Introduction
Nasopharyngeal carcinoma (NPC) is mainly prevalent
in Southern China and Southeast Asia (1). Radiation therapy (RT) is the primary
treatment for NPC. Intensity-modulated radiotherapy (IMRT), which
exhibits fewer side effects compared with conventional two- and
three-dimensional conformal radiation, is now widely used and
achieves a good local control rate (2). However, for locally advanced or
recurrent patients, treatment with IMRT alone often shows a poor
prognosis (3). Besides RT,
chemotherapy could also be administered as neoadjuvant, concurrent
or adjuvant and is beneficial to NPC treatment. Even with the
combination of RT and chemotherapy, local failure occurs in 7–13%
of patients following primary treatment for NPC (4). In addition to RT and the traditional
chemotherapy, targeted therapy has attracted increasing attention
for cancer treatment in the past few decades. Despite the success
in lung (5), breast (6–8)
and colorectal cancer (9,10), phase II studies of targeted
therapy showed insufficient results for NPC treatment. Drugs
involved in the studies, including gefitinib, sorafenib and
cetuximab, did not show a clear benefit to progression-free
survival (PFS) or overall survival (OS) (11–14). These results suggested that
traditional molecular targets, which are beneficial in other
tumors, may not work well in NPC treatment. Therefore, it is
necessary to discover new molecular targets in NPC treatment and
improve the prognosis.
SHP-1 is an SH2 domain containing protein tyrosine
phosphatase (PTP), which consists of 17 exons and 16 introns and
spans ~17 kb DNA (15,16). PTP SHP-1 expression increased in
NPC tissue and was associated with radiation resistance and local
recurrence (17). Knocking down
SHP-1 by siRNA resulted in higher radiosensitivity in the NPC and
lung cancer cell lines (18,19). According to these studies, SHP-1
may be a potential target in NPC treatment and silencing SHP-1 may
help improve the NPC prognosis.
The discovery of microRNAs (miRNAs or miRs)
established a new era for targeted therapy. miRNAs are small
non-coding RNAs that containing 19–25 nucleotides. These small
non-coding RNAs regulate the target gene expression at the
post-transcriptional level by binding to the 3′ or 5′-untranslated
region (UTR) and cause the degradation of target mRNA (20,21). By regulating oncogene and
anti-oncogene expression, miRNAs have important roles in tumor
initiation and progression (22,23). For that reason, miRNAs are closely
associated with the tumor behaviors, as they can be conveniently
detected in blood and other body fluid and they have great
potential to become new biomarkers to monitor tumor development and
therapeutic effect (24–26). As carcinogenesis is often
correlated to abnormal miRNAs expression, reconstructing the normal
miRNA network is a novel strategy in tumor treatment (27). A paradigm of miRNA therapeutics
has been reported by Duchaine and Slacks (28). The paradigm pointed out a ‘double
strategies’ concept: Using miRNAs as an anticancer agent or as
cancer therapeutic targets (29).
In the present study, miRNA was used as an
anticancer agent in NPC treatment. The miRNAs targeting SHP-1 were
used to treat NPC cells, explore how it affects NPC cell
proliferation and how it regulates SHP-1 expression.
Materials and methods
Cell culture
The NPC cell lines, CNE-1 and CNE-2, were purchased
from the Cell Bank of Sun Yat-sen University (Guangzhou, China).
5-8F was obtained from the Cell Bank of Xiangya University
(Changsha, China). C666-1, 6-10B, HNE-2, HNE-1 and HONE-1 were
obtained from the Cell Bank of JRDUN Biotechnology (Shanghai,
China). Cells were routinely cultured in RPMI-1640 (HyClone, Logan,
UT, USA) supplemented with 12% fetal bovine serum (FBS; Gibco,
Grand Island, NY, USA) and 1% penicillin/streptomycin (HyClone).
The cells were maintained at 37°C in a humidified incubator with 5%
CO2.
Lentivirus, miRNA mimics or inhibitor
transfection
miRNA mimics or inhibitors were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The control
scrambled sequence (SCR) was used as a negative control. The miRNA
mimics, inhibitors and SCR were transfected into NPC cells using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Lentiviruses containing the SHP-1 gene
(LP-H1802-Lv201-C0010) and negative oligomers (LP-Neg-Lv201-0200)
were purchased from GeneCopoeia (Guangzhou, China;
8.6×109 copies/ml). The two lentiviral stocks (50
µl; LP-H1802Lv201 and LP-NegLv201) were transfected into the
CNE-2 cells. Puromycin (2 µg/ml) was used to select the
lentiviral-transfected cells. Total protein was isolated, and SHP-1
expression was detected by western blotting.
MTT assay
Cells were seeded into 96-well culture plates and
incubated for 1, 2, 3, 4 and 5 days, respectively. The
serum-containing culture medium was exchanged for normal culture
medium not containing serum and 20 µl MTT (5 mg/ml; Sigma,
St. Louis, MO, USA) was added. The samples were incubated in the
dark for 4 h. The culture medium was removed, 150 µl
dimethyl sulfoxide (Sigma) was added and the sample was slowly
agitated for 15 min. The optical density (OD) at 490/630 nm was
tested using a microplate reader system (BioTek Instruments, Inc.,
Winooski, VT, USA).
Western blotting
Cells were harvested, lysed by the
radioimmunoprecipitation assay (Google Biotechnology Ltd. Co.,
Wuhan, China) and total protein was extracted. Protein
concentrations of the lysates were determined by the bicinchoninic
acid protein assay system (Google Biotechnology Ltd. Co.). Equal
amounts of protein were separated by 8–12% SDS-PAGE, and
transferred to a polyvinylidene fluoride membrane (Millipore,
Billerica, MA, USA). The membranes were blocked with 5% bovine
serum albumin and subsequently probed with either anti-SHP-1 (Cat.
no. 1606-1; monoclonal, rabbit, targeted against mouse, rat, human;
Epitomics, Burlingame, CA, USA) or anti-GAPDH (Cat. no. sc-25778;
polyclonal, rabbit, targeted against human; Santa Cruz
Biotechnology, Dallas, TX, USA) antibodies. Following washing, the
membrane was incubated with the appropriate horseradish peroxidase
secondary antibody (Cat. no. A24537; polyclonal, goat, targeted
against rabbit; Life Technologies, Carlsbad, CA, USA) and
visualized by chemiluminescence using a chemiluminescence kit
(Invitrogen), and the specific bands were recorded by a UV
transilluminator (Uvitec Ltd., Avebury House, Cambridge, UK). GAPDH
protein levels were used as a control to verify equal protein
loading.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted by TRIzol (Invitrogen) and
reverse transcription was used to generate cDNA, according to the
manufacturer’s instructions of the Takara RT-PCR kit (Takara Bio,
Shiga, Japan). Subsequently, qPCR was performed according to the
manufacturer’s instructions of SYBR-Green (Applied Biosystems,
Foster City, CA, USA) in a PCR amplifier (ABI Prism 7000; Applied
Biosystems). StepOne™ software v2.1 was used to analyze the data.
