Introduction
Malignant glioma is the most common primary brain
tumor affecting adults and is graded according to the World Health
Organization (WHO) 2007 pathological classifications as follows:
grade I, pilocytic astrocytoma is generally benign; grade II,
low-grade astrocytoma (LGA); grade III, anaplastic astrocytoma (AA)
is prone to transformation into grade IV; and grade IV,
glioblastoma (GBM) (1). Malignant
astrocytoma (MA) mainly includes AA and GBM, of which the latter,
accounts for 50–80% of all MA cases. The median survival of
patients with GBM is approximately 12–15 months despite current
therapeutic strategies.
Uncontrolled cell apoptosis is one of the most
important mechanisms for tumor pathogenesis and thereof therapeutic
resistance (2–5). The caspase family is involved in the
initiation of early apoptosis, signal transduction and later stage
apoptotic effects. One of the key members is caspase-8, an
apoptosis initiation factor which is closely related to the
occurrence and development of cancer (6). In the extrinsic death
receptor-mediated type I apoptotic pathway, Fas ligand and tumor
necrosis factor bind to the death receptors, which subsequently
interact with the adaptor protein, Fas-associated protein with
death domain (FADD) and pro-caspase-8, forming the death-inducing
signaling complex (DISC). Caspase-8 activation in the DISC leads to
cleavage of pro-caspase-3 and caspase-3 activation (7,8).
In the intrinsic mitochondrial-associated type II apoptotic
pathway, pro-caspase-9 is activated by cytochrome c, and
Smac is released from the damaged mitochondria. Pro-caspase-9
activation leads to the activation of key apoptotic effectors,
including caspase-3. Pro-caspase-8 activation can also cleave Bid
to trigger mitochondrial cytochrome c release for caspase-3
activation (9). Thus, caspase-3
activation is identified as the key biomarker of the apoptotic
pathway in the irreversible stage (10).
In cancer cells, the aberrant methylation of CpG
islands located in the promoter regions of genes that are
implicated in cell apoptosis, tumor cell migration, or DNA repair
process is frequently associated with transcriptional silencing or
repression, particularly in the tumor suppressor genes (11–14). For example, the methylation of
P16INK4 is widespread in multiple myeloma (15). Sun et al (16) found that the methylation of the
caspase-8 gene increased tumor susceptibility in many types of
cancer, including lung, esophageal, gastric, colorectal and breast
cancer. In sporadic colorectal cancers, the methylation status of
O6-methylguanine-DNA-methyltransferase (MGMT) differs
among tumor tissue, para-carcinoma tissue and normal tissue,
implying that specific gene defects resulting from MGMT epigenetic
loss are associated with colorectal cancer development (17). The aberrant methylation of the
p33ING1b gene, and in parallel, significantly reduced or
absent mRNA levels of the gene have been detected in ovarian cancer
samples (18). Moreover,
alterations of the 'epigenome' may contribute to biological
behaviors and classifications of human cancer (14). For example, the methylation rates
of the death-associated protein kinase (DAPK), MGMT and
Ras-association domain family 1, isoform A (RASSF1A) genes have
been shown to be significantly higher in high-grade cervical
squamous cell carcinomas (19).
Aberrant DNA methylation is involved in clear cell renal carcinoma
invasion and metastasis (20),
and affects the survival time of patients with gastric cancer
(21).
Nevertheless, the link between the aberrant gene
expression of anti-apoptotsc effectors, i.e., the caspase family
members, and the pathogenesis of human malignant glioma remains
unclear. In this study, we assessed caspase-8 gene expression
levels and methylation status in human normal brain, glioma tissue
and cancer cell lines, and found that downregulated capase-8 levels
were significantly associated with the grade of malignant glioma.
Moreover, we verified an association between the capase-8
methylation-related gene downregulation and an enhanced
anti-apoptotic activity in selected human cancer cells. Our data
thus suggest that caspase-8 gene methylation may serve as an
indicator for the early diagnosis of human malignant glioma.
Combination therapy with demethylation reagents may overcome
resistance in the same malignancy.
Materials and methods
Human glioma tissue specimens and cancer
cell lines
From August, 2010 to December, 2012, 66 glioma
tissue samples were obtained from patients who underwent resection
of cerebral gliomas. Normal brain tissue samples were obtained from
5 patients with brain trauma. This study was approved by the
Institutional Research Board of Harbin Medical University and a
signed consent form was obtained from each patient prior to
obtaining the samples. Histopathological examination revealed that
of the 66 patients, 15 had pilocytic astrocytoma, 8 had
oligodendroglioma, 13 had anaplastic oligoastrocytoma and 30 had
glioblastoma. According to the WHO classification of tumors of the
central nervous system (1), our
cases were graded as follows: 23 cases belonged to the grade I–II
low-level group and 43 cases belonged to the grade III–IV
high-level group. Of the 66 cases, 35 were males and 31 were
females. The age of the patients ranged from 5 to 65 years (mean
age, 36.7±15.2 years). None of the cancer patients had received
radiotherapy or chemotherapy prior to surgery (Table I). Furthermore, another set of 10
human glioblastoma (WHO grade IV) patient tissue samples were
collected at the same hospital and were randomly selected for
further analysis. The same patient selection and diagnosis
criteria, as well as the informed consent form, were applied to
this set of specimens. All specimens confirmed by neuropathological
diagnosis were stored at −80°C after freezing in liquid
nitrogen.
 | Table IAssociation between the expression of
caspase-8 and clinicopathological characteristics in patients with
human glioma (n=66). |
Table I
Association between the expression of
caspase-8 and clinicopathological characteristics in patients with
human glioma (n=66).
| Characteristic | No. | Expression of
caspase-8
|
|---|
| Negative | Positive | P-value |
|---|
| Age | | | | |
| <40 | 30 | 25 | 5 | >0.05 |
| ≥40 | 36 | 29 | 7 | |
| Gender | | | | |
| Male | 35 | 27 | 8 | >0.05 |
| Female | 31 | 27 | 4 | |
| Tumor volume | | | | |
| <25
cm3 | 30 | 24 | 6 | >0.05 |
| ≥25
cm3 | 36 | 30 | 6 | |
| Tumor grade | | | | |
| I–II | 23 | 15 | 8 | <0.05 |
| III–IV | 43 | 39 | 4 | |
In the present study, we used various human cancer
cell lines (originally obtained from ATCC and maintained in our
laboratory) as follows: the U87MG, RG, T98, LN229, LN18 and U343
glioma cell lines; the HT29, LoVo, HCT116, SW620 and SW1116
colorectal cancer cell lines; the DU145 and PC3 prostate cancer
cell lines; the HeLa cervical caner cells; the A431 human
epidermoid carcinoma cells; and the K562 leukemia cells. The cells
were grown in Dulbecco's modified Eagle's medium (DMEM) containing
10% fetal bovine serum (FBS). All cell lines were cultured in an
atmosphere of 5% CO2 at 37°C.
