Introduction
Type 2 diabetes mellitus (T2DM) is characterized by
the resistance of target tissues to insulin stimulation (1), which is usually associated with
hyperglycemia, dyslipidemia, obesity, hypertension, fatty liver,
atherosclerosis, certain cancers and cardiovascular diseases
(2). Insulin resistance occurs
when a normal dose of insulin is incapable of eliciting its
metabolic responses (3), which is
caused by multiple defects in intracellular events including an
impairment of the insulin signaling pathway (4–6).
T2DM patients also manifest adipocyte resistance to the
antilipolytic effects of insulin (7,8).
The peroxisome proliferator-activated receptor
(PPARs) family, belonging to the nuclear hormone receptor family,
consists of three isoforms, PPARα, PPARβ/δ and PPARγ (9). PPARγ, mainly expressed in adipose
tissue and vascular tissue/macrophages (10), affects various genes involved in
lipid and glucose homeostasis. PPARγ agonists increase insulin
sensitivity so that they are used for treatment of T2DM. In
addition, the PPARγ agonist promotes adipocyte differentiation and
controls mobilization of lipid into adipocytes by inducing the
expression of such lipid transport genes as adipocyte fatty
acid-binding protein (aP2), thereby reducing lipotoxicity
(11,12). However, several concerns, such as
the weight gain associated with increased excess fat, arise in
PPARγ agonist-treated T2DM patients (13). Accumulating evidence indicates
that the activation of PPARα predominantly expressed in the liver
(14), would stimulate lipid
consumption by enhancing the expression of fatty acid oxidation
genes, resulting in the amelioration of hyperlipidemia. PPARα
agonists have potent effects on the reduction of plasma
triglycerides (15). Due to the
distinct metabolic effects of PPARα and PPARγ agonists on insulin
sensitivity and lipid metabolism, development of novel drugs has
focused on dual PPARs that possess PPARγ and PPARα activities. It
has been proposed that the simultaneous activation of PPARα and
PPARγ would guarantee more desirable effects with alleviated
adverse effects (16–18). Numerous PPARα/γ dual agonists have
been identified and tested in obese and insulin-resistant
individuals; however, the majority of these drugs have shown
unexpected side effects, including weight gain, heart failure,
renal failure, urinary cancer and anemia (19,20). Therefore, the development of novel
PPARα/γ dual agonists with few adverse effects is urgently
required.
Recently, there has been a growing interest in the
therapeutic use of natural compounds to treat metabolic syndrome as
natural compounds may exert their diverse pharmacological
properties by interacting with multiple cellular targets. Recently,
we reported that amorphastilbol (APH) from Amorpha fruticosa
(AF) stimulates transcriptional activities of PPARα/γ (21), and improves glucose and lipid
metabolisms in the diabetic db/db mouse model
(22). To support the
anti-diabetic effects of APH and AF, the present study evaluated
their pharmacological properties in a high-fat-diet (HFD) mouse
model and their effects on insulin sensitivity.
Materials and methods
Cell culture and chemicals
3T3-L1 and C2C12 cells were obtained from American
Type Culture Collection (Manassas, VA, USA) and cultured in
Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS; HyClone
Laboratories, Logan, UT, USA) and 1% penicillin/streptomycin
(Invitrogen). APH was synthesized by Dr J Ham [Korea Institute of
Science and Technology (KIST) Gangneung Institute] for the in
vivo study. Rosiglitazone was purchased from Sigma-Aldrich Co.
(St. Louis, MO, USA).
Extract of AF
The fruit of AF (300 g) was extracted three times
with 95% ethanol and evaporated under vacuum at 40°C. The extract
(20 mg/ml) was reconstituted with 0.8% carboxymethylcellulose
solution.
Animal experiments
All the experiments were performed according to the
procedures approved by the KIST’s Institutional Animal Care and Use
Committee. Seven-week-old male C57BL/6 mice were purchased from the
Shizuoka Laboratory Animal Center (Shizuoka, Japan). The mice were
housed under conditions of 23±2°C and 55±5% humidity with standard
light cycles (12-h light/dark). The C57BL/6 mice were fed a regular
diet (10% kcal fat; 38057; Purina Mills Inc., Gray Summit, MO, USA)
or an HFD (65% kcal fat; 101556; Dyets Inc., Bethlehem, PA, USA)
for 8 weeks. The mice were orally administered 4 mg/kg
rosiglitazone, 200 mg/kg AF or 20 mg/kg APH once a day for 8 weeks
prior to the gene expression or blood biomarker analyses. Glucose
was measured by tail vein bleeds at the indicated time intervals
using an Accu-Chek glucometer (Roche Diagnostics GmbH, Mannheim,
Germany), and the serum insulin concentrations were determined by
the enzyme-linked immunosorbent assay (Shibayagi, Gunma, Japan). At
the end of the experimental period, epididymal white tissue/body
weight ratios were measured, and blood samples were obtained from
the abdominal aorta to determine plasma biomarker
concentrations.
Analysis of plasma biomarkers
After the experiment, blood was collected in tubes
containing 0.18 M ethylenediaminetetraacetic acid (EDTA) and
centrifuged at 5,000 × g for 5 min at 4°C. Following
centrifugation, the plasma was separated for the estimation of the
total cholesterol, low-density lipoprotein (LDL)-cholesterol,
triglycerides and free fatty acids. The total cholesterol levels
were measured by enzymatic methods using SICDIA L T-CHO reagents
(Eiken Chemical, Tokyo, Japan), and the LDL-cholesterol levels were
determined by enzymatic methods using L-Type LDL-C reagents (Wako
Pure Chemical Industries, Osaka, Japan). The triglyceride levels
were measured by GPO-HMMPS using the SICDIA L TG reagent, and free
fatty acids were measured by enzymatic methods using NEFAZYME-S
(from Eiken Chemical).
