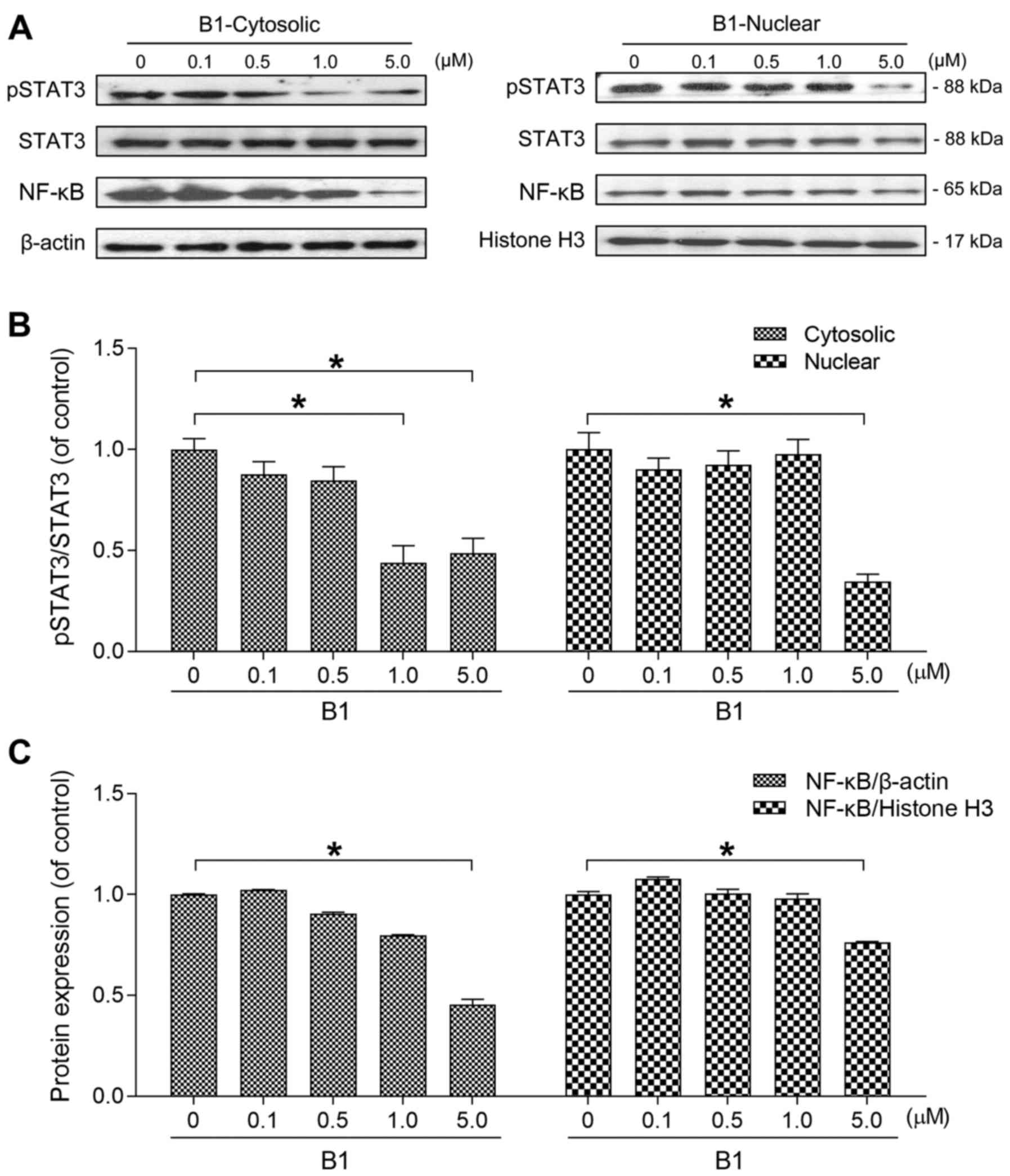
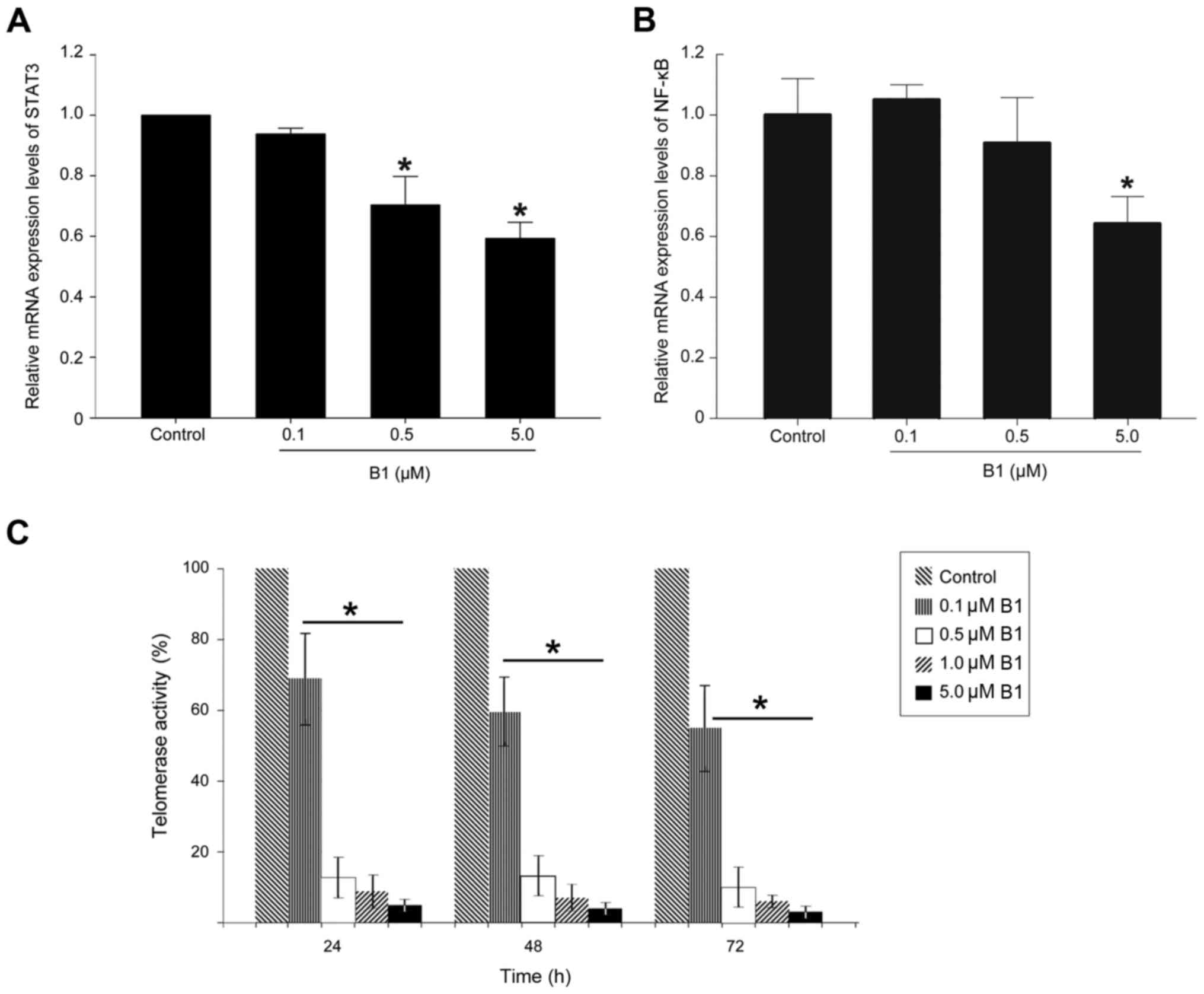
|
1
|
Cheng MH, Yang YC, Wong YH, Chen TR, Lee
CY, Yang CC, Chen SH, Yang IN, Yang YS, Huang HS, et al: B1, a
novel topoisomerase II inhibitor, induces apoptosis and cell cycle
G1 arrest in lung adenocarcinoma A549 cells. Anticancer Drugs.
23:191–199. 2012. View Article : Google Scholar
|
|
2
|
Cataldo VD, Gibbons DL, Pérez-Soler R and
Quintás-Cardama A: Treatment of non-small-cell lung cancer with
erlotinib or gefitinib. N Engl J Med. 364:947–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pande V: Understanding the Complexity of
Epigenetic Target Space. J Med Chem. 59:1299–1307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marks PA: Discovery and development of
SAHA as an anticancer agent. Oncogene. 26:1351–1356. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh BN, Zhang G, Hwa YL, Li J, Dowdy SC
and Jiang SW: Nonhistone protein acetylation as cancer therapy
targets. Expert Rev Anticancer Ther. 10:935–954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duvic M: Histone deacetylase inhibitors
for cutaneous T-cell lymphoma. Dermatol Clin. 33:757–764. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu L, Li S and Stohr BA: The role of
telomere biology in cancer. Annu Rev Pathol. 8:49–78. 2013.
View Article : Google Scholar
|
|
8
|
Sekaran V, Soares J and Jarstfer MB:
Telomere maintenance as a target for drug discovery. J Med Chem.
