Introduction
Osteoarthritis (OA), which affects weight-bearing
joints and pathologically alters the cartilage and subchondral
bone, is a chronic and degenerative disease that causes chronic
joint pain and dysfunction (1).
Pathologically, OA is characterized by the progressive degeneration
of articular cartilage, the abnormal reconstruction of subchondral
bone, and subchondral bone sclerosis. OA results from an imbalance
between the synthesis and degradation of the cartilage and
subchondral bone, secondary to effects by multiple factors. It was
previously considered that cartilage degeneration is the
disease-initiating factor. Following the progress made in basic
research, subchondral bone is now believed to be closely associated
with the onset and progression of OA (2), and has thus become a hotspot in the
research of OA pathogenesis.
During the development of OA, subchondral bone is
pathologically characterized by an abnormal increase in bone
turnover. The Wnt/β-catenin signaling pathway is mainly responsible
for cell proliferation, differentiation and apoptosis. In recent
years, Wnt/β-catenin signaling has been shown to play an important
role in maintaining the normal functions of osteocytes and
osteoblasts, and regulates bone metabolism. In osteogenesis,
Wnt/β-catenin inhibits the differentiation of mesenchymal stem
cells into adipocytes, instead promoting their differentiation into
osteoblasts. Indeed, Wnt/β-catenin induces osteoblast proliferation
and maturation (3), while
inhibiting apoptosis. In bone resorption, Wnt/β-catenin promotes
osteoprotegerin (OPG) secretion by osteoblasts (4) and simultaneously inhibits receptor
activator of nuclear factor-κB ligand (RANKL) secretion (5), which reduces the RANKL/OPG ratio and
suppresses bone resorption. In addition, Wnt/β-catenin inhibits
apoptosis and activates downstream target genes that regulate bone
metabolism (3–5). However, the regulatory effects of
the activated Wnt signaling pathway in osteocytes on other cells
are not yet fully understood. The activation of Wnt/β-catenin
signaling reduces bone resorption and increases osteogenesis.
Sclerostin the secreted protein product of the SOST
gene, is a negative regulator of osteoblasts and may be exclusively
osteocyte-derived (6). Sclerostin
is an antagonist for Wnt signaling in Xenopus embryos and
mammalian cells by binding to the extracellular domain of the Wnt
coreceptors, LRP5 and LRP6, and disrupting Wnt-induced Frizzled-LRP
complex formation. The loss of SOST function likely leads to the
hyperactivation of Wnt signaling that underlies bone overgrowth
observed in sclerosteosis patients (7–10).
We hypothesized that sclerostin production by osteocytes may
regulate the linear extent of formation and the induction or
maintenance of a lining cell phenotype on bone surfaces. In doing
so, sclerostin may act as a key inhibitory signal governing
skeletal microarchitecture. Thus, sclerostin is considered an
attractive therapeutic target for patients with bone metabolic
diseases (11).
In this study, we aimed to assess the levels of
β-catenin, transcription factor-4 (TCF-4) and sclerostin in samples
from patients with OA of different disease stages, and to clarify
the effect of sclerostin and Wnt/β-catenin signaling on OA.
Materials and methods
Sample collection
Fresh tibial plateau specimens were collected from
patients with primary OA who underwent total knee arthroplasty at
the Third Department of the General Hospital of Ningxia Medical
University, Yinchuan, China between 2013 and 2014. A total of 45
medial or lateral tibial plateau samples was collected from 32
patients with OA, including 1 male and 31 females. The median age
of the patients was 64.9 years, ranging from 51 to 74 years. OA was
diagnosed according to the American College of Rheumatology Society
diagnostic criteria (12), and
patients with OA secondary to other diseases, such as trauma and
connective tissue diseases, were excluded.
This study was approved by the Ethics Committee of
the General Hospital of Ningxia Medical University (no. 2015-005),
and informed consent was obtained from each patient prior to
enrolment.
Macroscopic observations
Tibial plateau material was collected during surgery
and immediately observed on a clean bench, for surface appearance
and color, and for the presence of cartilage ulcers, cartilage
fractures and osteophytes.
Sample processing
Bone and cartilage at the central load-bearing area
of the medial or lateral tibial plateau samples were preserved and
trimmed into blocks (2.0×2.0×1.0 cm). The bone and cartilage
tissues were divided into 2 parts by coronal sectioning. One part,
containing cartilage and subchondral bone, was fixed in 10% neutral
formalin for 24 h, decalcified in 10% ethylenediaminetetraacetic
acid (EDTA) at room temperature, paraffin-embedded, sliced at
4-µm-thick discontinuous sections, and submitted to Safranin
O and Fast Green staining or immunohistochemistry. The cartilage
was removed from the other part, and the subchondral bone was
wrapped with foil paper and stored at −80°C for the detection of
mRNA and protein levels.
Mankin scoring
As previously described (13), OA was classified into 3 stages as
follows: early-stage (mild degeneration, scores 0–6; n=15),
intermediate-stage (moderate degeneration, scores 7–9; n=13), or
late-stage (severe degeneration, scores 10–14; n=17).
Structural evaluation of cartilage and
subchondral bone
Three sections from each sample were used for
Safranin O and Fast Green staining (Hebei Bohai Biotechnology
Development Co., Ltd., Hebei, China), with 3 high power fields
(magnification, ×4) randomly selected per section for analysis. The
Image-Pro Plus 6.0 image analysis software (Media Cybernetics,
Bethesda, MD, USA) was employed to assess the structural parameters
of cartilage and subchondral bone with morphometrical methods. The
assessed parameters included: i) total articular cartilage (TAC),
namely the distance between the cartilage surface and bonding line;
ii) subchondral bone plate (SCP) thickness, namely the distance
from the bonding line to the interface between the subchondral bone
plate and trabecular bone; and iii) trabecular bone volume (BV/TV),
which was cacluated as follows: (trabecular bone area in the region
of measurement)/(trabecular bone area + marrow cavity area)
×100.
Immunohistochemistry
The sections were incubated at 60°C overnight and
deparaffinized. Antigen retrieval was performed with 0.1% trypsin
for 20 min, and the sections were incubated with 3%
H2O2 for 10 min, blocked with goat serum for
10 min, and treated at 37°C for 75 min with rabbit anti-human
β-catenin (1:100; ab32572) or anti-human sclerostin (1:50; ab63097)
(both from Abcam, Cambridge, MA, USA) monoclonal antibodies in
phosphate-buffered saline containing Tween-20 (PBST). Following 3
washes with PBST (5 min), the sections were incubated with
HRP-conjugated goat anti-rabbit IgG (1:2,000; ZB-2301; Beijing
Zhongshan Golden Bridge Biotechnology, Co., Ltd., Beijing, China)
at 37°C for 40 min. Visualization was carried out with
3,3′-diaminobenzidine (DAB), and hematoxylin used for
counterstaining. Routine dehydration and mounting were performed.
