Introduction
Gene-based and cell-based therapies are effective
approaches for engineering biological pacemakers. Stem cells are
candidates that are capable of carrying genes to achieve a
combination of gene- and cell-based precision therapy.
Adipose-derived stem cells (ADSCs) have many advantages; they are
available in abundance using a simple collection method and do not
cause non-immune rejection. ADSCs have multilineage differentiation
potential (1). Several studies
have shown that ADSCs possess the ability to differentiate into
cardiomyocytes in vitro and in vivo (2–4).
One study investigated the pacemaker activity of cells derived from
ADSCs in semi-solid methylcellulose medium (5).
The development of the sinoatrial node is regulated
by many transcription factors including those of the T-box (TBX)
family. TBX18 is a member of the TBX family. In the early stages of
embryonic development, epicardial TBX18+ mesenchymal
progenitor cells migrate to the head region of the sinoatrial node.
The gene tracer technique has shown that the majority of cells in
the head of the sinoartial node are derived from TBX18+
progenitor cells (6). A study
showed that TBX18+ mesenchymal progenitor cells are
capable of differentiating into ventricular septal and left
ventricular myocardial cells (7),
which remains controversial (8).
In homozygous TBX18-mutant and TBX18-deficient mouse fetuses, the
head of the sinoatrial node was found to be significantly reduced,
and the sinoatrial node and atrial myocardial layer were found to
be disorganized (6). However, no
abnormal changes were observed in the left ventricular
myocardium.
Kapoor et al (9) demonstrated that focal TBX18
transduction reprogrammed ventricular myocytes into pacemaker cells
that are indistinguishable from genuine sinoatrial node cells, both
physiologically and morphologically. In vivo experiments
showed that TBX18 produced biological pacemaker activity in the
ventricle and high septal region by percutaneous gene transfer
(9,10).
To date, no studies have reported the effects of
TBX18 on stem cells, to the best of our knowledge. This study used
rat ADSCs as seed cells. ADSCs were transfected with TBX18 and were
co-cultured with neonatal rat ventricular cardiomyocytes (NRVMs) in
order to examine whether ADSCs are capable of differentiating into
pacemaker-like cells in vitro.
Materials and methods
Isolation and culture of ADSCs
All experimental procedures were conducted in
accordance with the Institutional Guidelines for the Care and Use
of Laboratory Animals at Wuhan University (Wuhan, China) and
conformed to the National Institutes of Health Guide for the Care
and Use of Laboratory Animals. Ethics approval was provided by the
Ethics Committee of Wuhan University. One adult male Sprague Dawley
(SD) rat, aged 4 weeks, was anesthetized with an intraperitoneal
injection of 3% pentobarbital sodium (30 mg/kg). Adipose tissue was
bilaterally obtained from the inguen of the rat and washed with
sterile phosphate-buffered saline (PBS). The adipose tissue was
then cut into 1×1 mm3 pieces after the careful removal
of blood vessels and fascia. The tissue pieces were digested by
incubation with 1 mg/ml collagenase type I (Sigma, St. Louis, MO,
USA) for 1 h at 37°C with constant shaking. The cell suspension was
centrifuged at 1,000 × g for 10 min. The pellet was resuspended in
Dulbecco's modified Eagle's medium (DMEM)/F12 supplemented with 10%
fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA) and 1%
penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA). The cell
inoculation density was 5×106 nucleated cells/100-mm
tissue culture dish (11).
Following 48 h of culture in an incubator at 37°C with a 5%
CO2 atmosphere, the medium was removed and replaced with
fresh medium. When the cells reached 80–90% confluence, they were
passaged using 0.25% trypsin (Gibco). Cells of passages 3–5 were
used for all subsequent analyses.
Adenovirus construction and
purification
pHBAd-MCMV-GFP (Hanbio, Shanghai, China) was
digested with BamHI and NotI. The ORF sequence of the
human TBX18 gene (GenScript, Nanjing, China) was amplified by
polymerase chain reaction (PCR). After enzyme digestion, gel
extraction was performed. The digested fragment and vector were
ligated to form pHBAd-MCMV-GFP-TBX18, which was then transformed
into competent DH5α cells (Tiangen, Beijing, China). Positive
clones were identified by liquid sequencing. Bacteria in liquid in
the logarithmic growth phase were incubated at 37°C in LB culture
medium with shaking at 300 × g overnight. Large scale preperation
of recombinant plasmid was conducted using the Plasmid Midi
Preparation kit (Beijing CW Biotech Co., Ltd., Beijing, China).
Cells (293; from our laboratory) were transfected with
pHBAd-MCMV-GFP-TBX18 and the backbone vector pHBAd-BHG using
Lipofilter™ (both from Hanbio). The supernatant was harvested after
virus amplification. Ad-GFP and Ad-TBX18 were measured as 1X 1010
PFU/ml and were preserved at −80°C.
ADSCs transfected with TBX18
ADSCs of passages 3–5 were removed from the culture
dishes by digestion. A cell suspension was prepared and then
inoculated onto 6-well plates. When cell confluence reached 70–80%,
Ad-TBX18 in DMEM/F12 was added to the cells at different
multiplicity of infection (MOI) values. The control group was
treated with Ad-GFP. After a 2 h incubation period, the medium was
replaced with fresh complete medium. The cells were observed under
a light microscope and a fluorescent microscope (BX51 systems,
Olympus, Tokyo, Japan).
Flow cytometric analysis
ADSCs were transfected with different MOI values (0,
10, 20, 50, 80, 100 and 1,000). The transfected cells were digested
with 0.25% trypsin at 48 h and 7 days after transfection. The cell
suspension was centrifuged at 800 × g for 5 min and then washed
with PBS twice. The cell density was 1×106/ml.
Non-transfected cells served as a negative control. The percentage
of green fluorescent protein-positive cells was detected by flow
cytometric analysis (Becton-Dickinson, Franklin Lakes, NJ,
USA).
Isolation and culture of NRVMs
Cardiomyocytes were isolated from ten 1–2-day-old SD
rats and then washed three times with PBS at a temperature of 4°C.