57:521–538. 2014. View Article : Google Scholar
|
|
9
|
Thomas SJ, Snowden JA, Zeidler MP and
Danson SJ: The role of JAK/STAT signalling in the pathogenesis,
prognosis and treatment of solid tumours. Br J Cancer. 113:365–371.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furtek SL, Backos DS, Matheson CJ and
Reigan P: Strategies and approaches of targeting STAT3 for cancer
treatment. ACS Chem Biol. 11:308–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koliaraki V, Pasparakis M and Kollias G:
IKKβ in intestinal mesenchymal cells promotes initiation of
colitis-associated cancer. J Exp Med. 212:2235–2251. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y and Tergaonkar V: Noncanonical
functions of telomerase: Implications in telomerase-targeted cancer
therapies. Cancer Res. 74:1639–1644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar
|
|
14
|
Wang GW, Lv C, Shi ZR, Zeng RT, Dong XY,
Zhang WD, Liu RH, Shan L and Shen YH: Abieslactone induces cell
cycle arrest and apoptosis in human hepatocellular carcinomas
through the mitochondrial pathway and the generation of reactive
oxygen species. PLoS One. 9:e1151512014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Correia C, Lee SH, Meng XW, Vincelette ND,
Knorr KL, Ding H, Nowakowski GS, Dai H and Kaufmann SH: Emerging
understanding of Bcl-2 biology: Implications for neoplastic
progression and treatment. Biochim Biophys Acta. 1853:1658–1671.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lunardi S, Muschel RJ and Brunner TB: The
stromal compartments in pancreatic cancer: Are there any
therapeutic targets? Cancer Lett. 343:147–155. 2014. View Article : Google Scholar
|
|
17
|
Grivennikov SI and Karin M: Dangerous
liaisons: STAT3 and NF-kappaB collaboration and crosstalk in
cancer. Cytokine Growth Factor Rev. 21:11–19. 2010. View Article : Google Scholar
|
|
18
|
Wang XH, Liu BR, Qu B, Xing H, Gao SL, Yin
JM, Wang XF and Cheng YQ: Silencing STAT3 may inhibit cell growth
through regulating signaling pathway, telomerase, cell cycle,
apoptosis and angiogenesis in hepatocellular carcinoma: Potential
uses for gene therapy. Neoplasma. 58:158–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barneda-Zahonero B and Parra M: Histone
deacetylases and cancer. Mol Oncol. 6:579–589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z and Zhu WG: Targeting histone
deacetylases for cancer therapy: From molecular mechanisms to
clinical implications. Int J Biol Sci. 10:757–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhaskara S and Hiebert SW: Role for
histone deacetylase 3 in maintenance of genome stability. Cell
Cycle. 10:727–728. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Helleday T, Petermann E, Lundin C, Hodgson
B and Sharma RA: DNA repair pathways as targets for cancer therapy.
Nat Rev Cancer. 8:193–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhaskara S, Knutson SK, Jiang G,
Chandrasekharan MB, Wilson AJ, Zheng S, Yenamandra A, Locke K, Yuan
JL, Bonine-Summers AR, et al: Hdac3 is essential for the
maintenance of chromatin structure and genome stability. Cancer
Cell. 18:436–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Danial NN: BAD: Undertaker by night,
candyman by day. Oncogene. 27(Suppl 1): S53–S70. 2008. View Article : Google Scholar
|
|
25
|
Bolden JE, Shi W, Jankowski K, Kan CY,
Cluse L, Martin BP, MacKenzie KL, Smyth GK and Johnstone RW: HDAC
inhibitors induce tumor-cell-selective pro-apoptotic
transcriptional responses. Cell Death Dis. 4:e5192013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar
|
|
27
|
Frankowski H, Gu YH, Heo JH, Milner R and
Del Zoppo GJ: Use of gel zymography to examine matrix
metalloproteinase (gelatinase) expression in brain tissue or in
primary glial cultures. Methods Mol Biol. 814:221–233. 2012.
View Article : Google Scholar
|
|
28
|
Leinonen T, Pirinen R, Bohm J, Johansson R
and Kosma VM: Increased expression of matrix metalloproteinase-2
(MMP-2) predicts tumour recurrence and unfavourable outcome in
non-small cell lung cancer. Histology and histopathology.
23:693–700. 2008.PubMed/NCBI
|
|
29
|
Lee H, Kim JS and Kim E: Fucoidan from
seaweed Fucus vesiculosus inhibits migration and invasion of human
lung cancer cell via PI3K-Akt-mTOR pathways. PLoS One.
7:e506242012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Zhu S, Shen M, Liu J, Wang M, Li
C, Wang Y, Deng A and Mei Q: STAT3 is involved in esophageal
carcinogenesis through regulation of Oct-1. Carcinogenesis.
34:678–688. 2013. View Article : Google Scholar
|
|
31
|
Connelly L, Barham W, Onishko HM, Sherrill
T, Chodosh LA, Blackwell TS and Yull FE: Inhibition of NF-kappa B
activity in mammary epithelium increases tumor latency and
decreases tumor burden. Oncogene. 30:1402–1412. 2011. View Article : Google Scholar
|
|
32
|
Haybaeck J, Zeller N, Wolf MJ, Weber A,
Wagner U, Kurrer MO, Bremer J, Iezzi G, Graf R, Clavien PA, et al:
A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer
Cell. 16:295–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fan Y, Mao R and Yang J: NF-κB and STAT3
signaling pathways collaboratively link inflammation to cancer.