Primer sequences are shown in Table
I.
 | Table IPrimer sequences for PCR. |
Table I
Primer sequences for PCR.
| Genes | Sequences |
|---|
| miR-4649-3p | Forward:
5′-ACACTCCAGCTGGGTCTGAGGCCTGCCTC-3′ |
| Reverse:
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTGGGGA-3′ |
| U6 snRNA | Forward:
5′-CTTCGGCAGCACATATAC-3′ |
| Reverse:
5′-GGCCATGCTAATCTTCTC-3′ |
| SHP-1 | Forward:
5′-ACCATCATCCACCTCAAGTACC-3′ |
| Reverse:
5′-CTGAGCACAGAAAGCACGAA-3′ |
| β-actin | Forward:
5′-GATGAGATTGGCATGGCTTT-3′ |
| Reverse:
5′-CACCTTCACCGTTCCAGTTT-3′ |
Luciferase reporter assay
A 3′UTR fragment of SHP-1 containing the putative
miR-4649-3p binding site (1018–1024 nucleotides) was amplified by
PCR. The PCR product was subcloned into a p-GL3 vector (Promega,
Madison, WI, USA) immediately downstream to the luciferase gene
sequence. A p-GL3 construct containing the 3′UTR of SHP-1 with a
mutant sequence of the miR-4649-3p binding site was also
synthesized. All the constructs were verified by DNA sequencing.
Cells were seeded in 96-well plates and subsequently co-transfected
with 100 ng constructs with or without miR-4649-3p mimics or
inhibitors. At 48 h after transfection, luciferase activity was
detected using a dual-luciferase reporter assay system (Promega)
and normalized to Renilla activity.
Statistical analysis
Experimental data are expressed as the means ±
standard deviation from ≥3 independent experiments. Differences in
measured variables between experimental and control groups were
assessed using the t-test (SPSS 21.0 software; IBM Corp., Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
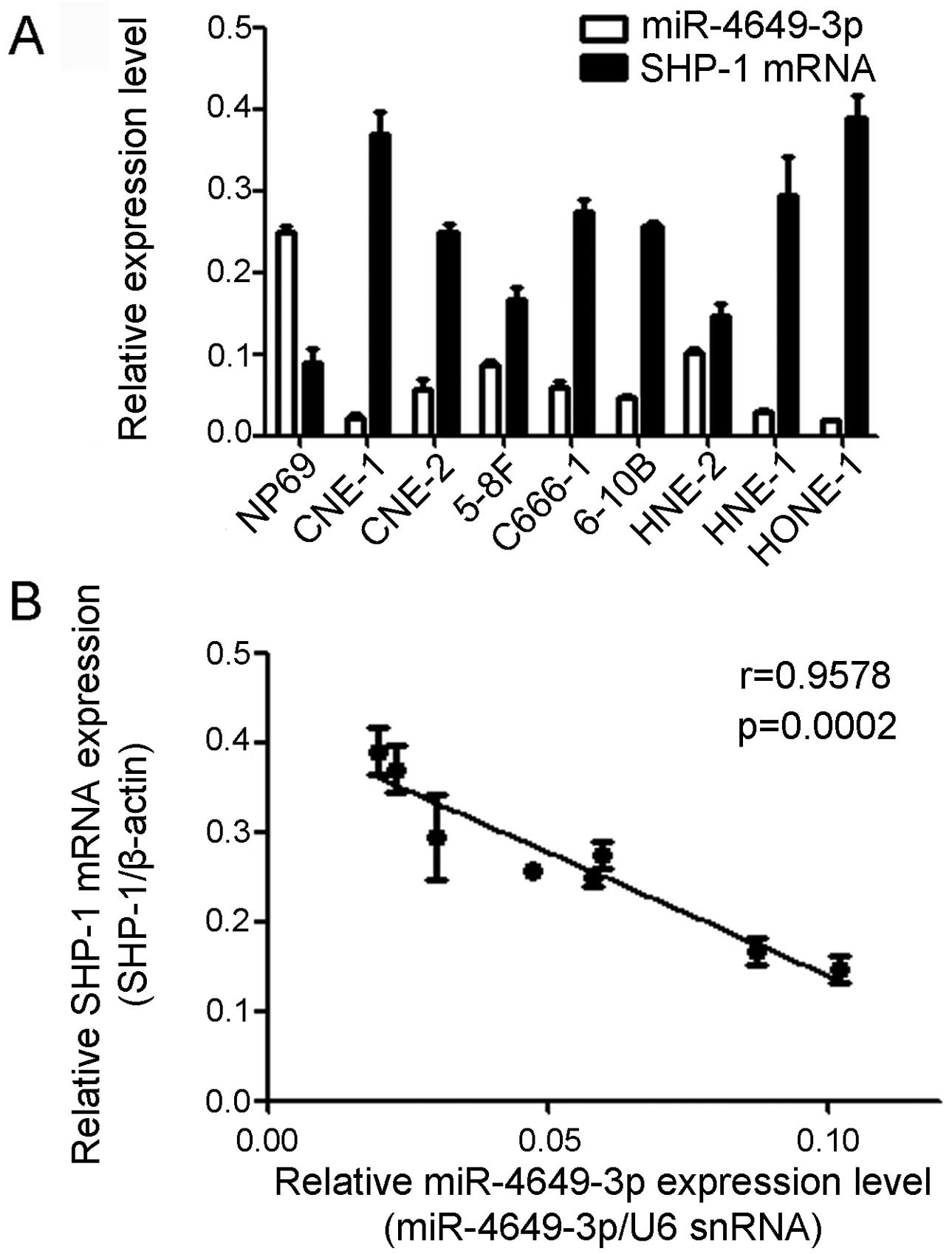
miR-4649-3p is downregulated in NPC cell
lines accompanied with SHP-1 upregulation
qPCR was used to detect the miR-4649-3p and SHP-1
mRNA expression level in the normal nasopharyngeal epithelia cell
line and 8 different NPC cell lines. Compared with the normal
nasopharyngeal epithelia NP69 cells, the miR-4649-3p expression
level was significantly decreased in the 8 NPC cell lines, CNE-1,
CNE-2, HNE-1, HNE-2, 5-8F, 6-10B, C666-1 and HONE-1. However, the
expression of SHP-1 mRNA was not the same as miR-4649-3p. The SHP-1
mRNA expression level in the NP69 cells was evidently lower
compared to the NPC cells. The Pearson’s correlation test was used
to analyze the association between miR-4649-3p and SHP-1 mRNA
expression in the 8 NPC cell lines. The results showed that
miR-4649-3p expression was inversely correlated to the SHP-1
expression level. The Pearson correlation parameter was r=−0.9578
(P=0.0002) (Fig. 1).