Heart tissue was cut into 1 mm3 pieces and digested with
0.125% trypsin at 37°C for 10 min and the supernatant was
discarded. The pieces were then digested with mixed liquor
containing 0.125% trypsin and 0.08% collagenase II (Sigma) five
times at 37°C for 5 min. The digested tissue pieces were then
centrifuged at 1,000 × g for 10 min. The pellets were resuspended
with fresh high-glucose DMEM supplemented with 10% FBS and 1%
penicillin/streptomycin, and filtered through a 200 mesh sieve. The
resuspended cells were then inoculated into a culture dish for 1.5
h to isolate the cardiomyocytes from the fibroblasts. After 1.5 h,
the cardiomyocytes were gently sucked out of the culture dish and
were seeded into 6-well plates. The cell concentration was adjusted
to 5×105/ml. During the first 48 h after seeding, 0.1
mmol/l bromodeoxyuridine (BrdU; Sigma) was used to inhibit the
mitosis of fibroblasts.
Co-culture of transfected ADSCs with
NRVMs
ADSCs were transfected with Ad-TBX18 and Ad-GFP for
48 h, and used to prepare a cell suspension, and then co-cultured
with NRVMs at a ratio of 1:5 or 1:10. The complete culture medium
was replaced every two days.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the transfected ADSCs
and co-culture systems after one week using TRIzol®
reagent (Invitrogen). Quantitative PCR was performed to evaluate
the mRNA expression of human TBX18 and hyper-polarization activated
cyclic nucleotide gated potassium channel 4 (HCN4). Isolated RNA (2
µg) was converted into cDNA using the First Strand cDNA
Synthesis kit (Toyobo, Tokyo, Japan). The primers used for PCR
amplification were synthesized by Invitrogen Biotechnology
(Shanghai, China) and are presented in Table I. RT-qPCR was performed using the
StepOne™ Real-Time PCR system (Life Technologies, Carlsbad, CA,
USA). The reactions were then conducted using the SYBR®
Premix Ex Taq TM II (Takara Bio, Japan). Semilog amplification
curves were analyzed using the 2-ΔΔCt comparative quantification
method and the expression of each gene was normalized to GAPDH.
 | Table IPCR primers used in this study. |
Table I
PCR primers used in this study.
| Gene | Primer | Reaction
condition | Product size
(bp) |
|---|
| H-TBX18 | Sense:
5′-ACGTCATCCGTAAAGACTGTGG-3′ | 33 cycles at 54°C
in 1 mM MgCl2 | 251 |
| Antisense:
5′-AGTCCGTAGTGATGGTCGCC-3′ | | |
| HCN4 | Sense:
5′-AACCTGGGGGCTGGACAGA-3′ | 33 cycles at 58°C
in 1 mM MgCl2 | 462 |
| Antisense:
5-′CTGGGCAGCCTGTGGAGAG-3′ | | |
| GAPDH | Sense:
5′-GCCATCAACGACCCCTTCAT-3′ | 33 cycles at 56°C
in 1 mM MgCl2 | 315 |
| Antisense:
5′-TTCACACCCATCACAAACAT-3′ | | |
Western blot analysis
TBX18-ADSCs and GFP-ADSCs co-cultured with NRVMs and
NRVMs cultured alone were plated on 6-well culture dishes. The
cells were harvested using RIPA lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). Equal amounts of protein were loaded
onto a gel for sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE), and the separated proteins were
transferred to a nitrocellulose membrane, and then incubated with
the primary antibodies against HCN4 (ab32675; Abcam, Cambridge, MA,