Protein Cell. 4:176–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
West AC and Johnstone RW: New and emerging
HDAC inhibitors for cancer treatment. J Clin Invest. 124:30–39.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song X, Wang J, Zheng T, Song R, Liang Y,
Bhatta N, Yin D, Pan S, Liu J, Jiang H, et al: LBH589 Inhibits
proliferation and metastasis of hepatocellular carcinoma via
inhibition of gankyrin/stat3/akt pathway. Mol Cancer. 12:1142013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin TY, Fenger J, Murahari S, Bear MD,
Kulp SK, Wang D, Chen CS, Kisseberth WC and London CA: AR-42, a
novel HDAC inhibitor, exhibits biologic activity against malignant
mast cell lines via down-regulation of constitutively activated
Kit. Blood. 115:4217–4225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chien W, Lee DH, Zheng Y, Wuensche P,
Alvarez R, Wen DL, Aribi AM, Thean SM, Doan NB, Said JW, et al:
Growth inhibition of pancreatic cancer cells by histone deacetylase
inhibitor belinostat through suppression of multiple pathways
including HIF, NFκB, and mTOR signaling in vitro and in vivo. Mol
Carcinog. 53:722–735. 2014. View Article : Google Scholar
|
|
38
|
He G and Karin M: NF-κB and STAT3 - key
players in liver inflammation and cancer. Cell Res. 21:159–168.
2011. View Article : Google Scholar
|
|
39
|
Carpenter RL and Lo HW: STAT3 target genes
relevant to human cancers. Cancers (Basel). 6:897–925. 2014.
View Article : Google Scholar
|
|
40
|
Song L, Rawal B, Nemeth JA and Haura EB:
JAK1 activates STAT3 activity in non-small-cell lung cancer cells
and IL-6 neutralizing antibodies can suppress JAK1-STAT3 signaling.
Mol Cancer Ther. 10:481–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jeannot V, Busser B, Vanwonterghem L,
Michallet S, Ferroudj S, Cokol M, Coll JL, Ozturk M and Hurbin A:
Synergistic activity of vorinostat combined with gefitinib but not
with sorafenib in mutant KRAS human non-small cell lung cancers and
hepatocarcinoma. Onco Targets Ther. 9:6843–6855. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Low KC and Tergaonkar V: Telomerase:
Central regulator of all of the hallmarks of cancer. Trends Biochem
Sci. 38:426–434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sharma V, Koul N, Joseph C, Dixit D, Ghosh
S and Sen E: HDAC inhibitor, scriptaid, induces glioma cell
apoptosis through JNK activation and inhibits telomerase activity.
J Cell Mol Med. 14:2151–2161. 2010. View Article : Google Scholar
|
|
45
|
Li CT, Hsiao YM, Wu TC, Lin YW, Yeh KT and
Ko JL: Vorinostat, SAHA, represses telomerase activity via
epigenetic regulation of telomerase reverse transcriptase in
non-small cell lung cancer cells. J Cell Biochem. 112:3044–3053.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim JS, Lee SC, Min HY, Park KH, Hyun SY,
Kwon SJ, Choi SP, Kim WY, Lee HJ and Lee HY: Activation of
insulin-like growth factor receptor signaling mediates resistance
to histone deacetylase inhibitors. Cancer Lett. 361:197–206. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee SC, Min HY, Jung HJ, Park KH, Hyun SY,
Cho J, Woo JK, Kwon SJ, Lee HJ, Johnson FM, et al: Essential role
of insulin-like growth factor 2 in resistance to histone
deacetylase inhibitors. Oncogene. 35:5515–5526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Min HY, Lee SC, Woo JK, Jung HJ, Park KH,
Jeong HM, Hyun SY, Cho J, Lee W, Park JE, et al: Essential role of
DNA methyltransferase 1-mediated transcription of insulin-like
growth factor 2 in resistance to histone deacetylase inhibitors.
Clin Cancer Res. 23:1299–1311. 2017. View Article : Google Scholar
|
|
49
|
Ceccacci E and Minucci S: Inhibition of
histone deacetylases in cancer therapy: Lessons from leukaemia. Br
J Cancer. 114:605–611. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen JB, Chern TR, Wei TT, Chen CC, Lin JH
and Fang JM: Design and synthesis of dual-action inhibitors
targeting histone deacetylases and 3-hydroxy-3-methylglutaryl
coenzyme A reductase for cancer treatment. J Med Chem.
56:3645–3655. 2013. View Article : Google Scholar : PubMed/NCBI
|