In the negative controls, the primary antibody was replaced with
PBST. For histological scoring, 3 high power fields (magnification,
×20) were randomly selected from the subchondral bone plate and
trabecular bone sections. This was performed independently by 2
experienced pathologists (Dr Anshan Han and Dr Haiping Tian;
Ningxia Medical University). The numbers of β-catenin- or
sclerostin-positive cells were counted, and positive-to-total cell
ratios were considered to be the relative protein expression
levels.
Reverse transcription-quantititave PCR
(RT-qPCR)
Six samples from each group were randomly selected
to assess the mRNA levels of target genes in the subchondral bone
specimens. In brief, the subchondral bone samples were thawed,
weighed and homogenized in liquid nitrogen. Subsequently, total RNA
was extracted from the tissues using the RNA pure tissue kit
(Beijing Kangwei Shiji Biotechnology, Co., Ltd., Beijing, China)
according to the manufacturer's instructions. Total RNA amounts and
purity were determined by UV spectrophotometry. Primers for
β-catenin, TCF-4 and sclerostin were designed and synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China) (Table I). Total RNA was reverse
transcribed into cDNA using the Super RT cDNA kit (Beijing Kangwei
Shiji Biotechnology, Co., Ltd.), according to the manufacturer's
instructions. Quantitative PCR (qPCR) reactions (25 µl) were
composed of cDNA (0.5 µl), SYBR-GreenER qPCR SuperMix
Universal (Beijing Kangwei Shiji Biotechnolo gy, Co., Ltd.) (12.5
µl), forward and reverse primers (0.5 µl each) and
diethyl-pyrocarbonate-treated water (11 µl). PCR was carried
out at 50°C (20 min), 95°C (10 min) and 40 cycles at 95°C (15 sec)
and 60°C (60 sec). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used as an
internal reference; the Ct value of GAPDH in early OA served as a
control. Finally, the relative mRNA levels of target genes were
assessed using the 2−ΔΔCt method.
 | Table ISequences of primers used for
RT-qPCR. |
Table I
Sequences of primers used for
RT-qPCR.
| Gene | Sequence (5′→3′) | Product size
(bp) |
|---|
| β-catenin |
TGGTGACAGGGAAGACATCA | 20 |
|
CCATAGTGAAGGCGAACTGC | 20 |
| TCF-4 |
CTCCGATGTCCACTTTCCAT | 20 |
|
CGCTTCCTCTATTTGCCATT | 20 |
| Sclerostin |
TTCTCCTTCGGGACCTCAAT | 20 |
|
TCTCTCACCTCTGCCCATT | 19 |
| GAPDH |
ACAACTTTGGTATCGTGGAAGG | 22 |
|
GCCATCACGCCACAGTTTC | 19 |
Western blot analysis
Five samples from each group were examined to
determine protein levels by western blot analysis. In brief, the
subchondral bone specimens were thawed, washed with pre-chilled
distilled water (to remove bone marrow cavity contents), weighed
and homogenized in liquid nitrogen. Total protein was extracted
using the total protein sample kit (Sigma-Aldrich, St. Louis, MO,
USA) according to the manufacturer's instructions, and the protein
concentration was determined using the bicinchoninic acid (BCA)
method. Subsequently, proteins were resolved by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and
transferred onto a polyvinylidene fluoride membrane at 300 mA for
90 min. After 3 washes in TBST (5 min each time), the membrane was
blocked in Tris-buffered saline-Tween-20 (TBST) containing 5%
non-fat milk for 1 h, and treated with rabbit anti-human monoclonal
antibodies raised against β-catenin (1:500), TCF-4 (1:1,000;
ab185736), sclerostin (1:300), or β-actin (1:2,000; ab8227) (all
from Abcam) in TBST at 4°C overnight. After 3 washes in TBST, the
membrane was incubated with HRP-conjugated goat anti-rabbit IgG
(1:2,000; Beijing Zhongshan Golden Bridge Biotechnology, Co., Ltd.)
at room temperature for 1 h. Visualization was carried out by
electrochemiluminescence, with the protein bands revealed on an
ALS4000 gel image analysis system (GE Healthcare Life Sciences,
Logan, UT, USA). Quantity One software (Bio-Rad Laboratories, Inc.)
was employed to assess the protein bands, with target protein
expression normalized to β-actin levels.
Statistical analysis
Statistical analysis was performed with SPSS version
20.0 software (IBM, Chicago, IL, USA). Data are the means ±
standard deviation (SD), and were compared by one-way analysis of
variance (ANOVA) followed by the Student-Newman-Keuls (SNK) test.
Pearson's correlation analysis was employed to evaluate the
associations of the protein and gene expression levels with the
Mankin scores. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
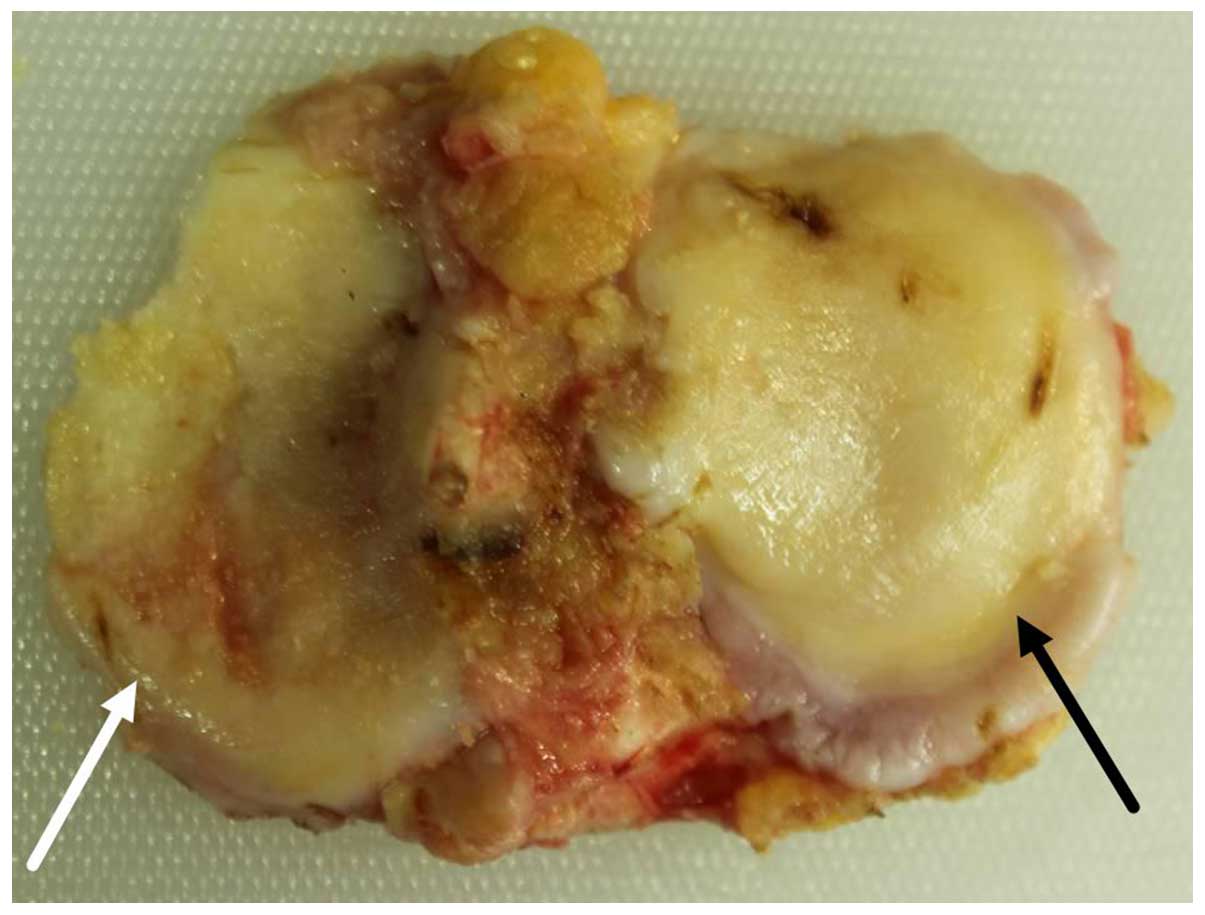
Macroscopic observations
The medial and lateral tibial plateaus exhibited
distinct degrees of degeneration. Patients with varus and valgus
deformities, presented with severe degeneration of the medial and
lateral tibial plateau, respectively. The tibial plateau samples
with severe degeneration exhibited a coarse surface, giant
fractures, extensive malacia, an altered cartilage thickness (in
severe cases), ivory-like subchondral bone and multiple osteophytes
at the edges. The tibial plateau samples with mild degeneration
were relatively plain, but exhibited superficial ulcers, malacia,
superficial fractures and a few osteophytes at the edges (Fig. 1).
