Introduction
Atherosclerosis, which is also known as
arteriosclerotic vascular disease (ASVD), refers to a specific form
of arteriosclerosis in which an artery wall thickens as a result of
the invasion and accumulation of white blood cells (WBCs) (foam
cells) and the proliferation of intimal smooth-muscle cells,
creating a fibrofatty plaque (1).
Atherosclerosis is considered the major cause of heart attack,
stroke and gangrene of the extremities, and is responsible for 50%
of all mortality across Western countries (1).
The pathogenesis and causes of atherosclerosis are
highly complex and remain exclusive to date. For a long period of
time, atherosclerosis was considered a metabolic disease and its
development was traditionally based on thecholesterol hypothesis
due to the accumulation of atherogenic lipoproteins in the blood
vessel wall (2,3). Atherosclerosis is associated with
other metabolic diseases, such as diabetes and dyslipoproteinemia
(4,5). However, in recent years, it was
discovered that inflammation may be a contributing factor for
atherosclerosis and this may thus provide new insight into the
mechanisms responsible for the disease (2,3).
In a previous review, it was suggested that
constituents of oxidatively modified (oxidized) low-density
lipoprotein (oxLDL) induce a local inflammatory response (6). Pro-inflammatory stimuli in
endothelial cells (ECs) trigger the expression of adhesion
molecules, such as P-selectin and vascular cell adhesion molecule-1
(VCAM-1), which results in the attachment of circulating monocytes
or lymphocytes (7–9). In macrophages, the expression of
scavenger receptors in response to inflammatory cytokines
subsequently increases, transforming them into lipid-laden foam
cells following the endocytosis of modified lipoprotein particles;
macrophage-derived foam cells drive lesion progression via the
continuation of the secretion of pro-inflammatory cytokines
(3). There are data to suggest a
central role for inflammation in both early atherogenesis and in
the progression of lesions (10).
Therefore, the circulating markers of inflammation are considered
as an indicator of atherosclerosis (10). On the other hand, the role of
inflammation also implies a potential therapeutic target for
atherosclerosis.
Ghrelin is a peptide hormone produced by
ghrelinergic cells in the gastrointestinal tract and acts as a
neuropeptide in the central nervous system (11). However, a recent study suggested
that ghrelin may be a potent anti-inflammatory mediator and a
promising therapeutic agent in the treatment of inflammatory
diseases or injury (12). It has
been shown that low ghrelin serum levels are significantly
associated with advanced carotid atherosclerosis in patients with
type 2 diabetes (13). Another
study also demonstrated that the administration of ghrelin
attenuated inflammation, oxidative stress, and apoptosis during and
after the development of non-alcoholic fatty liver disease
(14). Therefore, it is
interesting to note that ghrelin also plays a role in the prognosis
of atherosclerosis.
In this study, we demonstrate that the treatment of
human umbilical vein endothelial cells (HUVECs) with ghrelin
inhibits the oxLDL-induced inflammatory response via the
upregulation of uncoupling protein (UCP)2. Treatment of the HUVECs
with ghrelin inhibited the ubiquitin-mediated degradation of UCP2,
while its mRNA level was unaffected by ghrelin. Our data highlight
the potential use of ghrelin as an anti-atherosclerotic agent, as
it inhibited the oxLDL-induced inflammatory response in HUVECs. Our
data may also provide further insight into the pathogenesis of
atherosclerosis.
Materials and methods
Cells and chemicals
Human umbilical vein endothelial cells (HUVECs,
ATCC® CRL-1730™) were purchased from ATCC (Manassas, VA,
USA) and maintained in Kaighn's Modification of Ham's F-12 Medium
(ATCC® 30–2004™) supplemented with 10% fetal bovine
serum (Gibco, Carlsbad, CA, USA). oxLDL was purchased from
Cleveland HeartLab (Cleveland, OH, USA) and used to treat the cells
at a concentration of 50 µg/ml. Ghrelin was purchased from
Cayman Chemical (Ann Arbor, Michigan, USA). UCP2 siRNA (h)
(sc-42682) and scramble siRNA (control siRNA; sc-37007) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The transfection of the cells with siRNA was carried out
using Lipofectiamine 2000 (Invitrogen, Carlsbad, CA, USA) according
to the manufacturer's instructions.
Cell treatment
For the cell treatment with different drugs, ghrelin
was used to treating cells at doses of 1, 5, 10, 20, 40 and 50 nM
24 h prior oxLDL stimulation. The oxLDL was purchased from
Cleveland HeartLab and used to treat the cells at a concentration
of 50 µg/ml for 36 h. Mock cells were the cells without any
treatment.
