Introduction
Hepatic cancer is a common malignant tumor, which
ranks fifth in terms of global incidence and third in terms of
cancer-related mortality worldwide (1). Although surgery is the preferred
treatment option for patients with hepatic cancer, the overall
morbidity rate following resection is only about 22–42% (2). Therefore, the treatment of hepatic
cancer involves the joint application of various types of
non-surgical treatments. The implantation of radioactive iodine 125
seeds has been used widely in the treatment of hepatic cancer, with
profound effects. The process of particle implantation is performed
manually, and is done irrespective of the 'hot' and 'cold' spots of
radiation (3). Full conformal
radiotherapy is difficult to implement.
As a novel precious material, metal nanoparticles,
namely gold nanorods (GNRs) have unique optical properties
(4), and have a low toxicity and
good biocompatibility (5). GNRs
have been used as a radiation sensitizer (6). Currently, GNRs are being used in
combination with internal radiotherapy in targeted cancer therapy
and are becoming a hotspot in cancer treatment (7,8).
However, GNRs require certain surface modifications to ensure good
compatibility in order to provide the optimal effects from their
clinical application. Silica-coated gold nanorods
(GNRs@SiO2) are relatively easy to prepare, and may help
to maintain low levels of cytotoxicity. The silica surface is easy
for amination, as its surface amino integrates with the carboxyl of
folic acid (FA) to build a covalent bond, and this functions as a
bridge for the folate-conjugated GNRs. Research has shown that FA
receptors are highly expressed on the surface of malignant cancer
cells than on normal cells (9).
However, hepatic colorectal cancer cells have a surfaces rich in FA
receptors (10); FA are natural
ligands and a strong binding force for FA receptors. Therefore, FA
may be used as a tumor targeting factor, and FA may be efficiently
targeted into the liver cancer cells (11); thus, it may lay the foundation for
folate-targeted cancer therapy.
In this study, we investigated a new treatment
method. We used iodine 125 seeds to irradiate tumor cells, as well
as folic acid-conjugated silica-coated GNRs
(GNRs@SiO2-FA) to target folate receptors highly
expressed on the surface membrane of cancer cells, as described in
a previous study (4). The present
study aimed to investigate the apoptosis of the hepatocellular
carcinoma cell line, HepG2, induced by treatment with
GNRs@SiO2-FA in combination with radiotherapy. In
addition, we examined the involvement of apoptosis-related proteins
(e.g., Bax, Bcl-2, Ki67) in these effects.
Materials and methods
Materials
HepG2 cells were obtained from the cell bank of the
Type Culture Collection of Chinese Academy of Sciences (Shanghai,
China). Cell culture reagents were purchased from Gibco, Carlsbad,
CA, USA. Chlorauric acid (HAuCl4·3H2O),
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC),
N-hydroxysuccinimide (NHS), folic acid (FA) and
(3-aminopropyl)triethoxysilane (APTS) were purchased from Sigma
(St. Louis, MO, USA); cetyltrimethylammonium bromide (CTAB), silver
nitrate (AgNO3) and sodium borohydride
(NaBH4) were from Aladdin (Shanghai, China);
tetraethoxysilane (TEOS) and ascorbic acid were obtained from
Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2,5-Diphenyltetrazolium bromide (MTT) and cell apoptosis reagents
were purchased from BestBio (Shanghai, China). Polymerase chain
reaction (PCR)-related reagents and cell incubators were purchased
from Thermo Fisher Scientific, Inc. (Rockford, IL, USA). Anti-Bax
(ab77566), anti-Bcl-2 (15071s) and anti-Ki-67 (PB0065) antibodies
were purchased from Abcam (Cambridge, UK), Cell Signaling
Technology (Danvers, MA, USA) and Boster Biological Technology,
Ltd. (Wuhan, China), respectively.
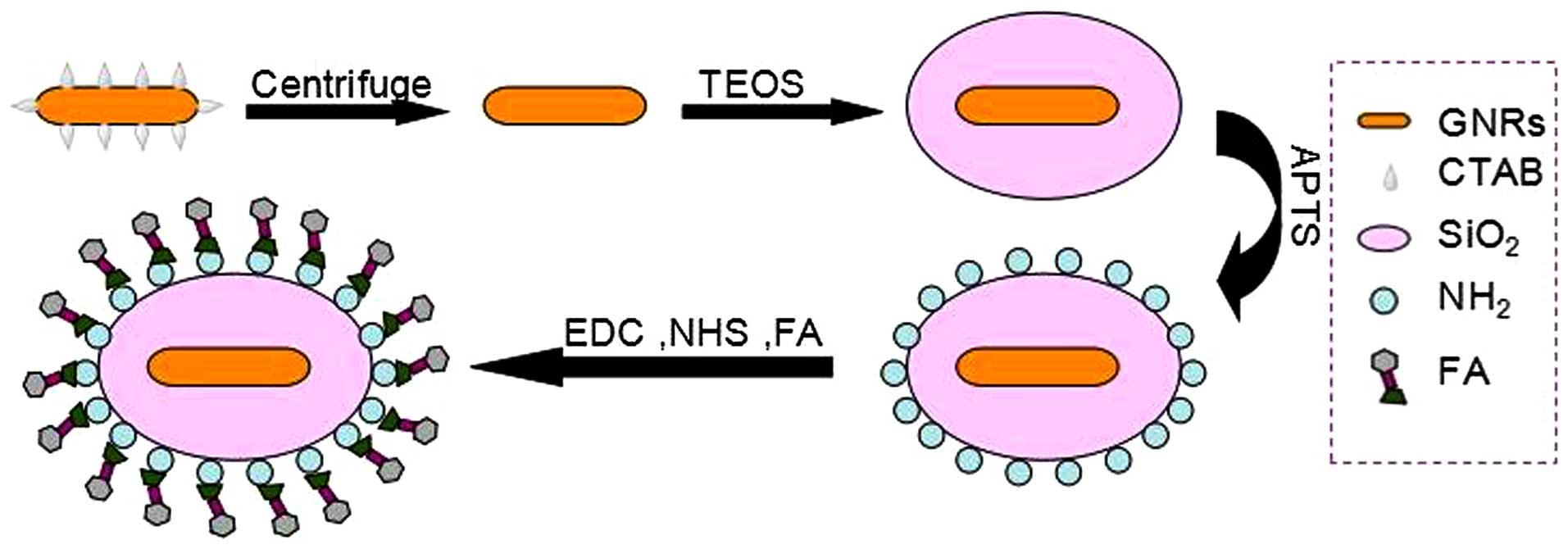
Synthesis and characterization of
GNRs@SiO2-FA
The gold nanorods (GNRs) were synthesized as
previously described (12).
Briefly, 0.6 ml ice-cold 0.01 M NaBH4 was mixed with 10
ml of aqueous solution containing 7.5 ml 0.2 M CTAB and 2.5 ml
0.001 M HAuCl4 under vigorous stirring. It was then kept
at 25°C for at least 2 h prior to use as a seed solution. The
growth solution contained 50 ml 0.2 M CTAB, 50 ml 0.001 M
HAuCl4, 1 ml 0.004 M AgNO3 and 0.7 ml 0.0778
M ascorbic acid. After gently mixing the growth solution, 80
µl of seed solution were added and kept at 30°C for 24 h to
obtain the GNRs.