USA) overnight at 4°C. The primary antibodies were detected by
incubating the membrane with horseradish peroxidase-conjugated
secondary antibodies (KPL, 14-16-06, 074-1506) raised in the
appropriate species, and then performing enhanced chemiluminescence
detection (ECL; Beyotime Institute of Biotechnology). The level of
GAPDH was used to normalize the signal intensities.
Immunostaining studies
TBX18-ADSCs and GFP-ADSCs were co-cultured with
NRVMs on gelatin-coated coverslips in 6-well culture dishes. The
cell cultures were washed with PBS and fixed with 4%
paraformaldehyde. Following permeabilization with 0.1% Triton
X-100, the cells were incubated with the primary antibody
anti-cardiac troponin I (cTnI; ab19615; 1:200; Abcam) overnight at
4°C. The secondary antibodies Cy3-conjugated Affinipure goat
anti-rabbit IgG (111-165-003) and FITC-AffiniPure F(ab′)2 Fragment
goat anti-mouse IgG (115-096-006) (both from Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA)were used to detect cTnI.
4′,6-Diamidino-2-phenylindole (DAPI) was used to visualize the
nuclei. The cells were observed under a fluorescent microscope
(Leica, Wetzlar, Germany). We randomly selected three visual fields
in three different cell isolates to observe the positive rates of
cTnI detection.
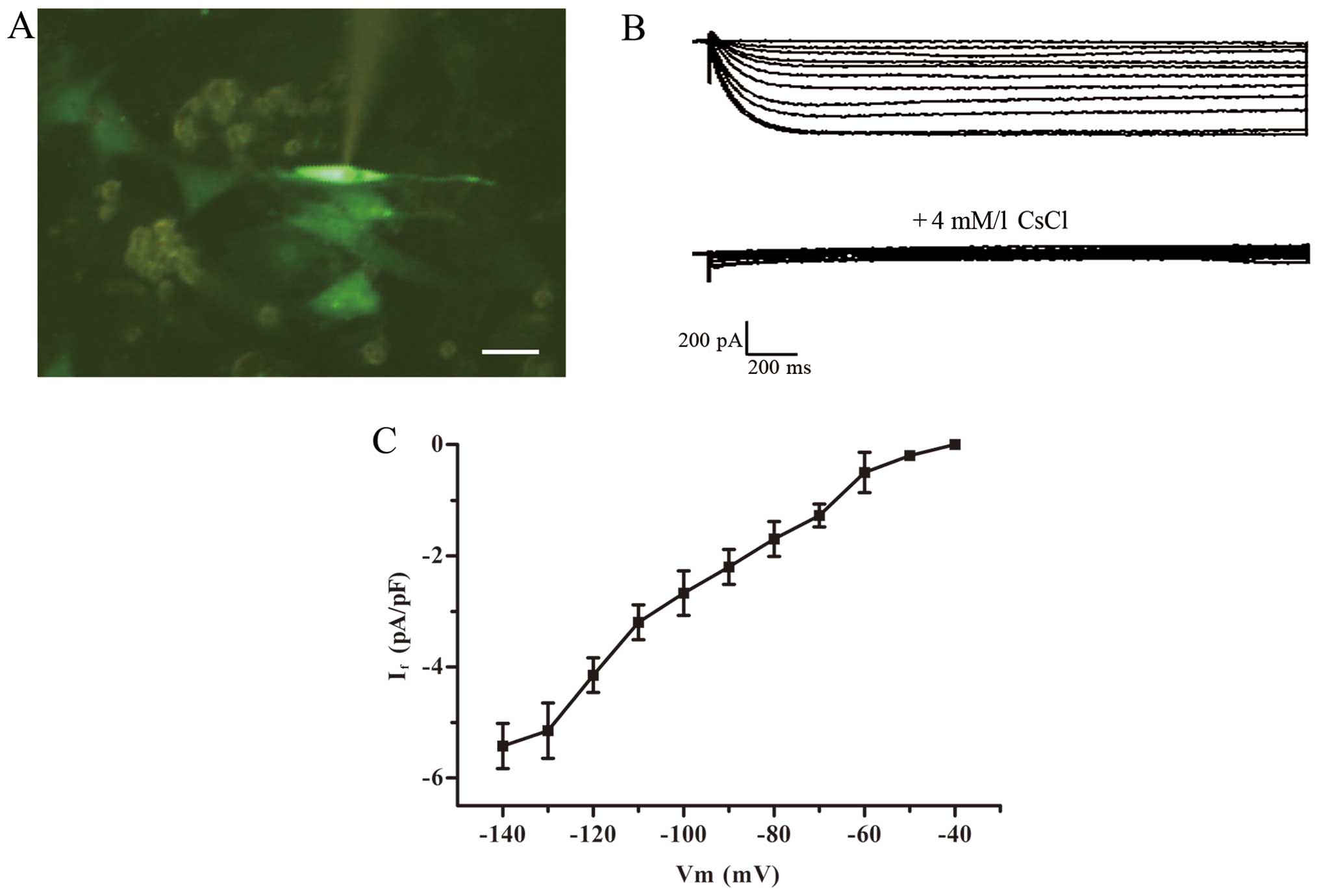
Electrophysiological recordings
Co-cultured systems were plated on gelatin-coated
coverslips in 24-well culture dishes. The whole cell patch clamp
technique was used to record the If current after 5–7
days of co-culture. The cells were previously incubated with 180
µmol/l 2-aminoethoxydiphenyl borate (2-APB; Sigma) for 15
min. 2-APB blocks Cx40, Cx43 and Cx45 and thus, blocks the
electrical conduction between cells (12,13). We selected smooth and plump cells
to record current. The impedance of the fluid filled electrode was
3 to 4 MΩ. The experiments were performed using an Axon patch-clamp
amplifier 700B (Molecular Devices, Sunnyvale, CA, USA). A digital
700AD/DA converter and 6.0.4 pClamp (both from Axon Instruments,
Union City, CA, USA) were used for recording and analyzing the
data. The bath solution has the following components (in mmol/l):
140 NaCl, 5.4 KCl, 1.0 MgCl2, 1.8 CaCl2, 1.0
BaCl2, 5.5 HEPES, 5.0 glucose (pH 7.3); the pipette
solution contained the following (in mmol/l): 20 KCl, 125
K-gluconate, 1.0 MgCl2, 5.0 NaCl, 10 HEPES, 5
K2ATP (pH 7.3). The whole cell recording mode was used
to record If. The Clampex program was applied to the
sample. The sampling frequency was 10 kHz, and the filtering rate
was 5 kHz. The resting potential was −30 mV and decreased to −140
mV with each sweep 10 mV, then returned to the resting potential.
CsCl (4 mmol/l) was administered to detect the change in
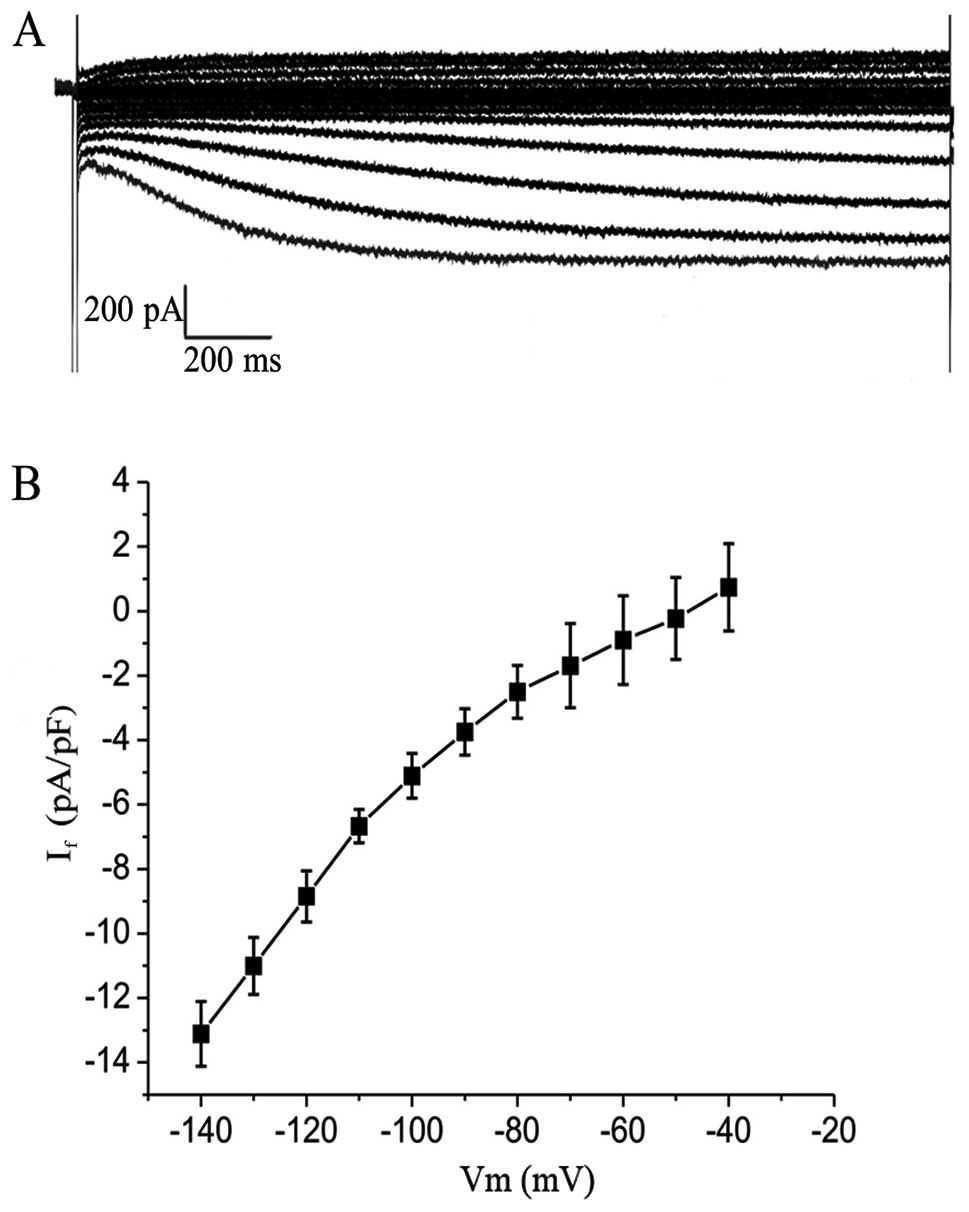
If. Commercial human induced pluripotent stem
cell-derived cardiomyocytes (hiPS-CMs) cell line (CDI, Madison, WI,
USA) were used to compared the macroscopic current using the whole
cell patch clamp technique with ADSCs. Cells were held at −30 mV
before eliciting a 2 sec hyperpolarization pulse from −140 to −40
mV to measure If.
