Introduction
Many common types of cancer have a strong tendency
to metastasize to the bones. Bone cancer pain (BCP) can be induced
by tumor metastasis, and can occur at any time during the course of
the disease. This severe and unmanageable pain severely affects the
functional status and quality of life of patients (1,2).
Currently, the management of BCP is largely based on the classical
'analgesic ladder' that was promulgated by the World Health
Organization in 1986. However, this traditional analgesic strategy
is not satisfactory, and is also associated with significant
side-effects (3). Hence, in BCP
research, a major goal is to identify novel analgesic targets and
therapies.
Currently, the cellular and molecular mechanisms
underlying chronic pain have progressed from the idea of the
dominant 'neuronal-based' theory to the idea of neuron-astrocyte
interaction (4). Accumulating
evidence has indicated that neuroglial cells, particularly
astrocytes and microglial cells, further contribute to neurological
disorders, including chronic pain (5,6).
Certain studies have suggested that spinal astrocytic activation
depends on afferent neuronal inputs from the injured side and the
release of chemical mediators. Both of pro-inflammatory cytokines,
such as interleukin (IL)-1β, IL-6, IL-8 and tumor necrosis factor-α
(TNF-α) that are released from activated neuroglia may provoke the
cascading activation of adjacent neurons (7,8),
and then facilitate pain transmission through its coupling to
neuronal glutamate receptors (9).
This bidirectional neuron-neuroglial signaling plays a key role in
astrocytic activation, cytokine production and the initiation and
maintenance of allodynia and hyperalgesia (10). Recently, it has been demonstrated
that the differential activation of mitogen-activated protein
kinase (MAPK) family members occurs in spinal neuroglial cells
following nerve injury and inflammation, and contributes to the
generation and maintenance of chronic pain (11). Particularly, the spinal activation
of c-Jun N-terminal kinase (JNK) in astrocytes produces the initial
factor chemokine (C-C motif) ligand (CCL2), which is pivotal for
the maintenance of pain behavior (12).
Phosphodiesterase (PDE), which degrades the cyclic
AMP (cAMP) and cyclic GMP (cGMP) phosphodiester bond, has the
ability to terminate their action. There are at least 11 PDE
subtypes, PDEs 5, 6 and 9 only hydrolyze cGMP, and PDEs 4, 7 and 8
only function on cAMP, whereas PDEs 1, 2, 3, 10 and 11 hydrolyze
both cAMP and cGMP (13). It has
been demonstrated that PDE4 is mainly predominantly expressed in
nerve and immune cells, and the inhibition of PDE4 in the central
nervous system (CNS) has been shown to exert anti-inflammatory and
anti-nociceptive effects (14).
Previous studies have found that rolipram (ROL), one of the
classical PDE4 inhibitors, exerts analgesic effects in inflammatory
pain and neuropathic pain models (15–17), However, the analgesic mechanisms
of action of ROL have not yet been fully clarified. Hence, in this
study, we aimed to test the hypothesis that ROL attenuates pain
behavior through the inhibition of spinal astro-cytic activation
via the JNK/CCL2 pathway in rats with BCP.
Materials and methods
Animal preparation
A total of 208 adult female Sprague-Dawley (SD) rats
(8 weeks old, weighing 180–220 g) and 16 young female SD rats (3
weeks old, weighing 80–100 g) provided by the Experimental Animal
Center of Xi'an Jiaotong university (Xi'an, China) were utilized in
all the experiments. The adult SD rats were used to establish bone
cancer pain models, and young female SD rats were used to culture
tumor cells in abdominal cavity. All rats were housed in standard
transparent plastic cages with a 12/12 h light/dark cycle (light on
at 08:00 a.m.) under a 22–25°C ambient temperature with food and
water ad libitum. Prior to being used in the experiments,
animals were allowed to acclimatize to the housing environment for
at least 7 days.
All experimental procedures received prior approval
from the Animal Use and Care Committee for Research and Education
of the Xi'an Jiaotong University and the National Institute for
Physiological Sciences Animal Care and Use Committee. All efforts
were made to minimize animal suffering and to reduce the number of
animals used.
Cell line, doses and timings for drug
administration
The 16 SD rats (weighing, 80–100 g) received an
intraperitoneal (i.p.) inoculation of Walker 256 mammary gland
carcinoma cells (2×106 cells/ml, 1 ml; CCL-38; ATCC,
Manassas, VA, USA). After 1 week, the tumor cells were extracted
from the cancerous ascitic fluid of the rats, and resuspended in a
concentration of 1×107 cells/ml in phosphate-buffered
saline (PBS) for inoculation.
ROL (Sigma-Aldrich, St. Louis, MO, USA) was diluted
with 0.9% saline containing a small amount of dimethyl sulfoxide
(DMSO) prior to administrtion, and was injected in a volume of 10
μl at the doses of 12.5, 25, 50 and 100 μg/kg.
Previous studies have demonstrated the anti-inflammatory and
anti-nociceptive effects of intraperitoneally and orally
administered ROL in the CNS and in rodent models (14–17). However, in this study, ROL was
administered via the intrathecal (i.t.) route due to the following
reasons: first, PDE4, the target of ROL, is widely expressed in the
CNS, including spinal neurons and glia (14). One of the advantages is that by
i.t. administration, ROL can directly come in contact with its
spinal target, PDE4. Second, the systemic administration of ROL has
some side-effects, such as nausea and emesis; however, the i.t.
injection largely reduces the dose of ROL and decreases the
drug-related side-effects (18).
This direct interaction would minimize the possible influence
induced by systemic administration and the potential analgesic
effect mediated by super-spinal mechanisms (11). Finally, spinal neurotoxicity is
the main concern of the i.t. administration of ROL. Thus, we
designed the rotarod test to identify the possible influence on
motor function, and the results revealed that all rats were free
from motor disabilities with the i.t. administration of ROL at the
dose of 12.5–100 μg/kg (p>0.05; Fig. 1D).
Similarly, the specific JNK inhibitor, SP600125
(S5567; Sigma-Aldrich), was also diluted with 0.9% saline
containing a small amount of DMSO before application, and injected
with 5 μg in a volume of 10 μl (12,19,20). SP600125 was administered daily
from days 14 to 20 according to the study by Hu et al
(21). They found that the spinal
expression of p-JNK was time-dependently upregulated after tumor
cell inoculation, and the increased exoression of p-JNK was first
evident at day 7. Subsequently, it reached the peak at day 14, and
was maintained at a high phosphorylation level up to day 21. In
order to better illustrate the connection between the JNK/CCL2
pathway and ROL, the determination of the optimal injection time
point of SP600125 was performed at day 14. The same volume of ROL
and SP600125 was injected to rats in both the Sham and BCP
groups.
Yang et al demonstrated that mechanical
allodynia and thermal hyperalgesia produced by tumor cell
inoculation would reach the minimum on day 14; thus, the time
points examined were days 2, 4, 7, 10, 12 and 14 in their study
(5). For investigating the
short-term analgesic effects of ROL, we designed the time points
according to the study of Wu et al, which demonstrated that
an effective analgesic effect could be observed at the minimum 6 h
after single bolus; thus, we designed once/hour for detecting
mechanical allodynia and thermal hyperalgesia (22). The time points of once/day from
days 7 to 14 after tumor cell inoculation were designed for the
long-term observation with a consecutive administration protocol
according to Liu et al (23).
BCP model surgery
The inoculation was performed as previously
described (5). Briefly, the rats
were anaesthetized with chloral hydrate (300 mg/kg, i.p.), the
right rear hindlimb was shaved in order to expose the skin over the
femoral-tibial joint. The intercondylar eminence of the right tibia
was exposed after cleaning the skin 3 times with iodine tincture
and 75% ethanol. A 22 gauge needle was drilled into the site of
place described previously (5),
and a 20-μl microinjection syringe (Hamilton, Reno, NV, USA)
containing a 10 μl suspension of tumor cells, was used to
slowly inject the tumor cells into the tibial cavity. The drilled
hole was sealed with bone wax (Johnson & Johnson, Rochester,
NY, USA) in order to prevent the tumor cells from leaking outside
the bone. For the sham-operated group, 10 μl PBS was used to
replace the tumor cells injected into the tibia.