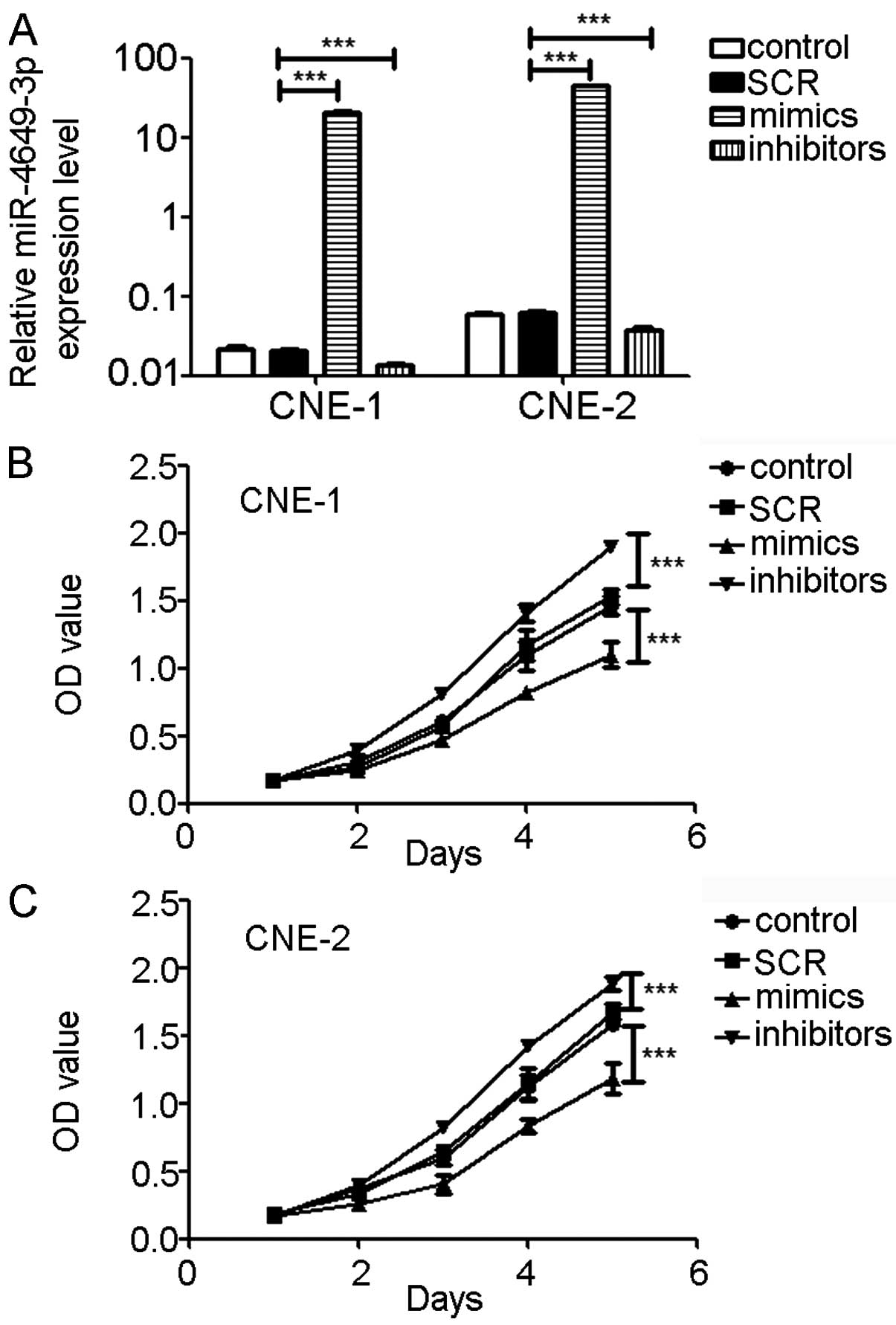
miR-4649-3p inhibits NPC cell
proliferation
To evaluate whether miR-4649-3p affects NPC cell
proliferation, miR-4649-3p mimics and inhibitors were used to
transfect CNE-1 and CNE-2 cells. SCR was used as a negative
control. NPC cells transfected with miR-4649-3p inhibitors showed a
40–45% decrease in miR-4649-3p expression. The miR-4649-3p
mimic-transfected cells showed a 900- to 1,000-fold increase of
miR-4649-3p expression. Three days after transfection, CNE-1 and
CNE-2 cells were seeded in 96-wells plates and incubated for
different times. Subsequently, cell proliferation, presented by the
OD value, was determined. Cell proliferations were significantly
inhibited in CNE-1 and CNE-2 cells treated with miR-4649-3p mimics.
However, miR-4649-3p inhibitors significantly promoted cell
proliferation (Fig. 2).