Statistical analysis
The reported data are expressed as the means ± SD.
The statistical significance of the differences between two groups
was determined using the Student's t-test. Comparisons among three
groups were made using one-way analysis of variance (ANOVA). A
P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
Optimum MOI value of transfection and
expression of the TBX18 gene
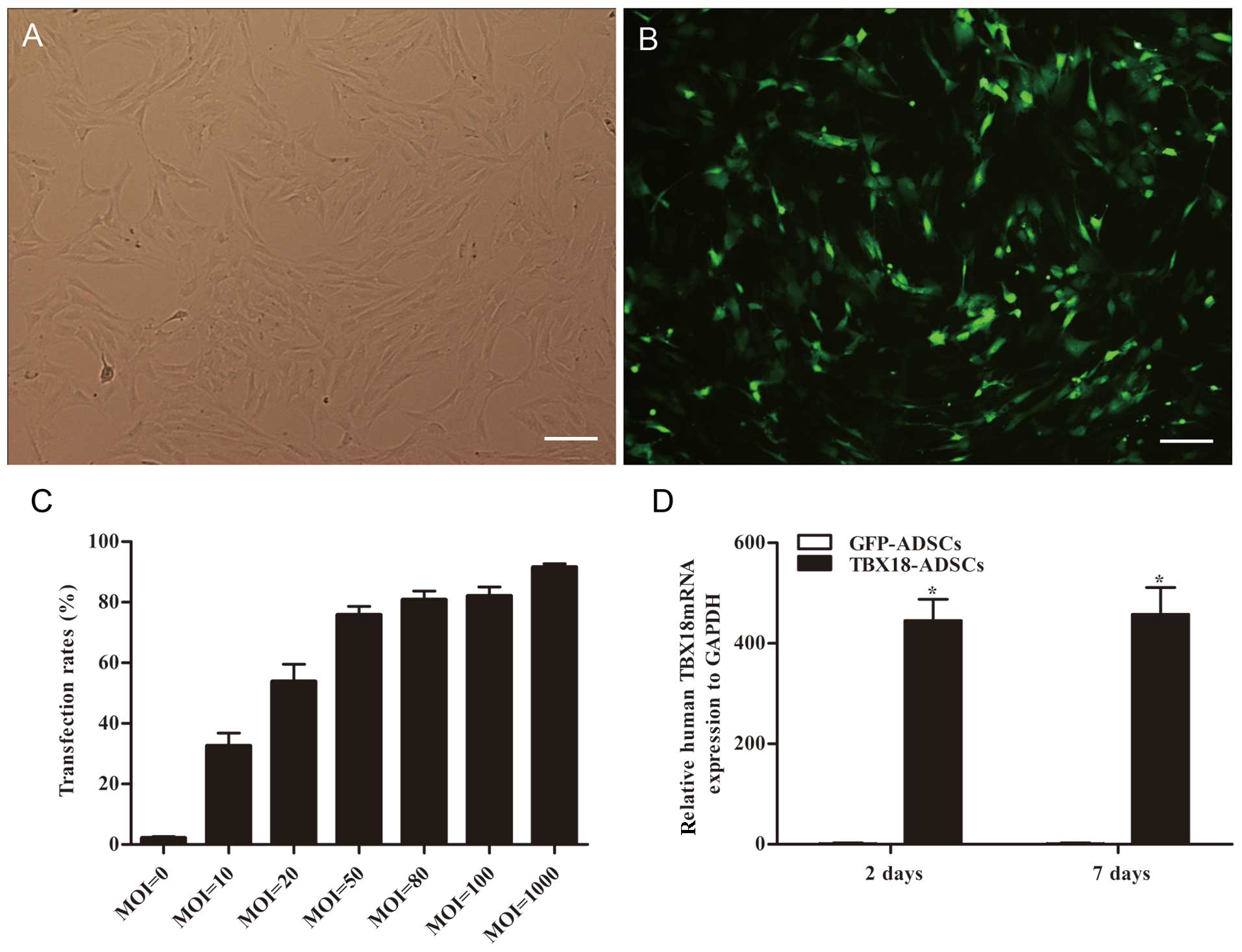
The ADSCs appeared fibroblast-like and showed
parallel growth (Fig. 1A). The
ADSCs were transfected with GFP and the TBX18 gene at different MOI
values. After successful transfection, the ADSCs exhibited green
fluorescence (Fig. 1B). Flow
cytometric analysis showed that the transfection efficiency was
>70% at a MOI value ≥50 (Fig.
1C). The transfection efficiency was 75.7±5.6% at an MOI of 50
(Fig. 1C). At an MOI of 80, the
fluorescent cells appeared as roundish cells with vacuoles in the
cytoplasm. This phenomenon was more severe at an MOI of 1000. The
optimum MOI value of transfection was 50 and was adopted in this
study. The mRNA expression of TBX18 in the TBX18-ADSCs group was
significantly higher than that in the control group at 48 h and 7
days after transfection (Fig.
1D). The results showed that TBX18 is stably expressed in
ADSCs.