The rats were divided into the following groups with
4 rats per group: i) the sham-operated group administered the
vehicle (0.9% saline) (sham + vehicle); ii) the sham-operated group
administered ROL (sham + ROL); iii) the rats with BCP administered
the vehicle (BCP + vehicle); and iv) the rats with BCP administered
ROL (BCP + ROL). For the initital determination of the optimal dose
of ROL, and for the behavioral tests, we used 8 rats per group.
Intrathecal administration
Intrathecal administration was performed under
chloral hydrate (300 mg/kg, i.p.) anesthesia. Briefly, a midline
incision (3 cm) was cut on the backs of the rats at the level of
the thoracic vertebrae. A pre-measured length of PE-10 tubing (ID
0.28 mm and OD 0.61 mm) was passed caudally from the T8 to the L3
level of the spinal cord, fixed at the back of the rats' ears
through subcutaneous tunnel, and 2 cm of the free end were exposed
in the upper thoracic region. The rats were allowed to recover for
a period of 3–5 days before being used in further experiments. Only
animals that were judged to have no neurological deficits and that
presented with complete paralysis of the tail and bilateral hind
legs after the administration of 2% lidocaine (10 μl)
through the intrathecal catheter were used for the following
experiments. The administration of ROL and/or SP600125 was
administered intrathecally.
Behavioral test
The Von Frey test was applied to evaluate mechanical
allodynia. Briefly, the rats were placed on an elevated wire-mesh
floor in plexiglas chambers and the middle of the hindlimb paw was
completely exposed. A series of Von Frey filaments were used to
prod the plantar surface of the hindlimb paw with spaced increments
ranging from 1 to 26 g (1, 2, 4, 6, 8, 10, 15 and 26 g; Stoelting
Co., Wood Dale, IL, USA). The whole process of data recorded was
determined according to the method and formula provided in the
study by Dixon (24).
The Hargreaves test was applied to evaluate thermal
hyperalgesia. In brief, the rats were placed individually in
plexiglas chambers on an elevated glass floor. The plantar surface
of the hindlimb paw was thermally stimulated by the radiant heat
source. The withdrawal latencies were recorded according to
previous studies (25). The
threshold value of basal paw withdrawal latency (PWL) was
approximately 16 sec. To prevent tissue thermal burn, the cut-off
value was set at 20 sec. Finally, the blinding method was
implemented strictly for the whole behavioral test (25).
Motor coordination was determined using a standard
rat rotarod test (Shanghai Mobiledatum Information Technology Co.,
Ltd., Shanghai, China), as previously described (26). Daily training for each rat was
performed at 30 min following the i.p. administration of ROL or the
vehicle for 7 days by placing the rats on the rotating drums and
measuring the time from the start of the acceleration period until
the rat fell off the drum. A cut-off latency of 30 sec was used for
all rotarod assessments. The time that the animal remained on the
rotarod was recorded and expressed as a percentage of its own
baseline value.
Immunohistochemical staining
The rats were deeply anesthetised with an overdose
of chloral hydrate (10%, 0.3 ml/100 g) and transcardially perfused
with 200 ml of 0.01 M PBS (pH 7.4), followed by 500 ml of 4%
paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The L4–L6
spinal cord segments with the spinal dorsal horn (SDH) were
harvested and dehydrated in 30% sucrose at 4°C. Transverse spinal
sections (30-μm-thick) were then cut using a cryostat
(CM3050S; Leica, Wetzlar, Germany). For double immunofluorescence,
the sections were incubated with rabbit anti-c-Fos (1:500;
ab209794; Abcam, Cambridge, MA, USA), mouse anti-glial fibrillary
acidic protein (GFAP) (1:400; #3670; Cell Signaling Technology,
Danvers, MA, USA) and goat anti-IBA-1 (1:500; MABN92; Millipore,
Billerica, MA, USA) antibodies, followed by FITC-conjugated
secondary antibodies [Alexa Fluor 594 donkey anti-rabbit IgG
(A21207), Alexa Fluor 488 donkey anti-mouse IgG (A21202) and Alexa
Fluor 488 donkey anti-goat IgG (A11055); 1:500; all from
Invitrogen, Carlsbad, CA, USA] for 2 h at room temperature. The
stained sections were observed and captured under a confocal
laserscanning microscope (FV1000; Olympus, Tokyo, Japan).
Western blot analysis
The L4-L6 spinal cord segments were rapidly
extracted from the anesthetized rats. The tissues were homogenized
in lysis buffer (Bio-Rad Laboratories, Hercules, CA, USA) which
contains a mixture of protease inhibitors and phenylmethylsulfonyl
fluoride (Roche Diagnostics, Basel, Switzerland). Equivalent
amounts of protein (10 μl) were loaded and separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE; Bio-Rad Laboratories), and transferred onto PVDF
membranes (Millipore). The membranes were incubated with the
primary antibodies overnight at 4°C, followed by HRP-conjugated
secondary antibodies for 2 h (1:1,000; #7074 and #7076; Cell
Signaling Technology). The secondary antibody signal was detected
by enhanced chemiluminescence (Millipore), and captured using the
ChemiDoc XRS system (Bio-Rad Laboratories). The following primary
antibodies were used: rabbit anti-p-JNK (1:1,000; ab4821), rabbit
anti-JNK (1:1,000; ab85139), rabbit anti-PDE4B (1:1,500; ab14611)
(all from Abcam), mouse anti-GFAP (1:2,000; #3670; Cell Signaling
Technology), goat anti-IBA1 (1:1,000; MABN92; Millipore) and mouse
anti-β-actin (1:10,000; A1978; Sigma-Aldrich).
Enzyme-linked immunosorbent assay
(ELISA)
The spinal cord tissues were collected according to
the same method used for western blot analysis. The spinal cord
tissues were homogenized in a pre-made lysis buffer which contained
protease and phosphatase inhibitors. The protein samples of L4–5
spinal tissues were collected according to the same method used for
western blot analysis. The spinal cord tissues were homogenized in
a pre-made lysis buffer which contained protease and phosphatase
inhibitors. Rat IL-1β (RLB00), IL-6 (R6000B), TNF-α (RTA00) and
CCL2 (MJE00) ELISA kits were purchased from R&D Systems
(Minneapolis, MN, USA). For each reaction in a 96-well plate, 100
μg proteins from spinal tissue and standard were used. The
expression levels of IL-1β, IL-6, TNF-α and CCL2 were determined by
ELISA according to the manufacturer's instructions. According to
the standard curve from each set of standard samples assayed, the
concentration of all spinal samples was calculated.
Statistical analysis
All data were collected and analyzed by experienced
researchers using blinded methods. Two-way ANOVA with Bonferroni
post hoc tests were used for the nociceptive behavioral and rotarod
data. One-way ANOVA with least significant difference (LSD) for
post hoc analysis were used for immunohistochemistry ELISA and
western blot analysis. All data are presented as the means ±
standard error of the mean (SEM). All statistical analyses were
performed using GraphPad Prism version 5.01 for Windows (GraphPad
Software, San Diego, CA, USA; www.graphpad.com). A value of p<0.05 was considered
to indicate a statistically significant difference in all
statistical analyses.