Next, the spherical core-shell silica-coated GNRs
(GNRs@ SiO2) were successfully prepared using the
sol-gel method. First, the obtained GNRs were washed with deionized
water twice to remove the excess CTAB and redispersed in 40 ml of
water. Subsequently, aqueous ammonia solution was added to obtain a
pH of approximately 10, and 8 ml of 10 mM TEOS/ethanol solution
were added to the solution. The reaction mixture was allowed to
react for 24 h under vigorous stirring. The resulting mesoporous
silica-coated GNRs (GNRs@SiO2) were redispersed in
absolute ethyl alcohol. Finally, the GNRs@SiO2 were
conjugated with folic acid (GNRs@SiO2-FA). EDC and NHS
were used to activate the carboxyl groups of folic acid (Fig. 1).
Cell culture
The human hepatocellular carcinoma cell line, HepG2,
was used in the experiments. The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) (both from Gibco) at 37°C in a 5% CO2
humidified incubator (Thermo Fisher Scientific, Inc.).
Cell viability assay
The HepG2 cells were cultured in a 96-well plate at
a density of 5×103 cells/ml for 24 h, and the culture
medium was then replaced with solutions containing either GNRs,
GNRs@SiO2, or GNRs@SiO2-FA, which were
pre-mixed with fresh DMEM supplemented with 10% FBS [gold (Au)
concentrations were 40, 20, 10, 5 and 2.5 ppm]. Untreated cells
served as the controls. The cells were cultured for a further 48 h,
and the culture medium was then replaced with 0.2 ml fresh culture
medium containing MTT assay reagent (4 mg/ml), followed by
incubation for a further 4 h. DMSO (100 µl) was added to
each well to dissolve the colored crystals. The optical density of
the colored product was measured at 490 nm using a microplate
reader (Bio-Tek ELx800; Bio-Tek Instruments Inc., Winooski, VT,
USA).
Transmission electron microscopy (TEM) to
determine the distribution of GNRs@SiO2-FA within
cells
The HepG2 cells were cultured in DMEM medium
supplemented with 10% FBS, under standard cell culture conditions
(5% CO2, 37°C) for 24 h. The cells were washed 3 times
with phosphate-buffered saline (PBS). The experimental group was
treated with 9 ml of DMEM medium and 1 ml of 40 µg/ml
GNRs@SiO2-FA solution, and the control group (untreated)
was treated with 10 ml DMEM medium. The cells in the experimental
group were then fixed with 2.5% glutaraldehyde, followed by
ethanol-acetone gradient dehydration and embedding in epoxy resin.
The cells were then visualized under a transmission electron
microscope (JEM-2100; JEOL, Tokyo, Japan).
Determination of targeting efficacy of
GNRs@SiO2-FA
The HepG2 cells were cultured in a 6-well plate at a
density of 5×104 cells/ml for 24 h. The cells were
divided into 2 groups: in one group, 100 µl of
GNRs@SiO2 were added to each well, and in the other
group, 100 µl of GNRs@SiO2-FA were added to each
well. The cells were then incubated in humidified air at 37°C with
5% CO2. The cultivation of cells was terminated after 1,
4, 8, 16 and 24 h, and this was followed by the collection of the
cells, the detection of Au elements by inductively coupled plasma
mass spectrometry (ICP-MS), and the calculation of the cell solid
Au element content per kilogram.
First, a total of 10 (2 ml) centrifuge tubes (mg)
were measured for electronic balance. Before the cells were
harvested, they were washed with PBS 3 times to remove the free
GNRs@ SiO2-FA and GNRs@SiO2. The cells were
then digested with 300 µl 0.25% trypsin. This was followed
by the addition of 500 µl DMEM and 1 ml PBS, and the cells
were then collected and placed within weighed 2 ml centrifuge
tubes. Next, using a thermostat (60 degrees), the centrifuge tubes
containing the cells were dried. They were then weighed for
electronic balance, and the quality of the cell solid material was
obtained by subtracting the previous record of quality. This was
followed by the additino of 500 µl aqua regia to each tube,
and the solid material was dissolved by ultrasound, with the
addition of triple-distilled water to a final volume of 10 ml.
Finally, the Au element was detected by ICP-MS and the Au element
content was caclulated per kg cell solids (mg/kg).
Apoptosis assay
HepG2 cells, cultured in vitro, were divided
into the following 4 groups: i) the control group (untreated), ii)
the GNRs@SiO2-FA group (40 µg/ml), iii) the
iodine 125 seeds group (9 grains, 0.8 mCi), and iv) the combination
group (with GNRs@SiO2-FA and iodine 125 seeds). The
cells in the 4 groups were collected and washed twice with cold
PBS. The cells were centrifuged at 2,000 rpm for 5 min and were
then resuspended in 100 µl of Annexin V binding buffer at a
density of 1×106 cells/ml. The cells were incubated with
5 µl of Alexa Fluor 488 Annexin V conjugate and 5 µl
of propidium iodide (PI) (both from BestBio) for 15 min at room
temperature in the dark; 400 µl of 1X binding buffer was
added to each sample tube, and the samples were immediately
analyzed using a flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA). Histograms and statistics were processed using FlowJo
software version 7.6.1.
Semi-quantitative reverse transcription
(RT)-PCR
Bax and Bcl-2 mRNA expression levels were measured
by RT-PCR. The HepG2 cells (1×106), which were treated
with GNRs in combination with iodine 125 seeds, were collected and
counted. The analysis was performed using the two-step RT-PCR kit
(Takara Co., Dalian, China). The purity of the total RNA was
determined using a UV spectrophotometer (UV-2450; Shimadzu Corp.,
Kyoto, Japan) at OD260/OD280 (>1.8). The amplification method
was as follows: Bax, 30 cycles; Bcl-2, 32 cycles; GAPDH, 32 cycles
(94°C, 30 sec; 56°C, 30 sec; 72°C, 1 min). The amplified product
was analyzed by gel electrophoresis on a 2% agarose gel and EB
staining (ImageJ software). The primer sequences and PCR product
sizes are listed in Table I.
 | Table IPrimers used for RT-PCR. |
Table I
Primers used for RT-PCR.
| Forward primer | Reverse primer | Amplified fragment
length (bp) |
|---|
| Bax |
5′-GCCCACCAGCTCTGAGCAGATCAT-3′ |
5′-CGGCAATCATCCTCTGCAGC-3′ | 209 |
| Bcl-2 |
5′-GACTTCGCCGAGATGTCCAG-3′ |
5′-CAGGTGCCGGTTCAGGTACT-3′ | 225 |
| GAPDH |
5′-AGAAGGCTGGGGCTCATTTG-3′ |
5′-AGGGGCCATCCACAGTCTTC-3′ | 258 |
Western blot analysis
For protein extraction, protein extraction solution
with protease inhibitor (1% Triton-X100, 0.1% SDS, 150 mM NaCl, 50
mM Tris-HCl pH 7.4, 1% deoxycholic acid, 2 mM EDTA pH 8.0) was
used, and the protein was quantified using a BCA Protein assay kit
(Thermo Fisher Scientific, Inc.) to plot a standard curve. The
protein samples were boiled for 5 min, loaded and subjected to
SDS-polyacrylamide gel electrophoresis (PAGE), and were
subsequently transferred onto a PVDF membrane. The membrane was
blocked with 5% BSA for 1 h at room temperature and probed with
rabbit antibodies against Bcl-2, Bax and caspase-3 (anti-caspase-3
antibody from Cell Signaling Technology) (all 1:1,000 dilution)
overnight at 4°C. The membrane was washed with Tris-HCl buffer
containing Tween-20 (TBST 20 mM Tris-HCl, pH 7.4, 150 mM NaCl and
0.05% Tween-20) and incubated in horseradish peroxidase
(HRP)-conjugated secondary goat anti-rabbit antibody (Cat. no.