Cardiomyogenic differentiation confirmed
by immunocytochemical analysis
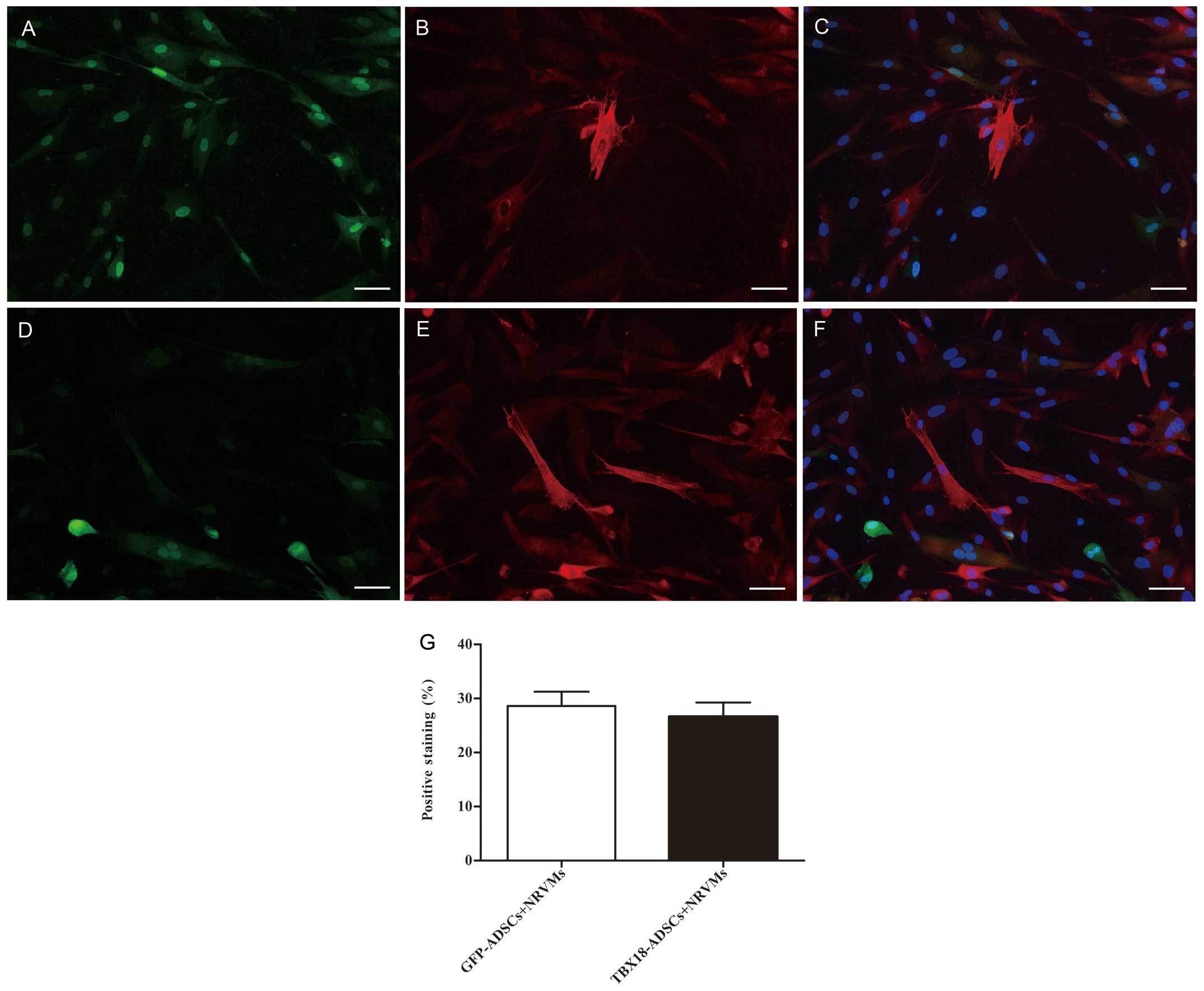
The expression of the myocardial specific marker
cTnI was detected by immunofluorescence at 7 days after
transfection and co-culture. The NRVMs and cardiac muscle-like
cells were all positive for cTnI expression. Cells positive for
cTnI expression were found in the GFP-ADSCs-NRVMs and the
TBX18-ADSCs-NRVMs groups (Fig.
2A–F). The number of positive cells was not significantly
different between the two groups. Fluorescence microscopy revealed
that the number of positively transfected ADSCs at one week was
28.6±5.8 and 26.6±5.9% in the GFP-ADSCs-NRVMs and
TBX18-ADSCs-NRVMs, respectively (Fig.
2G). cTnI expression was observed in transfected ADSCs that
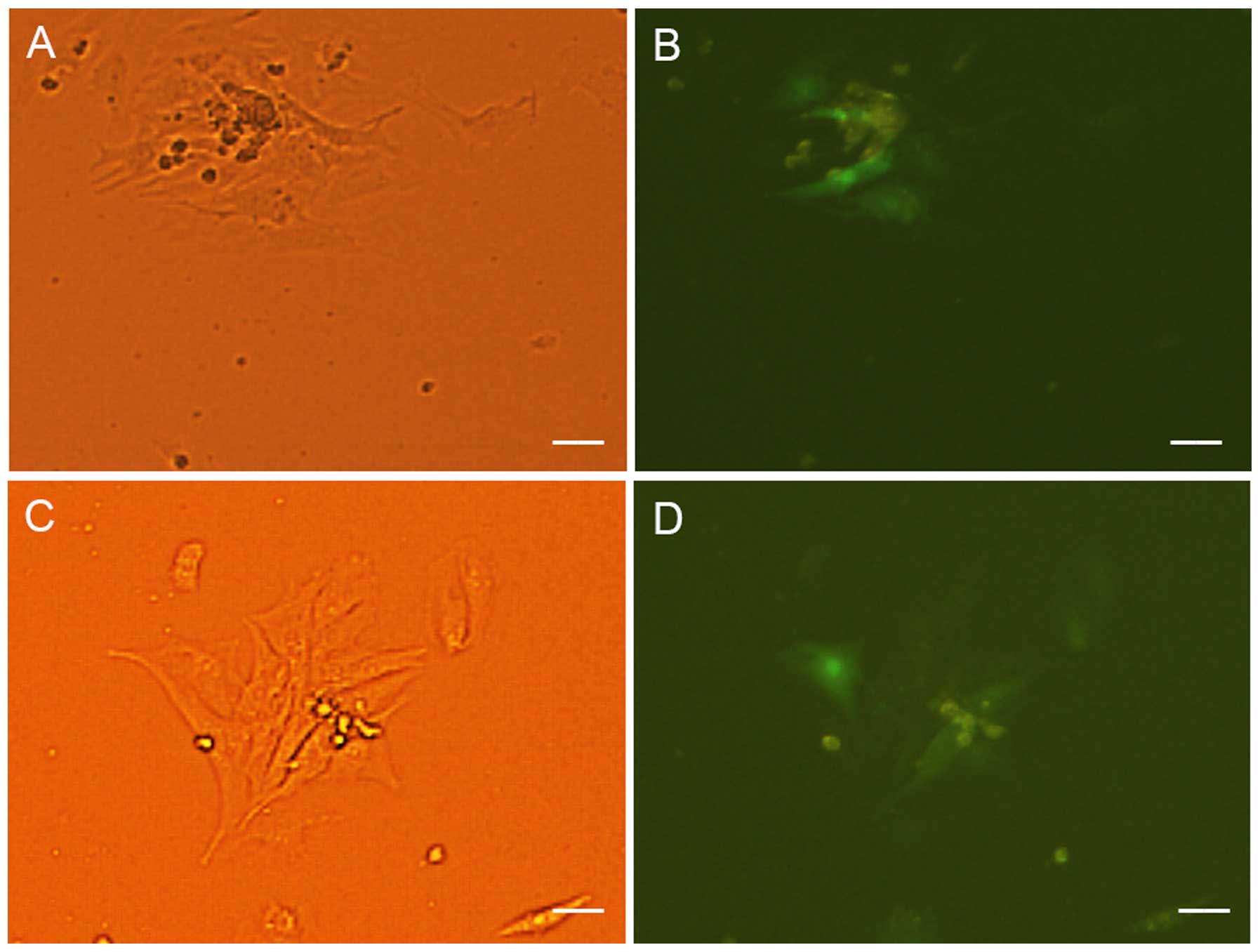
were not co-cultured with NRVMs. At 5–7 days of co-culture, the
transfected cells appeared to form connections with the adjacent
NRVMs and synchronous beating was generated (Fig. 3).