Extraction of total RNA, cDNA synthesis
and reverse transcription-PCR
TRIzol reagent (Invitrogen, Carlsbad, USA) was used
to extract total RNA from the tissues and cell lines. SuperScript
II transcription enzyme, oligonucleotide(dT), and random primers
(high capactiy cDNA reverse transcription kit; Applied Biosystems)
were incubated with total RNA for reverse transcription. The entire
process was carried out in accordance with the manufacturer's
instructions. The synthesized cDNA was stored at −70°C. The primers
used to amplify the caspase-8 gene and the internal reference
genes, glyceraldehyde-phosphate dehydrogenase (GAPDH) in tissues
and β-actin in cell lines, are listed in Table II. The size of the amplified
fragments for caspase-8, GAPDH and β-actin were 427, 240 and 336
bp, respectively. The 50 µl PCR reaction included 1X buffer,
1.5 mM MgCl2, 10 pmol bidirectional primers, 0.2 mM
deoxynucleoside triphosphates (dNTPs), 1 unit TaqDNA polymerase,
cDNA and double-distilled water. A PCR reaction without a template
was used as the negative control. The PCR reaction conditions were
as follows: denaturation at 94°C for 5 min; denaturation at 94°C
for 30 sec, annealing at 60°C for 45 sec, extension at 72°C for 45
sec, 28 cycles; extension at 72°C for 10 min. PCR products were
separated on a 1.2% agarose gel (with ethidium bromide staining).
The resultant gel image was analyzed using the AlphaImager gel
analysis system. Each analysis was repeated 3 times, and the mean
was obtained in order to reduce error. The level of caspase-8 mRNA
expression in the glioma and normal brain tissues was determined by
semi-quantitative RT-PCR. The semi-quantitative value was expressed
as an integrated optical density ratio (irOD), where irOD =
(average caspase-8 electrophoresis optical density × area)/(average
GAPDH electrophoresis optical density × area). A ratio of >0.5
was considered as positive expression, and a ratio of ≤0.5 was
considered as negative expression.
 | Table IIPrimer sequences used for RT-PCR and
MSP. |
Table II
Primer sequences used for RT-PCR and
MSP.
| Primer sequences
(5′→3′) |
|---|
| Casp-8 | F:
TCTGGAGCATCTGCTGTCTG |
| Casp-8 | R:
CCTGCCTGGTGTCTGAAGTT |
| GAPDH | F: TGATGACATCAAGAA
GGTGGTGAAG |
| GAPDH | R: TCCTTGGAGGCCAT
GTAGGCCAT |
| β-actin | F:
ATTGGCAATGAGCGGTTCCGC |
| β-actin | R:
CTCCTGCTTGCTGATCCACATC |
| Casp-8
methylated | F:
TAGGGGATTCGGAGATTGCGA |
| Casp-8
methylated | R:
CGTATATCTACATTCGAAACGA |
| Casp-8
unmethylated | F:
TAGGGGATTTGGAGATTGTGA |
| Casp-8
unmethylated | R:
CCATATATCTACATTCAAAACAA |
Extraction of DNA
The Qiagen DNeasy kit (Qiagen Co. Ltd., Shanghai,
China) was used to extract DNA from the tissues and cell lines.
Extracted DNA was first subjected to agarose gel electrophoresis to
detect whether there was any degradation. Subsequently, the quality
and quantity of the extracted DNA was determined using an
ultraviolet (UV) spectrophotometer (BioPhotometer plus; Eppendorf
Co., Ltd., Hamburg, Germany). An OD value of 260/280 nm between 1.8
and 2.0 was used as the quality standard.
Bisulfite modification
As per the reported literature (22), bisulfite modification was
performed as follows. First, 2 µg of DNA were diluted with
25 ml distilled water and heated for 5 min in a water bath
maintained at 95°C. The denaturation DNA was then mixed with 2%
low-melting-point agarose gel. Second, agarose beads were prepared
using paraffin sealing film. The beads were placed into microtubes,
and 1 ml of fresh sulfite solution (3.0 M sodium bisulfite, 1 mM
hydroquinone, pH 5.0) was added to each microtube. The samples were
kept at 55°C in the dark in a water bath for 16 h. The samples were
then washed twice for 15 min each using 1 ml wash buffer [10 mM
Tris-HCl, pH 8.0, 10 mM ethylenediaminetetraacetate (EDTA)] and
then washed 3 times for 15 min each using 1 ml 10 N NaOH solution
to remove acid. The samples were then neutralized with 200
µl of 1 N HCl 3 times, for 15 min each time. Finally, the
samples were washed twice with the wash buffer. Agarose beads were
cut into 4–6 equal-sized sections to be used as the
methylation-specific PCR (MSP) template.
Methylation-specific PCR assay and PCR
product sequencing
The primers used to detect the methylation status
were synthesized according to the procedures outlined in the study
by Hopkins-Donaldson et al (11). The primer sequences for caspase-8
methylation and demethylation are listed in Table II. The sizes of the amplified
products were 320 and 322 bp. PCR reaction conditions were in
accordance with the methods described in the study by
Hopkins-Donaldson et al (11). Known methylated DNA was used as
the positive control; the negative control was the PCR reaction
without a template. PCR products were separated on a 1.5% agarose
gel (with ethidium bromide staining) and then photographed and
analyzed under UV light. In addition, purified PCR products were
ligated to the plasmid pGEMT at 4°C at an appropriate molecular
ratio. Plasmids with high purity and quality were selected and
delivered to a gene sequencing company (BGI Life Technologies Co.,
Ltd., Beijing, China).