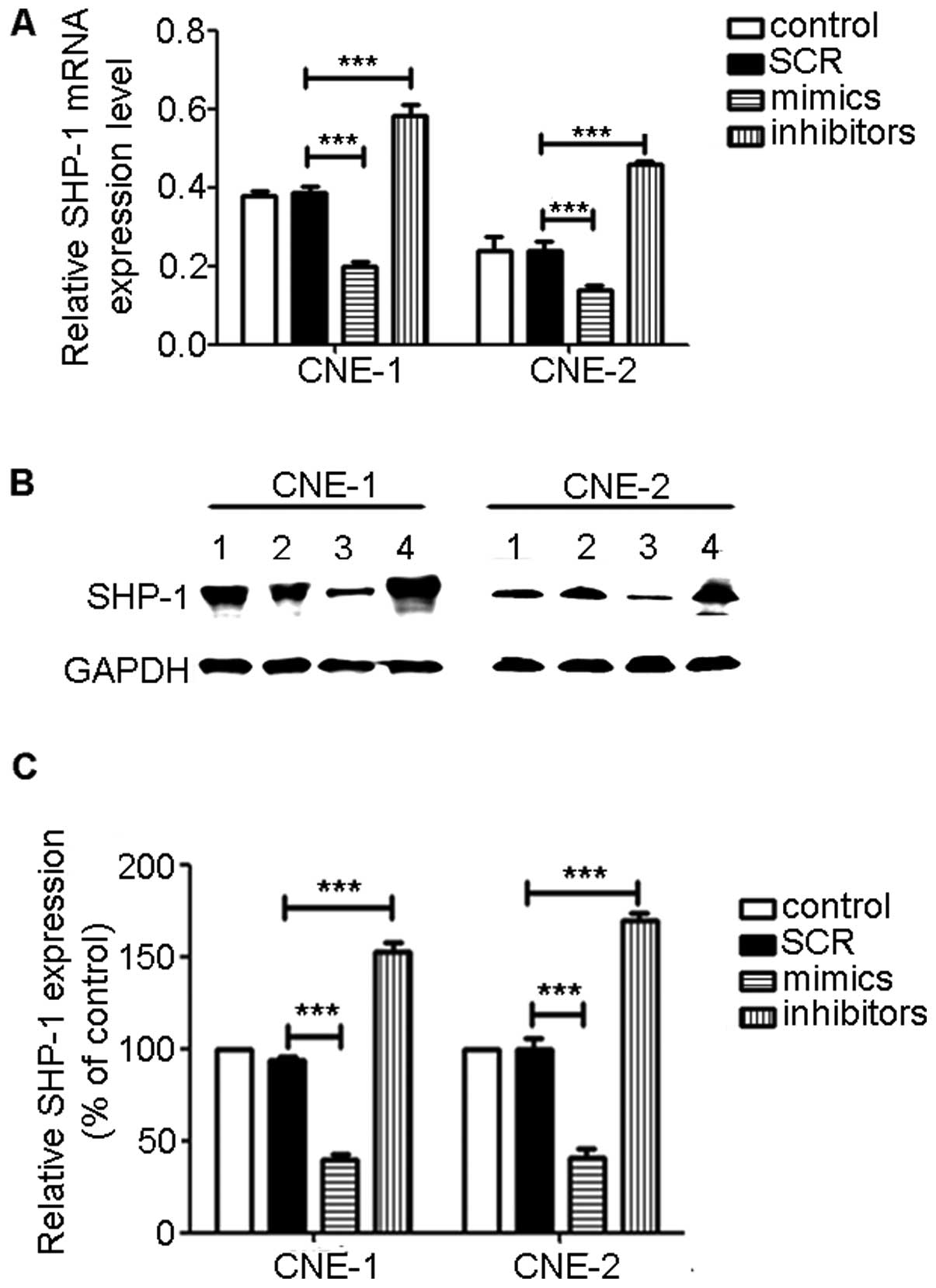
miR-4649-3p mimics downregulate SHP-1
expression
To investigate how miR-4649-3p affects SHP-1
expression, NPC cells were transfected with miR-4649-3p mimics and
inhibitors. After three days, total RNA and protein were extracted
and SHP-1 expression was examined by qPCR and western blot
analysis. Between the control and SCR group, SHP-1 mRNA and protein
expression did not have a significant difference. However, in the
miR-4649-3p mimic-transfected group, SHP-1 expression was inhibited
at the mRNA and protein level. In the miR-4649-3p
inhibitor-transfected group, the SHP-1 expression level was
evidently increased (Fig. 3).
miR-4649-3p directly targets SHP-1
3′UTR
To further understand how miR-4649-3p upregulated
SHP-1 expression, luciferase reporter assays were performed.
TargetScan human6.2 (www.targetscan.org) was used to predict the binding
site of SHP-1 (PTPN6) with miR-4649-3p. According to the binding
site sequence, a mutant sequence of the 3′UTR of SHP-1 was
constructed by changing certain base pairs. Subsequently, the
wild-type (wt) and mutant (mt) 3′UTR of SHP-1 were connected to a
luciferase reporter gene and the wt-3′UTR and mt-3′UTR vectors were
formed. The wt-3′UTR and miR-4649-3p mimics or inhibitors were
co-transfected into the NPC cells. Luciferase activity of the
miR-4649-3p mimics group was significantly suppressed while the
activity of miR-4649-3p inhibitors groups was clearly increased.
However, when the miR-4649-3p mimics or inhibitors was
co-transfected with mt-3′UTR, the impact of the miR-4649-3p mimics
or inhibitors on luciferase activity was abolished (Fig. 4).