Results
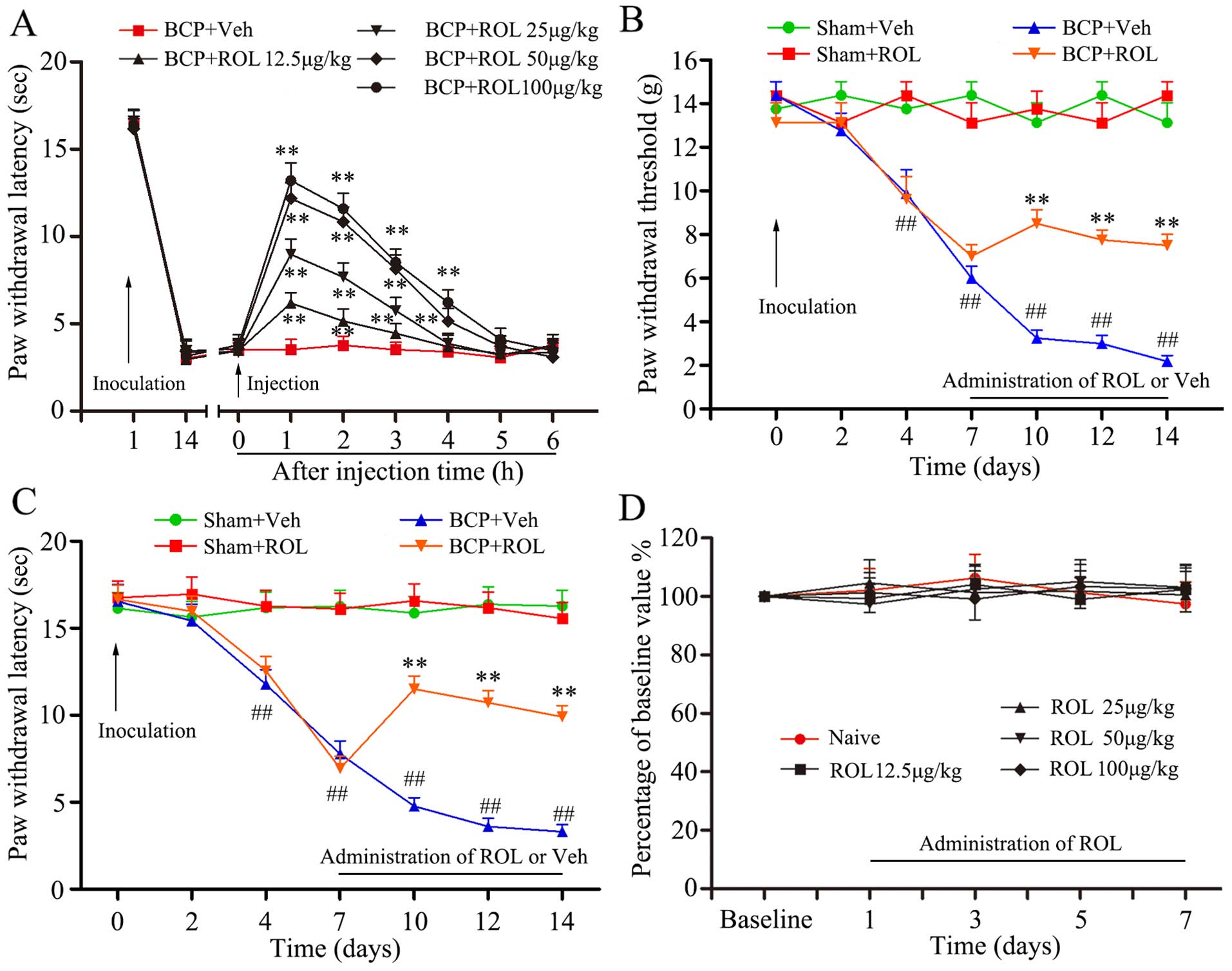
Intrathecal administration of ROL
attenuates mechanical allodynia and thermal hyperalgesia
The inoculation of tumor cells resulted in prominent
mechanical allodynia and thermal hyperalgesia, as demonstrated in
the BCP + vehicle group. In order to identify the potential
analgesic effects of ROL in BCP, different doses of ROL, were i.t.
injected on day 14 following tumor cell inoculation. Compared with
BCP + vehicle group, the i.t. administration of ROL significantly
elevated the PWL of the rats in a dose dependent manner
(p<0.01), and the dose of 100 μg/kg did not exert a more
prominent analgesic effect than the dose of 50 μg/kg
(Fig. 1A). Thus, we selected 50
μg/kg as the maximal dose for further experiments to
determine the continuous analgesic effects of ROL. Subsequently, at
dose of 50 μg/kg, ROL was administered daily by i.t.
injection from days 7 to 14 following tumor cell inoculation. The
results from the behavioral test revealed that there was no
apparent influence of the daily i.t. administration of ROL on paw
withdrawal thresholds (PWTs) and PWLs in the sham + ROL group,
indicating that the i.t. administration of ROL did not affect the
basal pain threshold. However, compared with the BCP + vehicle
group, the i.t. administration of ROL significantly elevated both
the PWTs and PWLs, as shown by the Von Frey test (p<0.01;
Fig. 1B) and Hargreaves test
(p<0.01; Fig. 1C),
respectively. From days 7 to 14 in the rats in the BCP + ROL group,
the i.t. administration of ROL attenuated mechanical allodynia and
thermal hyperalgesia. For an ideal analgesic agent, fewer
side-effects are always expected. Our results revealed that the
daily i.t. administration of ROL did not affect the motor
performance of the rats (ROL at the doses of 12.5, 25, 50 and 100
μg/kg) (p>0.05; Fig.
1D), thus confirming that ROL exerts anti-allodynic and
anti-hyperalgesic effects without any obvious measurable impairment
on motor functions.
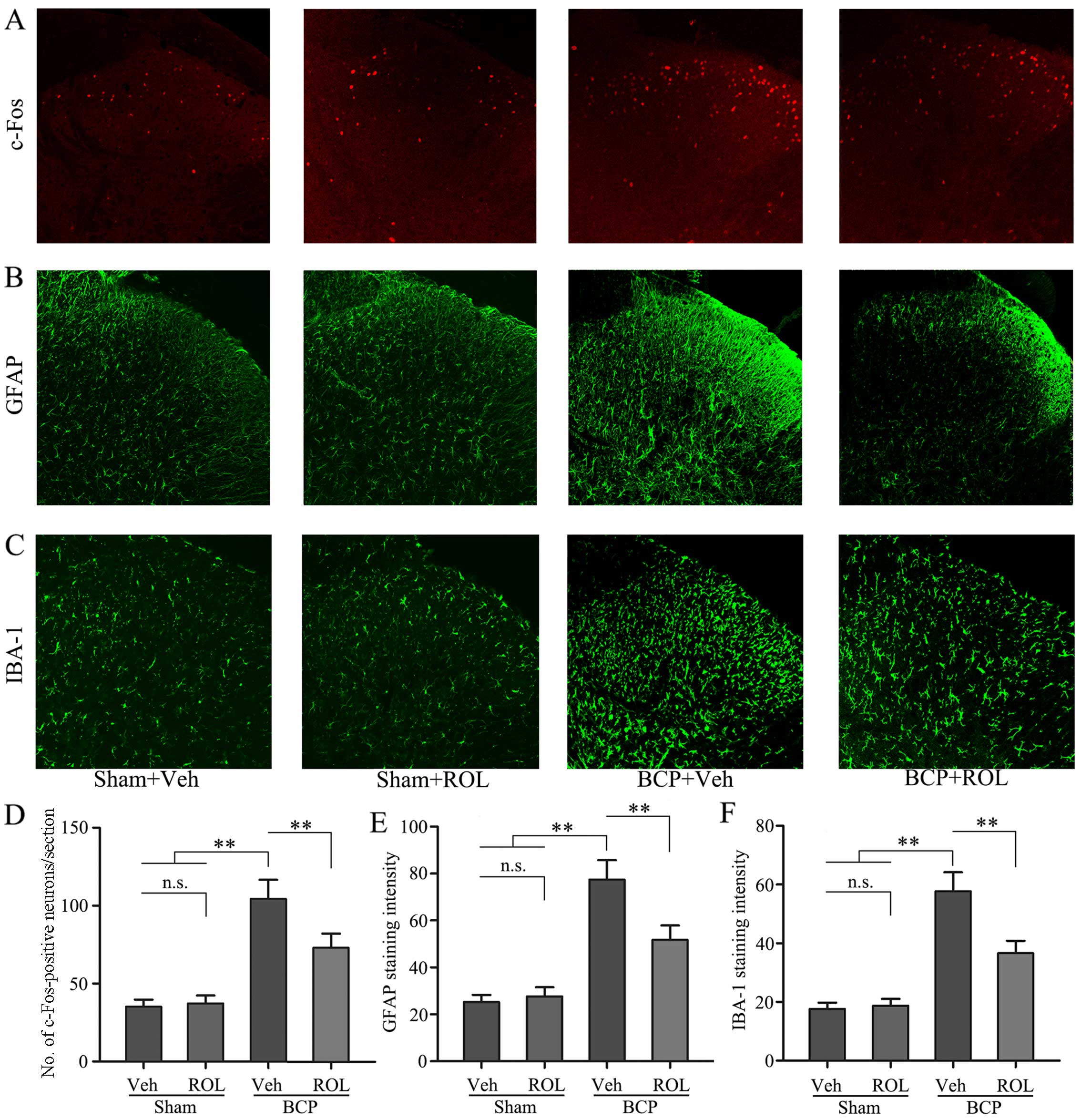
ROL inhibits PDE4B and neuron-astrocytic
activation
Immunohistochemical staining was used to illustrate
whether the analgesic effects of ROL at a dose of 50 μg/kg
are accompanied by spinal neuron-astrocytic activation in BCP.