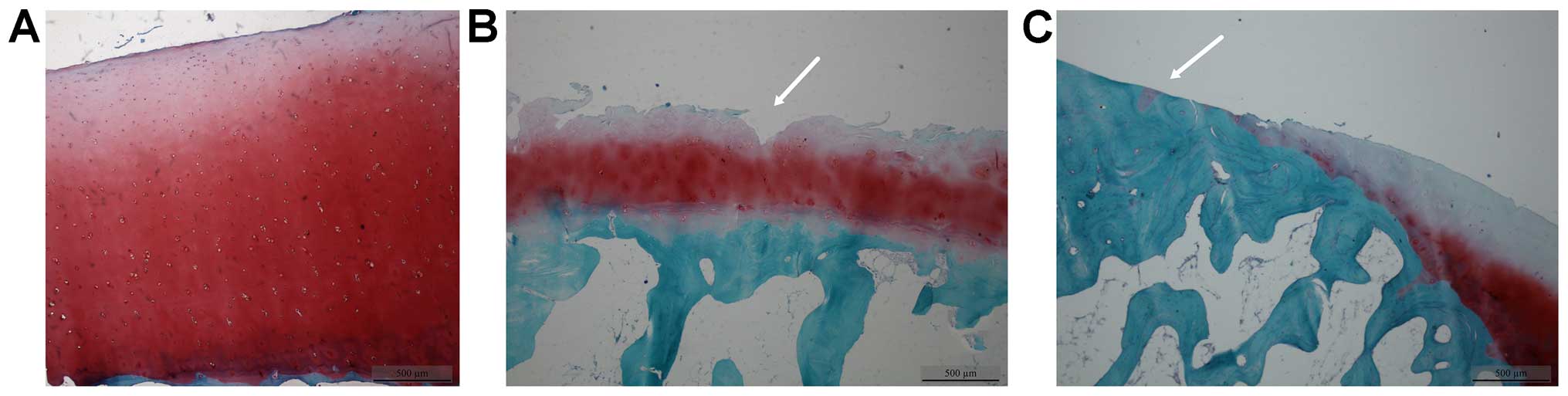
Safranin O/Fast Green staining
In the early-stage samples, the cartilage layer did
not exhibit any fractures; the chondrocytes had a normal size and
morphology, with the cartilage matrix evenly stained (Fig. 2A). In the intermediate-stage
samples, vertical fractures were observed deep in the cartilage
layer (Fig. 2B, white arrow),
with an abnormal chondrocyte morphology and arrangement; in
addition the proliferation and clustering of chondrocytes was
evident, with the cartilage matrix lightly stained (Fig. 2B). In the late-stage samples,
ivory-like subchondral bone (Fig.
2C, white arrow) was observed; the chondrocytes had an abnormal
morphology and had becom swollen, and were reduced in number; the
cartilage matrix was not stained (Fig. 2C). Safranin O/Fast green staining
grading scores were calculated according to the Mankin scoring
system (Table II).
 | Table IIStructural parameters of cartilage
and subchondral bone at different OA stages (mean ± SD). |
Table II
Structural parameters of cartilage
and subchondral bone at different OA stages (mean ± SD).
| Groups | Mankin | TAC
(µm) | SCP
(µm) | BV/TV (%) |
|---|
| Early stage
(n=15) | 3.3±1.2 | 2343.5±434.8 | 218.4±56.3 | 21.9±6.4 |
| Intermediate stage
(n=13) | 7.7±0.9 |
1158.2±477.8a | 433.4±208.9a | 35.3±12.2a |
| Late stage
(n=17) | 13.0±1.2 | 154.4±40.5a,b |
1223.2±291.4a,b | 49.7±5.0a,b |
Cartilage and subchondral bone structural
parameters
The TAC levels were reduced, while SCP thickness and
the BV/TV were increased with the increasing severity of OA.
Compared with the values obtained from the early-stage samples, the
TAC levels were decreased and SCP thickness and the BV/TV were
increased in the intermediate- and late-stage samples, exhibiting
statistically significant differences (P<0.05). Compared with
the values obtained from the intermediate-stage samples, the TAC
levels were decreased, and SCP thickness and the BV/TV were
increased in the late-stage samples, with statistically significant
differences (P<0.05) (Table
II).
