Introduction
Osteoarthritis (OA) is a progressive cartilage
degradation skeletal disease characterized by the synthesis and
degradation imbalance of extracellular matrix (ECM), resulting in a
gradual loss of cartilage integrity (1). The final common pathway of cartilage
degradation arises from the failure of chondrocytes to maintain the
homeostatic balance between ECM synthesis and degradation (2). Chondrocytes are the only cell type
resident in the cartilage and it has been demonstrated that the
increasing number of chondrocytes undergoing apoptosis is
significantly correlated with the severity of OA (3,4).
Recently, endoplasmic reticulum (ER) stress was found to have a
crucial role in apoptosis (5),
and is considered to be one of the most important risk factors for
the pathology during OA progression (6).
ER stress can initiate apoptosis by diverse stimuli.
Upon ER stress, three sensors, protein kinase RNA-like ER kinase
(Perk), activating transcription factor-6 (Atf6) and inositol
requiring protein-1 (Ire1), are primarily activated, leading to the
initiation of unfolded protein response (UPR). Persistent ER stress
or UPR occurs from failure to correct the balance, which activates
the cell apoptotic program (7,8),
leading to caspase-mediated apoptosis (9). ER stress has been shown to increase
chondrocyte apoptosis and to decrease the proteins of ECM,
including type II collagen and aggrecan, which cause a vicious
cycle of cartilage degradation (10,11). Additionally, it has been
demonstrated that microRNAs (miRNAs or miRs) are involved in
survival and apoptosis (12,13), and miR-34a plays a pivotal role in
chondrocyte apoptosis (14). The
effect of delaying chondrocyte apoptosis by regulating ER stress
may be one of the therapeutic strategies to treat OA.
Duhuo Jisheng decoction (DHJSD), a traditional
Chinese herbal formula, confers the effects of expelling
wind-dampness, relieving numbness and pain, nourishing the liver
and kidneys, invigorating qi-blood, and has been used for
treating OA and proved effective by relieving pain, reducing joint
stiffness, and improving mobility and quality of life (15). Previous studies have proven that
DHJSD has potential cooperation and polypharmacology against OA,
and could inhibit chondrocyte apoptosis by the
mitochondria-dependent signaling pathway (16). However, the mechanisms of how
DHJSD inhibit the chondrocyte apoptosis by ER stress remain to be
elucidated, which has limited its wider use. In the present study,
the effects and cellular mechanisms of DHJSD were investigated on
ER stress tunicamycin (TM)-induced chondrocyte apoptosis.
Furthermore, the potential mechanisms of action of DHJSD on ER
stress apoptosis were examined by measuring the expression of
miR-34a.
Materials and methods
Preparation of DHJSD aqueous extract
DHJSD is composed of 9 g of Radix Angelicae
Pubescentis and 6 g of Radix Gentianae Macrophyllae, Ramulus
Loranthi, Radix Saposhnikoviae, Herba Asari, Cortex Cinnamomi,
Poria Cocos, Rhizoma Chuanxiong, Radix Angelicae Sinensis, Radix
Achyranthis Bidentatae, Radix Rehmanniae Preparata, Radix Paeoniae
Alba, Cortex Eucommiae Ulmoidis, Panax ginseng and Radix
Glycyrrhizae. The herbs were identified by the teaching and
research section of Fujian University of Traditional Chinese
Medicine (TCM; Fuzhou, China) and the components were mixed and
extracted with the standard methods according to Chinese
Pharmacopoeia (China Pharmacopoeia and Committee, 2010). Herbs were
soaked in distilled water and boiled for 30 min twice, and the
extracts were filtered and concentrated. The concentrate filtrate
was dissolved in Dulbecco's modified Eagle's medium (HyClone,
Logan, UT, USA) at a concentration of 10 mg/ml, and was
subsequently filtered and stored at 4°C.
The quality control of the DHJSD extracts was
analyzed by the contents of polysaccharides and coumarins using an
ultraviolet (UV) spectrophotometer (Beckman Coulter, Inc., Brea,
CA, USA). Concentration gradients of the glucose standard solutions
(National Institutes for Food and Drug Control, China) and DHJSD
were prepared and subsequently measured at 490 nm using
phenol-sulphate colorimetry. A series concentration of osthole
standard solutions (National Institutes for Food and Drug Control,
China) and the DHJSD were dissolved in methanol, respectively, and
detected at 320 nm.
Isolation, identification and treatment
of chondrocytes
The present study was approved by the Institutional
Animal Care and Use Committee of Fujian University of TCM. Male
Sprague-Dawley rats (4-week-old) were from Super-BK Laboratory
Animal Co. (Shanghai, China). Chondrocytes were isolated from
articular cartilage and cultured as previously described (17). The second-passage (P2)
chondrocytes cultured until ~80% confluency were used in the
study.
Assessment of cell viability
Chondrocytes were seeded at a density of
5×104 cells/ml in 96-well plates (100 μl/well)
for 24 h. Subsequently, the medium was replaced with or without 2
μg/ml TM (Sigma-Aldrich, St. Louis, MO, USA) and a series of
concentrations of DHJSD (50, 100, 200, 300 and 400 μg/ml) in
the presence of TM, and incubated for 24, 48 or 72 h. Following
treatment, 100 μl 1%
3-(4,5-dimeth-ylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich) replaced the medium. After incubation at 37°C for 4
h, the supernatant was replaced with 150 μl dimethyl
sulfoxide and shaked for 10 min. The optical density (OD) was
analyzed by measuring at 490 nm using an enzyme-linked
immunosorbent assay reader (BioTek, Winooski, VT, USA).
Experimental design
The cells were assigned to 4 groups as follows:
untreated cells, TM-exposed chondrocytes, TM-exposed chondrocytes
treated with 200 μg/ml DHJSD, and TM-exposed chondrocytes
treated with 5 mM sodium 4-phenylbutyrate (PBA; Sigma-Aldrich),
which was diluted in PBS.