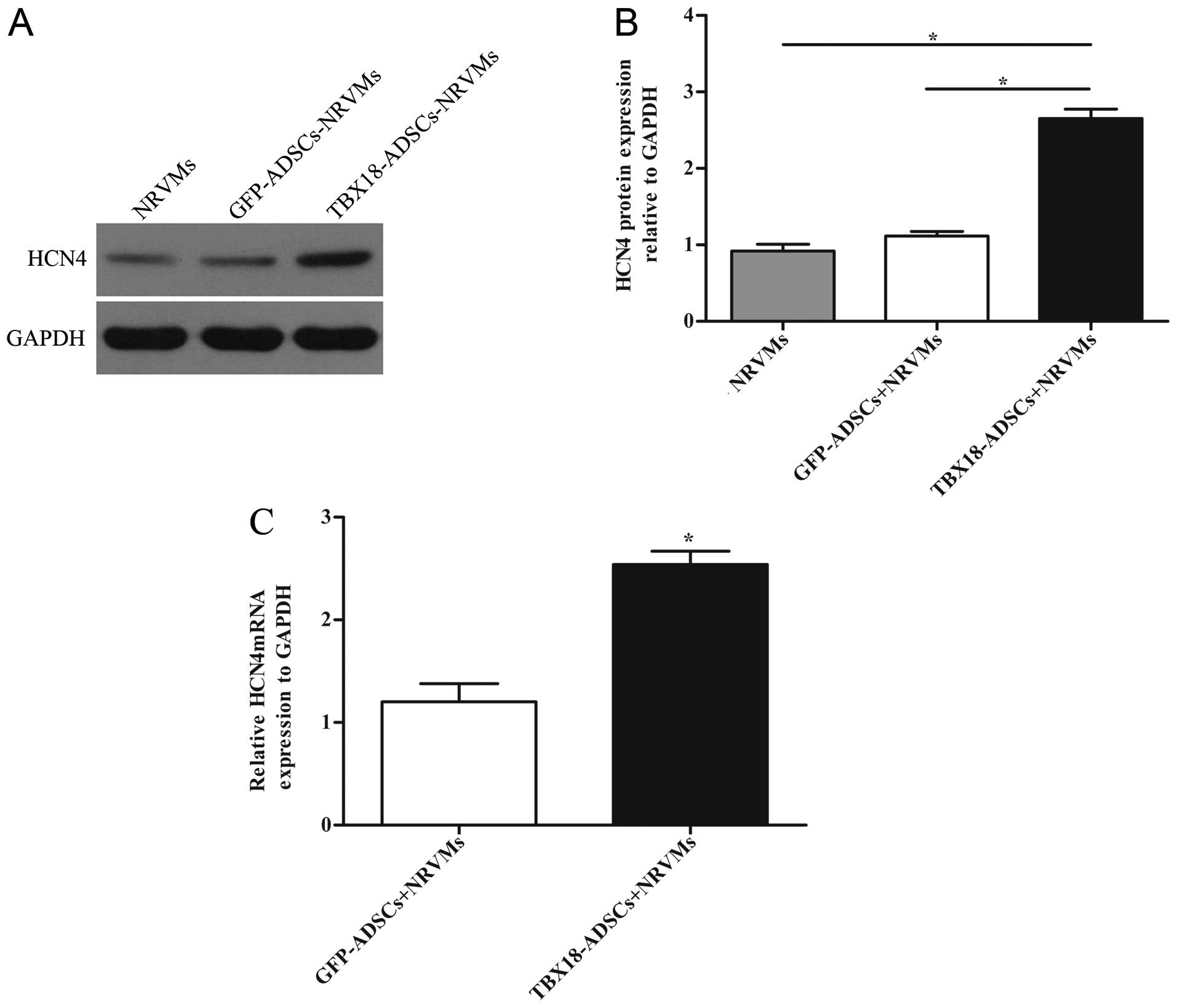
Expression of the pacemaker channel
HCN4
The expression of HCN4 was detected by RT-qPCR and
western blot analysis. NRVMs served as the positive control group.
The protein level of HCN4 in the TBX18 group was significantly
higher than that in the control group and NRVMs after 7 days of
co-culture (Fig. 4A and B). The
results of RT-qPCR revealed that the mRNA expression of HCN4 in the
TBX18 group was higher than that in the GFP group (Fig. 4C).
Patch clamp detection of If
current
Green fluorescent cells detected by the patch clamp
method were treated with 2-ABP to block electric coupling (Fig. 5A). The If current was
detected in the TBX18-ADSCs group (Fig. 5B), but not in the control group.