Caspase-8 activity assays
Caspase-8 activity in the HT29 and SW620 cells was
assayed after the start of treatment with 5-Aza-2-deoxycytidine
(5adc, 0.5 µM) for 24/48 h and in LN18 cells for 18/24/48 h.
The cells were collected and analyzed using the Caspase-8
Colorimetric assay kit (R&D Systems, Minneapolis, MN, USA). The
cells were lysed to collect their intracellular contents. The cell
lysate was tested for protease activity by the addition of a
caspase-specific peptide that was conjugated to the color reporter
molecule p-nitroanaline (pNA). The cleavage of the peptide by the
caspase releases the chromophore pNA, which was quantified
spectrophotometrically at a wavelength of 405 nm using a UV
spectrophotometer (BioPhotometer plus; Eppendorf Co., Ltd.). The
level of caspase enzymatic activity in the cell lysate was directly
proportional to the color reaction. 5-Aza-2-deoxycytidine was
purchased from Sigma-Aldrich (Shangai, China).
Annexin V/propidium iodide (PI) staining
and flow cytometry
The apoptotic cells were assayed after the use of
camptothecin (CPT; 50 µM), or the combination of CPT (50
µM) and 5adc (0.5 µM). These agents were respectively
used with the HT29, LN18 and SW620 cells for 24 h. Apoptotic cells
were assessed by Annexin V staining using the Annexin V-FITC
apoptosis detection kit (BD Bioscience, Beijing, China) according
to the manufacturer's instructions and analyzed by flow cytometry
using CellQuest (FACSAria I; BD Biosciences, Franklin Lakes, NJ,
USA). In the Annexin V assay, the cells with staining only for
Annexin V-FITC (early apoptosis) were located in the lower right
quadrant, whereas the cells with staining for both Annexin V and PI
(late apoptosis) were located in the upper right quadrant. The
percentage of positive cells for PI staining only in the top left
quadrant represented the necrotic population. CPT was purchased
from Sigma-Aldrich.
Statistical analysis
The statistical package for social sciences (SPSS)
19.0 statistical analysis software was used. The χ2 test
was performed on the count data, and analysis of variance (ANOVA)
and the t-test were performed on the measured data. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
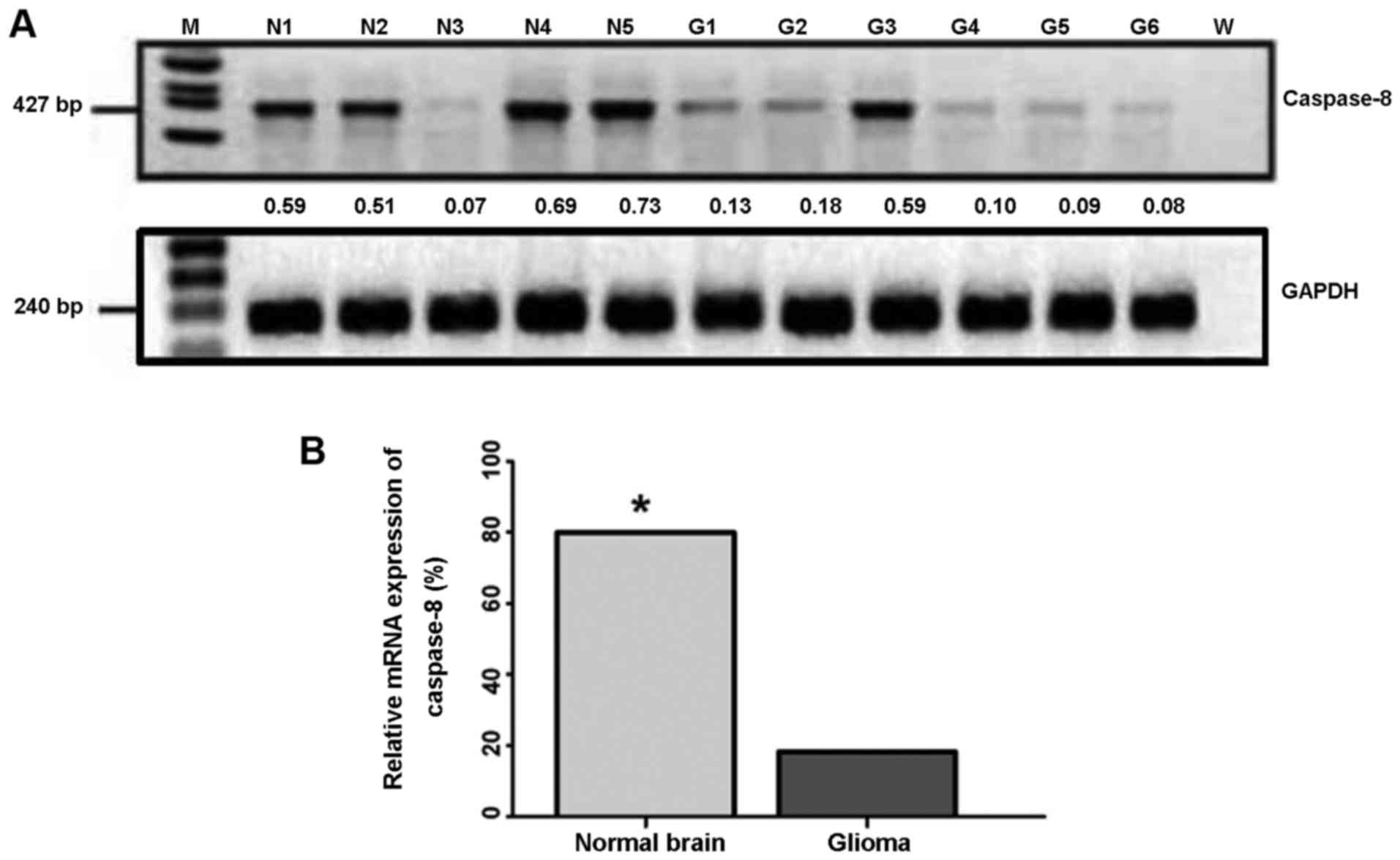
Downregulated mRNA expression of
caspase-8 correlates with the grade of human malignant glioma
We used RT-PCR to analyze the expression of
caspase-8 at the mRNA level in tissue of human gliomas and normal
brain specimens, and semi-quantified the expression levels using
the housekeeping gene, GAPDH, as the internal reference control.