Assessment of chondrocyte apoptosis by
4′,6-diamidino-2-phenylindole (DAPI) staining
Following treatment, cells were washed with
phosphate-buffered saline (PBS), and fixed with 4% neutral
formaldehyde at 4°C for 15 min. Subsequently, the cells were
stained in 5 μg/ml DAPI for 5 min and washed 3 times with
PBS, and were observed under a fluorescent microscope (FACSCalibur;
Becton-Dickinson, San Jose, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen, Grand Island, NY, USA). RNA (1 μg) was
reverse transcribed into cDNA according to the manufacturer's
instructions. The DNA bands were analyzed by gel electrophoresis
(1.5% agarose) and examined using a Gel Documentation System
(Bio-Rad, Hercules, CA, USA), subsequently normalized to that of
β-actin. Primer sequences were as follows: Binding protein (Bip)
forward, 5′-ATC AAC CCA GAT GAG GCT GTA GCA-3′ and reverse, 5′-AGA
CCT TGA TTG TTA CGG TGG GCT-3′; Atf4 forward, 5′-AAT GGC TGG CTA
TGG ATG GG-3′ and reverse, 5′-TGT CTG AGG GGG CTC CTT ATT AG-3′;
X-box binding protein-1 (Xbp1) forward, 5′-AGC ATA GGC CTG TCT GCT
TCA CTA-3′ and reverse, 5′-TGG TAA AGT CCA GCA CTT GGG AGT-3′;
Xbp1s forward, 5′-TCT GCT GAG TCC GCA GCA GG-3′ and reverse, 5′-CTC
TAA GAC TAG AGG CTT GG-3′; Bax forward, 5′-GGC GAT GAA CTG GAC
AAC-3′ and reverse, 5′-TCC CGA AGT AGG AAA GGA G-3′; Bcl-2 forward,
5′-TGG CAT CTT CTC CTT CCC-3′ and reverse, 5′-GGT ACA TCT CCC TGT
TGA CG-3′; caspase-9 forward, 5′-GCC TCA TCATCA ACA ACG-3′ and
reverse, 5′-CTG GTA TGG GAC AGC ATC T-3′; caspase-3 forward, 5′-GGA
CCT GTG GAC CTG AAA-3′ and reverse, 5′-GGG TGC GGT AGA GTA AGC-3′;
and β-actin forward, 5′-GAG AGG GAA ATC GTG CGT GAC-3′ and reverse,
5′-CAT CTG CTG GAA GGT GGA CA-3′.
Western blot analysis
Total protein was extracted from cells using
radioimmunoprecipitation assay buffer, and protein concentrations
were measured using a bicinchoninic acid kit. An equal amount of
protein was separated by electrophoresis on sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and was
transferred onto PVDF membranes. Following blocking with 5% non-fat
milk, membranes were incubated with primary antibodies against Bip,
Atf4, C/EBP-homologous protein (Chop), Xbp1, Bax, Bcl-2, caspase-3
and -9 (Cell Signaling Technology, Inc., Beverly, MA, USA) or
β-actin (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C
overnight followed by a horseradish peroxidase-conjugated secondary
antibody (Zhongshan Goldenbridge Biotech, Beijing, China). ECL was
used to make the blots visible, and blots were quantitated using a
Bio-Rad Chemi Doc XRS+ (Bio-Rad), normalizing to that of
β-actin.
RT-quantitative (q) PCR
RT-qPCR was used to detect the expression of
miR-34a. Total RNA was isolated according to the manufacturer's
instructions for the mirVana™ isolation kit (Invitrogen, Life
Technologies). Reverse transcription was performed with the TaqMan
microRNA reverse transcription kit and miRNA specific stem-loop RT
primers (both from Applied Biosystems, Foster City, CA, USA). The
expression of miR-34a was confirmed by the TaqMan Universal PCR
Master mix according to the manufacturer's instructions using a
7500 Real-time PCR System (both from Applied Biosystems). Fold
changes were calculated by the formula 2−ΔΔCt relative
to the expression in untreated cells and U6 was the endogenous
control (18).
Statistical analysis
Data was analyzed by one-way analysis of variance or
Student's t-test using SPSS 19.0 software (IBM Corp., Armonk, NY,
USA) and expressed as mean ± standard deviation from at least three
independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
Quality control of DHJSD
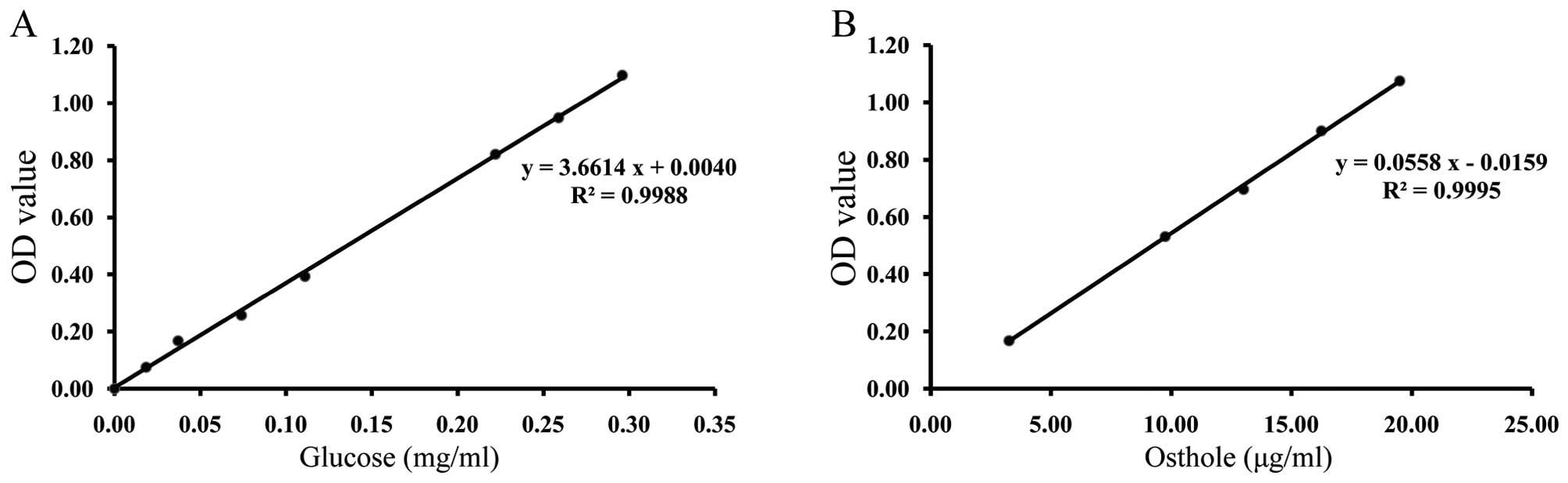
UV is used for content determination of one type of
material, which contains some of the same functional groups and has
the same absorption wavelength in the UV spectrum. According to the
regression equation of the glucose standard solutions (Fig. 1A) and osthole standard solutions
(Fig. 1B), the contents of
polysaccharides and coumarins were 1.69% and 0.0204%, respectively.
The extract quality of DHJSD could be controlled in this way.