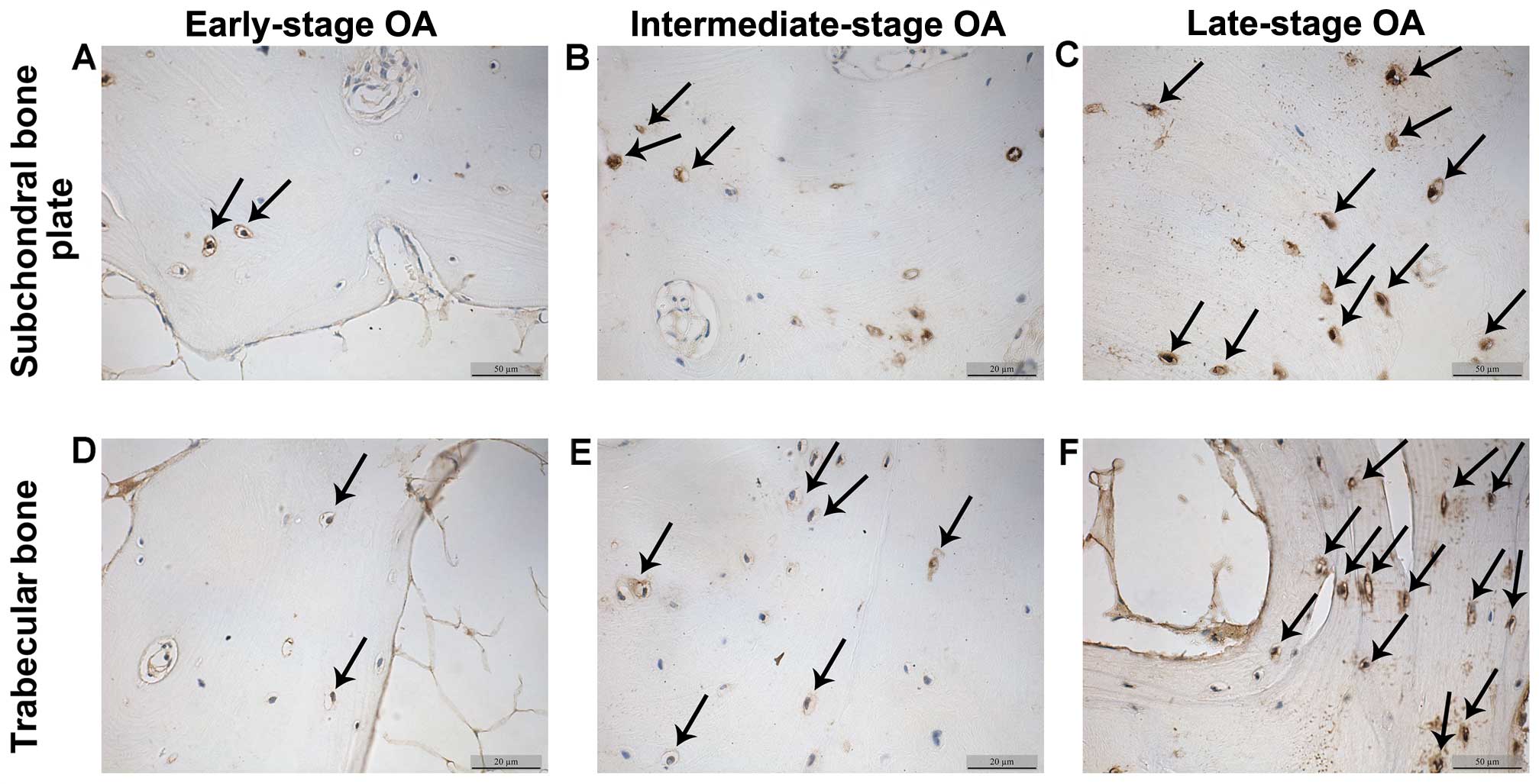
β-catenin and sclerostin levels detected
by immunohistochemistry
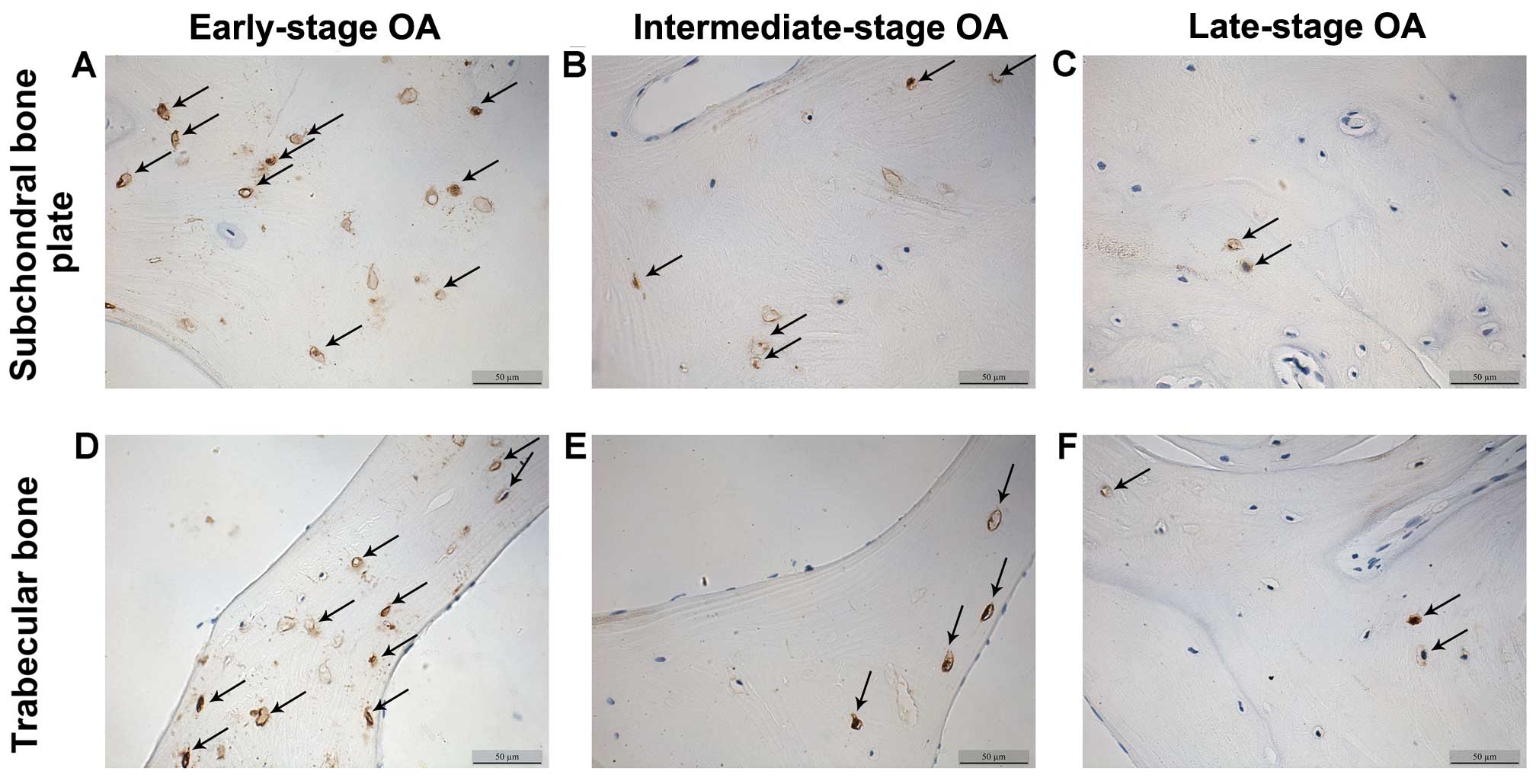
The β-catenin and sclerostin levels were observed in
the subchondral bone plate and trabecular bone based on the
corresponding positions (Fig. 3).