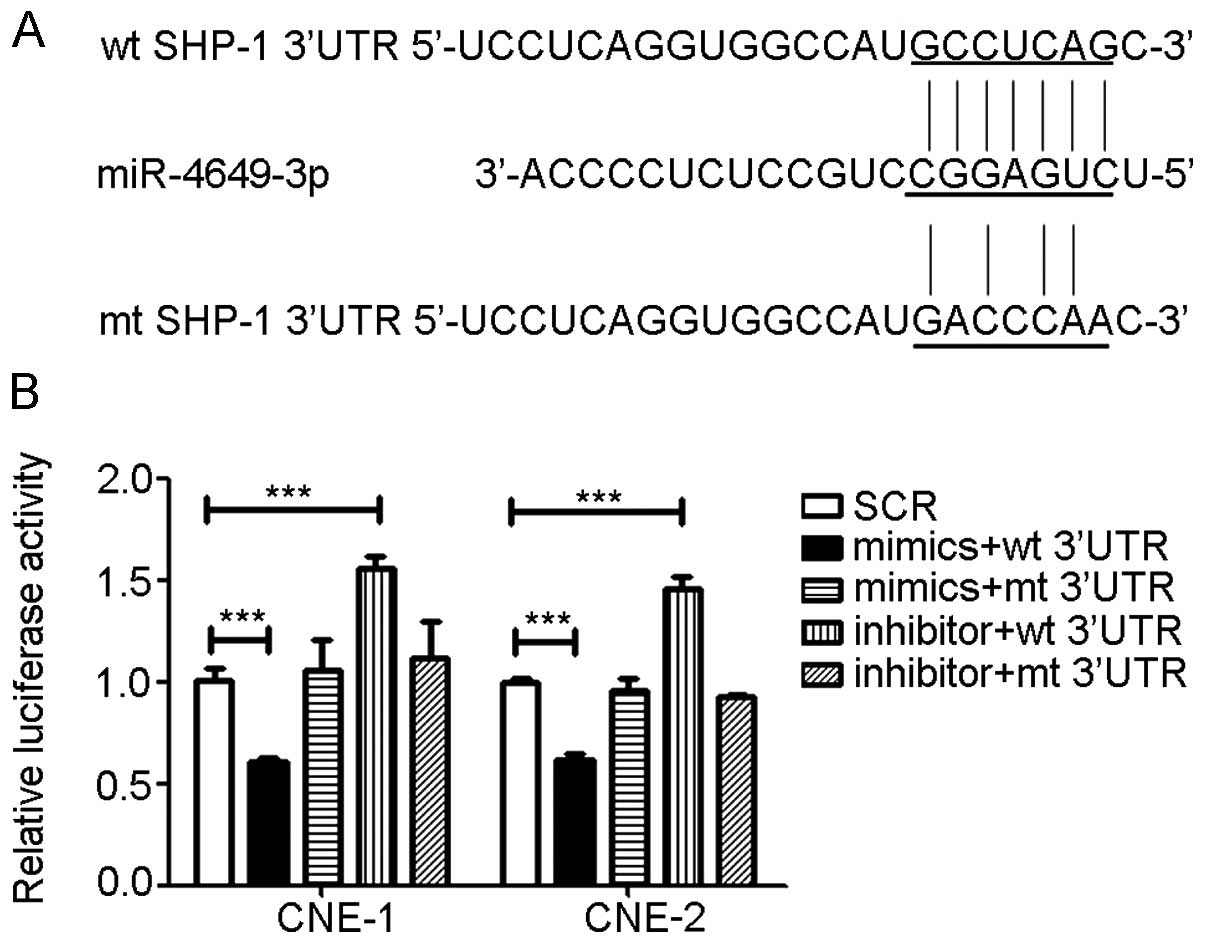 | Figure 4miR-4649-3p directly targets SHP-1
3′UTR. According to the binding site of SHP-1 3′UTR and
miR-4649-3p, mt SHP-1 3′UTR was constructed by replacing certain
base pairs. Subsequently, the luciferase reporter assay was used to
examine the binding of miR-4649-3p and SHP-1 3′UTR in CNE-1 and
CNE-2. When miR-4649-3p mimics and wt SHP-1 3′UTR were
co-transfected into nasopharyngeal carcinoma (NPC) cells,
luciferase activity was significantly inhibited. When
co-transfected with miR-4649-3p inhibitors and wt SHP-1 3′UTR,
luciferase activity was clearly increased. However, in the mt SHP-1
3′UTR groups, the effect of miR-4649-3p mimics and inhibitors was
abolished. (A) Sequence of miR-4649-3p, wt SHP-1 3′UTR and mt SHP-1
3′UTR. (B) Luciferase activity of CNE-1 and CNE-2. Data are
presented as mean ± standard deviation from three independent
experiments; ***P<0.001. SCR, control scrambled
sequence; UTR, untranslated region; wt, wild-type; mt, mutant. |
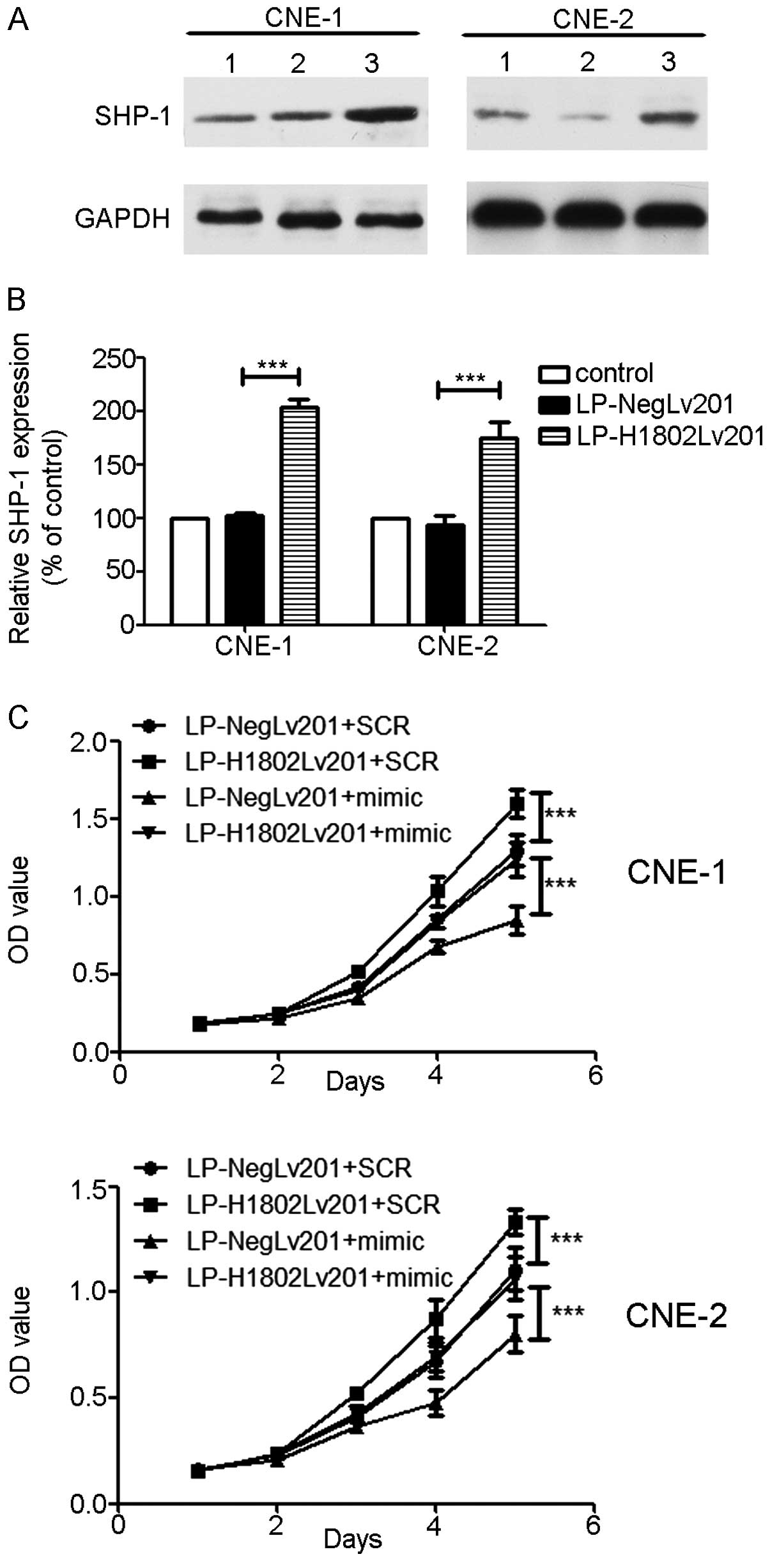
Overexpression of SHP-1 abolishes the
inhibition effect of miR-4649-3p
The lentivirus containing the SHP-1 gene
(LP-H1802lv201) was used to transfect the NPC cells, CNE-1 and
CNE-2, and SHP-1 overexpressed NPC cells were generated.
LP-Neglv201 was a negative control. The MTT assay was performed
again and an increasing SHP-1 expression level promoted cell
proliferation. However, transfection of the miR-4649-3p mimics into
NPC cells inhibited cell proliferation. When miR-4649-3p mimics and
LP-H1802lv201 were co-transfected into NPC cells, the influence of
SHP-1 on cell proliferation was abolished (Fig. 5)
Discussion
miRNAs have attracted increasing attention since
being discovered, particularly in the field of cancer diagnosis and
therapy. Since they can be detected in peripheral blood, body fluid
and even in feces, they are extremely convenient for clinical use.
Thus far, a significant number of studies have been performed
utilizing miRNAs, from tumor markers to cancer therapy (21,30–37,39). Traditional chemotherapeutic agents
have certain limitations, among which severe side effects and drug
resistance are the major ones. Through binding to target gene mRNA,
miRNAs can knockdown the oncogene expression. The strategy of using
miRNAs to inhibit oncogene expression provides a new therapeutic
approach with high selectivity and small side effects. Increasing
research has focused on using miRNAs as new therapeutic agents in
tumor therapy. Inhibiting NF-κB activation by miR-31 in breast
cancer promoted cell death and increased cell sensitivity to
ionizing radiation (38). Zhang
et al (39) found that
miR-451 can increase radiosensitivity of NPC cells by targeting
RAB14. Recently, certain investigators have tried to deliver miRNAs
in combination with traditional chemotherapeutic drugs in cancer