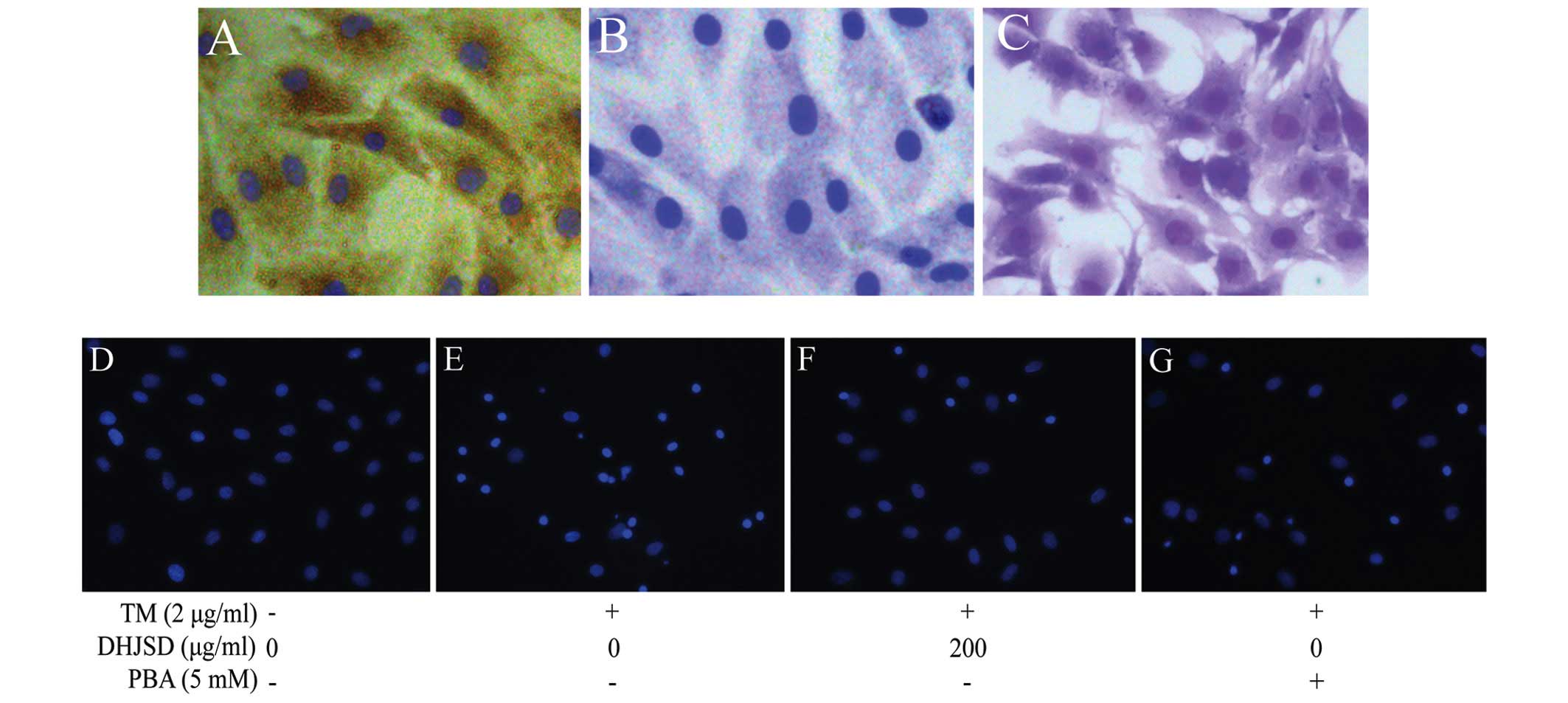
Morphology and identification of
chondrocytes
The morphology of newly isolated chondrocytes was
small, round and floating in the medium, and after a few days
culture, chondrocytes were connected during growth and showed an
irregular paving stone shape with clear boundaries and distinct
nuclei (17,19,20) (Fig.
2). Type II collagen has been identified as the characterized
form of collagen in articular cartilage, whereas proteoglycans
provide the function of articular cartilage. To characterize the
chondrocyte, the P2 chondrocytes cultured for 3 days were examined
by type II collagen immunohistochemical staining and toluidine blue
staining. The results showed that the brown-stained cytoplasm
represented a positive expression of type II collagen, while the
negative control did not stain brown by immunohistochemical
staining, and red/purple particles in the cytoplasm represent
proteoglycans by toluidine blue staining (21) (Fig.
3A–C). P2 chondrocytes are rich in ECM and show a typical
morphology of chondrocytes, and therefore, P2 chondrocytes were
used in the subsequent experiments.
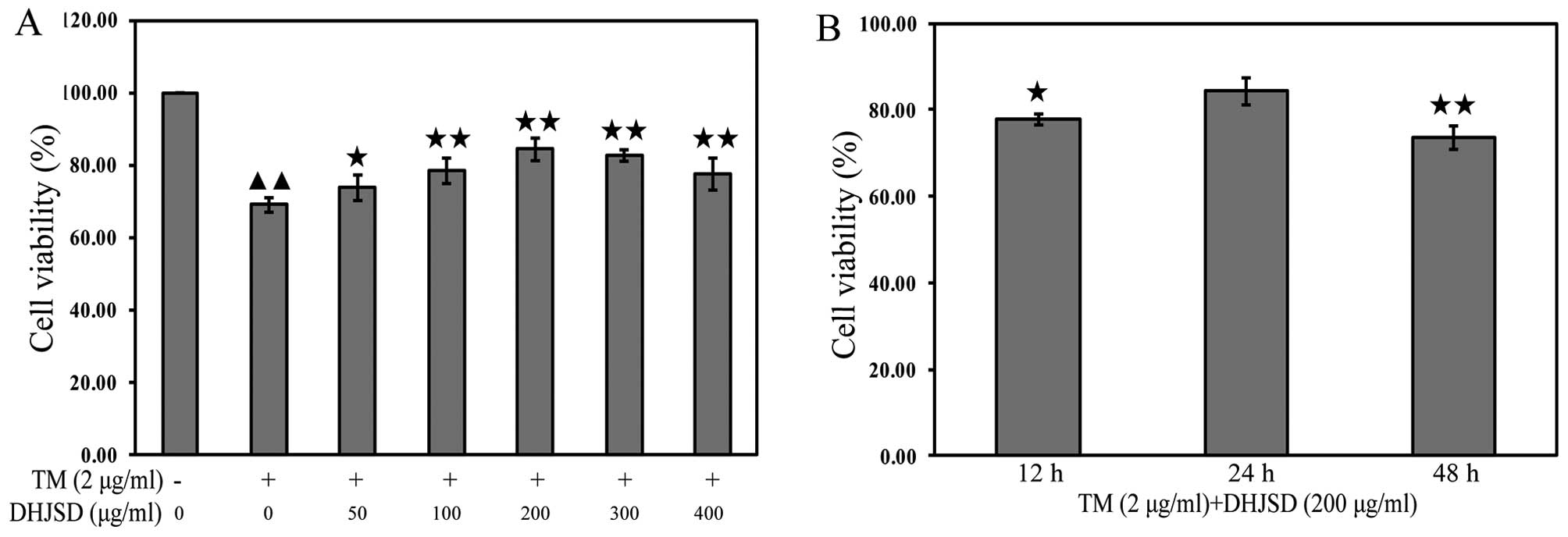
DHJSD enhances the viability chondrocytes
exposed to TM
The effects of DHJSD on TM-exposed chondrocytes were
evaluated by MTT assay. TM-exposed chondrocytes were treated with
various concentrations of DHJSD for different times. The results
showed that the viability of the TM-exposed chondrocytes was
significantly lower than that of the untreated cells (P<0.01),
and the viability of TM-exposed chondrocytes treated with DHJSD was
higher than that of the TM-exposed chondrocytes (P<0.01 and
P<0.05) (Fig. 4A). The
viability of TM-exposed chondrocytes treated with 200 μg/ml
DHJSD for 24 h was higher than that of the 12- and 48-h treatment
(P<0.01 and P<0.05) (Fig.