The results revealed that 80.0% of the normal brain tissue samples
(4/5) had an intermediate to a high level of caspase-8 expression
(positive) (Fig. 1A), whereas
only 18.2% of the human glioma tissue samples (12/66) had a high
level of caspase-8 expression (positive) (Fig. 1A). The remaining 54 glioma tissue
samples [81.8% (54/66)] had very low to null levels of caspase-8
expression (negative) (Table I).
Therefore, caspase-8 expression at the mRNA level in the human
glioma tissues was significantly lower than that in the normal
brain tissues (P<0.01; Fig.
1B).
To determine whether the expression of caspase-8 is
involved in the development of human glioma, we examined the
association between the caspase-8 expression level and
clinicopathological indexes using the χ2 test.
Statistical analysis revealed that the expression level of
caspase-8 was closely related to the tumor grade (P<0.05;
Table I); however, no significant
correlation was observed with gender, age or tumor size
(P>0.05).
Caspase-8 gene silencing is a result of
its gene methylation in human malignant glioma tissue
In order to investigate the mechanisms underlying
the downregulation of the caspase-8 gene in human glioma tissue, we
used MSP to detect the methylation status of the caspase-8 gene at
the CpG island. The results indicated that, among the 66 samples,
52 samples exhibited caspase-8 gene methylation at the CpG islands,
as confirmed by repeated experiments. Among the 52 samples, 9 of
them expressed medium to high levels of caspase-8 at the mRNA
level, whereas 43 samples expressed very low to null levels of
caspase-8; yet, 25 of the 43 samples exhibited a status of high
gene methylation at the CpG islands (Fig. 2A and B). Furthermore, statistical
analysis revealed that caspase-8 methylation was closely related to
its expression at the mRNA level in human glioma tissue (P<0.01;
Fig. 2B).
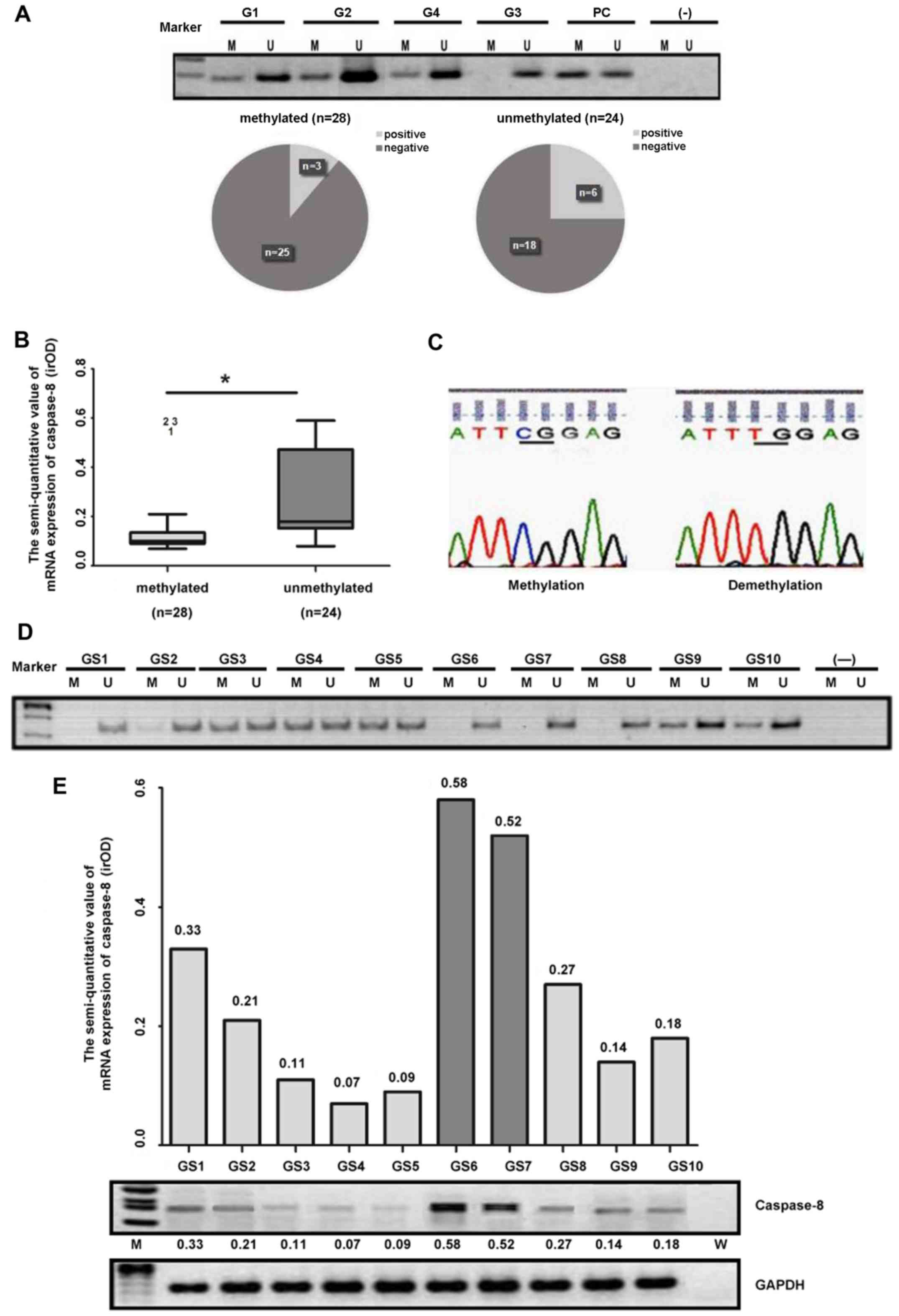 | Figure 2(A) Methylation status of caspase-8 in
human glioma. Marker, DNA marker; M, methylated; U, unmethylated;
G, glioma; PC, positive control; −, negative control. (B)
Semi-quantitative value of mRNA expression of caspase-8 (irOD) for
methylation and demethylation of caspase-8 in glioma (t-test).