Histology
Tissue samples of epididymal fat pads were fixed
with 4% buffered formalin and embedded in paraffin. Standard
sections of 5-μm were cut and stained with hematoxylin and
eosin (H&E), viewed with an optical microscope, and images were
captured (final magnification, ×100 or ×400).
Myotube formation and immunoblotting
C2C12 myoblast were cultured in DMEM until 90%
confluent. The cells were differentiated into myotubes with DMEM
containing 2% horse serum for 4 days, and were subsequently
incubated for 16 h in DMEM containing 2% bovine serum albumin and
10% FBS in the absence or presence of 0.75 mM palmitate to induce
insulin resistance. Subsequently, the AF- or APH-treated cells were
stimulated with 100 nM insulin for 10 min. Following stimulation,
cells were washed twice with phosphate-buffered saline (PBS) and
harvested. The following primary antibodies were used: mouse
monoclonal IgG anti-Akt (cs9272; 1:1,000), anti-phospho-Ser473 Akt
(cs4060; 1:1,000), anti-IRβ (cs3020; 1:1,000),
anti-phospho-Tyr1150/1151IRβ (cs3024; 1:1,000), anti-GSK3β (cs9832;
1:1,000), and anti-phospho-Ser9 GSK3β (cs9323; 1:1,000) were
purchased from Cell Signaling Technology (Beverly, MA, USA); mouse
polyclonal IgG anti-protein tyrosine phosphatase 1B (PTP1B)
(sc-1718; 1:1,000) was obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA) and mouse monoclonal IgG anti-β-actin (A1978;
1:3,000) was purchased from Sigma-Aldrich Co.
2-NBDG glucose uptake assay
The myotubes, which were obtained from the above
procedures, were stimulated with 100 nM insulin for 1 h. After
insulin stimulation, the myotubes were incubated with 50 μM
2-NBDG (Invitrogen) for 15 min and were subsequently washed with
PBS three times to remove free 2-NBDG. The fluorescence intensity
of cells containing 2-NBDG was measured on the Infinite M1000
microplate reader (Tecan Group Ltd., Männedorf, Switzerland) with
excitation at 485 nm and emission at 535 nm.
Gene expression analysis
Total RNA was isolated from mouse tissue using the
TRIzol reagent (Invitrogen) according to the manufacturer’s
instructions. The RNA concentration of each sample was determined
by spectrophotometry at 260 nm; the integrity of each RNA sample
was evaluated using the Agilent 2100 Bioanalyzer (Agilent
Technologies, Santa Clara, CA, USA). cDNA synthesis was performed
using 1 μg of total RNA in 20 μl with random primers
and Superscript II reverse transcriptase. Quantitative polymerase
chain reaction (PCR) analyses were performed with SYBR-Green
fluorescent dye using the 7500 Real-Time PCR system. Data analyses
were performed using 7500 System SDS software version 1.3.1
(Applied Biosystem, Foster City, CA, USA). The primer sets for
glucose-6-phosphatase (G6Pase) were 5′-ATGACTTTGGGATCCAGTCG-3′ and
5′-TGGAACCAGATGGGAAAGAG-3′; UCP3, 5′-ACAAAGGATTTGTGCCCTCC-3′ and
5′-CTTGCCTTGTTCAAAACGGA-3′; and GAPDH, 5′-TTGTTGCCATCAACGACCCC-3′
and 5′-GCCGTTGAATTTGCCGTGAG-3′.
PTP1B activity assay
PTP1B was purchased from Biomol Research
Laboratories, Inc. (Plymouth Meeting, PA, USA). The substrate,
para-nitrophenyl phosphate (pNPP), was obtained from New England
Biolabs, Inc. (Beverly, MA, USA). The reaction mixture (100
μl) containing 20 mM pNPP and 0.05 μg PTP1B in a
reaction buffer [50 mM citrate buffer (pH 6.0), 0.1 M NaCl, 1 mM
EDTA, and 1 mM dithiothreitol] was incubated at 37°C for 30 min
with or without the indicated extract or compound. The reaction was
quenched by the addition of 10 μl of 10 N NaOH. The amount
of resulting p-nitrophenol was estimated by measuring the
absorbance at 405 nm.
Statistics
The data are expressed as the mean ± standard
deviation. In the animal experiment (Fig. 1), the data are expressed as the
means ± standard error (SE). Differences between the mean values in
the two groups were analyzed using one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Anti-diabetic and anti-obesity effects of
APH in mice fed a HFD
APH has been recently reported as a novel PPARα/γ
dual agonist, which ameliorates glucose and lipid impairment in
db/db mice (22).