3B), suggesting that DHJSD enhanced TM-exposed chondrocyte
viability in a dose- and time-dependent manner. Therefore, 200
μg/ml DHJSD for 24 h was used in the following
experiments.
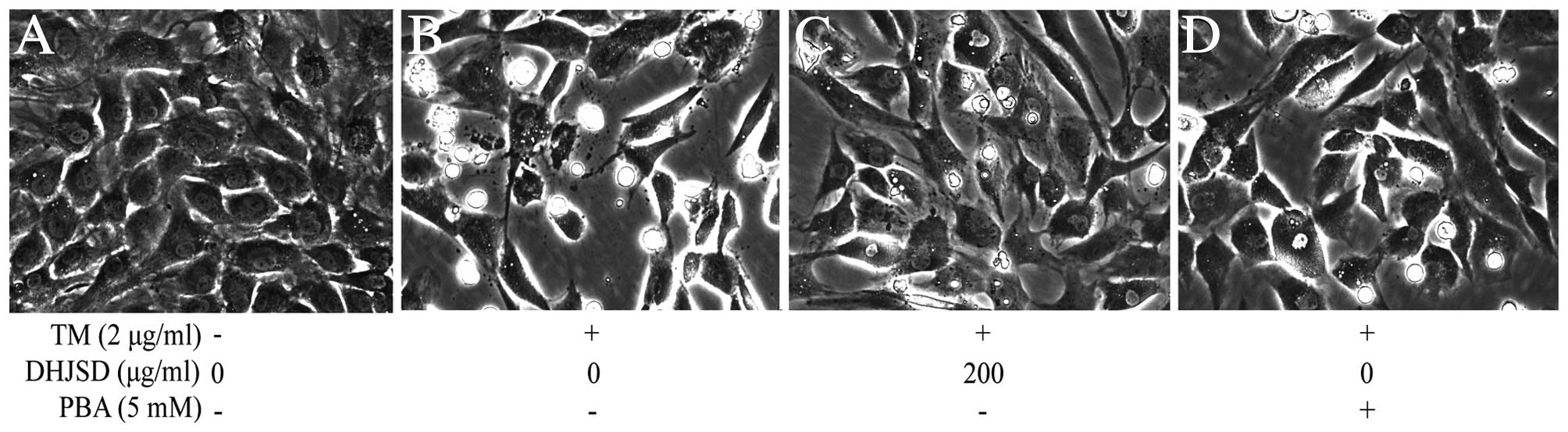
DHJSD inhibits morphological changes in
chondrocytes exposed to TM
The morphological changes of TM-exposed chondrocytes
treated with or without DHJSD or PBA (Sigma-Aldrich) were observed
by phase-contrast microscope (Fig.
5). The morphology of the untreated cells exhibited a healthy
status, while TM-exposed chondrocytes presented more apoptotic
cells that detached from each other and became bright, elongated
and shrunken, or floated in the medium compared to that of the
TM-exposed chondrocytes treated with DHJSD or PBA.
DHJSD reduces the apoptosis of TM-exposed
chondrocytes
To examine whether DHJSD enhanced TM-exposed
chondrocyte viability by inhibiting apoptosis, the cells were
determined by DAPI staining. The apoptotic cells exhibited typical
changes, such as staining bright blue and condensed or fragmented
nucleus. The typical changes were more observable in the TM-exposed
chondrocytes than that of the TM-exposed chondrocytes treated with
DHJSD or PBA (Fig. 3D–G).
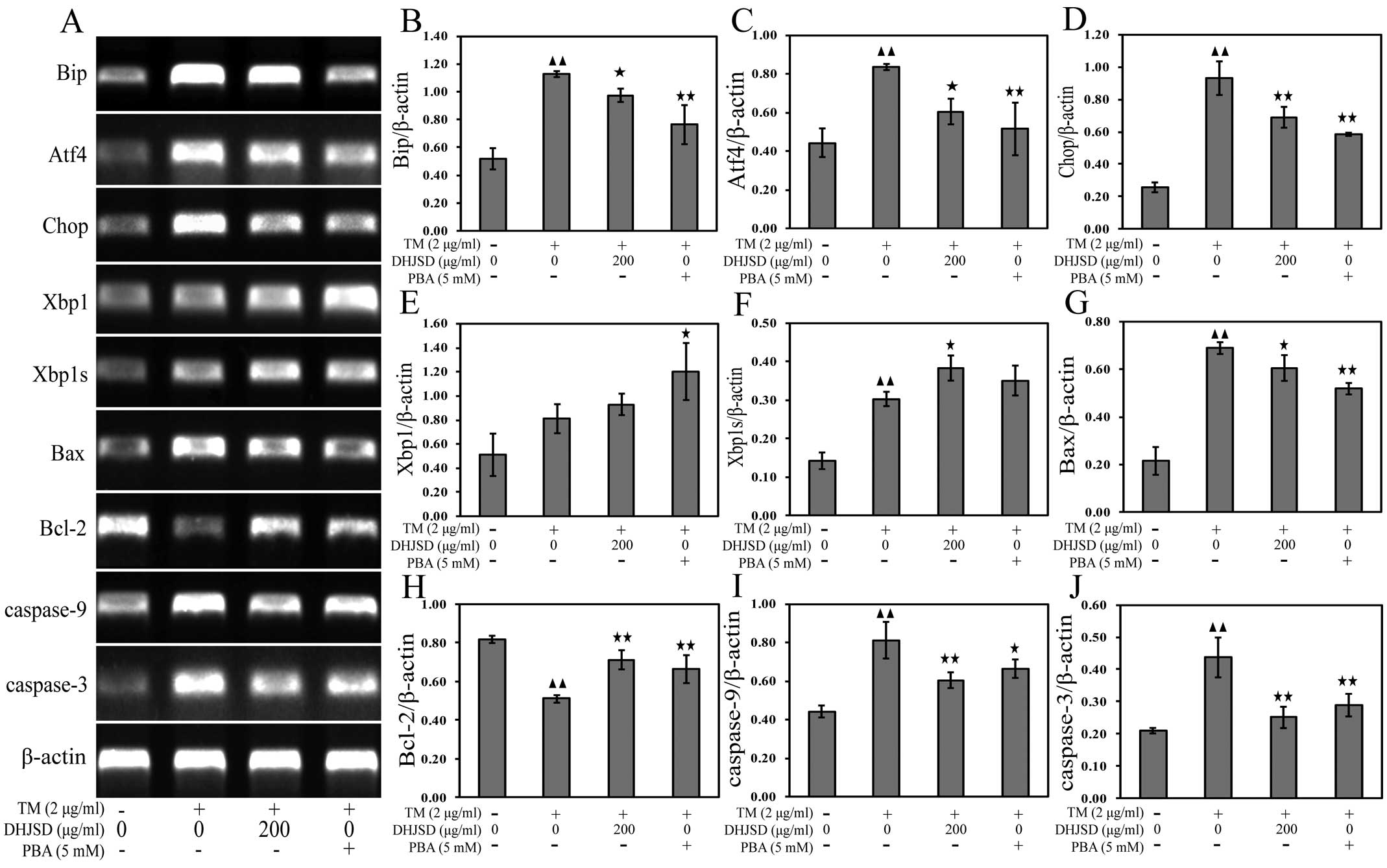
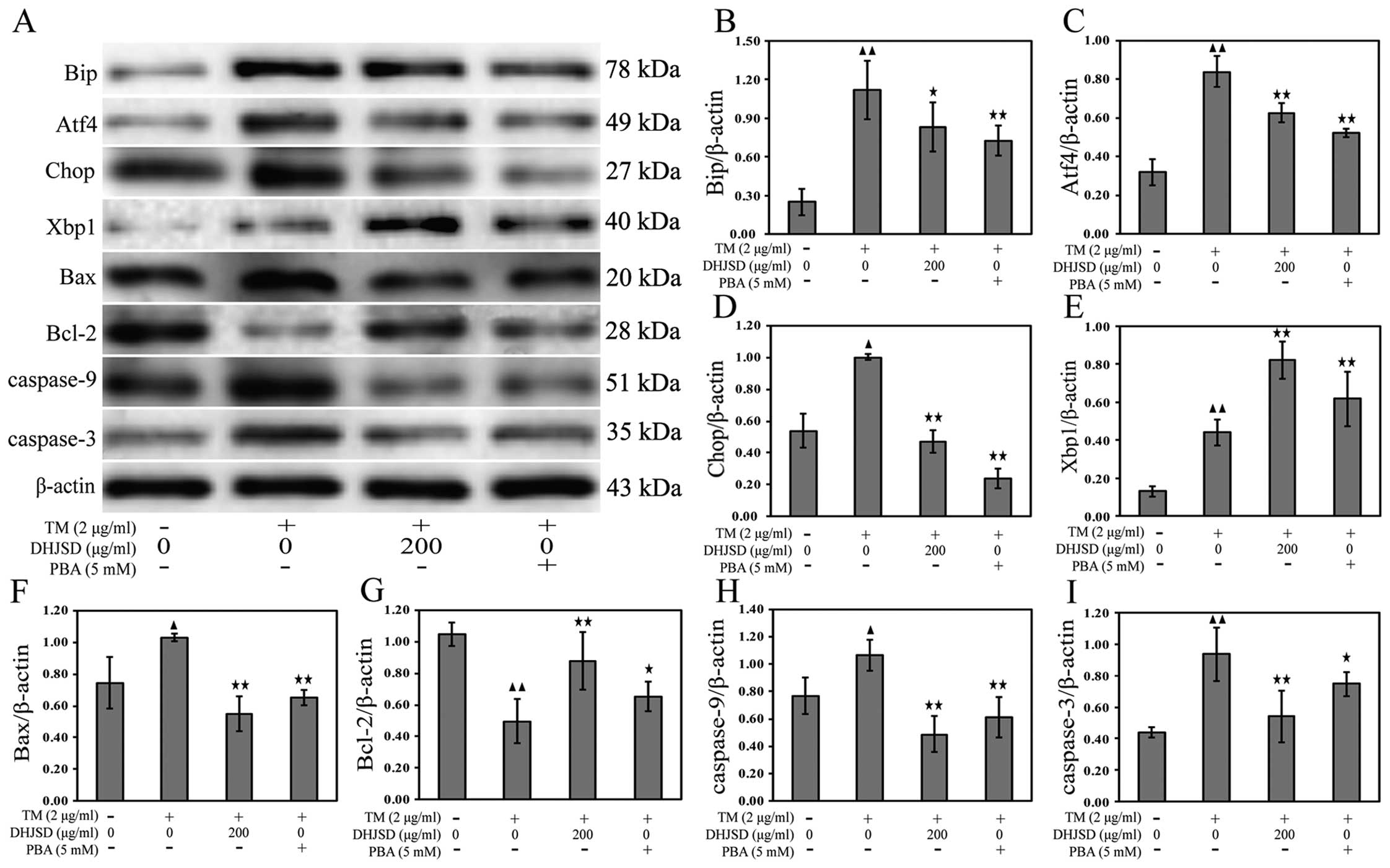