*P<0.01. (C) DNA sequence of caspase-8 methylation
and demethylation (methylated cytosine did not change after
bisulfite treatment due to the protection of the methylated group).
(D) DNA methylation status. (E) Caspase-8 expression at the mRNA
level in malignant glioma tissue samples from patients. GS,
glioblastoma; M, methylated; U, unmethylated; -, negative control;
M, marker; W, negative control. |
Additionally, PCR product sequencing with the MSP
assay confirmed that the CpG islands of the caspase-8 gene in the
methylation-positive samples were indeed methylated (Fig. 2C). Moreover, among a separate set
of WHO grade IV GBM patient samples that were further examined for
DNA methylation (Fig. 2D) and the
expression status of caspase-8 at the mRNA level (Fig. 2E), 6 out of a total of 10 GBM
samples had gene methylation at the CpG islands (Fig. 2D). In addition, the mRNA
expression levels of caspase-8 significantly correlated with the
gene methylation status (Fig. 2D and
E).
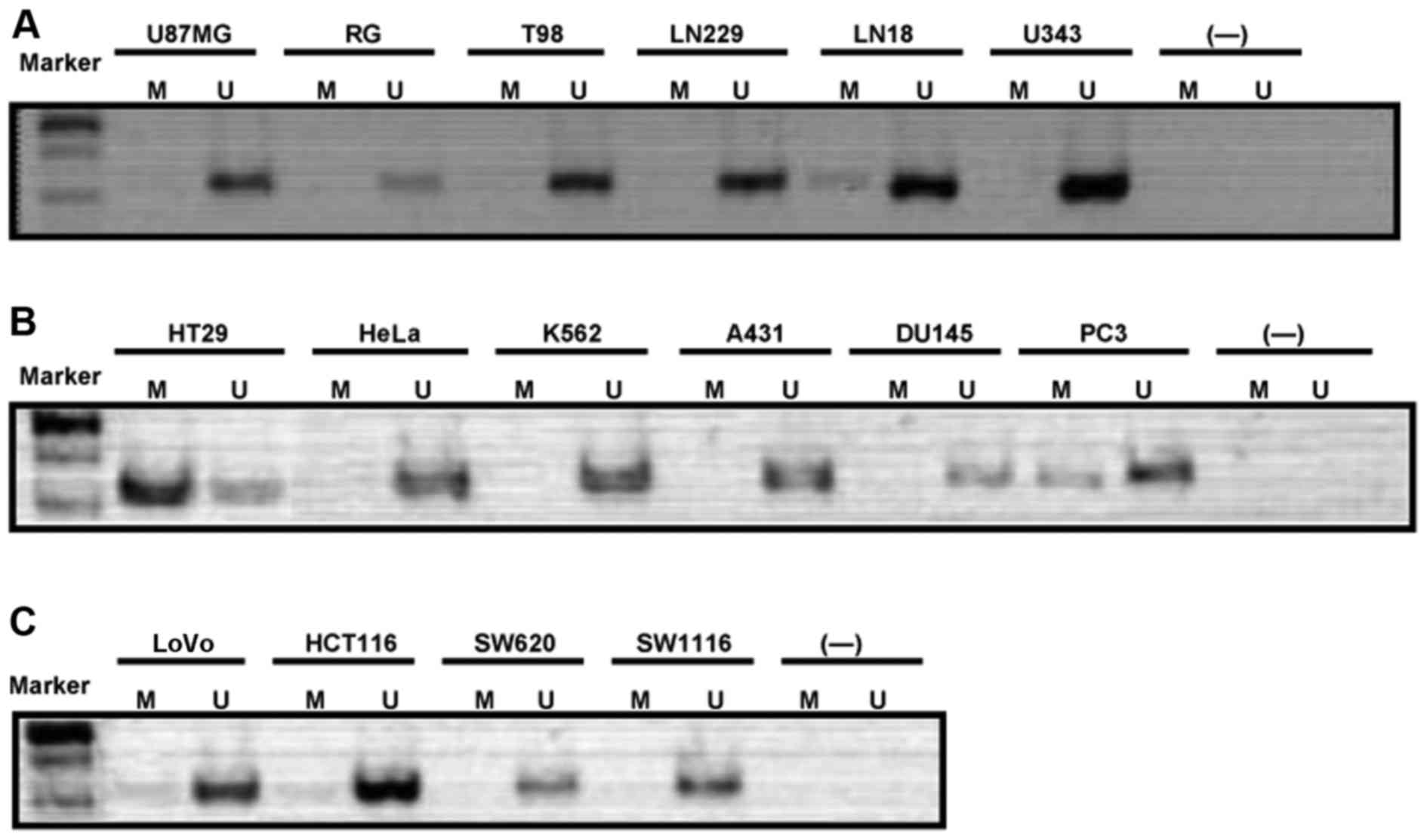
Methylation of caspase-8 gene promoter is
rare in human cancer cell lines
To verify the caspase-8 gene methylation status in
human cancer cells, we first detected the status in 6 glioma cell
lines and found that only the LN18 cells had relatively low
caspase-8 gene methylation (Fig.
3A). We then examined 6 cell lines of other cancer types,
including HT29 colorectal cancer cells, HeLa cervical cancer cells,
K562 myelogenous leukemia cells, A431 epidermoid carcinoma cells,
and DU145 and PC3 prostate cancer cell lines. The results revealed
that, among these 6 cancer cell lines, only the HT29 colorectal
cancer cells exhibited high caspase-8 gene methylation at the CpG
islands, whereas the PC3 prostate cancer cells appeared to have a
relatively low methylation status. The remaining cell lines were
not found to have a clear methylation (Fig. 3B). To further verify the caspase-8
gene methylation status, we examined 4 other colorectal cancer cell
lines, including LoVo, HCT116, SW620 and SW1116 cells. The data
indicated that these colorectal cancer cells hardly exhibited
caspase-8 gene methylation at the CpG islands (Fig. 3C).