The present study further evaluated the anti-diabetic and
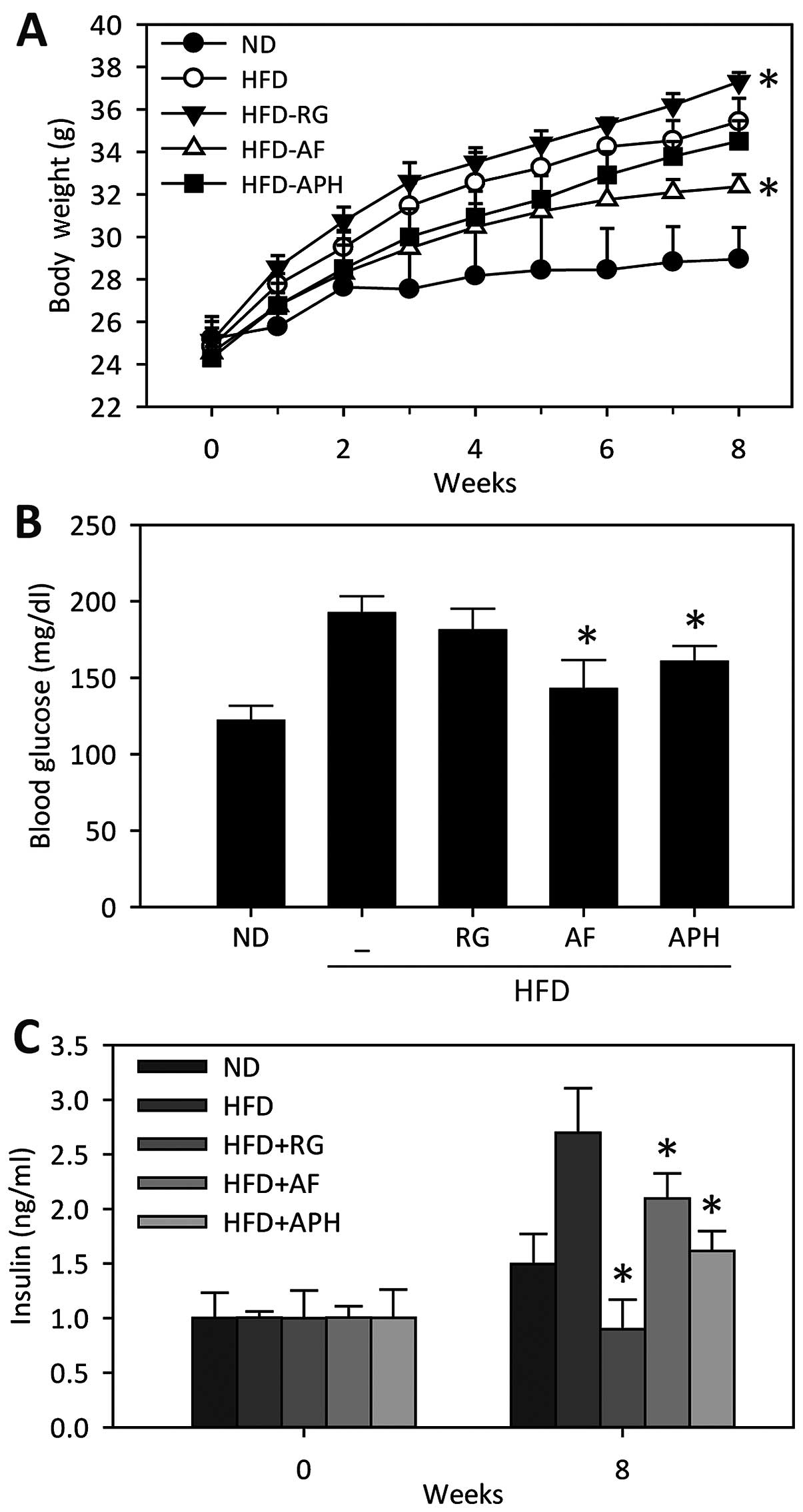
anti-obesity effects of APH in the HFD mouse model. Body weight and
blood glucose levels were significantly increased in mice fed a HFD
compared to mice fed a normal-diet (Fig. 1A and 1B). AF significantly attenuated the
weight gain in the mice fed a HFD, whereas the APH treatment showed
a weak effect on the HFD-induced weight gain (Fig. 1A). The blood glucose levels in the
mice fed a HFD significantly decreased with the AF and APH
treatments (Fig. 1B), which are
consistent with the results of the AF and APH treatments in the
db/db mouse model (22). Of note, the plasma insulin levels
in the mice fed a HFD increased (Fig.
1C), resulting in insulin resistance; however, this increase in
insulin levels significantly reduced by the AF and APH treatment,
respectively (Fig. 1C).
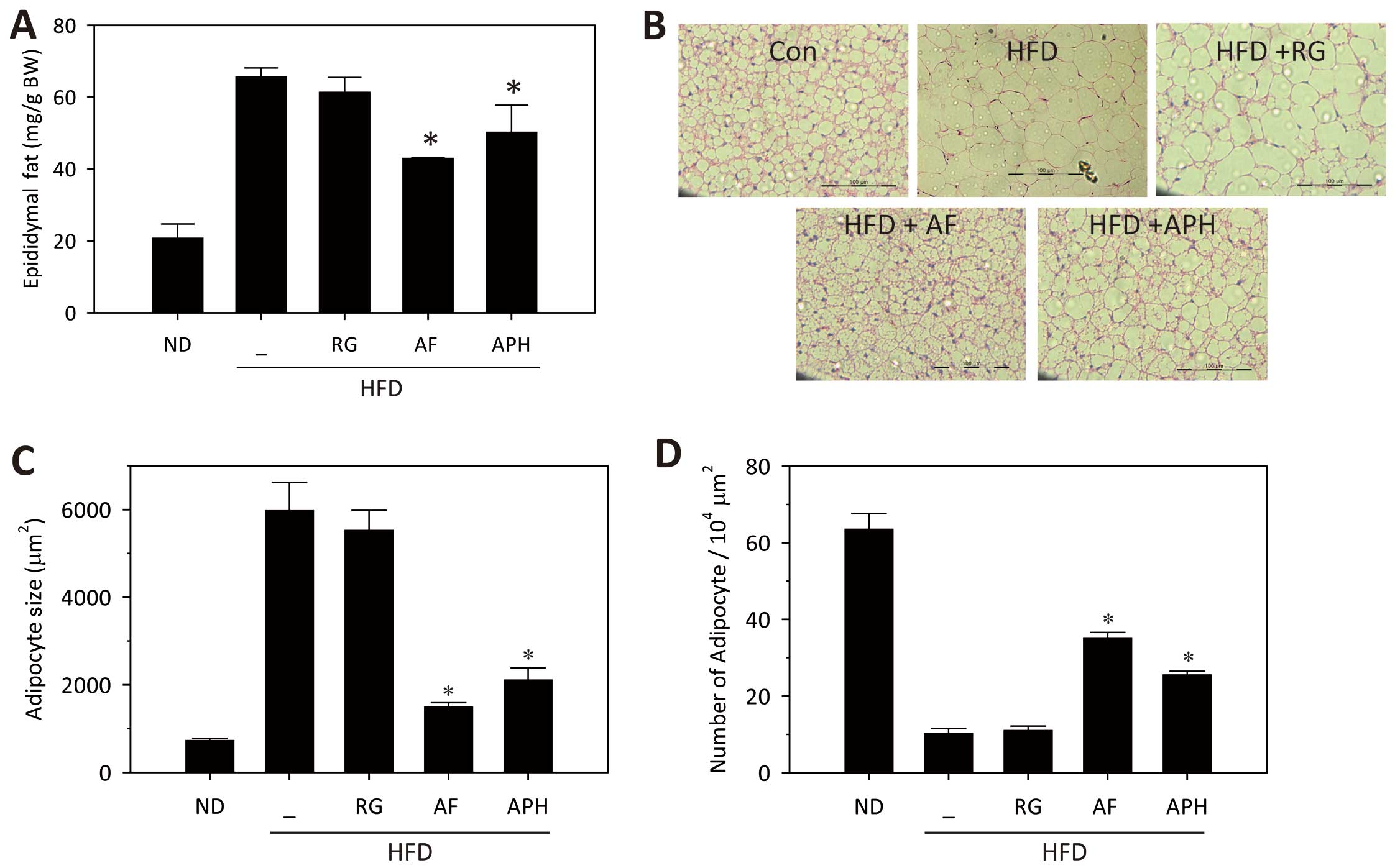
Subsequently, epididymal fat mass and adipocyte size and number was
measured in each groups. Epididymal fat mass was significantly
reduced by administration of either AF or APH (Fig. 2A), which was accompanied by
reduction of adipocyte size and number in epididymal fat tissues
(Fig. 2B–D). All these data