DHJSD inhibits ER stress in induced by
chondrocytes
To explore the role of DHJSD in chondrocytes of ER
stress, the mRNA and protein expressions were examined by RT-PCR
and western blot analysis, respectively. The results showed that
the mRNA expression levels of Xbp1 and Xbp1s were increased, and
the mRNA expression levels of Bip, Atf4 and Chop were decreased in
TM-exposed chondrocytes treated with DHJSD or PBA compared to that
in the TM-exposed chondrocytes (P<0.01 and P<0.05) (Fig. 6A–F). The protein levels were
similar to their respective mRNA expressions (P<0.01 and
P<0.05) (Fig. 7A–E),
suggesting that DHJSD regulated ER stress in TM-exposed
chondrocytes.
ER stress is known to induce the dysfunction of
mitochondria, leading to caspase activation and subsequent
apoptosis (22). A hallmark of
apoptosis is the activation of caspases and the Bcl-2 family has a
crucial role in regulating their engagement under ER stress
(23). Bcl-2 antagonizes whereas
Bax promotes ER stress-induced mitochondrial cytochrome c
release and caspase activation to induce apoptosis (24). To gain insight into the mechanisms
responsible for DHJSD on the apoptosis of TM-exposed chondrocytes,
Bax, Bcl-2, caspase-9 and caspase-3 mRNA and protein expressions
were detected by RT-PCR and western blot analysis, respectively.