49620; Cell Signaling Technology) (1:4,000 dilution) for 1 h at
room temperature. The blots were developed by adding
electrochemiluminescence (ECL) detection reagents.
Immunostaining for Bcl-2, Bax and Ki-67
cell markers
Both the treated and untreated HepG2 cells were
fixed with 4% paraformaldehyde for 15 min. The cells were
permeabilized for 10 min at room temperature in 0.4% Triton X-100
diluted in PBS. The fixed cells were incubated overnight at 4°C
with each of the primary antibodies: anti-Bax (1:100), anti-Ki-67
(1:100) and anti-Bcl-2 (1:50). On the following day, after 3 washes
with PBS, the secondary biotin-labeled antibody (SP9001; ZSGB-BIO,
Beijing, China) was used at 1:200. For color development,
streptavidin was labeled with HRP at 1:200, using the
streptavidin-biotin-peroxidase complex (SABC) method.
Immunohistochemical staining
Bax-positive staining (tan) was observed in the
cytoplasm, and Bcl-2-positive staining (tan) sd was observed in the
cytoplasm and cell membrane. To quantify protein expression in the
various samples, a scoring method was applied. A mean percentage of
positive cancer cells was determined from at least 5 areas at ×400
magnification and assigned to 1 of the following 5 categories, as
previously described (13): 0
point, <5%; 1 point, 5–25%; 2 point, 26–50%; 3 point, 51–75%;
and 4 point, >75%. Points for staining and percentages were
multiplied for a 10-point scale as follows: 0 point, negative (−);
1–3 points, weakly positive (+); 4–6 points, positive (++); and 7–9
points, strongly positive (+++). For cells that showed
heterogeneous staining, the predominant pattern was taken into
account for scoring. The percentage of positive cancer cells and
the staining intensity were multiplied to produce a weighted score
for each case. Cases with a weighted surviving score <1 were
considered to be negative.
Positive Ki-67 staining was indicated by the
presence of fine tan particles in the nucleus. Each slice was
observed at >10 typical views (at a high magnification, ×400),
counting at least 200 cells. The percentage of positive cells
indicated the Ki-67 cell proliferation index, which was termed
KI.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software for Windows. The cell survival rate was determined by MTT
assay and the differences in the apoptotic rate in the 4 groups was
analyzed by one-way ANOVA. P-values <0.05 were considered to
indicate statistically significant differences.
Results
Synthesis of GNRs@SiO2-FA and
cellular uptake
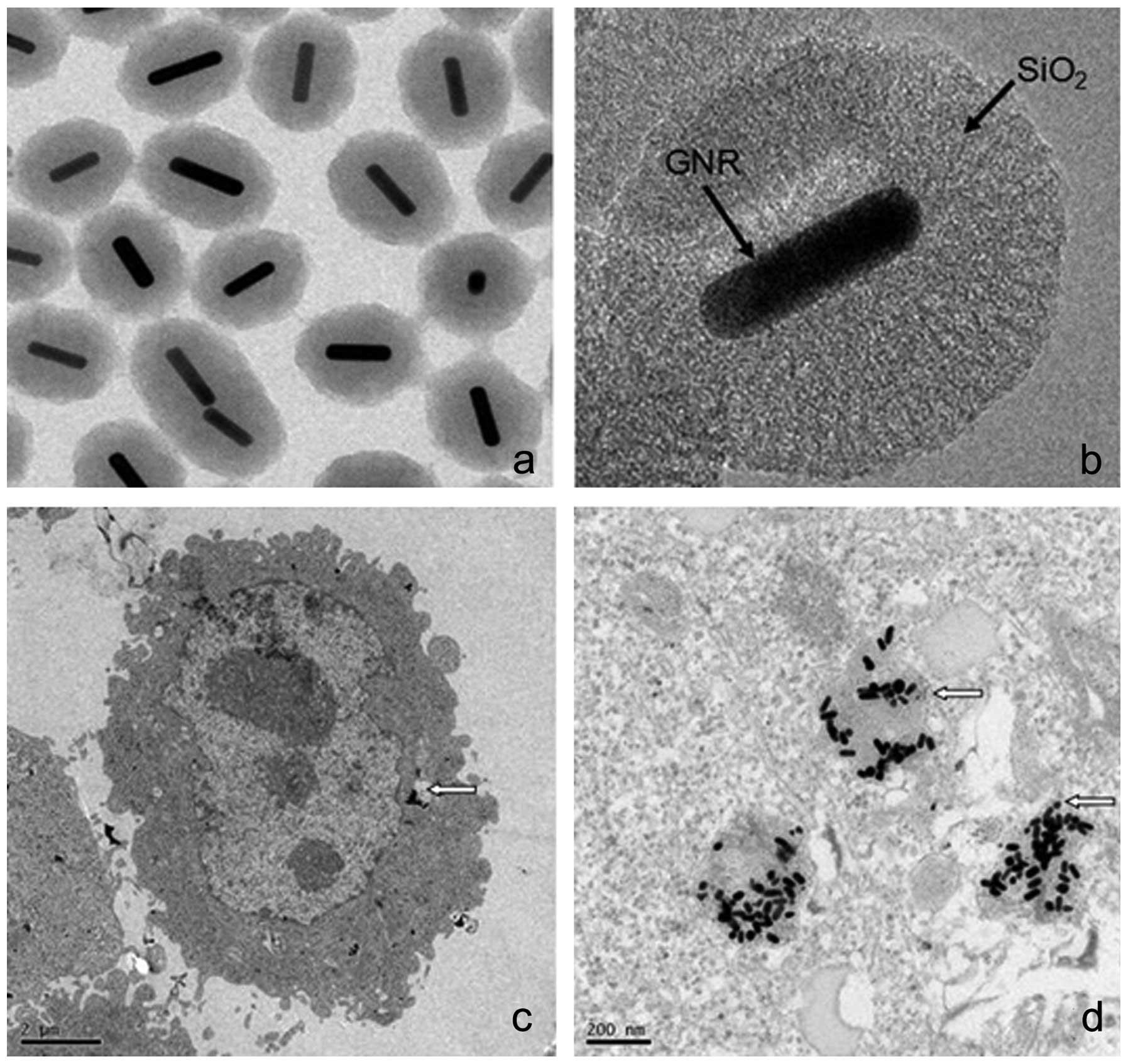
GNRs@SiO2-FA distribution in the cells
was determined by TEM. The prepared GNRs@SiO2-FA had an
ellipsoid shape. The GNRs were composed of a central rod,
approximately 40 nm long and 10 nm wide, with a coating of silica
on the surface. We demonstrated that GNRs@SiO2-FA
entered the cells in the form of nanoparticles. We also found that
following entry into the cell, the ellipsoid shape of the
GNRs@SiO2-FA was retained (Fig. 2).