support anti-diabetic and anti-obesity effects of AF, which is
driven, in part, by APH.
APH improves metabolic markers in the
liver, blood and white adipose tissue
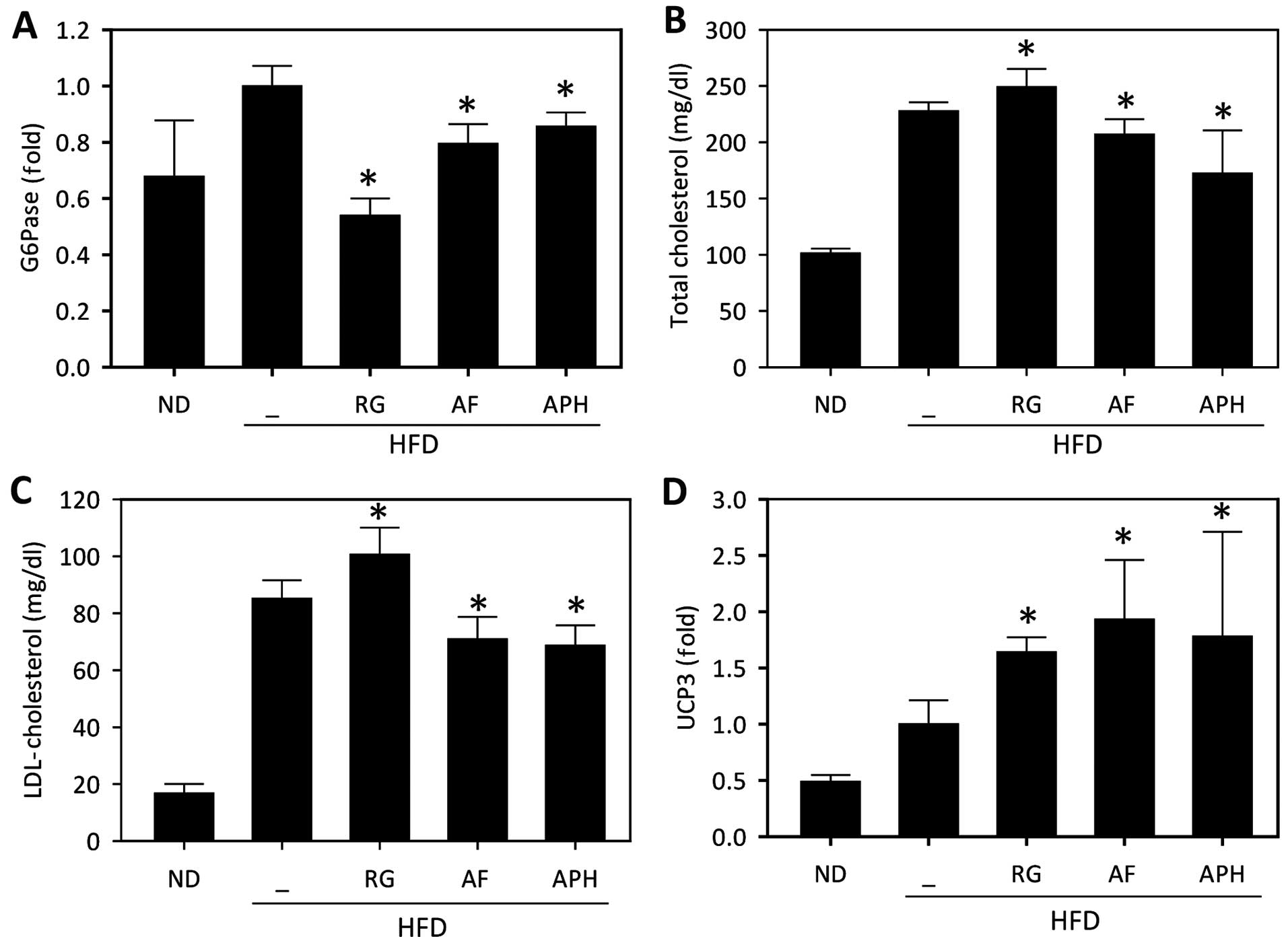
As hepatic gluconeogenesis gene expression is
markedly increased in diabetic animals and contributes to
hyperglycemia, the expression of G6Pase, one of the key enzymes in
gluconeogenesis, was measured in the liver tissue of mice fed a
HFD. The expression level of G6Pase significantly reduced in AF-
and APH-treated mice (Fig. 3A),
supporting the anti-diabetic effects of APH. Concomitantly, AF and
APH also markedly diminished the plasma cholesterol and LDL
cholesterol levels (Fig. 3B and
C), indicating that AF and APH restore cholesterol metabolism
in animals fed an HFD. The expression level of UCP3 was
subsequently analyzed in white adipose tissue, as UCP3 expression
is directly upregulated by PPARγ agonists (23,24). APH treatment significantly
increased in UCP3 expression in white adipose tissues (Fig. 3D), which may be associated with
the increased rate of lipid metabolism. All these data support that
APH exerts beneficial effects on glucose and lipid metabolism in
mice fed a HFD.