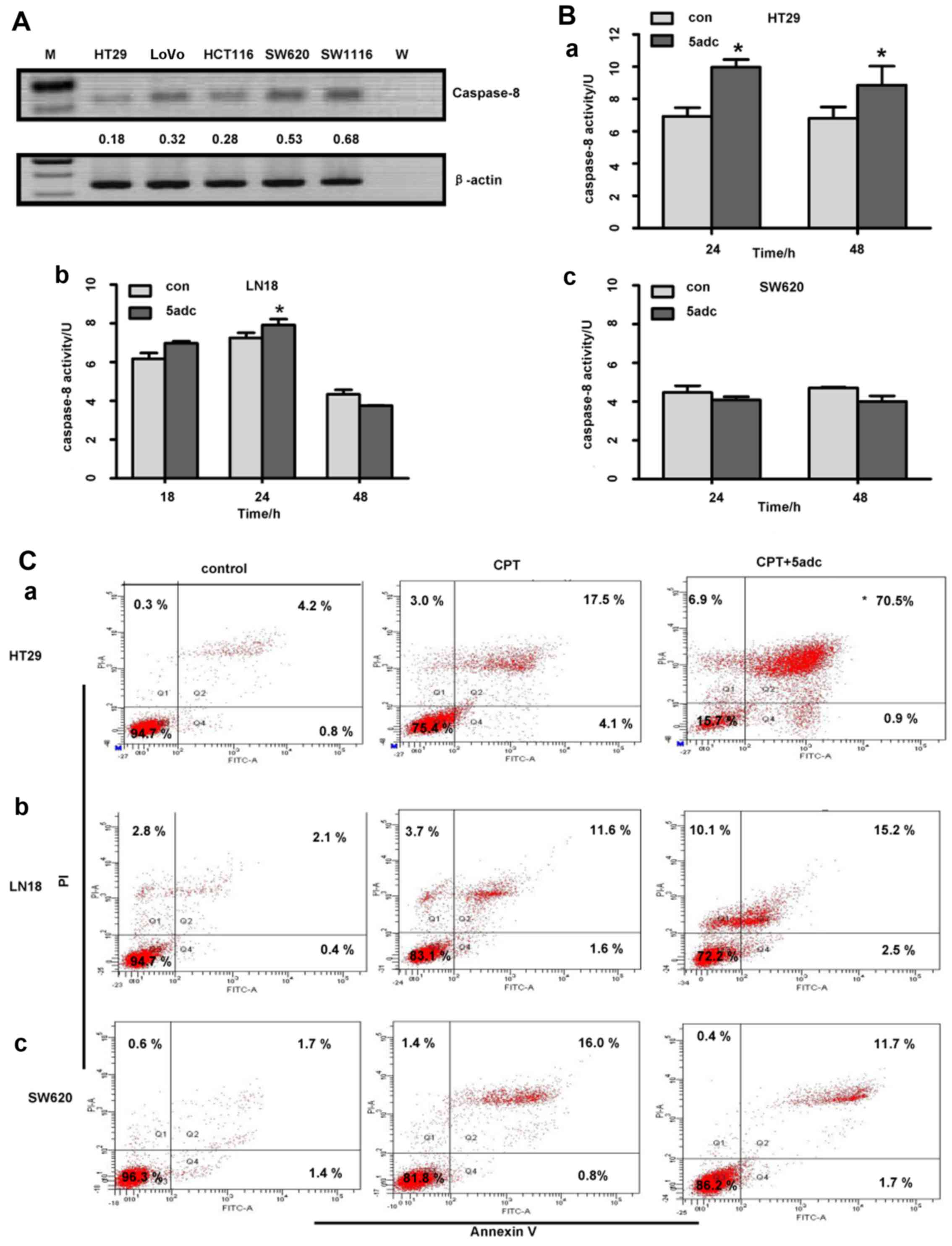
Anti-apoptotic activity and expression at
the mRNA level are highly relevant to caspase-8 gene methylation in
human cancer cells
We then used RT-PCR to analyze the expression of
caspase-8 at the mRNA level in 5 colorectal cancer cell lines and
to further semi-quantify the gene expression levels using the
housekeeping gene, β-actin, as the internal reference control. The
results revealed that the HT29 cells, in which the caspase-8 gene
was highly methylated, exhibited a lower level of caspase-8
expression (Fig. 4A); the other
colorectal cancer cell lines exhibited a higher level of caspase-8
expression, but relatively low gene methylation, as compared to the
HT29 cells (Figs. 3B and C and
4A).
We further verified whether caspase-8 protein kinase
activity was highly relevant to its gene methylation status by
treating the tumor cells with 5adc, a demethylation reagent. The
data indicated that treatment with 5adc restored caspase-8 protein
kinase activity in the HT29 and LN18 cells, but not in the SW620
cells. In the HT29 cells, the expression of caspase-8 was
upregulated at both 24 and 48 h (P<0.05; Fig. 4B, panel a); in the LN18 cell, the
upregulation occurred only at 24 h (P<0.05; Fig. 4B, panel b); however, in the SW620
cells, caspase-8 activity was not restored (Fig. 4B, panel c) following treatment
with 5adc.
To confirm the function of the upregulated caspase-8
protein activity by treatment with a demethylation agent, we
assayed the apoptotic cells in the HT29, LN18 and SW620 cell lines
following treatment with CPT (50 µM) alone, or in
combination with 5adc (0.5 µM) for 24 h. The apoptosis assay
carried out by flow cytometry revealed that the HT29 cell apoptotic
rate in the combination group was 79.3±8.07%, which was
significantly higher than that in the group treated with CPT alone
(18.67±3.52%; P<0.05; Fig. 4C,
panel a). These values (79.3±8.07% and 18.67±3.52%) are presented
as mean values of at least 3 experiments. In Fig. 4C, the values are presented as the
percentage of one experiment. However, no difference was observed
as regards the apoptotic rate of the LN18 and SW620 cells between
CPT treatment alone and the combination therapy (P>0.05;
Fig. 4C, panels b and c).