therapy. They found that the combination of miRNAs and
chemotherapeutic drugs was effective and could enhance the
sensitivity of cancer cells (40).
In the past few years, studies of miRNAs in NPC have
achieved significant advancements. A number of miRNAs have been
found to be associated with NPC formation, progression and other
malignant behaviors. The majority of these miRNAs have roles in NPC
pathology by modulating their target gene expression, including
cell proliferation, migration, invasion and radiosensitivity.
SHP-1 is an SH2 domain containing PTP, which has 17
exons and 16 introns and spans ~17 kb (15,16). It is strongly expressed in normal
hematopoietic cells and weakly expressed in certain hematological
malignancies. However, specific studies have found that SHP-1 is
highly expressed in certain epithelial carcinoma cells, such as
ovarian and breast cancer cell lines (41–45). It has been reported that SHP-1 was
involved in NPC initiation and progression. Knocking down SHP-1
enhances radiosensitivity in the NPC and NSCLC cells (17–19). The present study aimed to find a
way in which SHP-1 expression could be downregulated and thus
inhibit NPC malignant behaviors. The achievements reached in miRNA
research have offered a new insight.
As miRNAs can selectively regulate target gene
expression at a post-transcription level, our attention was focused
on miRNAs targeting SHP-1. First, the miRNAs that target SHP-1 were
predicted using TargetScan human6.2. Ten candidate miRNAs were
selected and tested. Among these, miR-4649-3p suppressed SHP-1
expression most significantly (data not shown). Thus, the present
study aimed to investigate the influence of miR-4649-3p on NPC cell
proliferation and SHP-1 expression. The miR-4649-3p and SHP-1
expression levels were examined in normal nasopharyngeal epithelia
NP69 cells and 8 NPC cells. Compared with the normal nasopharyngeal
epithelia cells, miR-4649-3p was downregulated whereas SHP-1 was
upregulated in NPC cell lines. Increasing miR-4649-3p expression
ectopically inhibited NPC cell proliferation. PCR and western blot
analysis showed that miR-4649-3p mimics suppressed SHP-1
expression. The luciferase reporter assay indicated that
miR-4649-3p suppressed SHP-1 expression by binding to SHP-1 3′UTR
and inhibiting SHP-1 mRNA translation. To verify that miR-4649-3p
inhibited NPC cell proliferation by targeting SHP-1, we ectopically
expressed SHP-1 in NPC cells and found that the effect of the
miR-4649-3p mimics was inversed. Therefore, ectopically expressing
miR-4649-3p in NPC cells may potentially be a new strategy in NPC
treatment.
Acknowledgments
The present study was supported by grants from the
National Natural Sciences Foundation of China (no. 81301976) and
the Natural Sciences Foundation of Hubei Province (no.