APH improves insulin sensitivity by
repressing PTP1B
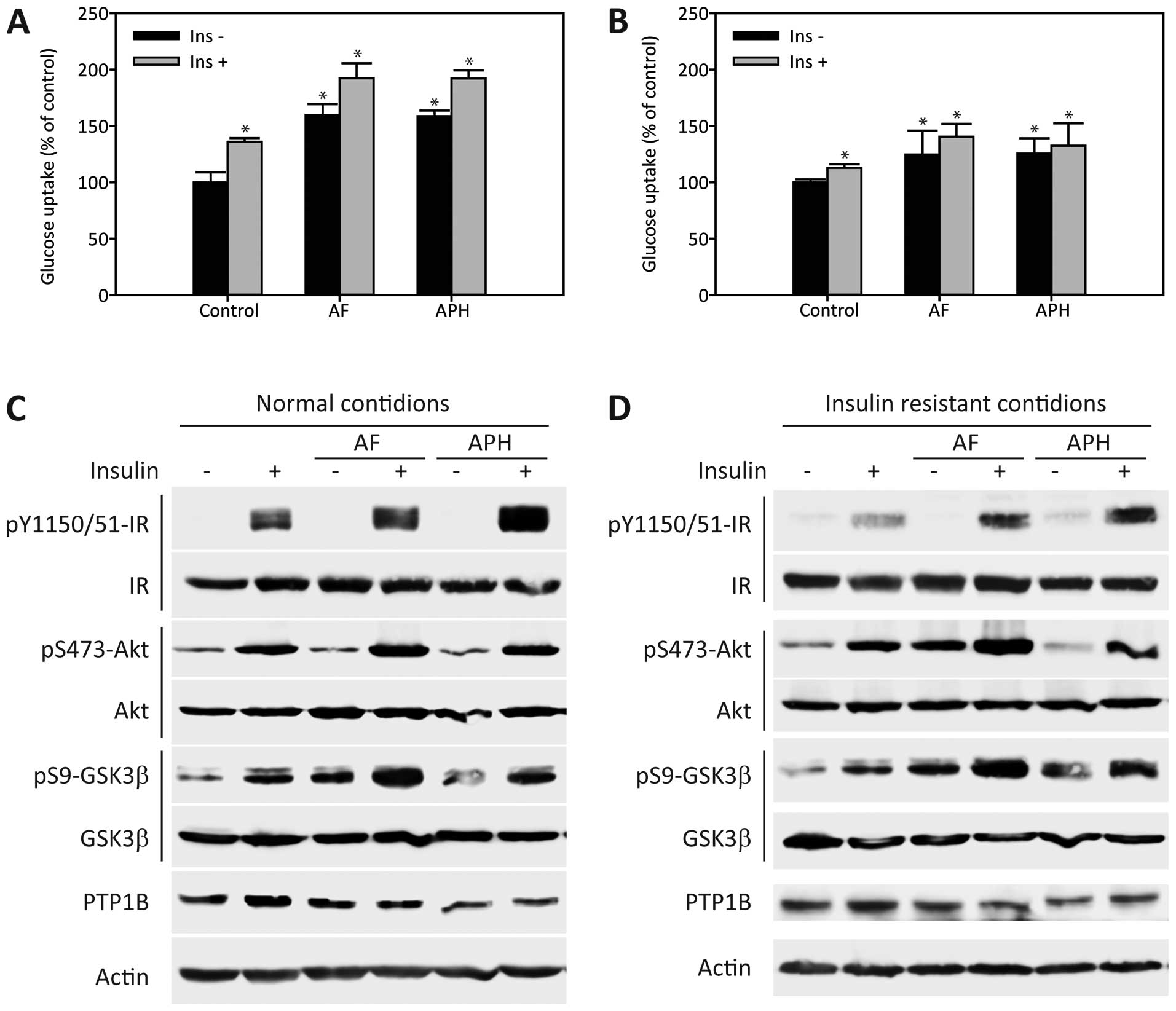
To further ascertain the positive effects of AF and
APH on insulin sensitivity, glucose uptake was first examined in
C2C12 myotubes under normal conditions. Glucose uptake in C2C12
myotubes in the absence or presence of insulin was significantly
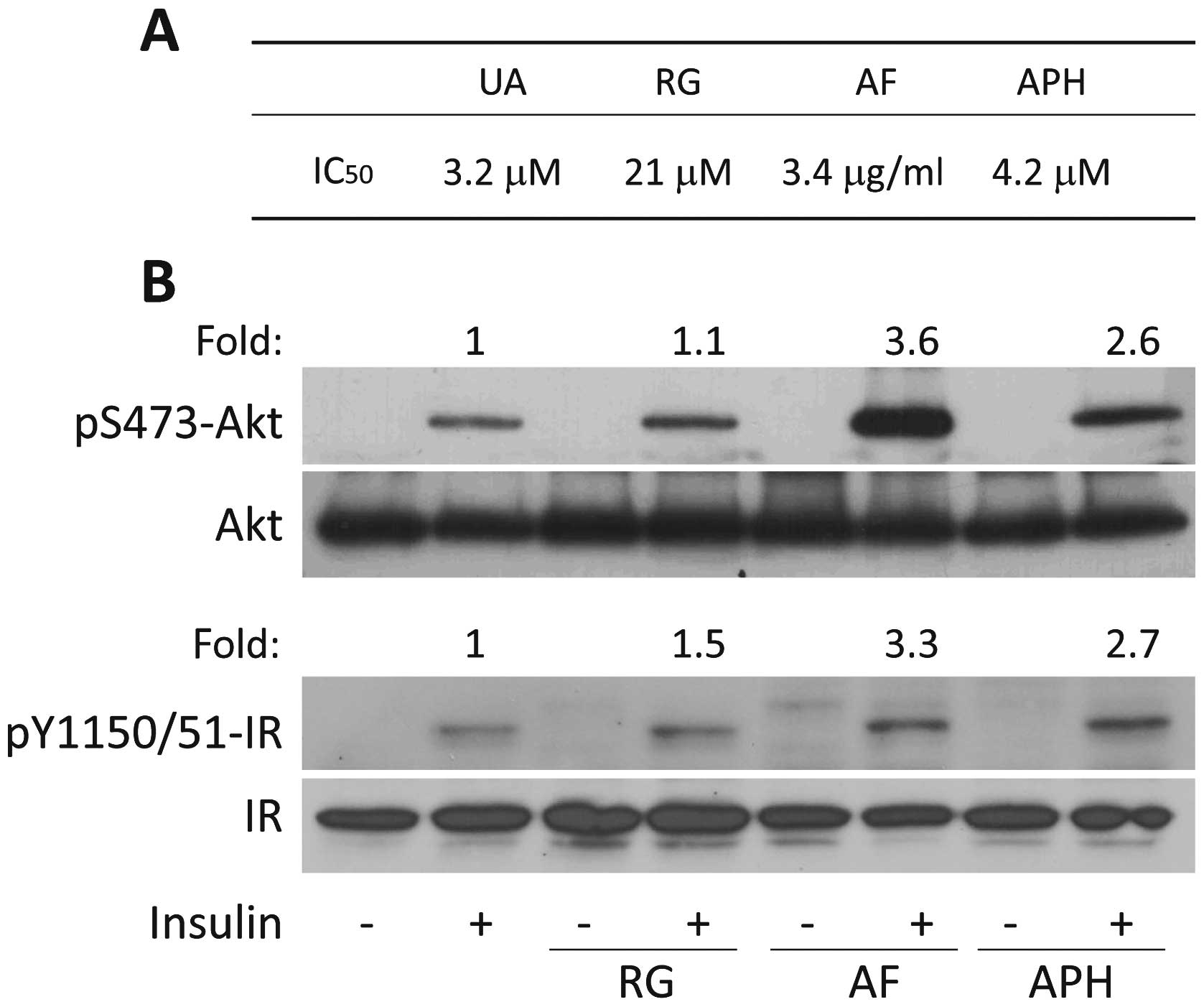
enhanced by AF and APH treatment under normal conditions (Fig. 4A). Correspondingly, AF and APH
application also enhanced the insulin-evoked phosphorylation of IR,
Akt and GSK-3β under normal conditions (Fig. 4C). Subsequently, the effects of AF
and APH on glucose uptake were investigated under palmitate-induced
insulin-resistant conditions. AF and APH also enhanced glucose
uptake under insulin-resistant conditions (Fig. 4B). The effects of AF and APH on
insulin sensitivity under insulin-resistant conditions were further
confirmed by elevated phosphorylation of these downstream proteins
in insulin signaling (Fig. 4D).
These effects were further confirmed by the observation that
insulin activation of the IR-Akt signaling axis was significantly
enhanced by AF and APH in 3T3-L1 adipocytes (Fig. 5B). To explore the underlying
mechanism responsible for improvement of insulin sensitivity by AF
and APH, the expression levels of PTP1B, a well-known negative
regulator of the insulin signaling pathway, were determined. AF and
APH treatment led to a significant decrease in PTP1B expression in
C2C12 myotubes, indicating that PTP1B may be a potential target of
AF and APH (Fig. 4C and D). In
addition, PTP1B activity was inhibited by AF and APH, with an
IC50 of 3.4 μg/ml and 4.2 μM,
respectively, whereas rosiglitazone has a weak effect on PTP1B
(IC50 21 μM) (Fig.
5A). All these results suggest that improvement of insulin
sensitivity by AF and APH are, at least in part, mediated by
repressing PTP1B.