Thus, we examined the expressoin of PDE4B, c-Fos, GFAP and IBA-1 on
day 14 in the different groups. The upregulation of PDE4B was
observed in the rats with BCP, and the i.t. administration of ROL
significantly decreased PDE4B expression in the BCP + ROL group
(p<0.01; Fig. 3A and C).
Compared with the sham-operated group, a significant increase in
neuron-astrocytic activation, as indicated by the upregulation of
c-Fos, GFAP and IBA-1 expression, predominantly in the superficial
layers of the SDH, was observed in the rats with BCP following
tumor cell inoculation. The consecutive daily i.t. administration
of ROL significantly decreased the levels of c-Fos, GFAP and IBA-1,
as indicated by immunohistochemistry (Fig. 2) and western blot analysis
(Fig. 3) in the BCP + ROL group
on day 14, compared with the BCP + vehicle group. There was no
apparent influence of the daily i.t. administration of ROL on
c-Fos, GFAP and IBA-1 expression in the sham + ROL group [all
p<0.01; c-Fos (Fig. 2A and D);
GFAP (Figs. 2B and E, and
3A and D); IBA-1 (Figs. 2C and F, and 3A and E)]. As expected, compared with
the BCP + vehicle group, ROL significantly inhibited
neuron-astrocytic activation on day 14 following tumor cell
implantation.
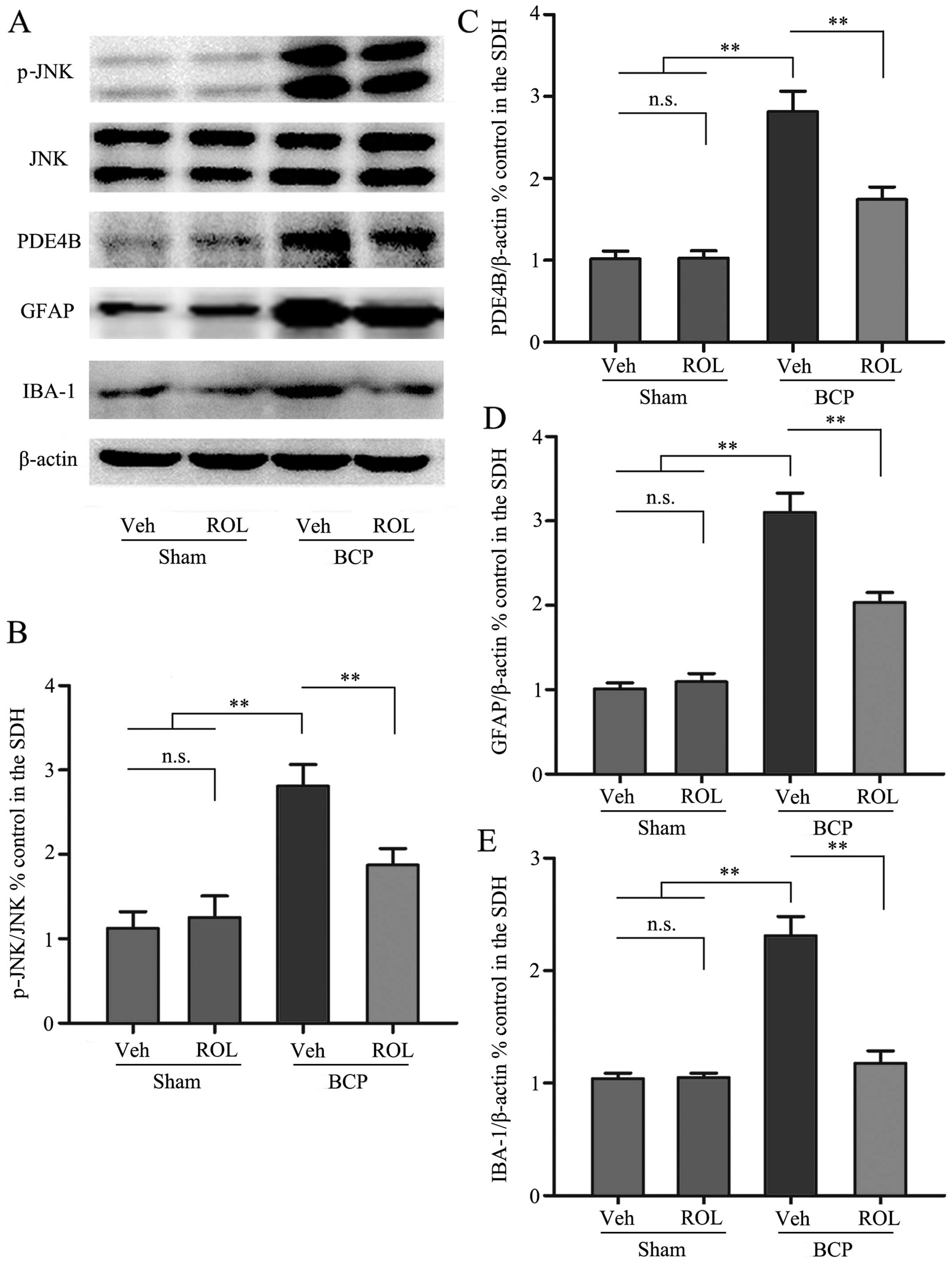 | Figure 3The spinal expression of p-c-Jun
N-terminal kinase (JNK), phosphodiesterase 4B (PDE4B), glial
fibrillary acidic protein (GFAP) and ionized calcium binding
adapter molecule-1 (IBA-1) was inhibited by the intrathecal
administration of rolipram (ROL). (A) Western blots showing the
protein levels of p-JNK, PDE4B, GFAP and IBA-1. (B-E)
Semi-quantitative assessment of the protein expression of p-JNK,
PDE4B, GFAP and IBA-1 follwing ROL administration. Tissues were
collected 14 days followig inoculation. **p<0.01;
n.s., no significance; BCP, bone cancer pain; Veh, vehicle; Sham,
sham-operated. Four rats were included in each group. |
p-JNK activation is decreased by the
administration of ROL
We further investigated whether the activation of
p-JNK could be detected following the administration of ROL at a
dose of 50 μg/kg. As shown by previous studies (12,27–29), almost all p-JNK-positive cells are
located on GFAP-positive astrocytes in many types of pain models.
In this study, western blot analysis was used to examine the effect
of the i.t. administration of ROL on p-JNK expression, and the
results revealed that the spinal expression of p-JNK was
significantly enhanced in the BCP + vehicle group compared with the
sham + vehicle group (p<0.01). However, ROL exerted a prominent
inhibitory effect on p-JNK expression as compared with the BCP +
vehicle group (p<0.01). However, ROL did not affect p-JNK
expression in the sham + ROL group compared with the sham + vehicle
group (Fig. 3A and B). These
results suggest that the i.t. administration of ROL effectively
inhibits the upregulation of p-JNK in rats with BCP.