β-catenin was expressed mainly in the cytoplasm and nucleus of the
osteocytes, while sclerostin was found mainly in osteocytes and in
the surrounding lacunae (Figs. 4
and 5). In the early-stage
samples, weak β-catenin signals were observed in the bone cells,
only in the subchondral bone plate (Fig. 4A and D). Strong positive
sclerostin signals were observed in the bone cells, in the
subchondral bone plate and trabecular bone (Fig. 5A and D). In the intermediate-stage
samples, β-catenin exhibited a higher expression compared with the
early-stage samples; the subchondral bone plate and trabecular bone
exhibited partial expression (Fig. 4B
and E). Sclerostin expression was weaker, with little
expression in the subchondral bone plate and trabecular bone
(Fig. 5B and E). In the
late-stage samples, β-catenin exhibited strong signals in bone
cells, both in the subchondral bone plate and trabecular bone
(Fig. 4C and F). Sclerostin
exhibited a low expression in bone cells, only in the subchondral
bone plate (Fig. 5C and F).
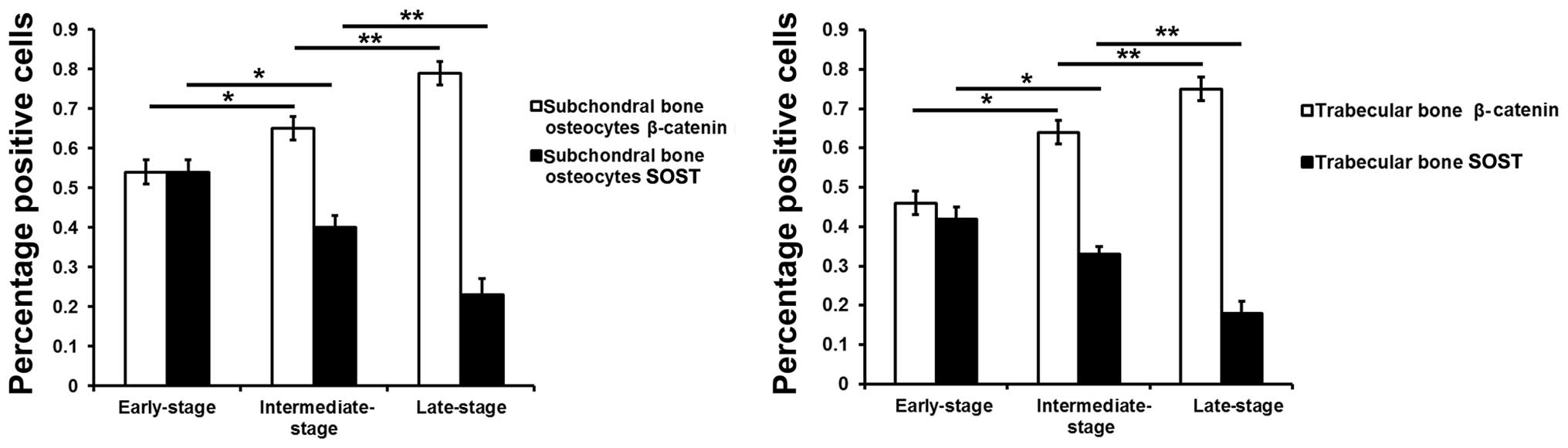
Fig. 6 represents the results of
the quantification of the immunohistochemistry data.
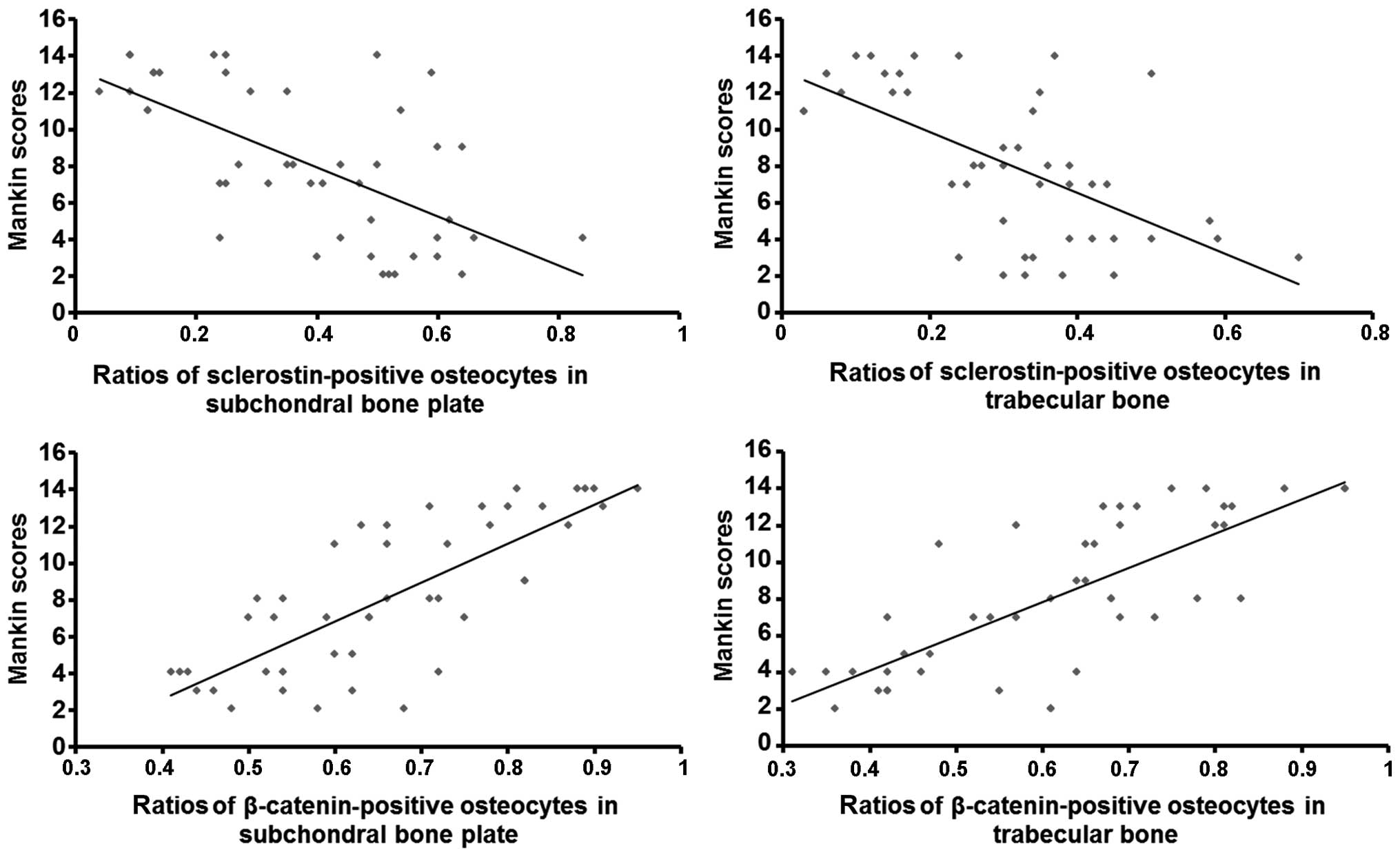
Correlation of β-catenin and sclerostin
levels in osteocytes with Mankin scores
Pearson's correlation analysis revealed that the
ratios of β-catenin-positive osteocytes in the subchondral bone
plate (r=0.775; P<0.01) and trabecular bone (r=0.769; P<0.01)
positively correlated with the Mankin scores, suggesting that
β-catenin protein expression in subchondral bone was positively
associated with the severity of degeneration (Fig. 7, bottom panels). Furthermore, the
ratios of sclerostin-positive osteocytes in the subchondral bone
plate (r=0.632; P<0.01) and trabecular bone (r=0.620; P<0.01)
negatively correlated with the Mankin scores, indicating that
sclerostin expression in subchondral bone was negatively associated
with the severity of degeneration (Fig. 7, top panels).