Discussion
Numerous oral anti-diabetic agents are currently
used for the treatment T2DM patients, such as insulin secretagogues
(sulfonylureas and meglitinides), metformin, thiazolidinediones
(rosiglitazone and pioglitazone), α-glucosidase inhibitors and
incretin-based therapies (exenatide and sitagliptin). Insulin
secretagogues increase insulin secretion from β cells through the
inhibition of the KATP channel and subsequently, decrease plasma
glucose. The incretin-based therapies control post-meal glucose
excursions by increasing insulin release and decreasing glucagon
secretion, leading to a decrease in plasma glucose (25,26). The anti-diabetic effects of these
two drug classes are dependent upon the actions of insulin. By
contrast, metformin and thiazolidinediones, which are classified as
insulin sensitizers, decrease hepatic glucose production and reduce
insulin resistance without affecting insulin secretion by the
pancreas. AMPK activation is associated with the pharmacological
actions of metformin (27),
whereas the transcriptional activation of PPARs is involved in the
effects of thiazolidin-ediones (11,12). Although insulin sensitizers are
considered as a better agent for the treatment of T2DM patients
with insulin resistance, the clinical use of thiazolidinediones,
particularly rosiglitazone, is currently challenged by their severe
adverse effects, including hepatotoxicity, weight gain,
dyslipidemia and the possible worsening of cardiovascular risk
(28,29). Thus, significant efforts are
concentrating on the development of novel insulin sensitizers
having less toxicity. Our recent study showed that the AF extract
has anti-diabetic and hypolipidemic effects via the dual agonistic
action on PPARα/γ. In addition, APH was identified as the active
ingredient in AF that contributes to its anti-diabetic and
hypolipidemic effects in db/db mice (22). In the present study, the
anti-diabetic and anti-obesity effects of AF and APH were
reconfirmed in the HFD mouse model. APH potently decreased fat cell
size and alleviated cholesterol abnormalities in mice fed an HFD,
thereby, at least partially, preventing weight gain. All these
effects of APH are extremely similar to those of AF, indicating
that APH is among major active components of AF.
Our recent study showed that AF and APH possess
potent plasma glucose-lowering capabilities, even though they have
relatively weak effects on PPARγ transactivation (22), which prompted the investigation of
other mechanisms of action. The tyrosine phosphorylation of IR
triggers the insulin signaling pathway, which is in turn
deliberately regulated by the tyrosine phosphatase, PTP1B (30–32). The overexpression of PTP1B
negatively regulates the insulin signaling pathway, leading to
insulin resistance, whereas the specific inhibition of PTP1B
enhances insulin signaling, which may improve insulin resistance
(32,33). The present data show that the
blood glucose-lowering effects of AF and APH are, at least in part,
mediated by repressing PTP1B action. PTP1B inhibition by AF and APH
led to an increase in the tyrosine phosphorylation of IRβ and
resulted in the activation of the Akt signaling pathway in C2C12
myotubes (Fig. 4) and 3T3L1
adipocytes (Fig. 5).
Consequently, glucose uptake was significantly enhanced by AF and
APH under normal and insulin-resistant conditions (Fig. 4).
In conclusion, AF has beneficial effects on glucose
and lipid metabolism in the improvement of metabolic disorders by
selectively activating PPARα and PPARγ and inhibiting PTP1B. All
the described effects of AF are driven, in part, by its active
component, APH. Therefore, further development of AF and APH as
anti-metabolic agents is strongly suggested to ameliorate glucose
and lipid abnormalities, insulin resistance and obesity.
Acknowledgments
The present study was supported by grants from the
National Research Foundation of Korea funded by the Korean
Government (MSIP) (no. 2011-0030074) and the Korea Institute of
Science and Technology, Republic of Korea (no. 2Z04371).
References
|
1
|
Rathmann W and Giani G: Global prevalence
of diabetes: Estimates for the year 2000 and projections for 2030.
Diabetes Care. 27:2568–2569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000.PubMed/NCBI
|
|
3
|
Saltiel AR: New perspectives into the
molecular pathogenesis and treatment of type 2 diabetes. Cell.
104:517–529. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bajaj M and Defronzo RA: Metabolic and
molecular basis of insulin resistance. J Nucl Cardiol. 10:311–323.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pendergrass M, Bertoldo A, Bonadonna R,
Nucci G, Mandarino L, Cobelli C and Defronzo RA: Muscle glucose
transport and phosphorylation in type 2 diabetic, obese
nondiabetic, and genetically predisposed individuals. Am J Physiol
Endocrinol Metab. 292:E92–E100. 2007. View Article : Google Scholar
|
|
6
|
Cusi K, Maezono K, Osman A, Pendergrass M,
Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR and Mandarino LJ:
Insulin resistance differentially affects the PI3-kinase- and MAP
kinase-mediated signaling in human muscle. J Clin Invest.
105:311–320. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Groop LC, Bonadonna RC, DelPrato S,
Ratheiser K, Zyck K, Ferrannini E and DeFronzo RA: Glucose and free
fatty acid metabolism in non-insulin-dependent diabetes mellitus.
Evidence for multiple sites of insulin resistance. J Clin Invest.
84:205–213. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bays H, Mandarino L and DeFronzo RA: Role
of the adipocyte, free fatty acids, and ectopic fat in pathogenesis
of type 2 diabetes mellitus: Peroxisomal proliferator-activated
receptor agonists provide a rational therapeutic approach. J Clin
Endocrinol Metab. 89:463–478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee CH, Olson P and Evans RM: Minireview:
Lipid metabolism, metabolic diseases, and peroxisome
proliferator-activated receptors. Endocrinology. 144:2201–2207.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vidal-Puig AJ, Considine RV, Jimenez-Liñan
M, Werman A, Pories WJ, Caro JF and Flier JS: Peroxisome
proliferator-activated receptor gene expression in human tissues.