Discussion
It has been demonstrated that caspase-8 is
frequently lost or silenced in human GBM (13) and a variety of human cancers
(23–25). In this study, we demonstrated that
the majority of our tested brain glioma samples exhibited a
decreased or null caspase-8 mRNA expression; the tumor samples
exhibited a significantly decreased caspase-8 expression as
compared to the normal brain tissue samples. Furthermore, we
demonstrated that caspase-8 expression at the mRNA level was
significantly downregulated in 39 out of 43 cases of high grade
glioma (WHO III–IV). In fact, a significantly decreased expression
of caspase-8 was closely associated with a higher tumor grade in
human glioma tissues, as previously demonstrated (26). Our data suggest that the
downregulated caspase-8 expression may be involved in human glioma
development and progression.
The mechanisms underlying the loss of gene
expression, include the loss of heterozygosity (LOH), point
mutation and CpG island methylation. Of these, CpG island
methylation plays a key role in the regulation of gene expression
in a variety of disease-related factors, including apoptotic
effectors (27). Previous human
glioma studies have indicated that gene mutation in caspase-8
occurs infrequently (28); no LOH
in glioma has been reported thus far. In our results, caspase-8
gene promoter methylation analysis in glioma tissue specimens
revealed that within the 43 cases of caspase-8 mRNA negative
expression, 58.1% had a high methylation. Our data are in
accordance with those of other related studies, which indicated
that CpG island abnormal methylation contributed to the
downregulation on the transcriptional activity of the caspase-8
gene (13,23–25). Thus far, however, the mechanisms
that are involved in the development and progression of human
glioma are not fully understood and appear highly complex. There
may be other factors that contribute to caspase-8 downregulation or
gene damage, leading to an anti-apoptotic effect in tumors, e.g.,
LOH (29,30).
To our surprise, whereas we tried to elucidate the
mechanisms of caspase-8 gene methylation and downregulated
expression in vitro with malignant glioma cells, we did not
observe the same phenomena as those in human glima tissue. Only 1
of the 6 tested malignant glioma cells, the LN18 cells, had a minor
methylation at the caspase-8 gene promoter. Such a lack of
caspase-8 gene methylation was further observed in 10 other
immortal cell lines of other cancer types, including 5 colorectal
cancer cell lines and 5 cell lines of other cancer types, among
which we only found that the HT29 colorectal cancer cells had a
high level of caspase-8 gene methylation. These data indicate that
there may be a significant difference in anti-apoptotic properties
between immortal tumor cell lines in vitro and tumor cells
in vivo. For example, the tumor cell lines in vitro
may adapt different routes of anti-apoptotic features as the tumor
microenvironment in vivo may be an essential element for
tumor development and progression in vivo (31,32). Further investigations are
warranted to confirm whether our in vitro model used to
elucidate the mechanisms and aid in the development novel treatment
strategies for cancer patients was reliable.
Nevertheless, taking advantage of a varied caspase-8
methlytion status in the HT29 colorectal cancer cells, the LN18
malignant glioma cells and the SW620 colorectal cancer cells, in
which caspase-8 had a respectively high, low or null level of
methylation, we confirmed that a high level of caspase-8 gene
methylation was significantly associated with its gene
downregulation, reduced kinase activity and increased
anti-apoptotic properties in tumor cells. Therefore, we indirectly
verified that an increased caspase-8 gene methylation may be highly
relevant to the development and progression of human malignant
glioma in vivo. However, the limitations of this study may
be as follows: i) the number of glioma tissue samples was
relatively small; ii) we provide indirect evidence that a high
methylation status of caspase-8 is closely associated with
anti-apoptotic mechanisms in human glioma; iii) the occurrence and
development of human glioma is complex and involves multi-gene and
functional pathways. Thus, further studies are warranted to confirm
our findings and to further determine the mechanisms responsible
for glioma malignancy.
Acknowledgments
The present study was supported by grants from the
Natural Science Foundation of China (nos. 91229112, 30772238 and
81472367 to H.R. and 81402054 to Y.-C.D.), the Heilongjiang
Province and China Postdoctoral Projects (nos. LBH-Z14138,
2014M560272 and 2015T80371 to Y.-C.D.); the Natural Science
Foundation of Heilongjiang Province (no.s41400298-9-15057 to
Y.-C.D.).
References
|
1
|
Fuller GN and Scheithauer BW: Symposium:
The 2007 revised world health organization (WHO) Classification of
tumors of the central nervous system: newly codified entities.
Brain Pathol. 17:304–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Varela M, Ranuncolo SM, Morand A, Lastiri
J, De Kier Joffé EB, Puricelli LI and Pallotta MG: EGF-R and
PDGF-R, but not bcl-2, overexpression predict overall survival in
patients with low-grade astrocytomas. J Surg Oncol. 86:34–40. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kajiwara Y, Yamasaki F, Hama S, Yahara K,
Yoshioka H, Sugiyama K, Arita K and Kurisu K: Expression of
survivin in astrocytic tumors: Correlation with malignant grade and
prognosis. Cancer. 97:1077–1083. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sarkar C, Karak AK, Nath N, Sharma MC,
Mahapatra AK, Chattopadhyay P and Sinha S: Apoptosis and
proliferation: Correlation with p53 in astrocytic tumours. J
Neurooncol. 73:93–100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grenet J, Teitz T, Wei T, Valentine V and
Kidd VJ: Structure and chromosome localization of the human CASP8
gene. Gene. 226:225–232. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boldin MP, Goncharov TM, Goltsev YV and
Wallach D: Involvement of MACH, a novel MORT1/FADD-interacting
protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell.