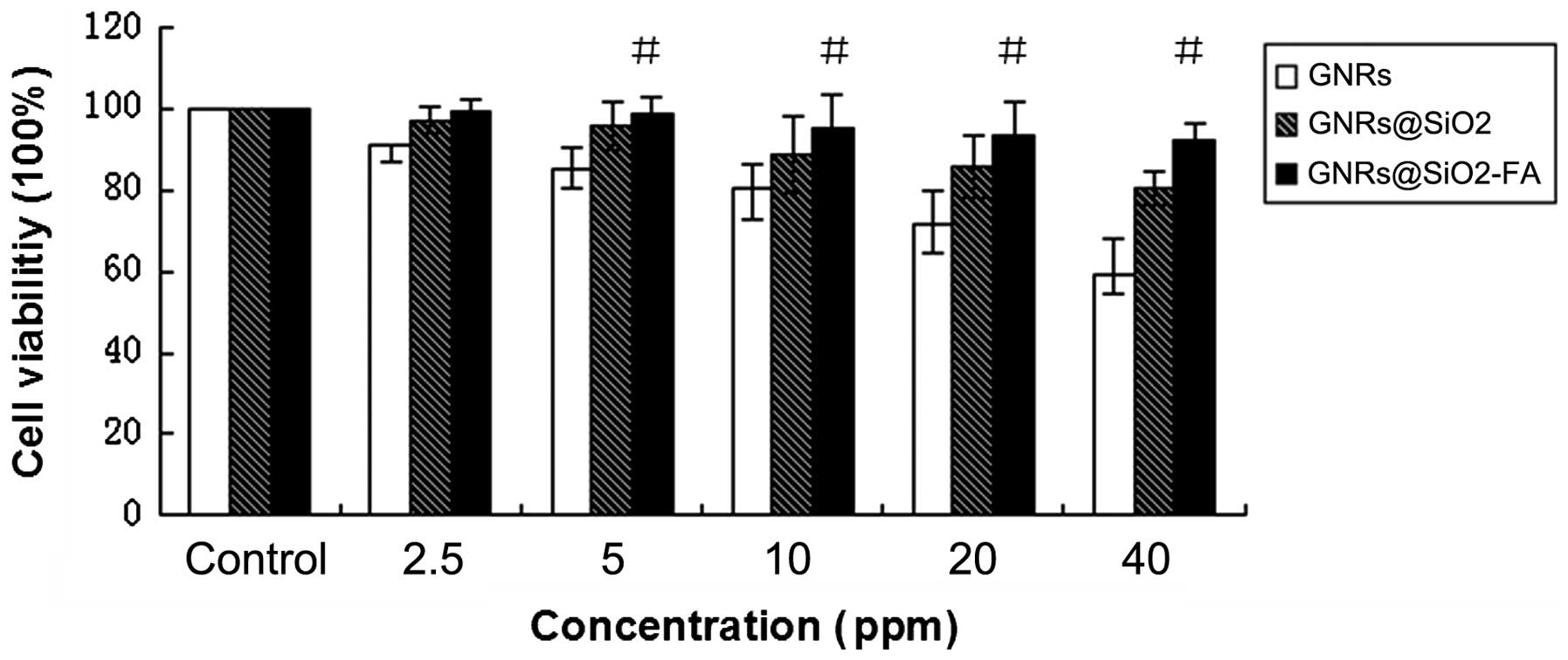
GNRs decrease cell viability at the
concentration >5 ppm
The results of MTT assay revealed that cell
viability decreased as the GNR concentration increased in the
culture medium. The cell survival rates in each group were as
follows: GNRs group were 59.1, 71.8, 80.5, 85.3 and 91.1%; the
GNRs@SiO2 group were 80.3, 85.7, 88.8, 95.8 and 97.1%;
and the GNRs@SiO2-FA group were 92.3, 93.6, 95.4, 98.7
and 99.4%, respectively compared to treatment with 2.5, 5, 10, 20
and 40 ppm of the
GNRs/GNRs@SiO2/GNRs@SiO2-FA. When the
concentration of the Au element was ≥5 ppm, all 3 groups (GNRs,
GNRs@SiO2 and GNRs@SiO2-FA) exhibited
statistically significant differences compared to the untreated
controls (P<0.05). The cell growth inhibitory effects of
GNRs@SiO2-FA were not as prominent as those of the GNRs
and GNRs@SiO2. The GNRs alone had the most prominent
inhibitory effect on cell growth (Fig. 3).
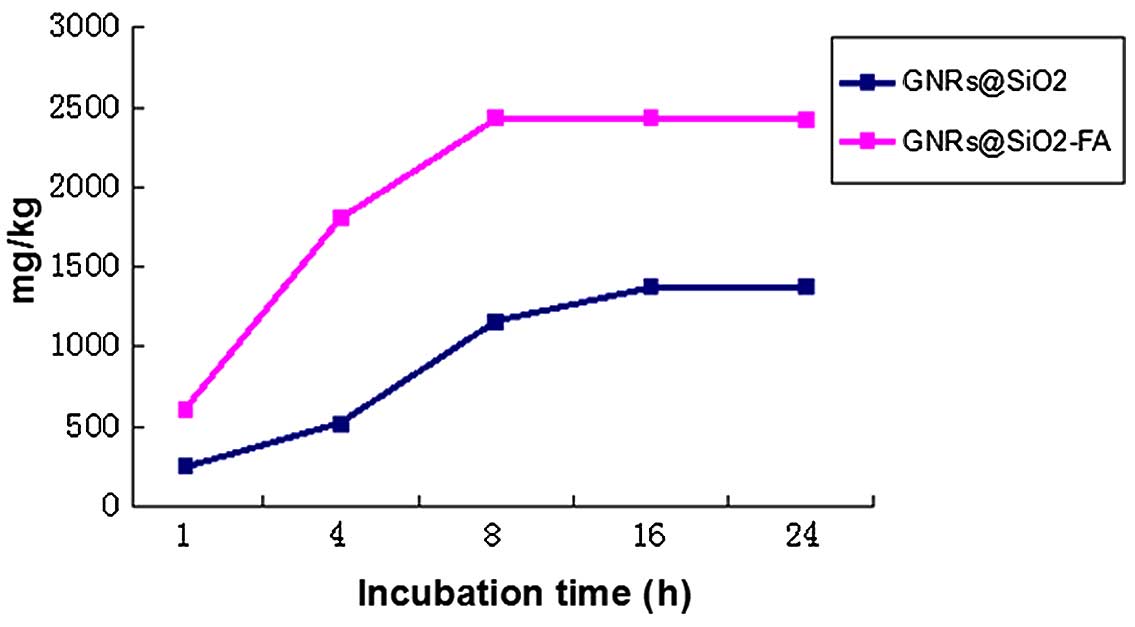
Targeting efficacy of
GNRs@SiO2-FA
As shown in Fig.