Effects of obesity, weight loss, and regulation by insulin and
glucocorticoids. J Clin Invest. 99:2416–2422. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lehrke M and Lazar MA: The many faces of
PPARgamma. Cell. 123:993–999. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Evans RM, Barish GD and Wang YX: PPARs and
the complex journey to obesity. Nat Med. 10:355–361. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fonseca V: Effect of thiazolidinediones on
body weight in patients with diabetes mellitus. Am J Med. 115(Suppl
8A): 42S–48S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bünger M, Hooiveld GJ, Kersten S and
Müller M: Exploration of PPAR functions by microarray technology -
a paradigm for nutrigenomics. Biochim Biophys Acta. 1771:1046–1064.
2007. View Article : Google Scholar
|
|
15
|
Bajaj M, Suraamornkul S, Hardies LJ, Glass
L, Musi N and DeFronzo RA: Effects of peroxisome
proliferator-activated receptor (PPAR)-alpha and PPAR-gamma
agonists on glucose and lipid metabolism in patients with type 2
diabetes mellitus. Diabetologia. 50:1723–1731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pickavance LC, Brand CL, Wassermann K and
Wilding JP: The dual PPARalpha/gamma agonist, ragaglitazar,
improves insulin sensitivity and metabolic profile equally with
pioglitazone in diabetic and dietary obese ZDF rats. Br J
Pharmacol. 144:308–316. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reifel-Miller A, Otto K, Hawkins E, Barr
R, Bensch WR, Bull C, Dana S, Klausing K, Martin JA,
Rafaeloff-Phail R, et al: A peroxisome proliferator-activated
receptor alpha/gamma dual agonist with a unique in vitro profile
and potent glucose and lipid effects in rodent models of type 2
diabetes and dyslipidemia. Mol Endocrinol. 19:1593–1605. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harrity T, Farrelly D, Tieman A, Chu C,
Kunselman L, Gu L, Ponticiello R, Cap M, Qu F, Shao C, et al:
Muraglitazar, a novel dual (alpha/gamma) peroxisome
proliferator-activated receptor activator, improves diabetes and
other metabolic abnormalities and preserves beta-cell function in
db/db mice. Diabetes. 55:240–248. 2006. View Article : Google Scholar
|
|
19
|
Mittra S, Sangle G, Tandon R, Sharma S,
Roy S, Khanna V, Gupta A, Sattigeri J, Sharma L, Priyadarsiny P, et
al: Increase in weight induced by muraglitazar, a dual
PPARalpha/gamma agonist, in db/db mice: Adipogenesis/or oedema? Br
J Pharmacol. 150:480–487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adeghate E, Adem A, Hasan MY, Tekes K and
Kalasz H: Medicinal chemistry and actions of dual and pan PPAR
modulators. Open Med Chem J. 5(Suppl 2): 93–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim T, Lee W, Jeong KH, Song JH, Park SH,
Choi P, Kim SN, Lee S and Ham J: Total synthesis and dual PPARα/γ
agonist effects of amorphastilbol and its synthetic derivatives.
Bioorg Med Chem Lett. 22:4122–4126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee W, Ham J, Kwon HC, Kim YK and Kim SN:
Anti-diabetic effect of amorphastilbol through PPARα/γ dual
activation in db/db mice. Biochem Biophys Res Commun. 432:73–79.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsuda J, Hosoda K, Itoh H, Son C, Doi K,
Hanaoka I, Inoue G, Nishimura H, Yoshimasa Y, Yamori Y, et al:
Increased adipose expression of the uncoupling protein-3 gene by
thiazolidinediones in Wistar fatty rats and in cultured adipocytes.
Diabetes. 47:1809–1814. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Emilsson V, O’Dowd J, Wang S, Liu YL,
Sennitt M, Heyman R and Cawthorne MA: The effects of rexinoids and
rosiglitazone on body weight and uncoupling protein isoform
expression in the Zucker fa/fa rat. Metabolism. 49:1610–1615. 2000.
View Article : Google Scholar
|
|
25
|
Campbell RK: Clarifying the role of
incretin-based therapies in the treatment of type 2 diabetes
mellitus. Clin Ther. 33:511–527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahrén B: The future of incretin-based
therapy: Novel avenues - novel targets. Diabetes Obes Metab.
13(Suppl 1): 158–166. 2011. View Article : Google Scholar
|
|
27
|
Zhou G, Myers R, Li Y, Chen Y, Shen X,
Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al: Role of
AMP-activated protein kinase in mechanism of metformin action. J
Clin Invest. 108:1167–1174. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nissen SE and Wolski K: Effect of
rosiglitazone on the risk of myocardial infarction and death from
cardiovascular causes. N Engl J Med. 356:2457–2471. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kermani A and Garg A:
Thiazolidinedione-associated congestive heart failure and pulmonary
edema. Mayo Clin Proc. 78:1088–1091. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alonso A, Sasin J, Bottini N, Friedberg I,
Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J and Mustelin
T: Protein tyrosine phosphatases in the human genome. Cell.
117:699–711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moller DE: New drug targets for type 2
diabetes and the metabolic syndrome. Nature. 414:821–827. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tonks NK: Protein tyrosine phosphatases:
From genes, to function, to disease. Nat Rev Mol Cell Biol.
7:833–846. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elchebly M, Payette P, Michaliszyn E,
Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J,
Chan CC, et al: Increased insulin sensitivity and obesity
resistance in mice lacking the protein tyrosine phosphatase-1B
gene. Science. 283:1544–1548. 1999. View Article : Google Scholar : PubMed/NCBI
|