitric oxide neurotoxicity in
neurodegenerative diseases. Front Biosci. 9:763–776. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han IO, Kim KW, Ryu JH and Kim WK: p38
mitogen-activated protein kinase mediates lipopolysaccharide, not
interferon-gamma, -induced inducible nitric oxide synthase
expression in mouse BV2 microglial cells. Neurosci Lett. 325:9–12.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim M, Li YX, Dewapriya P, Ryu B and Kim
SK: Floridoside suppresses pro-inflammatory responses by blocking
MAPK signaling in activated microglia. BMB Rep. 46:398–403. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Le W, Rowe D, Xie W, Ortiz I, He Y and
Appel SH: Microglial activation and dopaminergic cell injury: an in
vitro model relevant to Parkinson’s disease. J Neurosci.
21:8447–8455. 2001.PubMed/NCBI
|
|
20
|
Minghetti L and Levi G: Microglia as
effector cells in brain damage and repair: focus on prostanoids and
nitric oxide. Prog Neurobiol. 54:99–125. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sawada M, Kondo N, Suzumura A and
Marunouchi T: Production of tumor necrosis factor-alpha by
microglia and astrocytes in culture. Brain Res. 491:394–397. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akassoglou K, Bauer J, Kassiotis G, et al:
Oligodendrocyte apoptosis and primary demyelination induced by
local TNF/p55TNF receptor signaling in the central nervous system
of transgenic mice: models for multiple sclerosis with primary
oligodendrogliopathy. Am J Pathol. 153:801–813. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Botchkina GI, Meistrell ME III, Botchkina
IL and Tracey KJ: Expression of TNF and TNF receptors (p55 and p75)
in the rat brain after focal cerebral ischemia. Mol Med. 3:765–781.
1997.
|
|
24
|
Meda L, Cassatella MA, Szendrei GI, et al:
Activation of microglial cells by beta-amyloid protein and
interferon-gamma. Nature. 374:647–650. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sriram K, Matheson JM, Benkovic SA, Miller
DB, Luster MI and O’Callaghan JP: Mice deficient in TNF receptors
are protected against dopaminergic neurotoxicity: implications for
Parkinson’s disease. FASEB J. 16:1474–1476. 2002.PubMed/NCBI
|
|
26
|
Rothwell N, Allan S and Toulmond S: The
role of interleukin 1 in acute neurodegeneration and stroke:
pathophysiological and therapeutic implications. J Clin Invest.
100:2648–2652. 1997. View Article : Google Scholar
|
|
27
|
Chao CC, Hu S, Molitor TW, Shaskan EG and
Peterson PK: Activated microglia mediate neuronal cell injury via a
nitric oxide mechanism. J Immunol. 149:2736–2741. 1992.PubMed/NCBI
|
|
28
|
Jeohn GH, Kim WG and Hong JS: Time
dependency of the action of nitric oxide in
lipopolysaccharide-interferon-gamma-induced neuronal cell death in
murine primary neuron-glia co-cultures. Brain Res. 880:173–177.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iravani MM, Kashefi K, Mander P, Rose S
and Jenner P: Involvement of inducible nitric oxide synthase in
inflammation-induced dopaminergic neurodegeneration. Neuroscience.
110:49–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li FQ, Wang T, Pei Z, Liu B and Hong JS:
Inhibition of microglial activation by the herbal flavonoid
baicalein attenuates inflammation-mediated degeneration of
dopaminergic neurons. J Neural Transm. 112:331–347. 2005.
View Article : Google Scholar
|
|
31
|
Dawson VL and Dawson TM: Nitric oxide in
neuronal degeneration. Proc Soc Exp Biol Med. 211:33–40. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qin L, Liu Y, Wang T, et al: NADPH oxidase
mediates lipopolysaccharide-induced neurotoxicity and
proinflammatory gene expression in activated microglia. J Biol
Chem. 279:1415–1421. 2004. View Article : Google Scholar
|
|
33
|
Wang T, Qin L, Liu B, et al: Role of
reactive oxygen species in LPS-induced production of prostaglandin
E2 in microglia. J Neurochem. 88:939–947. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Farber JL: Mechanisms of cell injury by
activated oxygen species. Environ Health Perspect. 102(Suppl 10):
S17–S24. 1994. View Article : Google Scholar
|
|
35
|
Estevez AG and Jordan J: Nitric oxide and
superoxide, a deadly cocktail. Ann NY Acad Sci. 962:207–211. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie Z, Wei M, Morgan TE, et al:
Peroxynitrite mediates neurotoxicity of amyloid beta-peptide1–42-
and lipopolysaccharide-activated microglia. J Neurosci.
22:3484–3492. 2002.PubMed/NCBI
|
|
37
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bhat NR, Zhang P, Lee JC and Hogan EL:
Extracellular signal-regulated kinase and p38 subgroups of
mitogen-activated protein kinases regulate inducible nitric oxide
synthase and tumor necrosis factor-alpha gene expression in
endotoxin-stimulated primary glial cultures. J Neurosci.
18:1633–1641. 1998.PubMed/NCBI
|
|
39
|
Choi Y, Lee MK, Lim SY, Sung SH and Kim
YC: Inhibition of inducible NO synthase, cyclooxygenase-2 and
interleukin-1beta by torilin is mediated by mitogen-activated
protein kinases in microglial BV2 cells. Br J Pharmacol.
156:933–940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park JS, Woo MS, Kim DH, et al:
Anti-inflammatory mechanisms of isoflavone metabolites in
lipopolysaccharide-stimulated microglial cells. J Pharmacol Exp
Ther. 320:1237–1245. 2007. View Article : Google Scholar
|
|
42
|
Song Y, Qu R, Zhu S, Zhang R and Ma S:
Rhynchophylline attenuates LPS-induced pro-inflammatory responses
through downregulation of MAPK/NF-kappaB signaling pathways in
primary microglia. Phytother Res. 26:1528–1533. 2012.PubMed/NCBI
|