2012FFB02324).
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
RT
|
radiation therapy
|
|
IMRT
|
intensity-modulated radiotherapy
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
UTR
|
untranslated region
|
|
SCR
|
control scrambled sequence
|
References
|
1
|
Wee JT, Ha TC, Loong SL and Qian CN: Is
nasopharyngeal cancer really a ‘Cantonese cancer’? Chin J Cancer.
29:517–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ng WT, Lee MC, Hung WM, Choi CW, Lee KC,
Chan OS and Lee AW: Clinical outcomes and patterns of failure after
intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int
J Radiat Oncol. 79:420–428. 2011. View Article : Google Scholar
|
|
3
|
Tham IW, Hee SW, Yeo RM, Salleh PB, Lee J,
Tan TW, Fong KW, Chua ET and Wee JT: Treatment of nasopharyngeal
carcinoma using intensity-modulated radiotherapy-the national
cancer centre singapore experience. Int J Radiat Oncol.
75:1481–1486. 2009. View Article : Google Scholar
|
|
4
|
Stoker SD, van Diessen JN, de Boer JP,
Karakullukcu B, Leemans CR and Tan IB: Current treatment options
for local residual nasopharyngeal carcinoma. Curr Treat Options
Oncol. 14:475–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee CK, Brown C, Gralla RJ, Hirsh V,
Thongprasert S, Tsai CM, Tan EH, Ho JC, Chu da T and Zaatar A:
Impact of EGFR inhibitor in non-small cell lung cancer on
progression-free and overall survival: A meta-analysis. J Natl
Cancer I. 105:595–605. 2013. View Article : Google Scholar
|
|
6
|
Dahabreh IJ, Linardou H, Siannis F,
Fountzilas G and Murray S: Trastuzumab in the adjuvant treatment of
early-stage breast cancer: A systematic review and meta-analysis of
randomized controlled trials. Oncologist. 13:620–630. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, et al: Trastuzumab plus adjuvant chemotherapy for operable
HER2-positive breast cancer. N Engl J Med. 353:1673–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marty M, Cognetti F, Maraninchi D, Snyder
R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Antón A, Lluch A,
et al: Randomized phase II trial of the efficacy and safety of
trastuzumab combined with docetaxel in patients with human
epidermal growth factor receptor 2-positive metastatic breast
cancer administered as first-line treatment: The M77001 study
group. J Clin Oncol. 23:4265–4274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng AL, Li J, Vaid AK, Ma BB, Teh C, Ahn
JB, Bello M, Charoentum C, Chen LT, de Lima Lopes G Jr, et al:
Adaptation of international guidelines for metastatic colorectal
cancer: An asian consensus. Clin Colorectal Cancer. 13:145–155.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adachi T, Hinoi T, Egi H, Shimomura M and
Ohdan H: Oxaliplatin and molecular-targeted drug therapies improved
the overall survival in colorectal cancer patients with synchronous
peritoneal carcinomatosis undergoing incomplete cytoreductive
surgery. Surg Today. Aug 26–2014.Epub ahead of print.
|
|
11
|
Chua DT, Wei WI, Wong MP, Sham JS,
Nicholls J and Au GK: Phase II study of gefitinib for the treatment
of recurrent and metastatic nasopharyngeal carcinoma. Head Neck.
30:863–867. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma B, Hui EP, King A, To KF, Mo F, Leung
SF, Kam M, Lo YM, Zee B, Mok T, Ahuja A, et al: A phase II study of
patients with metastatic or locoregionally recurrent nasopharyngeal
carcinoma and evaluation of plasma Epstein-Barr virus DNA as a
biomarker of efficacy. Cancer Chemother Pharmacol. 62:59–64. 2008.
View Article : Google Scholar
|
|
13
|
Elser C, Siu LL, Winquist E, Agulnik M,
Pond GR, Chin SF, Francis P, Cheiken R, Elting J, McNabola A, et
al: Phase II trial of sorafenib in patients with recurrent or
metastatic squamous cell carcinoma of the head and neck or
nasopharyngeal carcinoma. J Clin Oncol. 25:3766–3773. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan AT, Hsu MM, Goh BC, Hui EP, Liu TW,
Millward MJ, Hong RL, Whang-Peng J, Ma BB, To KF, et al:
Multicenter, phase II study of cetuximab in combination with
carboplatin in patients with recurrent or metastatic nasopharyngeal
carcinoma. J Clin Oncol. 23:3568–3576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Banville D, Stocco R and Shen SH: Human
protein tyrosine phosphatase 1C (PTPN6) gene structure: Alternate
promoter usage and exon skipping generate multiple transcripts.
Genomics. 27:165–173. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Evren S, Wan S, Ma XZ, Fahim S, Mody N,
Sakac D, Jin T and Branch DR: Characterization of SHP-1 protein
tyrosine phosphatase transcripts, protein isoforms and phosphatase
activity in epithelial cancer cells. Genomics. 102:491–499. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng G, Cao R, Xue J, Li P, Zou Z, Huang J
and Ding Q: Increased expression of SHP-1 is associated with local
recurrence after radiotherapy in patients with nasopharyngeal
carcinoma. Radiol Oncol. 48:40–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng G, Cao RB, Li YH, Zou ZW, Huang J and
Ding Q: Alterations of cell cycle control proteins SHP-1/2, p16,
CDK4 and cyclin D1 in radioresistant nasopharyngeal carcinoma
cells. Mol Med Rep. 10:1709–1716. 2014.PubMed/NCBI
|
|
19
|
Cao R, Ding Q, Li P, Xue J, Zou Z, Huang J
and Peng G: SHP1-mediated cell cycle redistribution inhibits
radiosensitivity of non-small cell lung cancer. Radiat Oncol.
8:1782013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li G, Qiu Y, Su Z, Ren S, Liu C, Tian Y
and Liu Y: Genome-wide analyses of radioresistance-associated miRNA
expression profile in nasopharyngeal carcinoma using next
generation deep sequencing. PloS One. 8:e844862013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li G, Liu Y, Su Z, Ren S, Zhu G, Tian Y
and Qiu Y: MicroRNA-324-3p regulates nasopharyngeal carcinoma
radioresistance by directly targeting WNT2B. Eur J Cancer.