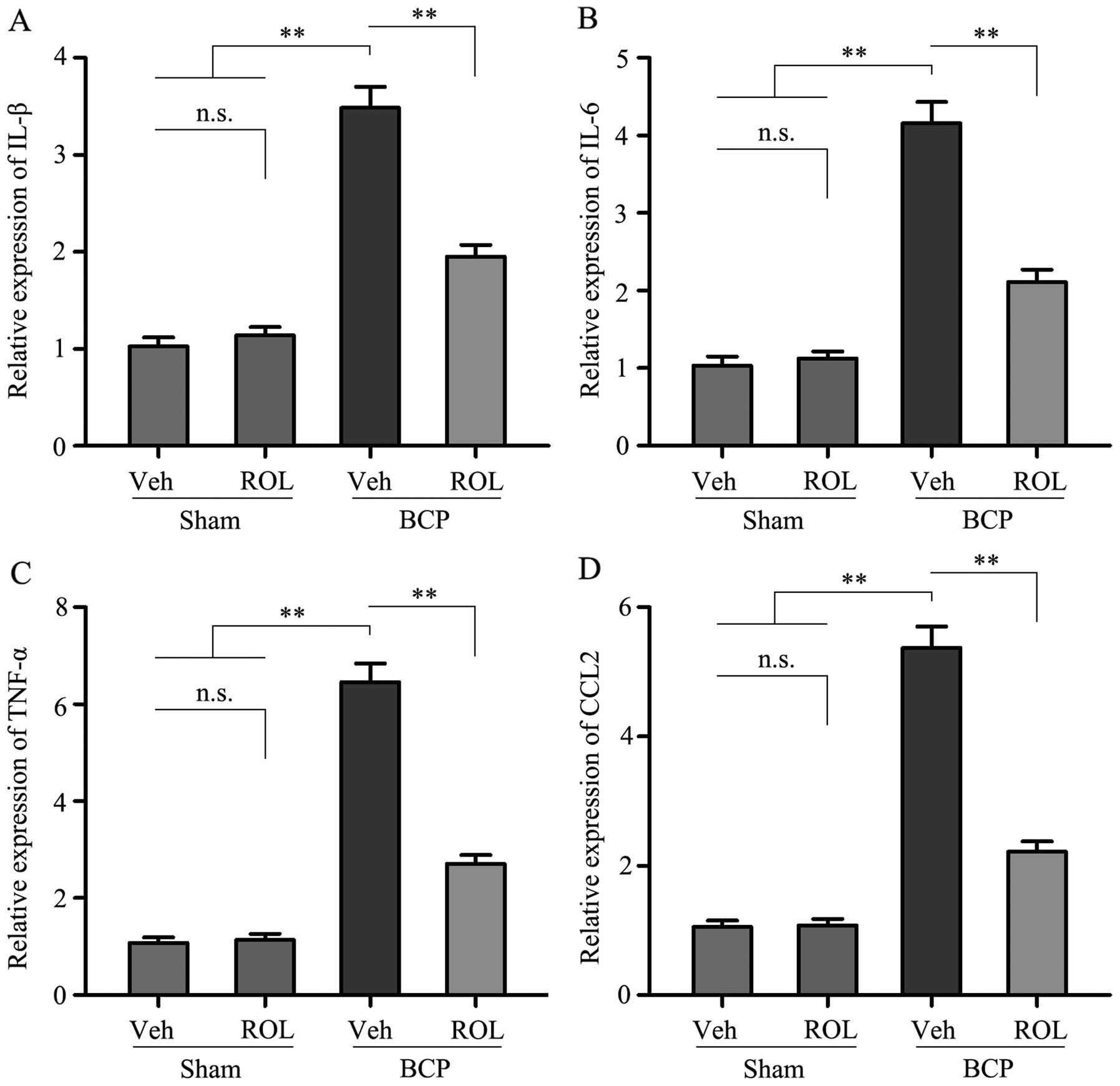
ROL attenuates the upregulation of
pro-inflammatory cytokines and CCL2 in rats with BCP
In order to investigate which possible intracellular
signal transduction pathway plays a role in the analgesic effects
of ROL at a dose of 50 μg/kg in rats with BCP, we detected
the levels of the pro-inflammatory cytokines, IL-1β, IL-6 and
TNF-α, as well as those of CCL2 in the rats in each group. Compared
with sham + vehicle group, a significant increase in the levels of
IL-1β, IL-6, TNF-α and CCL2 was observed in the BCP + vehicle
group. However, following the i.t. administration of ROL for 7
consecutive days, the expression levels of these above-mentioned
factors were significantly inhibited in the BCP + ROL group,
compared with the BCP + vehicle group (p<0.01; Fig. 4). Furthermore, the i.t.
administration of ROL did not alter the expression levels of spinal
pro-inflammatory cytokines and CCL2 in the sham + ROL group
(Fig. 4). These results suggest
that ROL effectively inhibits the upregulation of pro-inflammatory
cytokines and CCL2 in rats with BCP.
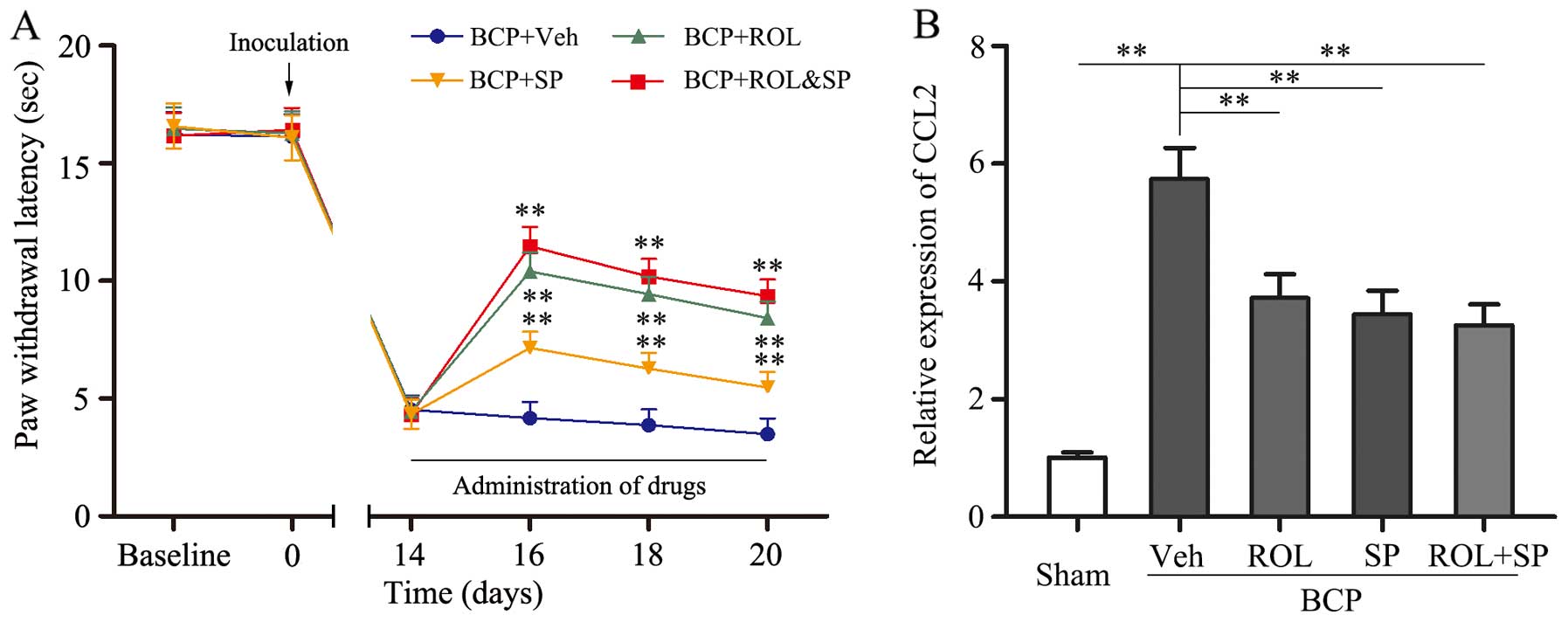
The analgesic effects of ROL are mediated
via the inhibition of the CCL2/JNK pathway in astrocytes in rats
with BCP
It was found that the inhibition of
neuron-astrocytic activation by ROL contributes to BCP; however, we
wished to determine which intracellular signal transduction pathway
contributes to these effects. SP600125, a specific JNK inhibitor,
was i.t. injected into the spinal cord of the rats. The consecutive
i.t. administration of SP600125 (5 μg) for 7 days
significantly attenuated thermal hyperalgesia compared with the BCP
+ vehicle group (p<0.01; Fig.
5). Following the co-administration of ROL (50 μg/kg)
and SP600125 (5 μg) to the rats with BCP, no significant
difference was observed in the analgesic effects compared to the
BCP + ROL group. Furthermore, similar results were observed with
CCL2 expression; the co-administration of ROL and SP600125 did not
exert any further inhibitory effects on CCL2 expression in the rats
with BCP than the administration of ROL (50 μg/kg) alone
(Fig. 5). Thus, the analgesic
effects of ROL appear to be dependent mainly on the inhibition of
the activation of the CCL2/JNK pathway.