4, the cellular uptake rate of GNRs@SiO2-FA was
2.65-fold higher than that of GNRs@SiO2 at 4 h, and the
cellular uptake rate in both groups reached a plateau at subsequent
time points (16 and 24 h. This indicated that we had successfully
prepared GNRs@SiO2-FA with a high targeting ability, and
with the ability to bind with folate receptors within a short
period of time. This experiment also revealed that nanoparticles
can enter cells by endocytosis; however, entry by endocytosis is
less efficient compared with the folate receptor-mediated entry of
nanoparticles into cells.
GNRs@SiO2-FA significantly
increase the apoptosis of HepG2 cells
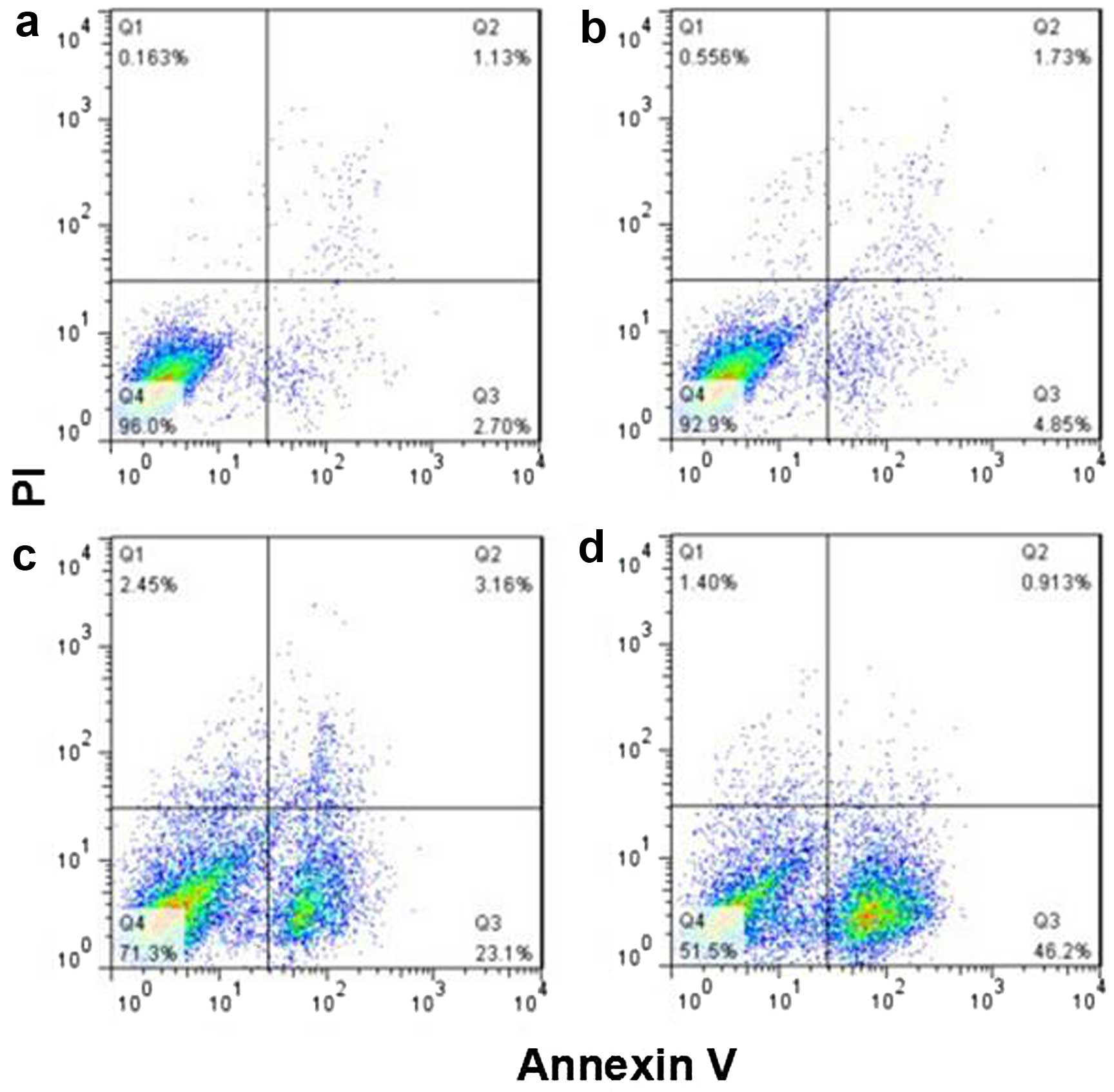
The apoptotic rates of the HepG2 cells were higher
in the GNRs@SiO2-FA group and the iodine 125 seeds group
than in the control group (P<0.05). The apoptotic rate was also
significantly higher in the combination group than in either the
GNRs@SiO2-FA group or iodine 125 seed group (P<0.05)
(Fig. 5 and Table II).
 | Table IIApoptotic rate of HepG2 cells. |
Table II
Apoptotic rate of HepG2 cells.
| Groups | n | Apoptotic rate
(%) |
|---|
| Control group | 10 | 5.91±1.52 |
|
GNRs@SiO2-FA group | 10 | 10.16±3.18 |
| Iodine 125 seeds
group | 10 | 20.78±4.79a |
| Combination
group | 10 | 33.41±8.00a |
| F-value | | 99.82 |
| P-value | | 0.00 |
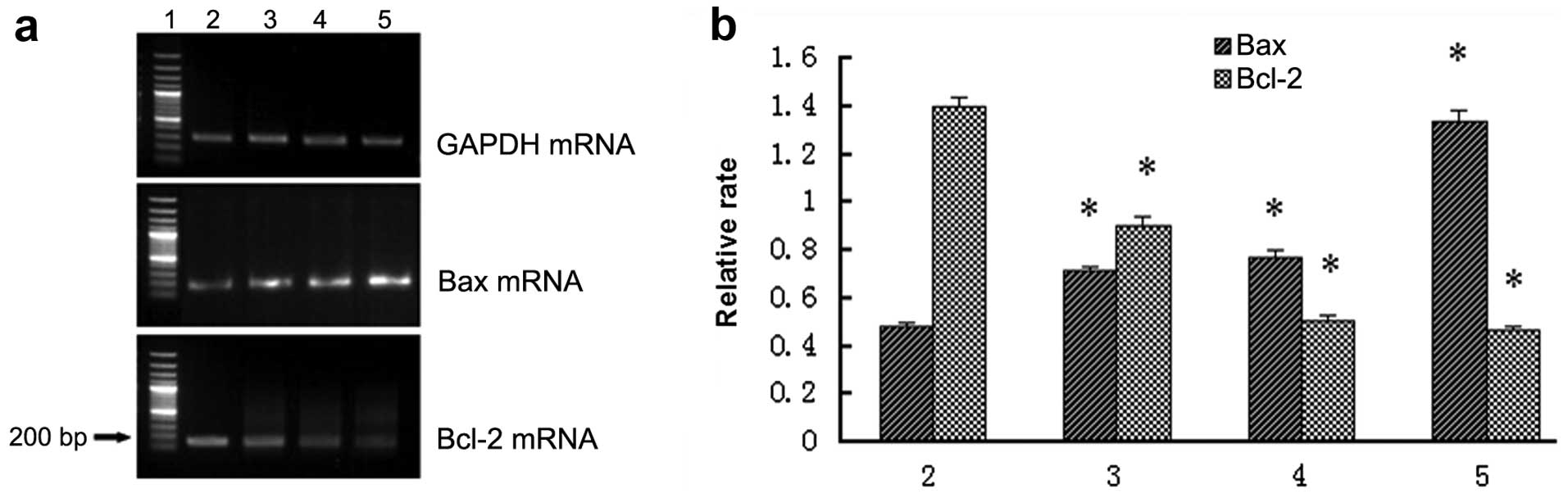
GNRs@SiO2-FA alters Bax and
Bcl-2 mRNA expression in HepG2 cells
RT-PCR revealed that the mRNA expression of Bax
significantly increased, and the mRNA expression of Bcl-2
significantly decreased in the combination group to a greater
extent compared with either the GNRs@SiO2-FA group or
the iodine 125 seed group (Fig.