The TBX18-ADSCs had an inward current (~1/20 cells) that was
activated by the hyperpolarizing steps from −40 mV. The maximum
activation potential of the If current was ~−140 mV. The
maximum current density of active voltage was −5.43±1.36 pA/pF
(n=4) (Fig. 5C). The current was
completely inhibited by 4 mmol/l CsCl.Macroscopic If
currents in a single beating hiPSCM (Fig. 6A) showed a greater If
current of −13.1±1.0 pA/pF (n=4) (Fig. 6B) compared with the
TBX18-ADSCs.
Discussion
The constructed vector of biological pacing mainly
contained mature cardiomyocytes and stem cells. Different
interventions were then applied to the carrier in order to achieve
biological pacing. Recently, related studies have examined the
effect of the early embryonic transcription factor TBX18 on the
mature myocardium (9,10). In the present study, we found that
rat ADSCs transfected with the TBX18 gene are capable of
differentiating into pacemaker-like cells after being co-cultured
with NRVMs. This design of this study took into account the
developmental mechanism of the sinoatrial node and the cellular
origin of sinoatrial node formation. We performed a direct
intervention at the beginning of stem cell differentiation by
transfecting cells with the transcription factor TBX18. The
transfected cells were co-cultured with NRVMs to induce
differentiation into pacemaker-like cells. We used this method due
to the fact that at the beginning of differentiation more stem
cells are inclined to differentiate into sinoatrial node cells.
Some researchers have achieved biological pacing
using transcription factors such as short stature homeobox 2
(SHOX2) and TBX3. Previous researchers have shown that both SHOX2
and TBX3 increased the efficiency of the differentiation of
embryonic stem cells into pacemaker-like cells (14,15). TBX18 delivered to cardiomyocytes
may induce fully functional pacemaker cells, whereas TBX3 only
induces pacemaker-like cells (16). One study found that bone marrow
mesenchymal stromal cells transfected with the Shox2 gene produced
pacemaker-like cells in vitro (17). TBX18, TBX3 and SHOX2 are all
embryonic transcription factors that regulate the development of
the sinoatrial node. TBX18 is upstream of TBX3 and SHOX2 (6). This study also found that the TBX18
gene induces the formation of pacemaker-like cells. SHOX2, TBX18
and TBX3 may play a synergistic role in the regulation of HCN4
expression.
In this study, ADSCs were successfully transfected
with the TBX18 gene. The transfected ADSCs were directly
co-cultured with NRVMs at a 1:10 or 1:5 ratio in order to induce
differentiation of the stem cells. Some studies have shown that
direct contact with adult stem cells is essential for them to
successfully differentiate into myocardial cells (3,18,19). In the co-culture system, cells
were induced to differentiate by cell factors, chemical substances,
electrical activity and mechanical stretch (20). The expression of myocardial
markers was higher after direct contact than after indirect contact
of the two cell types (3). In
this study, we found that ADSCs and NRVMs became connected to each
other and that the TBX18-ADSCs and GFP-ADSCs generated synchronized
beating with the syncytial NRVMs. This is consistent with previous
findings (21). We also found
that there was no significant difference between the two groups in
the myocardial-like differentiation rate. The results showed that
TBX18 does not affect the differentiation of ADSCs into
cardiomyocytes.
In the present study, HCN4 expression was detected
in the TBX18-ADSCs group by RT-qPCR and western blot analysis.
HCN1, HCN2 and HCN4 are the main subtypes of the HCN gene in the
heart (22). Sinoatrial node
cells contain the highest proportion of HCN4 which plays an
important role in phase 4 automatic depolarization of pacemaker
cells (23). Previous studies
have shown that ADSCs are capable of producing myocardial-like
cells after being co-cultured with NRVMs (3,24).
However, there was no expression of pacemaker-related markers. We
found that TBX18-ADSCs expressed HCN4. The voltage-clamp data
showed that TBX18-ADSCs generated an If-like current
after being co-cultured with NRVMs for 5–7 days, and CsCl
significantly decreased the If current. The occurrence
of an If current provides further evidence confirming
the presence of pacemaker-like cells derived from TBX18-ADSCs. A
previous study showed that ADSCs are capable of differentiating
into pacemaker cells in methylcellulose medium; however, the
differentiation rate reported was very low (5). The researchers found several
pacemaker cells with phase 4 automatic depolarization. In this
study, the underlying mechanism responsible for this
differentiation may involve upregulation of the HCN4 promoter by
TBX18 (9), which indirectly
increases the expression of HCN4 and enhances the efficiency of the
differentiation of ADSCs into pacemaker-like cells. In the present
study, phase 4 automatic depolarization was not observed in
TBX18-ADSCs. The first possible explanation for this negative
finding is that the patch clamp method was performed after blocking
electrical conduction. A recent study indicated that an
If current derived from stem cells flows into adjacent
cardiomiocytes to depolarize cardiomyocytes to the threshold for a
new action potential (25). When
electrical conduction is blocked, automatic depolarization cannot
occur. Secondly, it may due to the fact that automatic
depolarization not only requires the participation of If
current but also Ca2+ channels T-type
(ICa,T), Ca2+ channels L-type
(ICa,L) and Na+/Ca2+ exchanger
(INCX) (26). Another
reason may be the early stage of myocardial differentiation or the
immature cardiac fact; that TBX18-ADSCs show lower expression of If
current compared with pure hiPSCMs as demonstrated in a previous
study of inward rectified potassium channels (IK1)
(27).
In conclusion, this study successfully demonstrated
that ADSCs transfected with the human TBX18 gene induced
differentiation into pacemaker-like cells. TBX18 may enrich the
efficiency of pacemaker-like cell differentiation by promoting the
expression of HCN4. Taken together, these findings suggest that we
may construct the biological pacing by regulating the upstream
surface-related ion channel proteins.
References
|
1
|
Taha MF and Hedayati V: Isolation,
identification and multipotential differentiation of mouse adipose
tissue-derived stem cells. Tissue Cell. 42:211–216. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palpant NJ, Yasuda S, MacDougald O and
Metzger JM: Non-canonical Wnt signaling enhances differentiation of
Sca1+/c-kit+ adipose-derived murine stromal
vascular cells into spontaneously beating cardiac myocytes. J Mol
Cell Cardiol. 43:362–370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi YS, Dusting GJ, Stubbs S,
Arunothayaraj S, Han XL, Collas P, Morrison WA and Dilley RJ:
Differentiation of human adipose-derived stem cells into beating
cardiomyocytes. J Cell Mol Med. 14:878–889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai X, Yan Y, Song YH, Seidensticker M,
Rabinovich B, Metzele R, Bankson JA, Vykoukal D and Alt E: Both
cultured and freshly isolated adipose tissue-derived stem cells
enhance cardiac function after acute myocardial infarction. Eur
Heart J. 31:489–501. 2010. View Article : Google Scholar
|
|
5
|
Planat-Bénard V, Menard C, André M, Puceat
M, Perez A, Garcia-Verdugo JM, Pénicaud L and Casteilla L:
Spontaneous cardiomyocyte differentiation from adipose tissue
stroma cells. Circ Res. 94:223–229. 2004. View Article : Google Scholar
|
|
6
|
Wiese C, Grieskamp T, Airik R, Mommersteeg
MT, Gardiwal A, de Gier-de Vries C, Schuster-Gossler K, Moorman AF,
Kispert A and Christoffels VM: Formation of the sinus node head and
differentiation of sinus node myocardium are independently
regulated by Tbx18 and Tbx3. Circ Res. 104:388–397. 2009.