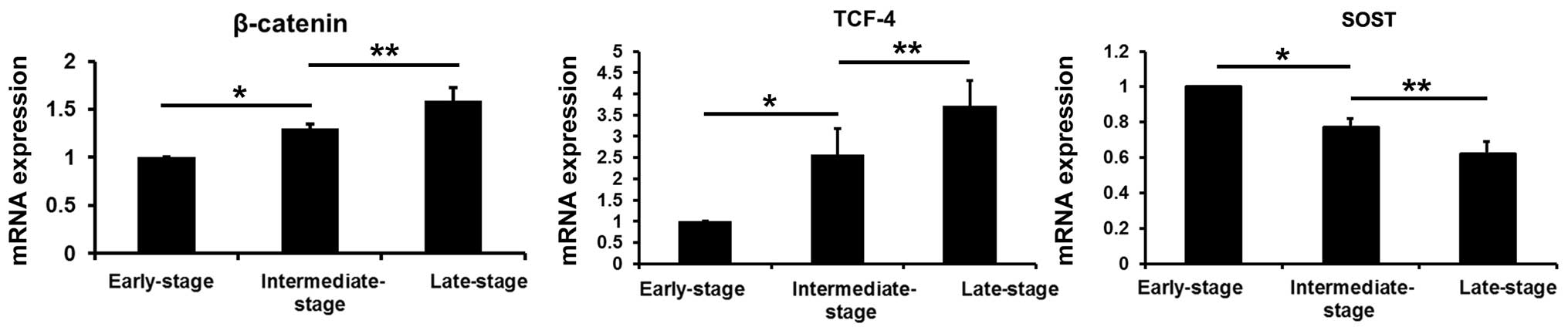
β-catenin, TCF-4 and sclerostin mRNA
levels in subchondral bone
Compared with the values obtained from the
early-stage samples, the mRNA levels of β-catenin and TCF-4 were
increased in the intermediate- and late-stage samples (P<0.05),
while the sclerostin levels were decreased (P<0.05). Compared
with the values obtained from the intermediate-stage samples, the
β-catenin and TCF-4 mRNA levels were increased in the late-stage
samples (P<0.05), and the sclerostin levels were decreased
(P<0.05) (Fig. 8).
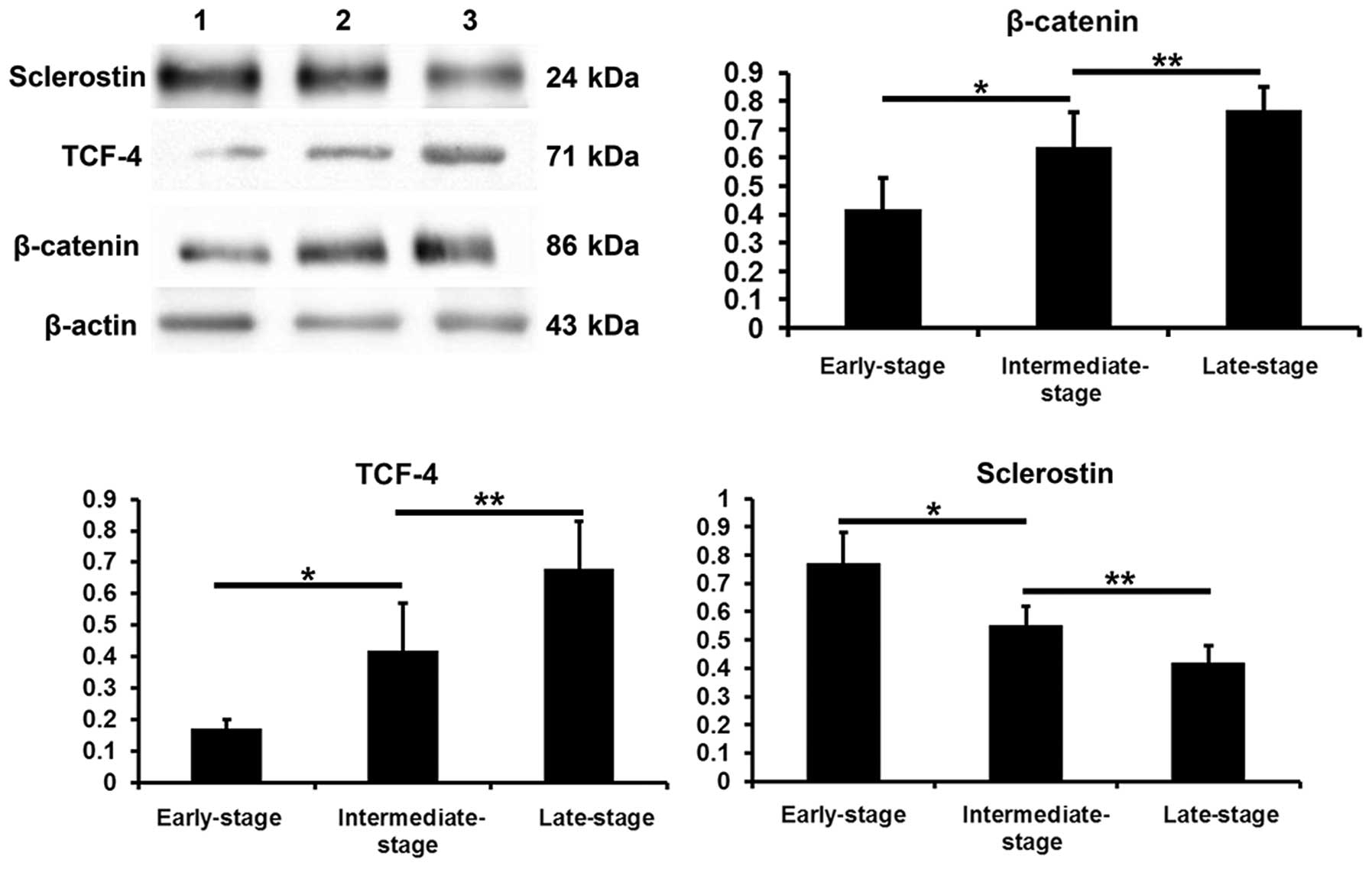
β-catenin, TCF-4 and sclerostin protein
levels in subchondral bone determined by western blot analysis
Compared with the values obtained from the
early-stage samples, the β-catenin and TCF-4 protein levels were
increased in the intermediate- and late-stages samples (P<0.05),
while the sclerostin levels were decreased (P<0.05). Compared
with the intermediate-stage samples, the β-catenin and TCF-4
protein levels were increased in the late-stage samples
(P<0.05), while the sclerostin levels were decreased (P<0.05)
(Fig. 9).