85:803–815. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muzio M, Chinnaiyan AM, Kischkel FC,
O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M,
Gentz R, et al: FLICE, a novel FADD-homologous ICE/CED-3-like
protease, is recruited to the CD95 (Fas/APO-1) death--inducing
signaling complex. Cell. 85:817–827. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng Y, Lin Y and Wu X: TRAIL-induced
apoptosis requires Bax-dependent mitochondrial release of
Smac/DIABLO. Genes Dev. 16:33–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blanchard H, Donepudi M, Tschopp M,
Kodandapani L, Wu JC and Grütter MG: Caspase-8 specificity probed
at subsite S(4): Crystal structure of the caspase-8-Z-DEVD-cho
complex. J Mol Biol. 302:9–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hopkins-Donaldson S, Ziegler A, Kurtz S,
Bigosch C, Kandioler D, Ludwig C, Zangemeister-Wittke U and Stahel
R: Silencing of death receptor and caspase-8 expression in small
cell lung carcinoma cell lines and tumors by DNA methylation. Cell
Death Differ. 10:356–364. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McKee AE and Thiele CJ: Targeting caspase
8 to reduce the formation of metastases in neuroblastoma. Expert
Opin Ther Targets. 10:703–708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ashley DM, Riffkin CD, Muscat AM, Knight
MJ, Kaye AH, Novak U and Hawkins CJ: Caspase 8 is absent or low in
many ex vivo gliomas. Cancer. 104:1487–1496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jones PA: DNA methylation and cancer.
Oncogene. 21:5358–5360. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galm O, Wilop S, Reichelt J, Jost E,
Gehbauer G, Herman JG and Osieka R: DNA methylation changes in
multiple myeloma. Leukemia. 18:1687–1692. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun T, Gao Y, Tan W, Ma S, Shi Y, Yao J,
Guo Y, Yang M, Zhang X, Zhang Q, et al: A six-nucleotide
insertion-deletion polymorphism in the CASP8 promoter is associated
with susceptibility to multiple cancers. Nat Genet. 39:605–613.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen L, Kondo Y, Rosner GL, Xiao L,
Hernandez NS, Vilaythong J, Houlihan PS, Krouse RS, Prasad AR,
Einspahr JG, et al: MGMT promoter methylation and field defect in
sporadic colorectal cancer. J Natl Cancer Inst. 97:1330–1338. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen DH, Chan KY, Khoo US, Ngan HY, Xue
WC, Chiu PM, Ip P and Cheung AN: Epigenetic and genetic alterations
of p33ING1b in ovarian cancer. Carcinogenesis.
26:855–863. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JH, Choi YD, Lee JS, Lee JH, Nam JH
and Choi C: Assessment of DNA methylation for the detection of
cervical neoplasia in liquid-based cytology specimens. Gynecol
Oncol. 116:99–104. 2010. View Article : Google Scholar
|
|
20
|
Hildebrandt MA, Gu J, Lin J, Ye Y, Tan W,
Tamboli P, Wood CG and Wu X: Hsa-miR-9 methylation status is
associated with cancer development and metastatic recurrence in
patients with clear cell renal cell carcinoma. Oncogene.
29:5724–5728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park SY, Kook MC, Kim YW, Cho NY, Jung N,
Kwon HJ, Kim TY and Kang GH: CpG island hypermethylator phenotype
in gastric carcinoma and its clinicopathological features. Virchows
Arch. 457:415–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kitazawa S, Kitazawa R and Maeda S:
Identification of methylated cytosine from archival formalin-fixed
paraffin-embedded specimens. Lab Invest. 80:275–276. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hopkins-Donaldson S, Bodmer JL, Bourloud
KB, Brognara CB, Tschopp J and Gross N: Loss of caspase-8
expression in highly malignant human neuroblastoma cells correlates
with resistance to tumor necrosis factor-related apoptosis-inducing
ligand-induced apoptosis. Cancer Res. 60:4315–4319. 2000.PubMed/NCBI
|
|
24
|
Hopkins-Donaldson S, Bodmer JL, Bourloud
KB, Brognara CB, Tschopp J and Gross N: Loss of caspase-8
expression in neuroblastoma is related to malignancy and resistance
to TRAIL-induced apoptosis. Med Pediatr Oncol. 35:608–611. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Teitz T, Wei T, Valentine MB, Vanin EF,
Grenet J, Valentine VA, Behm FG, Look AT, Lahti JM and Kidd VJ:
Caspase 8 is deleted or silenced preferentially in childhood
neuroblastomas with amplification of MYCN. Nat Med. 6:529–535.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saggioro FP, Neder L, Stávale JN,
Paixão-Becker AN, Malheiros SM, Soares FA, Pittella JE, Matias CC,
Colli BO, Carlotti CG Jr, et al: Fas, FasL, and cleaved caspases 8
and 3 in glioblastomas: A tissue microarray-based study. Pathol Res
Pract. 210:267–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones PA and Baylin SB: The fundamental
role of epigenetic events in cancer. Nat Rev Genet. 3:415–428.
2002.PubMed/NCBI
|
|
28
|
Gonzalez-Gomez P, Bello MJ, Inda MM,
Alonso ME, Arjona D, Amiñoso C, Lopez-Marin I, de Campos JM, Sarasa
JL, Castresana JS, et al: Deletion and aberrant CpG island
methylation of caspase-8 gene in medulloblastoma. Oncol Rep.
12:663–666. 2004.PubMed/NCBI
|
|
29
|
Knudson AG Jr: Mutation and cancer:
Statistical study of retinoblastoma. Proc Natl Acad Sci USA.
68:820–823. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ebinger M, Senf L, Wachowski O and
Scheurlen W: Promoter methylation pattern of caspase-8,
P16INK4A, MGMT, TIMP-3, and E-cadherin in
medulloblastoma. Pathol Oncol Res. 10:17–21. 2004. View Article : Google Scholar
|
|
31
|
Adams DJ, Waud WR, Wani MC, Manikumar G,
Flowers JL, Driscoll TA and Morgan LR: BACPTDP: A water-soluble
camptothecin pro-drug with enhanced activity in hypoxic/acidic
tumors. Cancer Chemother Pharmacol. 67:855–865. 2011. View Article : Google Scholar
|
|
32
|
Xia S, Lal B, Tung B, Wang S, Goodwin CR
and Laterra J: Tumor microenvironment tenascin-C promotes
glioblastoma invasion and negatively regulates tumor proliferation.
Neuro Oncol. 18:507–517. 2016. View Article : Google Scholar
|