The results showed that the expression of Bcl-2 was increased, and
the expression levels of Bax, caspase-9 and caspase-3 were
decreased in TM-exposed chondrocytes treated with DHJSD or PBA
compared to that in the TM-exposed chondrocytes (P<0.01 and
P<0.05) (Fig. 6A and G–J). The
protein levels were similar to their respective mRNA expression
levels (P<0.01 and P<0.05) (Fig. 7A and F–I), indicating that DHJSD
inhibits apoptosis of TM-exposed chondrocytes by regulating ER
stress.
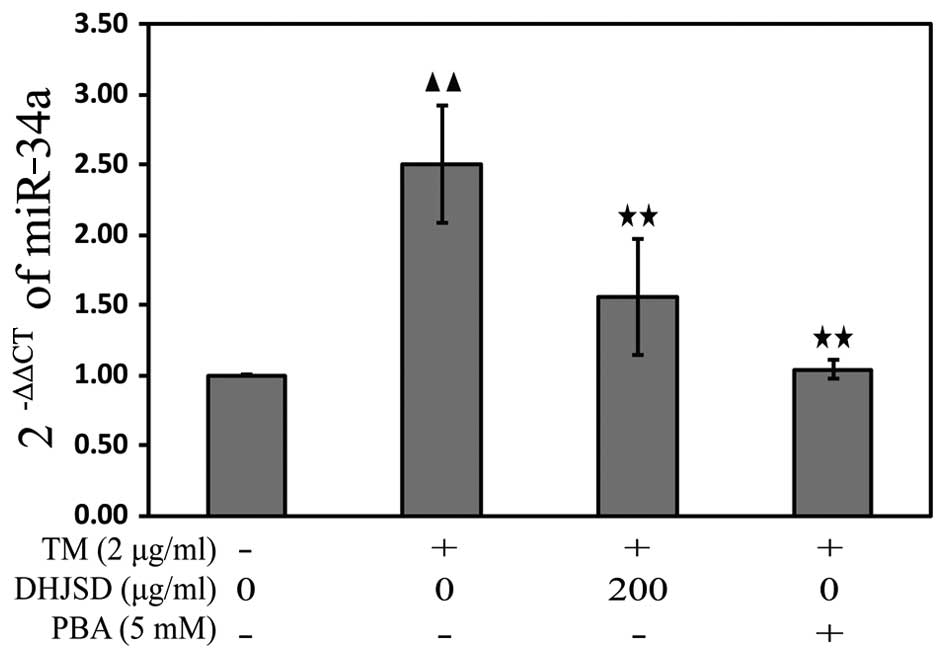
DHJSD inhibits ER stress in the
TM-exposed chondrocytes by downregulating miR-34a
miRNAs, a class of endogenous non-coding RNAs,
regulate gene expression by binding to the 3′-untranslated region
in their target mRNAs, resulting in either translational repression
or degradation of target mRNA expression. To explore the mechanisms
of DHJSD on the apoptosis of TM-exposed chondrocytes, the miR-34a
expression was analyzed by the TaqMan microRNA assay. The results
showed that the expression of miR-34a was downregulated in the
TM-exposed chondrocytes treated with DHJSD or PBA compared with
that in the TM-exposed chondrocytes (P<0.01) (Fig. 8), implying that DHJSD inhibits ER
stress TM-exposed chondrocyte apoptosis by downregulating
miR-34a.
Discussion
Natural products have been proved effective in
treating OA by decreasing joint pain and dysfunction, and
preventing and delaying the cartilage degeneration (25,26). DHJSD contains a number of natural
products with numerous chemical compounds that have been deemed to
have multi-target agents and multi-components exerting their
therapeutic function. However, the biological mechanisms of how
DHJSD improves the clinical consequences of OA are not fully
understood. The present results verified that DHJSD has multiple
pathways to inhibit chondrocyte apoptosis.
To control the extract quality of DHJSD, UV was used
to test the glucose and osthole concentrations, which belongs to
polysaccharides and coumarins, respectively. Therefore, the extract
quality of DHJSD was insured every time in the present study. The
chondrocytes were identified by type II collagen
immunohistochemical staining and toluidine blue staining, and the
results indicated that the chondrocytes cultured in vitro
can be established.
TM, an ER stress inducer, is an inhibitor of
N-linked glycosylation and the formation of
N-glycosidic protein-carbohydrate linkages (27). Therefore, TM-exposed chondrocytes
were used as the ER stress apoptosis model. The effect of DHJSD on
the viability of TM-exposed chondrocytes was examined by the MTT
assay, and the results revealed that DHJSD increased cell
viability. In addition, PBA, an ER stress inhibitor, aids in
protein folding at the molecular level and prevents misfolded
protein aggregation. Therefore, PBA was used as a positive control
and the morphology changes of TM-exposed chondrocytes showed that
TM-exposed chondrocyte survival could be enhanced by DHJSD or PBA.
For further study, DAPI staining was used to explore whether DHJSD
increased TM-exposed chondrocyte viability and enhanced TM-exposed
chondrocyte survival by inhibiting apoptosis, and the results
showed that DHJSD or PBA reduced TM-exposed chondrocyte apoptosis.
It remains to be determined whether DHJSD reduced TM-exposed
chondrocyte apoptosis by regulating ER stress.
The ER is a sophisticated lumen where protein
synthesis, folding and maturation occur. Perturbation of these
processes in the pathological states results in ER stress, and
activate a complex signaling network (28). Bip, an ER chaperone protein,
alleviates ER stress and maintains ER function by binding to the
incompletely folded proteins or are unfolded to prevent the
interaction of these proteins with surrounding molecules, and whose
expression induces ER stress (29). During ER stress, Bip away from the
UPR sensors Perk (the first responses of the cell to ER stress),
Atf6 and Ire1, results in the three sensors phosphorylation
(27). Perk is responsible for
phosphorylating the translation initiation factor, eIF2α, that
enhances the translation of Atf4 (30). Atf4 induces the pro-apoptotic
events by activating Chop, a key factor of ER stress, whose
overexpression evokes cell apoptosis (31,32), and all the three sensors
phosphorylation can induce transcription of Chop in response to ER
stress. By contrast, upon activation of the UPR, Ire1
autophosphorylation activates and serves as endoribonuclease, which
removes 26 ribonucleotides from the Xbp1 mRNA that undergoes
transcription by Atf6 activation, allowing production of the Xbp1
protein into Xbp1 spliced form (Xbp1s) mRNA to generate a more
potent transcription factor, Xbp1s, a key transcriptional regulator
that restores ER function by refolding or reducing misfolded
proteins that have accumulated in ER lumen (33). The present results showed that
DHJSD or PBA regulated ER stress by decreasing Bip, Atf4 and Chop,
and helped to restore ER function by increasing Xbp1 and Xbp1s.