Discussion
During the development of OA, the subchondral bone
is pathologically characterized by an abnormally increased bone
turnover. Bone resorption overwhelms osteogenesis, resulting in
transient osteoporosis in early-stage OA. With disease progression,
compensatory bone formation is observed, with bone formation
greater than resorption, finally resulting in increased bone mass
and bone sclerosis. The abnormally high bone turnover yields new
bones with incomplete mineralization and the loss of cartilage
protection; this results in cartilage degeneration, accelerating
the process of OA (1). Of note,
matrix metalloproteinases may enter the deep cartilage through new
blood vessels and articular calcified cartilage, resulting in the
abnormal metabolism of cartilage cells, and inducing cartilage
degeneration and matrix degradation (2,13).
Osteogenesis increases as a compensation and
overwhelms bone resorption, resulting in increased bone mass and
bone sclerosis in intermediate- and late-stage OA (14–17). The OPG/RANKL/RANK system is an
important downstream signaling pathway that regulates the balance
between bone resorption and osteogenesis. In early-stage OA, the
OPG/RANKL ratio decreases, and OA is characterized by increased
bone resorption and transient osteoporosis. The progression of OA
can increase the OPG/RANKL ratio, characterized by enhanced
osteogenesis and bone sclerosis. OPG and RANKL are secreted mainly
by bone osteocytes and osteoblasts, and may both constitute
important factors in determining osteoclast generation and activity
(18), as well as subchondral
bone turnover. Thus, the regulation of osteocyte and osteoblast
differentiation, proliferation and apoptosis is considered a key
target in therapeutic attempts to modulate bone metabolism.
The Wnt/β-catenin signaling is mainly involved in
cell proliferation, differentiation and apoptosis. In recent years,
studies have demonstrated that Wnt/β-catenin signaling plays an
important role in maintaining normal osteocyte and osteoblast
function, and bone metabolism. The following findings have been
demonstrated: i) as regards bone formation, Wnt/β-catenin signaling
restrains mesenchymal stem cells from differentiating into
adipocytes, and promotes osteoblast differentiation, proliferation
and maturation (3); in addition,
Wnt/β-catenin signaling inhibits osteoblast apoptosis (4). ii) As regards bone absorption,
Wnt/β-catenin signaling promotes OPG secretion and inhibits RANKL
expression in osteoblasts (5),
thus reducing the RANKL/OPG ratio and inhibiting bone resorption.
iii) Wnt/β-catenin signaling inhibits bone cell apoptosis (19), activating downstream target genes,
and regulating bone metabolism; however, the regulatory mechanisms
of action of the Wnt/β-catenin pathway signaling require further,
more in-depth investigations. Thus, the activation of Wnt/β-catenin
signaling reduces bone resorption and increases bone formation.
β-catenin is a classic hub molecule in the Wnt/β-catenin signaling
pathway. It is distributed in the cell membrane, cytoplasm and
nucleus (19), and its expression
is associated with the activation of the Wnt/β-catenin signaling
pathway. TCF-4, a transcription factor, and serves as an active
bridge connecting upstream and downstream effectors; it has been
shown to promote cell apoptosis (20). Sclerostin is specifically secreted
by osteocytes and may negatively regulate bone mass (7,8,21,22). Generally, after osteocytes are
embedded in the bone matrix, they begin to secrete sclerostin,
which then affects osteocytes in an autocrine manner or osteoblasts
via a paracrine mechanism (23).
Sclerostin binds to the co-receptor, LRP5/6, on the cell membrane
to inhibit the Wnt/β-catenin signaling pathway (9,10).
There is evidence to indicate that sclerostin, as a mechanics
sensitive protein, may bridge the mechanical sensation and
osteogenesis (24–26).
In the present study, fresh medial or lateral tibial
plateau samples were collected from patients with primary OA during
total knee arthroplasty and scored according to the Mankin scoring
system. Subsequently, the structural parameters of the cartilage
and subchondral bone were measured. Of note, the TAC levels
decreased, while SCP thickness and the BV/TV increased with the
progression of OA, corroborating previous studies (16). These findings suggest abnormal
subchondral bone reconstruction, excessive osteogenesis, and
increased bone mass and sclerosis during OA progression, as
reported in previous studies (27–29). In addition, immunohistochemistry
revealed more β-catenin-positive and less sclerostin-positive cells
with the increasing Mankin scores. As shown by western blot
analysis and RT-qPCR, the β-catenin and TCF-4 levels increased,
while the sclerostin levels decreased with the increasing Mankin
scores, both at the gene and protein levels.
Our findings demonstrated that the expression levels
of key Wnt/β-catenin signaling components (specifically, β-catenin
and TCF-4) increased with the increasing OA pathological stage, and
were positively associated with the severity of degeneration.
However, the levels of sclerostin, an antagonist of the
Wnt/β-catenin signaling pathway, were reduced with the increasing
pathological stage of OA, and negatively correlated with the
severity of degeneration.
Thus, we hypothesized that during the progression of
OA, sclerostin expression is reduced as a result of multiple
factors that activate the Wnt/β-catenin signaling pathway, thus
increasing osteogenesis and reducing bone resorption. This cascade
of signaling events may result in osteogenesis overwhelming bone
resorption, a condition characterized by increased bone mass and
sclerosis, which promotes the progression of OA.
Wnt signaling is considered a therapeutic target for
diseases characterized by abnormal bone density. However, the
activation of Wnt signaling is also closely related to
tumorigenesis. Thus, the targeted regulation of the Wnt signaling
pathway in bone tissues is key in modulating bone reconstruction
(30). In adults, sclerostin is
specifically expressed in bone tissues, and systemic modulation may
affect Wnt/β-catenin signaling in bone tissues, but apparently has
no impact on other tissues (7,8,21).
A sclerostin monoclonal antibody has been used in
the treatment of post-menopausal osteoporosis and other diseases in
animal experiments and clinical trials, with favorable results
(31). Although therapy targeting
sclerostin for the treatment of bone density-related diseases (such
as post-menopausal osteoporosis) has achieved effective results,
whether this strategy would play an important role in OA prevention
and therapy remains unclear. If safe and effective, therapy
targeting sclerostin may significantly aid in the prevention and
treatment of OA, thereby yielding health, social and economic
benefits. Our results revealed a reduced sclerostin expression in
the subchondral bone and the activation of the Wnt/β-catenin
signaling pathway during the progression of OA.
Based on these findings, the inhibition of
osteogenesis may serve as a therapeutic strategy for the treatment
of OA. In future studies, the systemic or intra-articular
administration of recombinant sclerostin may be employed to
increase sclerostin expression in the subchondral bone of patients
with OA. Other studies are also required to address abnormal
osteogenesis, subchondral bone and OA progression to assess the
efficacy and safety of such therapies.