Discussion
ROL, a well-characterized PDE4 inhibitor, is an
effective choice in the treatment of respiratory diseases, such as
chronic obstructive pulmonary disease (COPD) and asthma, and has
also been widely investigated in research field of spinal cord
injury, Alzheimer's disease, memory deficits and analgesia
(30–33). To the best of our knowledge, this
is the first study to demonstrate the beneficial analgesic effects
of ROL in a model of BCP. In this study, we demonstrated that
progressive nociceptive behavior could be induced by tumor cell
inoculation with significant neuron-astrocytic activation in the
SDH. ROL exerted a potent analgesic effect as a single injection or
as a continuous injection, and significantly attenuated nociceptive
behaviors. Additionally, the neuron-astrocytic activation and the
JNK pathway were markedly inhibited, and the release of
pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) was also
suppressed. We also found that the combined analgesic effects of
ROL and the JNK inhibitor, SP600125, on nociceptive behaviors did
not differ from those observed with ROL administration alone.
Finally, the expression of CCL2, which is known to be the
downstream chemokine of the JNK pathway, was significantly
decreased by treatment with ROL. Based on the above-mentioned
evidence, our results indicate that ROL, a potential specific
candidate for the management of pro-inflammatory factor production
and the development of BCP, can attenuate nociceptive behavior
wholly or mainly by inhibiting the activation of the JNK/CCL2
signaling pathway in rats with BCP.
The main function of PDE4 is degrading the cAMP
phosphodiester bond and terminating its functions (13,34). It has been demonstrated that PDE4
is most predominantly expressed in glial cells and immune cells in
the CNS, and inhibitors of PDE4 exhibit anti-depressant,
anti-asthmatic, anti-inflammatory properties (35). In particular, there is evidence to
indicate that PDE4B plays a crucial role in neuro-inflammation.
PDE4B has been shown to be upregulated with pain behavior following
L5 nerve ligation in a model of neuropathic pain, and the i.t.
administration of PDE4B siRNA was shown to attenuate pain behavior
with the inhibition of PDE4B (36). Furthermore, ROL has ability to act
upon the CNS and increase the level of the cAMP predominantly in
both nerve and immune tissues, and then activate protein kinase A
(PKA) (37). Activated PKA can
suppress the activation of nuclear factor-κB and the increased
activity of N-methyl-D-aspartate receptor, and can then decrease
the production of many pro-inflammatory cytokines, such as
inflammatory cytokines (TNF-α, IL-1 and IL-6), chemotaxis and
cytotoxicity. However, the increased expression of pro-inflammatory
cytokines (IL-1β, IL-6 and TNF-α) is closely associated with many
types of pain models (38), and
plays an important role in the progression and maintenance of pain.
In this study, an increase in the levels of IL-1β, IL-6 and TNF-α
was detected in rats with BCP with the activation of astrocytes and
microglia, and ROL attenuated hyperalgesia by inhibiting the levels
of IL-1β, IL-6 and TNF-α, and subsequently, astrocyte and
microglial activation was also suppressed. In addition, ROL had
been reported to exert analgesic effects in chemotherapy-induced
neuropathic pain in rats (17).
Based on the above-mentioned evidence, we proved that ROL inhibited
the increase in the levels of IL-1β, IL-6, TNF-α in rats with BCP,
and then decreased neuroglial activation, exerting an analgesic
effect. Therefore, ROL may be a potent candidate analgesic agent
for BCP.
It is known to all that spinal neuronal
sensitization may be dominant in the pain 'initiation phase', while
neuroglial cells, particularly astrocytes play a pivotal role in
the maintenance of BCP, acting as the main immune cells in the CNS
(23,39). Activated neuroglia produce
numerous mediators, such as pro-inflammatory cytokines and
chemokines which results in the cascading activation of surrounding
neuroglia that enhance neuronal activity; furthermore, these
activated pro-inflammatory cytokines and chemokines can maintain
the sustained activation of astrocytes (40). It has recently been found that
blocking the activation of spinal astrocytes with a glial metabolic
inhibitor impedes the exaggerated pain induced by peripheral tissue
inflammation, nerve injury and spinal cord impairment (41). In the present study, we observed
the alleviation of nociceptive behaviors following the
administration of ROL. In addition, we found that the expression of
GFAP and IBA-1 in the SDH was significantly reduced, and this
result indicated that astrocytic and microglial activation was
suppressed. According to these results, we suggest that the
analgesic effects of ROL may be possibly associated with the
suppression of neuroglial cell activation.
JNK, a key intracellular kinase of the MAPK pathway,
plays an important role in chronic pain and astrocytic activation
(42,43). The overexpression of p-JNK can be
detected in many types of pain models. In this study, following the
i.t. injection of SP600125, a JNK selective inhibitor, thermal
hyperalgesia was significantly attenuated. Additionally, another
JNK inhibitor, D-JNKI-1, has also shown potential analgesic effects
in spinal nerve ligation (SNL)-induced neuropathic pain (44). In this study, tumor cell
inoculation promoted the increase in p-JNK expression in the SDH,
and the increase in p-JNK expression was inhibited by the i.t.
injection of ROL with the alleviation of nociceptive behaviors.
Subsequently, the co-administration of ROL and SP600125 did not
exert more potent analgesic effects than the administration of ROL
alone. These results suggest that the analgesic effects of ROL may
be associated with the inhibition of spinal p-JNK expression. A
number of studies have confirmed that pro-inflammatory cytokines,
particularly IL-1β, IL-6 and TNF-α, induced by nerve injury and
inflammation, are essential initiators for the spinal activation of
p-JNK in astrocytes underlying the maintenance of many types of
chronic pain (45–47). Similarly, in our study, the levels
of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) in the SDH
were decreased by the i.t. injection of ROL, indicating that the
analgesic effects of ROL are associated with the inhibition of
spinal p-JNK activation via the suppression of the inflammatory
cascade reaction.