6).
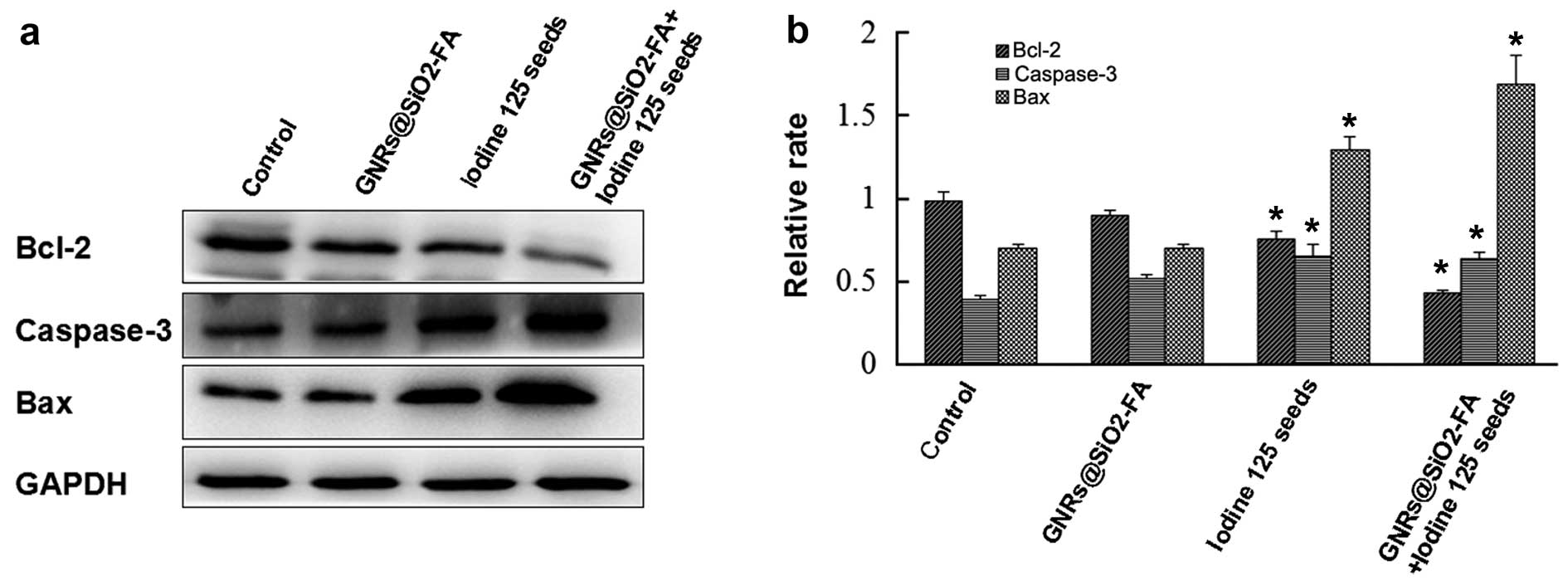
GNRs@SiO2-FA alters the
protein expression of apoptosis-related proteins
Western blot analysis revealed that the expression
of the apoptosis-related proteins, Bax and caspase-3, was
significantly upregulated in the combination group to a greater
extent compared with either the GNRs@SiO2-FA group or
the iodine 125 seed group. The expression of Bcl-2 was, however,
downregulated (Fig. 7).
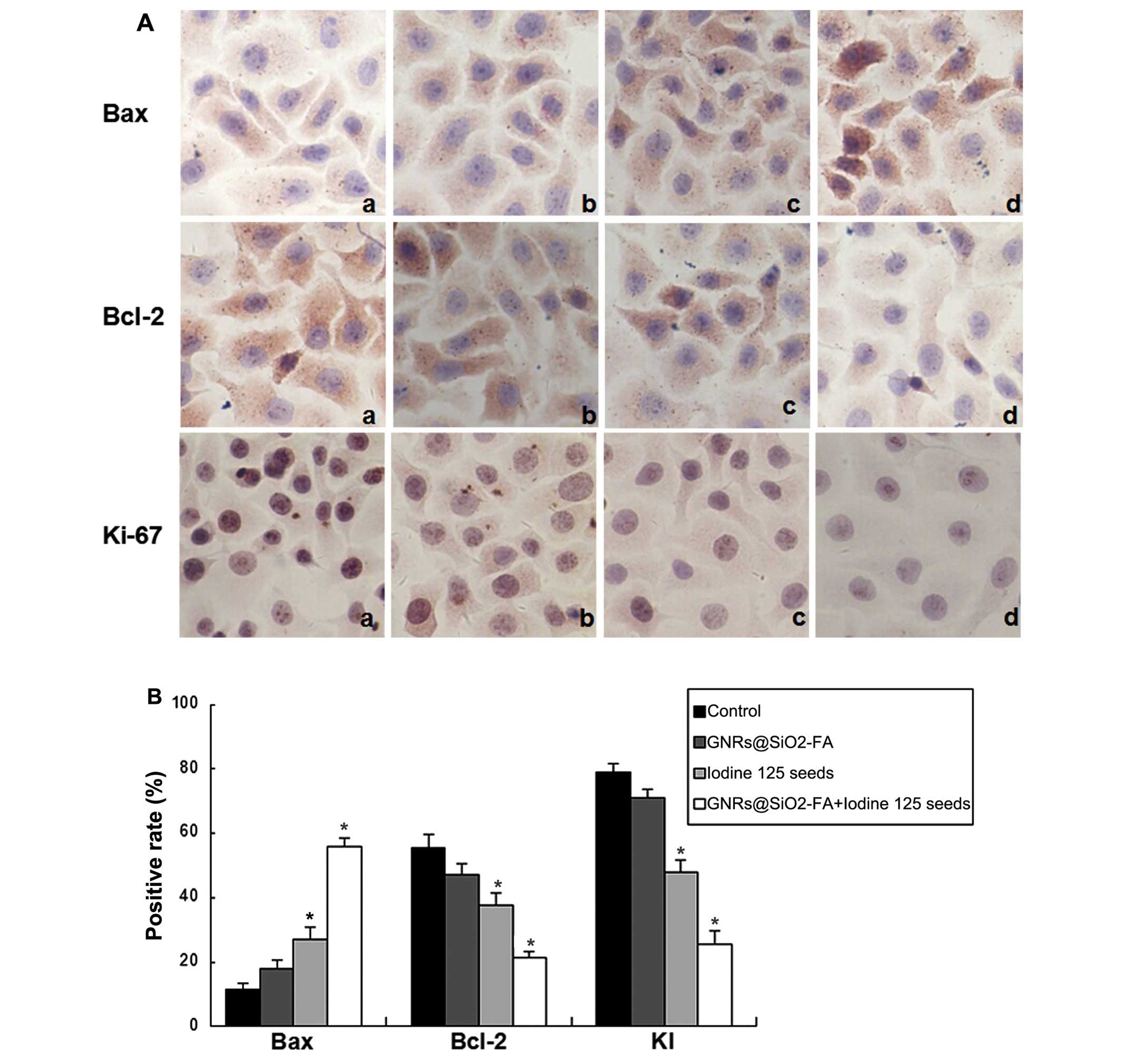
GNRs@SiO2-FA alters the
protein expression of apoptosis-related proteins as shown by
immunohistochemical staining Bax protein expression in HepG2
cells
In the 4 groups of HepG2 cells, the percentage of
Bax-positive cells was found to be 0.95±0.18, 1.51±0.44, 4.29±0.86
and 7.55±1.40 in the control, GNRs@SiO2-FA, iodine 125
seed and the combination group, respectively. Compared with the
GNRs@SiO2-FA and iodine 125 seeds groups, the protein
expression level of Bax was significantly increased in the
combination group (P<0.05) (Fig.