Mitochondria are recognized as the central regulator
of apoptotic cell death, and ER-mitochondrial cross talk may
mediate stress signals between these compartments (34). Accumulating evidence indicates
that ER stress results in apoptosis by regulating the Bcl-2 family
proteins that regulate the release of calcium from the ER and the
release of pro-apoptotic factors from mitochondria (35–37). Bax, one of the Bcl-2 family
proteins is recruited to the ER surface and the mitochondria to
induce apoptosis, whereas the anti-apoptotic Bcl-2 can prevent ER
stress-mediated apoptosis (36).
Bax and pro-apoptotic Bcl-2 are switched on by the Ire1 pathway,
and Chop can repress the pro-survival gene Bcl-2 (38–40). Caspases, a family of cysteine
proteases, act as common death effector molecules in various forms
of apoptosis and are involved in initiating and completing the
final execution of the cell (32). Caspase-12 is important in the
context of ER stress-mediated apoptosis and its activation cleaves
pro-caspase-9, which in turn activates caspase-3, thus leading to
cell death (41). The present
results demonstrated that DHJSD or PBA inhibited ER stress
apoptosis by increasing Bcl-2, whereas decreasing Bax, caspase-9
and caspase-3.
miRNAs regulate protein expression by degrading
target mRNA or inhibiting translation, resulting in complementary
matching between miRNAs and specific sites in target mRNAs
(42), correlate with human
disease and have a potential as therapeutic targets (43–45). Emerging evidence suggests miRNAs
control the balance of pro-survival and pro-apoptotic signals by
acting at different steps in each arm of the pathway to regulate ER
stress (46). To identify the
possible mechanisms, the expression of miR-34a was examined by
RT-qPCR and results showed that miR-34a was markedly downregulated
in TM-exposed chondrocytes treated with DHJSD or PBA.
In conclusion, the present results expand the
significant roles of DHJSD in reducing chondrocyte apoptosis by
inhibiting the ER stress apoptotic pathway, suggesting it may be a
potential drug for the treatment of OA. However, due to the
limitations in vitro and the exact mechanism implicated in
the regulation of ER stress in chondrocyte by DHJSD remains
unclear. Further studies are required to confirm the effects in
vivo, and a small interfering RNA inhibitor could be used to
confirm the precise mechanisms.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81373818), the
Special Research Fund for Doctor Discipline in College
(20123519110001), the Young and Middle-aged Core Personnel Training
Project of Fujian Province Health Department (grant no.
2014-ZQN-JC-29), and the Young-aged Teacher Educational and
Scientific Research Project of Fujian Province (grant no. JA12165),
the Developmental Fund of Chen Keji Integrative Medicine (grant
nos. CKJ2014014, CKJ2014001 and CKJ2015009), the Key Project of
Fujian Provincial Department of Science and Technology (grant no.
2014Y0064) and the Natural Science Foundation of Fujian Province
(grant no. 2014J01357).
References
|
1
|
Bijlsma JW, Berenbaum F and Lafeber FP:
Osteoarthritis: An update with relevance for clinical practice.
Lancet. 377:2115–2126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loeser RF: Aging and osteoarthritis: The
role of chondrocyte senescence and aging changes in the cartilage
matrix. Osteoarthritis Cartilage. 17:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mistry D, Oue Y, Chambers MG, Kayser MV
and Mason RM: Chondrocyte death during murine osteoarthritis.
Osteoarthritis Cartilage. 12:131–141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas CM, Fuller CJ, Whittles CE and
Sharif M: Chondrocyte death by apoptosis is associated with
cartilage matrix degradation. Osteoarthritis Cartilage. 15:27–34.
2007. View Article : Google Scholar
|
|
5
|
Gorman AM, Healy SJ, Jäger R and Samali A:
Stress management at the ER: Regulators of ER stress-induced
apoptosis. Pharmacol Ther. 134:306–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takada K, Hirose J, Senba K, Yamabe S,
Oike Y, Gotoh T and Mizuta H: Enhanced apoptotic and reduced
protective response in chondrocytes following endoplasmic reticulum
stress in osteoarthritic cartilage. Int J Exp Pathol. 92:232–242.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bernales S, Papa FR and Walter P:
Intracellular signaling by the unfolded protein response. Annu Rev
Cell Dev Biol. 22:487–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim R, Emi M, Tanabe K and Murakami S:
Role of the unfolded protein response in cell death. Apoptosis.
11:5–13. 2006. View Article : Google Scholar
|
|
10
|
Yang L, Carlson SG, McBurney D and Horton
WE Jr: Multiple signals induce endoplasmic reticulum stress in both
primary and immortalized chondrocytes resulting in loss of
differentiation, impaired cell growth, and apoptosis. J Biol Chem.
280:31156–31165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang L, McBurney D, Tang SC, Carlson SG
and Horton WE Jr: A novel role for Bcl-2 associated-athanogene-1
(Bag-1) in regulation of the endoplasmic reticulum stress response
in mammalian chondrocytes. J Cell Biochem. 102:786–800. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lafferty-Whyte K, Cairney CJ, Jamieson NB,
Oien KA and Keith WN: Pathway analysis of senescence-associated
miRNA targets reveals common processes to different senescence
induction mechanisms. Biochim Biophys Acta. 1792:341–352. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abouheif MM, Nakasa T, Shibuya H, Niimoto
T, Kongcharoensombat W and Ochi M: Silencing microRNA-34a inhibits
chondrocyte apoptosis in a rat osteoarthritis model in vitro.
Rheumatology (Oxford). 49:2054–2060. 2010. View Article : Google Scholar
|
|
15
|
Zheng CS, Xu XJ, Ye HZ, Wu GW, Li XH,
Huang SP and Liu XX: Computational approaches for exploring the
potential synergy and polypharmacology of Duhuo Jisheng decoction
in the therapy of osteoarthritis. Mol Med Rep. 7:1812–1818.
2013.PubMed/NCBI
|
|
16
|
Liu F, Liu G, Liang W, Ye H, Weng X, Lin
P, Li H, Chen J, Liu X and Li X: Duhuo Jisheng decoction treatment
inhibits the sodium nitroprussiate induced apoptosis of
chondrocytes through the mitochondrial dependent signaling pathway.
Int J Mol Med. 34:1573–1580. 2014.PubMed/NCBI
|
|
17
|
Li H, Li X, Liu G, Chen J, Weng X, Liu F,
Xu H, Liu X and Ye H: Bauhinia championi (Benth.) Benth.
polysaccharides upregulate Wnt/β-catenin signaling in chondrocytes.