It has been proven that CCL2 is produced by
astrocytes via the JNK-mediated signaling pathway in neuropathic
pain and subsequently mediates this pain via CCR2 receptors in
neurons (12). The administration
of a JNK inhibitor (D-JNKI-1) has been shown to inhibit the
production of CCL2 in TNF-α or IL-1β-induced inflammatory
conditions in neuropathic pain (44). Additionally, in mice, the
overexpression of CCL2 is mainly found in astrocytes and enhances
nociceptive responses (48). In
this study, the analgesic effects of ROL were mediated partly by
the downregulation of CCL2. Tumor cell inoculation increased the
expression of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α)
and PDE4B expression, and was associated with the activation of the
JNK/CCL2 pathway. We hypothesize that ROL attenuated BCP through
the following mechanisms: the administration of ROL antagonized the
changes in astrocyte and microglial cell morphology and reduced
pro-inflammatory cytokine production. Subsequently, JNK activation
was inhibited accompanied by the decrease of astrocyte-secreted
CCL2, and finally, nociceptive responses were attenuated by the
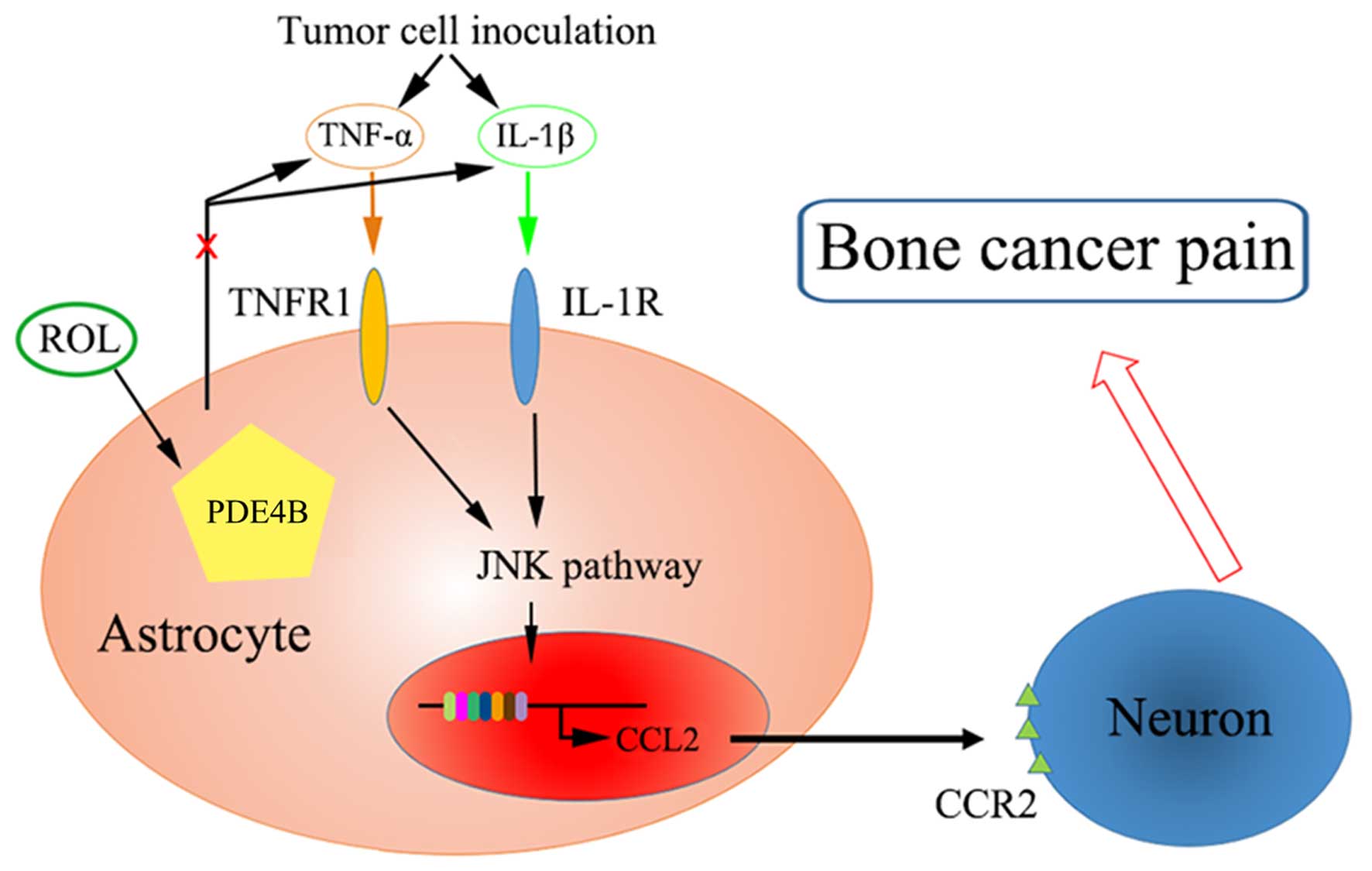
reduction of the sensitivity of neurons (Fig. 6).
The i.t. administration if ROL exerted potent
anti-inflammatory and anti-nociceptive effects in rats with BCP.
However, further investigations and clinical testing are required
to confirm our findings. ROL exerted long-acting analgesic effects.
Thus, this notable characteristic makes ROL good candidate for use
in the long-term treatment of chronic pain, particularly BCP.
Possibly, in the beginning and/or advanced stage of chronic pain
treatment, other short-onset analgesics, such as morphine, can be
used simultaneously with ROL. After a few days, ROL or PDE4
inhibitor could be used as the only treatment, thereby avoiding the
side-effects of long-term morphine treatment in clinical practice.
This may be a novel strategy for the treatmetn of chronic pain.
In conclusion, the present study suggested that the
i.t. administration of ROL attenuated nociceptive behaviors in BCP
rats through the inhibition of pro-inflammatory cytokines (IL-1β,
IL-6 and TNF-α). Furthermore, the suppression of the
astrocyte-related JNK/CCL2 pathway was involved in the analgesic
effects of ROL. Thus, we suggest that ROL may be a potential
specific candidate with which to regulate and attenuate the
development of BCP. However, there is still a great barrier between
animal reasearch and human application. Further experimental
studies on the effective safe dosage for human, pharmacokinetics
and specific activation pathways are warranted in order to
determine the effective use of ROL in the treatment and management
of chronic pain.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81371112 and 81171050)
and the Program for Shaanxi Province Key Research Team of Science
and Technology Innovation (no. 2012KCT-14).
Abbreviations:
|
BCP
|
bone cancer pain
|
|
ROL
|
rolipram
|
|
SDH
|
spinal dorsal horn
|
|
GFAP
|
glial fibrillary acidic protein
|
|
JNK/CCL2 pathway
|
c-Jun N-terminal kinase/chemokine (C-C
motif) ligand 2 pathway
|
|
IBA-1
|
ionized calcium binding adapter
molecule-1
|
References
|
1
|
Lozano-Ondoua AN, Symons-Liguori AM and
Vanderah TW: Cancer-induced bone pain: Mechanisms and models.
Neurosci Lett. 557:52–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bennett MI, Rayment C, Hjermstad M, Aass
N, Caraceni A and Kaasa S: Prevalence and aetiology of neuropathic
pain in cancer patients: A systematic review. Pain. 153:359–365.
2012. View Article : Google Scholar
|
|
3
|
Mantyh PW: Cancer pain and its impact on
diagnosis, survival and quality of life. Nat Rev Neurosci.
7:797–809. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ji RR, Berta T and Nedergaard M: Glia and
pain: Is chronic pain a gliopathy? Pain. 154(Suppl 1): S10–S28.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Y, Li H, Li TT, Luo H, Gu XY, Lü N,
Ji RR and Zhang YQ: Delayed activation of spinal microglia
contributes to the maintenance of bone cancer pain in female Wistar
rats via P2X7 receptor and IL-18. J Neurosci. 35:7950–7963. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Remeniuk B, Sukhtankar D, Okun A,
Navratilova E, Xie JY, King T and Porreca F: Behavioral and
neurochemical analysis of ongoing bone cancer pain in rats. Pain.
156:1864–1873. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oprée A and Kress M: Involvement of the
proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta,
and IL-6 but not IL-8 in the development of heat hyperalgesia:
Effects on heat-evoked calcitonin gene-related peptide release from
rat skin. J Neurosci. 20:6289–6293. 2000.PubMed/NCBI
|
|
8
|
Chichorro JG, Lorenzetti BB and Zampronio
AR: Involvement of bradykinin, cytokines, sympathetic amines and
prostaglandins in formalin-induced orofacial nociception in rats.
Br J Pharmacol. 141:1175–1184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou L, Huang J, Gao J, Zhang G and Jiang
J: NMDA and AMPA receptors in the anterior cingulate cortex
mediates visceral pain in visceral hypersensitivity rats. Cell
Immunol. 287:86–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo W, Miyoshi K, Dubner R, Gu M, Li M,
Liu J, Yang J, Zou S, Ren K, Noguchi K and Wei F: Spinal 5-HT3
receptors mediate descending facilitation and contribute to
behavioral hypersensitivity via a reciprocal neuron-glial signaling
cascade. Mol Pain. 10:352014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang XW, Hu S, Mao-Ying QL, Li Q, Yang CJ,
Zhang H, Mi WL, Wu GC and Wang YQ: Activation of c-jun N-terminal
kinase in spinal cord contributes to breast cancer induced bone
pain in rats. Mol Brain. 5:212012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang J, Zhu C, Li ZH, Liu XY, Sun SK,
Zhang T, Luo ZJ, Zhang H and Li WY: Inhibition of the spinal
astrocytic JNK/MCP-1 pathway activation correlates with the
analgesic effects of tanshinone IIA sulfonate in neuropathic pain.
J Neuroinflammation. 12:572015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Houslay MD and Adams DR: PDE4 cAMP
phosphodiesterases: Modular enzymes that orchestrate signalling
cross-talk, desensitization and compartmentalization. Biochem J.
370:1–18. 2003. View Article : Google Scholar
|
|
14
|
Pearse DD and Hughes ZA: PDE4B as a
microglia target to reduce neuroinflammation. Glia. 64:1698–1709.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar A, Jain NK and Kulkarni SK:
Analgesic and anti-inflammatory effects of phosphodiesterase
inhibitors. Indian J Exp Biol. 38:26–30. 2000.
|
|
16
|
Francischi JN, Yokoro CM, Poole S, Tafuri
WL, Cunha FQ and Teixeira MM: Anti-inflammatory and analgesic
effects of the phosphodiesterase 4 inhibitor rolipram in a rat
model of arthritis. Eur J Pharmacol. 399:243–249. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HK, Kwon JY, Yoo C and Abdi S: The
analgesic effect of rolipram, a phosphodiesterase 4 inhibitor, on
chemotherapy-induced neuropathic pain in rats. Anesth Analg.