8 and Table III).
 | Table IIIThe integral value of Bax, Bcl-2, and
KI in HepG2 cells in the different groups. |
Table III
The integral value of Bax, Bcl-2, and
KI in HepG2 cells in the different groups.
| Groups | n | Bax | Bcl-2 | KI |
|---|
| Control group | 10 | 0.95±0.18 | 9.01±1.02 | 75.59±6.29 |
|
GNRs@SiO2-FA group | 10 | 1.51±0.44 | 8.39±0.47 | 67.09±5.46 |
| Iodine 125 seeds
group | 10 | 4.29±0.86a | 3.81±1.28a | 46.84±7.90a |
| Combination
group | 10 | 7.55±1.40a | 1.70±0.83a | 27.90±5.83a |
| F-value | | 74.49 | 84.60 | 109.98 |
| P-value | | 0.00 | 0.00 | 0.00 |
Bcl-2 protein expression in HepG2
cells
In the 4 groups of HepG2 cells, the percentage of
Bcl-2-positive cells was 9.01±1.02, 8.39±0.47, 3.81±1.28 and
1.70±0.83 in the control, GNRs@SiO2-FA, iodine 125 seed
and the combination group, respectively. The protein expression
level of Bcl-2 was much lower in the combination group than in the
GNRs@SiO2-FA and iodine 125 seeds groups (P<0.05)
(Fig. 8 and Table III).
Ki-67 protein expression in HepG2
cells
The cells in the control group were plump in shape.
When the nuclei were hyper-chromatic, the results were positive.
The protein expression level of Ki-67 was significantly decreased
in the combination group compared with the GNRs@SiO2-FA
and iodine 125 seeds groups (P<0.05), and the cell nuclei were
stained lightly. In the 4 groups of cells, the Ki-67 proliferation
index (KI) was 75.59±6.29, 67.09±5.46, 46.84±7.90 and 27.90±5.83%
in the control, GNRs@SiO2-FA, iodine 125 seed and the
combination group, respectively, which was significantly different
between the 4 groups (P<0.05) (Fig. 8).
Discussion
In China, liver cancer is one of the malignant
tumors with a high incidence, which has an insidious onset, rapid
progression and alarmingly high mortality rates (14). The comprehensive treatment of
hepatic cancer combining multiple methods, such as surgery,
radiotherapy and chemotherapy may help in antagonizing the tumor
(15). As normal hepatic tissues
are poorly tolerant to radiation, it is not possible to increase
the external irradiation dose used in the treatment of hepatic
cancer; hence, the curative effect of external radiation therapy is
poor. Compared with external radiation therapy, brachytherapy using
iodine 125 seed implantation has the highest local dose and has a
high dose close to the seed source and a steep fall in the dose for
the surrounding tissues. After enough doses, it continues to kill
the tumor cells; therefore, tumor tissue is damaged more
thoroughly. However, the shortcoming of iodine 125 seed implant
brachytherapy is that is is applied manually to existing cold and
hot spots (3,16) idicated by radiotherapy
sensitization methods to achieve absolutely conformal
radiotherapy.
This study investigated the GNRs@SiO2-FA
containing the element Au, which has a high atomic number that
enables it to infiltrate into cancer cells (17,18). High atomic number materials, such
GNRs enter cancer cells, producing stronger photoelectric
absorption effects on cancer cells than on the surrounding normal
cells. GNRs@SiO2-FA, in combination with radiotherapy,
acts as a radiosensitization agent, thereby enhancing the efficacy
of radiotherapy. This type of combination therapy has a more
prominent photoelectric absorption effect, accelerating the DNA
chain rupture, and eventually leading to cell death (19,20).
Cell apoptosis is known as programmed cell death
(PCD), and multiple genes are involved in this process. A previous
study demonstrated that the delivery of programmed cell death
protein 4 (Pdcd4) in mice with liver cancer significantly
suppressed tumor growth, induced apoptosis suppressed proliferation
and angiogenesis (21). The
coordinated action of apoptosis and anti-poptotic genes determines
the initiation or inhibition of apoptosis (22). In this study, we selected Bcl-2,
Bax, caspase-3 and Ki-67 as a testing index, observing the effects
of GNRs@SiO2-FA in combination with radiotherapy on
their expression. Mitochondria are central to apoptotic processes
and can even decide cell fate. The mitochondrial membrane regulates
the apoptosis of Bcl-2 family proteins, and the combination of the
protein state will adjust the mitochondrial membrane potential,
alter the expression of Cyt C (23) and Smac/DIABLO (24) proteins, as well as the release of
AIF, activating the caspase pathway and lead to cell apoptosis; the
mitochondria exerts this effect on promoting apoptosis through
Bax/Bcl-2 and the regulation of caspases (25). Bcl-2 (26) is a type of mitochondrial
transmembrane protein that can promote cell survival and inhibit
apoptosis; it can prevent mitochondrial apoptosis before Cyt C
blocks apoptosis, and members of the Bcl-2 family can be inserted
in the mitochondrial outer membrane formation channels, thereby
activating apoptosis. Ki-67 (27)
is a type of cell cycle-related proliferating nuclear antigen that
is used as an independent prognostic indicator for curing hepatic
cancer and achieving total survival. A decrease in Ki-67 expression
(28) has been shown to suppress
cancer cell proliferation. Electron microscopy revealed that the
GNRs were mostly in the cytoplasm (Fig. 2c) after entering the cells and are
likely to be located in the mitochondria (Fig. 2d). The mitochondria may absorb
GNRs and accept iodine 125 particle irradiation simultaneously, and
mitochondrial damage leads to apoptosis.