Int J Mol Med. 32:1329–1336. 2013.PubMed/NCBI
|
|
18
|
Hu G, Huang K, Yu J, Gopalakrishna-Pillai
S, Kong J, Xu H, Liu Z, Zhang K, Xu J, Luo Y, et al: Identification
of miRNA signatures during the differentiation of hESCs into
retinal pigment epithelial cells. PLoS One. 7:e372242012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu F, Li X, Cai L, Li H, Chen J, Wong X,
Xu H, Zheng C, Liu X and Ye H: Achyranthes bidentata
polysaccharides induce chon-drocyte proliferation via the promotion
of the G1/S cell cycle transition. Mol Med Rep. 7:935–940.
2013.PubMed/NCBI
|
|
20
|
Li X, Peng J, Wu M, Ye H, Zheng C, Wu G,
Xu H, Chen X and Liu X: BMP2 promotes chondrocyte proliferation via
the Wnt/β-catenin signaling pathway. Mol Med Rep. 4:621–626.
2011.PubMed/NCBI
|
|
21
|
Li X, Du M, Liu X, Chen W, Wu M, Lin J and
Wu G: Millimeter wave treatment promotes chondrocyte proliferation
by upregulating the expression of cyclin-dependent kinase 2 and
cyclin A. Int J Mol Med. 26:77–84. 2010.PubMed/NCBI
|
|
22
|
Zong WX, Li C, Hatzivassiliou G, Lindsten
T, Yu QC, Yuan J and Thompson CB: Bax and Bak can localize to the
endoplasmic reticulum to initiate apoptosis. J Cell Biol.
162:59–69. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gupta S, Cuffe L, Szegezdi E, Logue SE,
Neary C, Healy S and Samali A: Mechanisms of ER Stress-Mediated
Mitochondrial Membrane Permeabilization. Int J Cell Biol.
2010:1702152010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hua B, Abbas E, Hayes A, Ryan P, Nelson L
and O'Brien K: Reliability of Chinese medicine diagnostic variables
in the examination of patients with osteoarthritis of the knee. J
Altern Complement Med. 18:1028–1037. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Wei S, Liu T, Pang J, Gao N, Ding
D, Duan T, Cao Y, Zheng Y and Zhan H: Effectiveness, medication
patterns, and adverse events of traditional chinese herbal patches
for osteoarthritis: A systematic review. Evid Based Complement
Alternat Med. 2014:3431762014.PubMed/NCBI
|
|
27
|
Lin JH, Li H, Yasumura D, Cohen HR, Zhang
C, Panning B, Shokat KM, Lavail MM and Walter P: IRE1 signaling
affects cell fate during the unfolded protein response. Science.
318:944–949. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yeung BH, Kwan BW, He QY, Lee AS, Liu J
and Wong AS: Glucose-regulated protein 78 as a novel effector of
BRCA1 for inhibiting stress-induced apoptosis. Oncogene.
27:6782–6789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
DuRose JB, Scheuner D, Kaufman RJ,
Rothblum LI and Niwa M: Phosphorylation of eukaryotic translation
initiation factor 2α coordinates rRNA transcription and translation
inhibition during endoplasmic reticulum stress. Mol Cell Biol.
29:4295–4307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hotamisligil GS: Endoplasmic reticulum
stress and the inflammatory basis of metabolic disease. Cell.
140:900–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sovolyova N, Healy S, Samali A and Logue
SE: Stressed to death - mechanisms of ER stress-induced cell death.
Biol Chem. 395:1–13. 2014. View Article : Google Scholar
|
|
33
|
Yoshida H, Matsui T, Yamamoto A, Okada T
and Mori K: XBP1 mRNA is induced by ATF6 and spliced by IRE1 in
response to ER stress to produce a highly active transcription
factor. Cell. 107:881–891. 2001. View Article : Google Scholar
|
|
34
|
Ouyang YB, Xu LJ, Emery JF, Lee AS and
Giffard RG: Overexpressing GRP78 influences Ca2+
handling and function of mitochondria in astrocytes after
ischemia-like stress. Mitochondrion. 11:279–286. 2011. View Article : Google Scholar :
|
|
35
|
Chipuk JE, Moldoveanu T, Llambi F, Parsons
MJ and Green DR: The BCL-2 family reunion. Mol Cell. 37:299–310.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Scorrano L, Oakes SA, Opferman JT, Cheng
EH, Sorcinelli MD, Pozzan T and Korsmeyer SJ: BAX and BAK
regulation of endoplasmic reticulum Ca2+: A control
point for apoptosis. Science. 300:135–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rodriguez DA, Zamorano S, Lisbona F,
Rojas-Rivera D, Urra H, Cubillos-Ruiz JR, Armisen R, Henriquez DR,
Cheng EH, Letek M, et al: BH3-only proteins are part of a
regulatory network that control the sustained signalling of the
unfolded protein response sensor IRE1α. EMBO J. 31:2322–2335. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hetz C, Bernasconi P, Fisher J, Lee AH,
Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A,
Glimcher LH, et al: Proapoptotic BAX and BAK modulate the unfolded
protein response by a direct interaction with IRE1α. Science.
312:572–576. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rodriguez D, Rojas-Rivera D and Hetz C:
Integrating stress signals at the endoplasmic reticulum: The BCL-2
protein family rheostat. Biochim Biophys Acta. 1813:564–574. 2011.
View Article : Google Scholar
|
|
40
|
Chen Y and Brandizzi F: IRE1: ER stress
sensor and cell fate executor. Trends Cell Biol. 23:547–555. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morishima N, Nakanishi K, Takenouchi H,
Shibata T and Yasuhiko Y: An endoplasmic reticulum stress-specific
caspase cascade in apoptosis. Cytochrome c-independent activation
of caspase-9 by caspase-12. J Biol Chem. 277:34287–34294. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar :
|
|
43
|
Tili E, Michaille JJ, Costinean S and
Croce CM: MicroRNAs, the immune system and rheumatic disease. Nat
Clin Pract Rheumatol. 4:534–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
O'Connell RM, Rao DS, Chaudhuri AA and
Baltimore D: Physiological and pathological roles for microRNAs in
the immune system. Nat Rev Immunol. 10:111–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dai R and Ahmed SA: MicroRNA, a new
paradigm for understanding immunoregulation, inflammation, and
autoimmune diseases. Transl Res. 157:163–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bartoszewska S, Kochan K, Madanecki P,
Piotrowski A, Ochocka R, Collawn JF and Bartoszewski R: Regulation
of the unfolded protein response by microRNAs. Cell Mol Biol Lett.
18:555–578. 2013. View Article : Google Scholar : PubMed/NCBI
|