121:822–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rock EM, Benzaquen J, Limebeer CL and
Parker LA: Potential of the rat model of conditioned gaping to
detect nausea produced by rolipram, a phosphodiesterase-4 (PDE4)
inhibitor. Pharmacol Biochem Behav. 91:537–541. 2009. View Article : Google Scholar
|
|
19
|
Sanna MD, Ghelardini C and Galeotti N:
Blockade of the spinal BDNF-activated JNK pathway prevents the
development of anti-retroviral-induced neuropathic pain.
Neuropharmacology. 105:543–552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Q, Wang J, Duan MT, Han SP, Zeng XY
and Wang JY: NF-κB, ERK, p38 MAPK and JNK contribute to the
initiation and/or maintenance of mechanical allodynia induced by
tumor necrosis factor-alpha in the red nucleus. Brain Res Bull.
99:132–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu XM, Liu YN, Zhang HL, Cao SB, Zhang T,
Chen LP and Shen W: CXCL12/CXCR4 chemokine signaling in spinal glia
induces pain hypersensitivity through MAPKs-mediated
neuroinflammation in bone cancer rats. J Neurochem. 132:452–463.
2015. View Article : Google Scholar
|
|
22
|
Wu HH, Yin JB, Zhang T, Cui YY, Dong YL,
Chen GZ and Wang W: Inhibiting spinal neuron-astrocytic activation
correlates with synergistic analgesia of dexmedetomidine and
ropivacaine. PLoS One. 9:e923742014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu S, Liu YP, Song WB and Song XJ:
EphrinB-EphB receptor signaling contributes to bone cancer pain via
Toll-like receptor and proinflammatory cytokines in rat spinal
cord. Pain. 154:2823–2835. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dixon WJ: Efficient analysis of
experimental observations. Annu Rev Pharmacol Toxicol. 20:441–462.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamm RJ, Pike BR, O'Dell DM, Lyeth BG and
Jenkins LW: The rotarod test: An evaluation of its effectiveness in
assessing motor deficits following traumatic brain injury. J
Neurotrauma. 11:187–196. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mei XP, Zhang H, Wang W, Wei YY, Zhai MZ,
Wang W, Xu LX and Li YQ: Inhibition of spinal astrocytic c-Jun
N-terminal kinase (JNK) activation correlates with the analgesic
effects of ketamine in neuropathic pain. J Neuroinflammation.
8:62011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao YJ and Ji RR: Activation of JNK
pathway in persistent pain. Neurosci Lett. 437:180–183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li ZY, Zhang YP, Zhang J, Zhang SB, Li D,
Huang ZZ and Xin WJ: The possible involvement of JNK activation in
the spinal dorsal horn in bortezomib-induced allodynia: the role of
TNF-α and IL-1β. J Anesth. 30:55–63. 2016. View Article : Google Scholar
|
|
30
|
Wang ZZ, Zhang Y, Liu YQ, Zhao N, Zhang
YZ, Yuan L, An L, Li J, Wang XY, Qin JJ, et al: RNA
interference-mediated phosphodiesterase 4D splice variants
knock-down in the prefrontal cortex produces antidepressant-like
and cognition-enhancing effects. Br J Pharmacol. 168:1001–1014.
2013. View Article : Google Scholar :
|
|
31
|
García-Osta A, Cuadrado-Tejedor M,
García-Barroso C, Oyarzábal J and Franco R: Phosphodiesterases as
therapeutic targets for Alzheimer's disease. ACS Chem Neurosci.
3:832–844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang ZZ, Yang WX, Zhang Y, Zhao N, Zhang
YZ, Liu YQ, Xu Y, Wilson SP, O'Donnell JM, Zhang HT and Li YF:
Phosphodiesterase-4D knock-down in the prefrontal cortex alleviates
chronic unpredictable stress-induced depressive-like behaviors and
memory deficits in mice. Sci Rep. 5:113322015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang Z and Mancini JA: Phosphodiesterase
4 inhibitors for the treatment of asthma and COPD. Curr Med Chem.
13:3253–3262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bolger GB, Dunlop AJ, Meng D, Day JP,
Klussmann E, Baillie GS, Adams DR and Houslay MD: Dimerization of
cAMP phosphodiesterase-4 (PDE4) in living cells requires interfaces
located in both the UCR1 and catalytic unit domains. Cell Signal.
27:756–769. 2015. View Article : Google Scholar :
|
|
35
|
Christiansen SH, Selige J, Dunkern T,
Rassov A and Leist M: Combined anti-inflammatory effects of
β2-adrenergic agonists and PDE4 inhibitors on astrocytes by
upregulation of intracellular cAMP. Neurochem Int. 59:837–846.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji Q, Di Y, He X, Liu Q, Liu J, Li W and
Zhang L: Intrathecal injection of phosphodiesterase 4B-specific
siRNA attenuates neuropathic pain in rats with L5 spinal nerve
ligation. Mol Med Rep. 13:1914–1922. 2016.
|
|
37
|
Nunes AR, Sample V, Xiang YK, Monteiro EC,
Gauda E and Zhang J: Effect of oxygen on phosphodiesterases (PDE) 3
and 4 isoforms and PKA activity in the superior cervical ganglia.
Adv Exp Med Biol. 758:287–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Perez-Aso M, Montesinos MC, Mediero A,
Wilder T, Schafer PH and Cronstein B: Apremilast, a novel
phosphodiesterase 4 (PDE4) inhibitor, regulates inflammation
through multiple cAMP downstream effectors. Arthritis Res Ther.
17:2492015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Old EA, Clark AK and Malcangio M: The role
of glia in the spinal cord in neuropathic and inflammatory pain.
Handb Exp Pharmacol. 227:145–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alfonso Romero-Sandoval E and Sweitzer S:
Nonneuronal central mechanisms of pain: Glia and immune response.
Prog Mol Biol Transl Sci. 131:325–358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Romero-Alejo E, Puig MM and Romero A:
Inhibition of astrocyte activation is involved in the prevention of
postoperative latent pain sensitization by ketamine and gabapentin
in mice. J Pharmacol Pharmacother. 7:22–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li W, Zhang Y, Xing C and Zhang M:
Tanshinone IIA represses inflammatory response and reduces
radiculopathic pain by inhibiting IRAK-1 and NF-κB/p38/JNK
signaling. Int Immunopharmacol. 28:382–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gao YJ, Zhang L, Samad OA, Suter MR,
Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q and Ji RR: JNK-induced
MCP-1 production in spinal cord astrocytes contributes to central
sensitization and neuropathic pain. J Neurosci. 29:4096–4108. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu Y, Jiang BC, Cao DL, Zhang ZJ, Zhang X,
Ji RR and Gao YJ: TRAF6 upregulation in spinal astrocytes maintains
neuropathic pain by integrating TNF-α and IL-1β signaling. Pain.
155:2618–2629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu C, Liu Y, Sun B, Sun Y, Hou B, Zhang Y,
Ma Z and Gu X: Intrathecal injection of JWH-015 attenuates bone
cancer pain via time-dependent modification of pro-inflammatory
cytokines expression and astrocytes activity in spinal cord.
Inflammation. 38:1880–1890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pillarisetti S: Targeting interleukin-1β
for pain. CNS Neurol Disord Drug Targets. 10:571–575. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Narita M, Shimamura M, Imai S, Kubota C,
Yajima Y, Takagi T, Shiokawa M, Inoue T, Suzuki M and Suzuki T:
Role of interleukin-1beta and tumor necrosis factor-alpha-dependent
expression of cyclooxygenase-2 mRNA in thermal hyperalgesia induced
by chronic inflammation in mice. Neuroscience. 152:477–486. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pevida M, González-Rodríguez S, Lastra A,
García-Suárez O, Hidalgo A, Menéndez L and Baamonde A: Involvement
of spinal chemokine CCL2 in the hyperalgesia evoked by bone cancer
in mice: A role for astroglia and microglia. Cell Mol Neurobiol.
34:143–156. 2014. View Article : Google Scholar
|