References
|
1
|
Felson DT: Developments in the clinical
understanding of osteoarthritis. Arthritis Res Ther. 11:2032009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Felson DT and Neogi T: Osteoarthritis: is
it a disease of cartilage or of bone? Arthritis Rheum. 50:341–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song L, Liu M, Ono N, Bringhurst FR,
Kronenberg HM and Guo J: Loss of wnt/β-catenin signaling causes
cell fate shift of preosteoblasts from osteoblasts to adipocytes. J
Bone Miner Res. 27:2344–2358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen B, Li XD, Liu DX, Wang H, Xie P, Liu
ZY, Hou GQ, Chang B and Du SX: Canonical Wnt signaling is required
for Panax notoginseng saponin-mediated attenuation of the RANKL/OPG
ratio in bone marrow stromal cells during osteogenic
differentiation. Phytomedicine. 19:1029–1034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spencer GJ, Utting JC, Etheridge SL,
Arnett TR and Genever PG: Wnt signalling in osteoblasts regulates
expression of the receptor activator of NFkappaB ligand and
inhibits osteoclastogenesis in vitro. J Cell Sci. 119:1283–1296.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moester MJ, Papapoulos SE, Löwik CW and
van Bezooijen RL: Sclerostin: current knowledge and future
perspectives. Calcif Tissue Int. 87:99–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Bezooijen RL, Roelen BA, Visser A, van
der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE,
ten Dijke P and Löwik CW: Sclerostin is an osteocyte-expressed
negative regulator of bone formation, but not a classical BMP
antagonist. J Exp Med. 199:805–814. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poole KE, van Bezooijen RL, Loveridge N,
Hamersma H, Papapoulos SE, Löwik CW and Reeve J: Sclerostin is a
delayed secreted product of osteocytes that inhibits bone
formation. FASEB J. 19:1842–1844. 2005.PubMed/NCBI
|
|
9
|
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang
J, Harris SE and Wu D: Sclerostin binds to LRP5/6 and antagonizes
canonical Wnt signaling. J Biol Chem. 280:19883–19887. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Semënov M, Tamai K and He X: SOST is a
ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem.
280:26770–26775. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Altman RD: Criteria for classification of
clinical osteoarthritis. J Rheumatol Suppl. 27:10–12.
1991.PubMed/NCBI
|
|
12
|
Zhang W, Moskowitz RW, Nuki G, Abramson S,
Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty
M, et al: OARSI recommendations for the management of hip and knee
osteoarthritis, Part II: OARSI evidence-based, expert consensus
guidelines. Osteoarthritis Cartilage. 16:137–162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bobinac D, Spanjol J, Zoricic S and Maric
I: Changes in articular cartilage and subchondral bone
histomorphometry in osteoarthritic knee joints in humans. Bone.
32:284–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burr DB: Anatomy and physiology of the
mineralized tissues: role in the pathogenesis of osteoarthro sis.
Osteoarthritis Cartilage. 12(Suppl A): S20–30. 2004. View Article : Google Scholar
|
|
15
|
Prasadam I, van Gennip S, Friis T, Shi W,
Crawford R and Xiao Y: ERK-1/2 and p38 in the regulation of
hypertrophic changes of normal articular cartilage chondrocytes
induced by osteoarthritic subchondral osteoblasts. Arthritis Rheum.
62:1349–1360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sanchez C, Deberg MA, Piccardi N, Msika P,
Reginster JY and Henrotin YE: Osteoblasts from the sclerotic
subchondral bone downregulate aggrecan but upregulate
metalloproteinases expression by chondrocytes. This effect is
mimicked by interleukin-6, -1beta and oncostatin M pre-treated
non-sclerotic osteoblasts. Osteoarthritis Cartilage. 13:979–987.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karsdal MA, Leeming DJ, Dam EB, Henriksen
K, Alexandersen P, Pastoureau P, Altman RD and Christiansen C:
Should subchondral bone turnover be targeted when treating
osteoarthritis? Osteoarthritis Cartilage. 16:638–646. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trouvin AP and Goëb V: Receptor activator
of nuclear factor-κB ligand and osteoprotegerin: maintaining the
balance to prevent bone loss. Clin Interv Aging. 5:345–354.
2010.
|
|
19
|
Bodine PV: Wnt signaling control of bone
cell apoptosis. Cell Res. 18:248–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma B, Zhong L, van Blitterswijk CA, Post
JN and Karperien M: T cell factor 4 is a pro-catabolic and
apoptotic factor in human articular chondrocytes by potentiating
nuclear factor κB signaling. J Biol Chem. 288:17552–17558. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Winkler DG, Sutherland MK, Geoghegan JC,
Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR,
Staehling-Hampton K, et al: Osteocyte control of bone formation via
sclerostin, a novel BMP antagonist. EMBO J. 22:6267–6276. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Bezooijen RL, Bronckers AL, Gortzak
RA, Hogendoorn PC, van der Wee-Pals L, Balemans W, Oostenbroek HJ,
Van Hul W, Hamersma H, Dikkers FG, et al: Sclerostin in mineralized
matrices and van Buchem disease. J Dent Res. 88:569–574. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krause C, Korchynskyi O, de Rooij K,
Weidauer SE, de Gorter DJ, van Bezooijen RL, Hatsell S, Economides
AN, Mueller TD, Löwik CW and ten Dijke P: Distinct modes of
inhibition by sclerostin on bone morphogenetic protein and Wnt
signaling pathways. J Biol Chem. 285:41614–41626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robling AG, Niziolek PJ, Baldridge LA,
Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido
TM, Harris SE, et al: Mechanical stimulation of bone in vivo
reduces osteocyte expression of Sost/sclerostin. J Biol Chem.
283:5866–5875. 2008. View Article : Google Scholar
|
|
25
|
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang
J, Li Y, Feng G, Gao X and He L: Sclerostin mediates bone response
to mechanical unloading through antagonizing Wnt/beta-catenin
signaling. J Bone Miner Res. 24:1651–1661. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bonewald LF and Johnson ML: Osteocytes,
mechanosensing and Wnt signaling. Bone. 42:606–615. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burr DB: The importance of subchondral
bone in the progression of osteoarthritis. J Rheumatol Suppl.
70:77–80. 2004.PubMed/NCBI
|
|
28
|
Goldring MB and Goldring SR: Articular
cartilage and subchondral bone in the pathogenesis of
osteoarthritis. Ann N Y Acad Sci. 1192:230–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Madry H, van Dijk CN and Mueller-Gerbl M:
The basic science of the subchondral bone. Knee Surg Sports
Traumatol Arthrosc. 18:419–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Funck-Brentano T, Bouaziz W, Marty C,
Geoffroy V, Hay E and Cohen-Solal M: Dkk-1-mediated inhibition of
Wnt signaling in bone ameliorates osteoarthritis in mice. Arthritis
Rheumatol. 66:3028–3039. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ominsky M, Warmington K, Asuncion F, Tan
H, Grisanti M, Geng Z, Stephens T, Henry A, Lawson A, Lightwood D,
et al: Sclerostin monoclonal antibody treatment increases bone
strength in aged osteopenic ovariectomized rats. J Bone Miner Res.
21(Suppl 1): S442006.
|