49:2596–2607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Pu J, Qi T, Qi M, Yang C, Li S,
Huang K, Zheng L and Tong Q: MicroRNA-145 inhibits the growth,
invasion, metastasis and angiogenesis of neuroblastoma cells
through targeting hypoxia-inducible factor 2 alpha. Oncogene.
33:387–397. 2014. View Article : Google Scholar
|
|
23
|
Si ML, Zhu S, Wu H, Lu Z, Wu F and Mo YY:
miR-21-mediated tumor growth. Oncogene. 26:2799–2803. 2007.
View Article : Google Scholar
|
|
24
|
Cheng G: Circulating miRNAs: Roles in
cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev.
81:75–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Y, Gu X, Zhou M, Xiang J and Chen Z:
Serum microRNAs: A new diagnostic method for colorectal cancer.
Biomed Rep. 1:495–498. 2013.
|
|
26
|
Berger F and Reiser MF: Micro-RNAs as
potential new molecular biomarkers in oncology: Have they reached
relevance for the clinical imaging sciences? Theranostics.
3:943–952. 2013. View Article : Google Scholar
|
|
27
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duchaine TF and Slack FJ: RNA interference
and micro RNA-oriented therapy in cancer: rationales, promises, and
challenges. Curr Oncol. 16:61–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tagliaferri P, Rossi M, Di Martino MT,
Amodio N, Leone E, Gulla A, Neri A and Tassone P: Promises and
challenges of MicroRNA-based treatment of multiple myeloma. Curr
Cancer Drug Targets. 12:838–846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Orang AV and Barzegari A: MicroRNAs in
colorectal cancer: from diagnosis to targeted therapy. Asian Pac J
Cancer Prev. 15:6989–6999. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Long Z, Wang B, Tao D, Huang Y and Tao Z:
Hypofractionated radiotherapy induces miR-34a expression and
enhances apoptosis in human nasopharyngeal carcinoma cells. Int J
Mol Med. 34:1388–1394. 2014.PubMed/NCBI
|
|
32
|
Li G, Wang Y, Liu Y, Su Z, Liu C, Ren S,
Deng T, Huang D, Tian Y and Qiu Y: miR-185-3p regulates
nasopharyngeal carcinoma radioresistance by targeting WNT2B in
vitro. Cancer Sci. 105:1560–1568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang C, Fang X, Li W, Shi Q, Wu L, Chen
X, Huang Z, Wu P, Wang Z and Liao Z: Influence of recombinant
lentiviral vector encoding miR-15a/16-1 in biological features of
human nasopharyngeal carcinoma CNE-2Z cells. Cancer Biother
Radiopharm. 29:422–427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li XH, Qu JQ, Yi H, Zhang PF, Yi HM, Wan
XX, He QY, Ye X, Yuan L, Zhu JF, et al: Integrated analysis of
differential miRNA and mRNA expression profiles in human
radioresistant and radiosensitive nasopharyngeal carcinoma cells.
PLoS One. 9:e877672014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang JX, Qian D, Wang FW, Liao DZ, Wei
JH, Tong ZT, Fu J, Huang XX, Liao YJ and Deng HX: MicroRNA-29c
enhances the sensitivities of human nasopharyngeal carcinoma to
cisplatin-based chemotherapy and radiotherapy. Cancer Lett.
329:91–98. 2013. View Article : Google Scholar
|
|
36
|
Qu C, Liang Z, Huang J, Zhao R, Su C, Wang
S, Wang X, Zhang R, Lee MH and Yang H: MiR-205 determines the
radioresistance of human nasopharyngeal carcinoma by directly
targeting PTEN. Cell Cycle. 11:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen ZX, Sun AM, Chen Y, Liu Y, Zhan JF,
Chen LH and Yuan YW: Effects of radiosensitivity and X-ray dose on
miR-7 expression in nasopharyngeal carcinoma. Nan Fang Yi Ke Da Xue
Xue Bao. 30:1810–1812. 2010.In Chinese.
|
|
38
|
Tong L, Yuan Y and Wu S: Therapeutic
microRNAs targeting the NF-kappa B signaling circuits of cancers.
Adv Drug Deliv Rev. 81:1–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang T, Sun Q, Liu T, Chen J, Du S, Ren
C, Liao G and Yuan Y: MiR-451 increases radiosensitivity of
nasopharyngeal carcinoma cells by targeting ras-related protein 14
(RAB14). Tumor Biol. 35:12593–12599. 2014. View Article : Google Scholar
|
|
40
|
Gandhi NS, Tekade RK and Chougule MB:
Nanocarrier mediated delivery of siRNA/miRNA in combination with
chemotherapeutic agents for cancer therapy: current progress and
advances. J Control Release. 194:238–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Delibrias CC, Floettmann JE, Rowe M and
Fearon DT: Downregulated expression of SHP-1 in Burkitt lymphomas
and germinal center B lymphocytes. J Exp Med. 186:1575–1583. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oka T, Yoshino T, Hayashi K, Ohara N,
Nakanishi T, Yamaai Y, Hiraki A, Sogawa CA, Kondo E, Teramoto N, et
al: Reduction of hematopoietic cell-specific tyrosine phosphatase
SHP-1 gene expression in natural killer cell lymphoma and various
types of lymphomas/leukemias: combination analysis with cDNA
expression array and tissue microarray. Am J Pathol. 159:1495–1505.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sato K, Horiuchi M, Yo R and Nakarai I: A
long survival case of small cell lung cancer synchronized with
renal cancer. Kyobu Geka. 44:251–253. 1991.In Japanese. PubMed/NCBI
|
|
44
|
Amin HM, Hoshino K, Yang H, Lin Q, Lai R
and Garcia-Manero G: Decreased expression level of SH2
domain-containing protein tyrosine phosphatase-1 (SHP-1) is
associated with progression of chronic myeloid leukaemia. J Pathol.
212:402–410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
López-Ruiz P, Rodriguez-Ubreva J, Cariaga
AE, Cortes MA and Colás B: SHP-1 in cell-cycle regulation.
Anticancer Agents Med Chem. 11:89–98. 2011. View Article : Google Scholar : PubMed/NCBI
|