To the best of our knowledge, there have been
limited studies published to date on the administration of GNRs in
combination with iodine 125 particles. Our in vitro
experiments confirmed that GNRs in combination with iodine 125
particles exerted marked synergistic anticancer effects. This study
provides the experimental basis for hepatic cancer therapy using
GNRs in combination with iodine 125 particles at the molecular
level. However, a more detailed investigation is warranted in order
to elucidate the specific biological mechanisms involved.
Acknowledgments
Dr B. Gao acknowledges the University of Science and
Technology of China and the National Science Foundation grant no.
81071240 for their financial assistance.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ciacio O, Voron T, Pittau G, Lewin M,
Vibert E, Adam R, Sa Cunha A, Cherqui D, Schielke A, Soubrane O, et
al: Interest of preoperative immunonutrition in liver resection for
cancer: study protocol of the PROPILS trial, a multicenter
randomized controlled phase IV trial. BMC Cancer. 14(980)2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herron B, Herron A, Howell K, Chin D and
Roads L: A Review of radiation therapy's role in early-stage breast
cancer and an introduction to electronic brachytherapy. Cancer
Treatment - Conventional and Innovative Approaches. Rangel L:
InTech. 223–238. 2013. View
Article : Google Scholar
|
|
4
|
Liu W, Zhu Z, Deng K, Li Z, Zhou Y, Qiu H,
Gao Y, Che S and Tang Z: Gold nanorod@chiral mesoporous silica
core-shell nanoparticles with unique optical properties. J Am Chem
Soc. 135:9659–9664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu XG and Gao XH: Multilayer coating of
gold nanorods for combined stability and biocompatibility. Phys
Chem Chem Phys. 13:10028–10035. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gui C and Cui DX: Functionalized gold
nanorods for tumor imaging and targeted therapy. Cancer Biol Med.
9:221–233. 2012.
|
|
7
|
Xu W, Luo T, Li P, Zhou C, Cui D, Pang B,
Ren Q and Fu S: RGD-conjugated gold nanorods induce
radiosensitization in melanoma cancer cells by downregulating
alpha(v)beta(3) expression. Int J Nanomedicine. 7:915–924.
2012.
|
|
8
|
Mackey MA, Ali MR, Austin LA, Near RD and
El-Sayed MA: The most effective gold nanorod size for plasmonic
photothermal therapy: theory and in vitro experiments. J Phys Chem
B. 118:1319–1326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Franzen SA: A comparison of peptide and
folate receptor targeting of cancer cells: from single agent to
nanoparticle. Expert Opin Drug Deliv. 8:281–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
D'Angelica M, Ammori J, Gonen M, Klimstra
DS, Low PS, Murphy L, Weiser MR, Paty PB, Fong Y, Dematteo RP, et
al: Folate receptor-alpha expression in resectable hepatic
colorectal cancer metastases: patterns and significance. Mod
Pathol. 24:1221–1228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leamon CP: Folate-targeted drug strategies
for the treatment of cancer. Curr Opin Investig Drugs. 9:1277–1286.
2008.PubMed/NCBI
|
|
12
|
Pastoriza-Santos I, Perez-Juste J and
Liz-Marzan LM: Silica-coating and hydrophobation of CTAB-stabilized
gold nanorods. Chem Mater. 8:2465–2467. 2006. View Article : Google Scholar
|
|
13
|
Lo Muzio L, Staibano S, Pannone G,
Mignogna MD, Mariggiò A, Salvatore G, Chieffi P, Tramontano D, De
Rosa G and Altieri DC: Expression of the apoptosis inhibitor
survivin in aggressive squamous cell carcinoma. Exp Mol Pathol.
70:249–254. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ribas J, Bettayeb K, Ferandin Y, Knockaert
M, Garrofé-Ochoa X, Totzke F, Schächtele C, Mester J,
Polychronopoulos P, Magiatis P, et al: 7-Bromoindirubin-3′-oxim
induces caspase-independent cell death. Oncogene. 25:6304–6318.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang P, Dong B, Yu X, Yu D, Cheng Z, Su
L, Peng J, Nan Q and Wang H: Computer-aided dynamic simulation of
microwave-induced thermal distribution in coagulation of liver
cancer. IEEE Trans Biomed Eng. 48:821–829. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Skowronek J: Low-dose-rate or
high-dose-rate brachytherapy in treatment of prostate cancer -
between options. J Contemp Brachytherapy. 5:33–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roeske JC, Nunez L, Hoggarth M, Labay E
and Weichselbaum RR: Characterization of the theorectical radiation
dose enhancement from nanoparticles. Technol Cancer Res Treat.
6:395–401. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho SH, Jones BL and Krishnan S: The
dosimetric feasibility of gold nanoparticle-aided radiation therapy
(GNRT) via brachy-therapy using low-energy gamma-/x-ray sources.
Phys Med Biol. 54:4889–4905. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Conde J, Doria G and Baptista P: Noble
metal nanoparticles applications in cancer. J Drug Deliv.
2012(751075)2012. View Article : Google Scholar
|
|
20
|
Jones B, Krishnan S and Cho SH: Estimation
of microscopic dose enhancement factor around gold nanoparticles by
Monte Carlo calculations. Med Phys. 37:3809–3816. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JH, Minai-Tehrani A, Kim YK, Shin JY,
Hong SH, Kim HJ, Lee HD, Chang SH, Yu KN, Bang YB, et al:
Suppression of tumor growth in H-ras12V liver cancer mice by
delivery of programmed cell death protein 4 using galactosylated
poly(ethylene glycol)-chitosan-graft-spermine. Biomaterials.
33:1894–1902. 2012. View Article : Google Scholar
|
|
22
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goodsell DS: The molecular perspective:
Bcl-2 and apoptosis. Stem Cells. 20:355–361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adrain C, Creagh EM and Martin SJ:
Apotosis-associated release of Smac/DIABLO from mitochondrial
requires active caspases and is blocked by Bcl-2. EMBO J.
20:6627–6663. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang H, Zhan HJ, Wang BG, Pan Y and Hao
XS: Change in expression of apoptosis genes after hyperthermia,
chemotherapy and radiotherapy in human colon cancer transplanted
into nude mice. World J Gastroenterol. 13:4365–4371.
2007.PubMed/NCBI
|
|
26
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stroescu C, Dragnea A, Ivanov B, Pechianu
C, Herlea V, Sgarbura O, Popescu A and Popescu I: Expression of
p53, Bcl-2, VEGF, Ki67 and PCNA and prognostic significance in
hepato-cellular carcinoma. J Gastrointestin Liver Dis. 17:411–417.
2008.PubMed/NCBI
|
|
28
|
Pichu S, Krishnamoorthy S, Shishkov A,
Zhang B, McCue P and Ponnappa BC: Knockdown of Ki-67 by
dicer-substrate small interfering RNA sensitizes bladder cancer
cells to curcumin-induced tumor Inhibition. PLoS One. 7:e485672012.
View Article : Google Scholar : PubMed/NCBI
|