View Article : Google Scholar
|
|
7
|
Zhou B, Ma Q, Rajagopal S, Wu SM, Domian
I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, et
al: Epicardial progenitors contribute to the cardiomyocyte lineage
in the developing heart. Nature. 454:109–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Christoffels VM, Grieskamp T, Norden J,
Mommersteeg MT, Rudat C and Kispert A: Tbx18 and the fate of
epicardial progenitors. Nature. 458:E8–E10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kapoor N, Liang W, Marbán E and Cho HC:
Direct conversion of quiescent cardiomyocytes to pacemaker cells by
expression of Tbx18. Nat Biotechnol. 31:54–62. 2013. View Article : Google Scholar
|
|
10
|
Hu YF, Dawkins JF, Cho HC, Marbán E and
Cingolani E: Biological pacemaker created by minimally invasive
somatic reprogramming in pigs with complete heart block. Sci Transl
Med. 6:245ra942014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang J, Song T, Wu P, Chen Y, Fan X, Chen
H, Zhang J and Huang C: Differentiation potential of human
mesenchymal stem cells derived from adipose tissue and bone marrow
to sinus node-like cells. Mol Med Rep. 5:108–113. 2012.
|
|
12
|
Bai D, del Corsso C, Srinivas M and Spray
DC: Block of specific gap junction channel subtypes by
2-aminoethoxydiphenyl borate (2-APB). J Pharmacol Exp Ther.
319:1452–1458. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harks EG, Camiña JP, Peters PH, Ypey DL,
Scheenen WJ, van Zoelen EJ and Theuvenet AP: Besides affecting
intracellular calcium signaling, 2-APB reversibly blocks gap
junctional coupling in confluent monolayers, thereby allowing
measurement of single-cell membrane currents in undissociated
cells. FASEB J. 17:941–943. 2003.PubMed/NCBI
|
|
14
|
Ionta V, Liang W, Kim EH, Rafie R,
Giacomello A, Marbán E and Cho HC: SHOX2 overexpression favors
differentiation of embryonic stem cells into cardiac pacemaker
cells, improving biological pacing ability. Stem Cell Rep.
4:129–142. 2015. View Article : Google Scholar
|
|
15
|
Jung JJ, Husse B, Rimmbach C, Krebs S,
Stieber J, Steinhoff G, Dendorfer A, Franz WM and David R:
Programming and isolation of highly pure physiologically and
pharmacologically functional sinus-nodal bodies from pluripotent
stem cells. Stem Cell Rep. 2:592–605. 2014. View Article : Google Scholar
|
|
16
|
Bakker ML, Boink GJ, Boukens BJ, Verkerk
AO, van den Boogaard M, den Haan AD, Hoogaars WM, Buermans HP, de
Bakker JM, Seppen J, et al: T-box transcription factor TBX3
reprogrammes mature cardiac myocytes into pacemaker-like cells.
Cardiovasc Res. 94:439–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng Y, Luo S and Zhiyuan S: Canine bone
marrow mesenchymal stromal cells modified with Shox2 gene rebuild
biological pacemakers in vitro. Heart. 99:A42013.
|
|
18
|
Ball SG, Shuttleworth AC and Kielty CM:
Direct cell contact influences bone marrow mesenchymal stem cell
fate. Int J Biochem Cell Biol. 36:714–727. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukuhara S, Tomita S, Yamashiro S,
Morisaki T, Yutani C, Kitamura S and Nakatani T: Direct cell-cell
interaction of cardiomyocytes is key for bone marrow stromal cells
to go into cardiac lineage in vitro. J Thorac Cardiovasc Surg.
125:1470–1480. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schuleri KH, Boyle AJ and Hare JM:
Mesenchymal stem cells for cardiac regenerative therapy. Handbook
Exp Pharmacol. 180:195–218. 2007. View Article : Google Scholar
|
|
21
|
Zhu Y, Liu T, Song K, Ning R, Ma X and Cui
Z: ADSCs differentiated into cardiomyocytes in cardiac
microenvironment. Mol Cell Biochem. 324:117–129. 2009. View Article : Google Scholar
|
|
22
|
Stieber J, Hofmann F and Ludwig A:
Pacemaker channels and sinus node arrhythmia. Trends Cardiovasc
Med. 14:23–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi W, Wymore R, Yu H, Wu J, Wymore RT,
Pan Z, Robinson RB, Dixon JE, McKinnon D and Cohen IS: Distribution
and prevalence of hyperpolarization-activated cation channel (HCN)
mRNA expression in cardiac tissues. Circ Res. 85:e1–e6. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi YS, Matsuda K, Dusting GJ, Morrison
WA and Dilley RJ: Engineering cardiac tissue in vivo from human
adipose-derived stem cells. Biomaterials. 31:2236–2242. 2010.
View Article : Google Scholar
|
|
25
|
Chauveau S, Brink PR and Cohen IS: Stem
cell-based biological pacemakers from proof of principle to
therapy: a review. Cytotherapy. 16:873–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lakatta EG, Maltsev VA and Vinogradova TM:
A coupled SYSTEM of intracellular Ca2+ clocks and
surface membrane voltage clocks controls the timekeeping mechanism
of the heart's pacemaker. Circ Res. 106:659–673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Doss MX, Di Diego JM, Goodrow RJ, Wu Y,
Cordeiro JM, Nesterenko VV, Barajas-Martínez H, Hu D, Urrutia J,
Desai M, et al: Maximum diastolic potential of human induced
pluripotent stem cell-derived cardiomyocytes depends critically on
I(Kr). PLoS One. 7:e402882012. View Article : Google Scholar : PubMed/NCBI
|