Reverse transcription-quantitative PCR
(RT-qPCR)
The RNA isolation from the HUVECs was conducted
using TRIzol reagent (Invitrogen) according to manufacturer's
instructions. The synthesis of cDNA was conducted using AMV reverse
transcriptase (Promega, Madison, WI, USA) according to instructions
provided by the manufacturer. Quantitative PCR (qPCR) detection for
the transcripts of target genes with SYBR-Green mix (Life
Technologies, Carlsbad, CA, USA) was carried out as previously
described (15,16). The conditions for qPCR cycle were
as follows: 98°C for 5 min for denaturation, 98°C for 30 sec, 72°C
for 1 min, then repeated for 40 cycles. Transcripts of ribosomal
protein L32 (RPL32) were also amplified from the same sample to
serve as an internal control for normalization purposes. Relative
gene expression was quantified by the 2−ΔΔCT method as
previously described (17). The
primers for qPCR detection are listed in Table I.
 | Table IPrimers used in this study. |
Table I
Primers used in this study.
| Primer name | Primer sequence
(5′→3′) |
|---|
| IL-6 | F:
GACAACTTTGGCATTGTGG |
| R:
ATGCAGGGATGATGTTCTG |
| CCL-2 | F:
CCCCAGTCACCTGCTGTTAT |
| R
AGATCTCCTTGGCCACAATG |
| ICAM-1 | F:
GGCCTCAGTCAGTGTGA |
| R
AACCCCATTCAGCGTCA |
| VCAM-1 | F:
TACTCCCGTCATTGAGGATATTGG |
| R
CTCCTTCACACACATAGACTCC |
| RPL32 | F:
CAACTGGCCATCAGAGTCAC |
| R
GTGCACATGAGCTGCCTACT |
Determination of reactive oxygen species
(ROS) generation
The dihydroethidium (DHE) fluorescence-based assay
was used to determine ROS generation and was conducted as
previously described (18).
Briefly, cells with the indicated treatments were first washed
twice with PBS, 0.5 µM DHE was then added to the cells and
the cells were incubated under 37°C for 20 min. After the
incubation, cells were washed again twice with PBS to remove the
free DHE probe. DHE-derived fluorescence at 570 nm which was
generated by each group was recorded by the VICTOR™ X5 Multilabel
Plate Reader (PerkinElmer, Waltham, MA, USA).
Western blot analysis
The cells subjected to the indicated treatments were
lysed by the Laemmli sample buffer as previously described
(16,19). The protein of lysate was then
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and western blot analysis was performed
as previously described (19).
Briefly, following SDS-PAGE, the separated proteins were
transferred onto PVDF membranes and probed with rabbit anti-UCP2
antibody and mouse anti-ubiquitin (sc-166553) (both from Santa Cruz
Biotechnology, Inc.). Specific reactions were detected using goat
anti-rabbit IgG or goat anti-mouse IgG conjugated with horseradish
peroxidase (Sigma, St. Louis, MO, USA) and revealed by a
chemiluminescence substrate (Bio-Rad Laboratories, Hercules, CA,
USA). The membranes were also blotted with tubulin antibody
(sc-33749; Santa Cruz Biotechnology, Inc.) to normalize protein
loading. The chemiluminescence signal was recorded using the
ChemiDoc MP imaging system (Bio-Rad Laboratories). The luminescence
signal was captured and analyzed using the Image Lab Program
(version 6.1).
Protein half-life assay
Cycloheximide is a translation inhibition drug which
is generally used to determine protein half-life by monitoring the
protein degradation speed. Briefly, 100 µg cycloheximide
(Sigma) was added to cells seeding in 12-well plates for the
indicated times (12, 24 and 36 h). The cells were then harvested
for SDS-PAGE and western blot analysis was performed to determine
the level of UCP2.
Protein ubiquitination assay
To examine the ubiquitination status of UPC2, the
immunoprecipitation (IP) for UCP2 was conducted first as previously
described with modifications (20). Briefly, the HUVECs subjected to
the indicated treatment were lysed with lysis buffer (50 mM Tris pH
7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100) followed by the
addition of ubiquitin aldehyde (Boston Biochem, Inc., Cambridge,
MA, USA), a specific inhibitor of ubiquitin C-terminal hydrolases,
at a final concentration of 2.53 µM. The lysate was
clarified by centrifugation at 14,000 × g for 5 min at 4°C.
Following centrifugation, the cell lysate was incubated with UCP2
antibody (Santa Cruz Biotechnology, Inc.) followed by incubation
with protein G-agarose (KPL, Inc., Gaithersburg, MD, USA). To
detect the ubiquitination UCP2, The IP samples containing UCP2 were
subjected to western blot analysis with ubiquitin antibody (Santa
Cruz Biotechnology, Inc.).
Statistical analysis
The experimental results were plotted and analyzed
for statistical significance using the Excel program (Microsoft,
Seattle, WA, USA). Data are represented as the means ± SD.
Differences in indicators between treatment samples, such as the
cellular RNA level between the groups in the presence or absence of
oxLDL or ghrelin, were assessed using the Student's t-test. A
two-tailed P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
Ghrelin inhibits the oxLDL-induced
inflammation response in HUVECs
Recent studies have suggested that ghrelin may be a
potent anti-inflammatory mediator and that the administration of
ghrelin attenuates inflammation, oxidative stress and apoptosis
during and after the development of non-alcoholic fatty liver
disease (12,14). Combined with previous observations
that the ghrelin serum levels are significantly associated with
advanced carotid atherosclerosis in patients with type 2 diabetes
(13), we wished to determine
whether ghrelin inhibits the oxLDL-induced inflammatory response in
HUVECs. In order to examine our hypothesis, in the present study,
the HUVECs were treated with 50 nM ghrelin for 24 h, and were then
stimulated by oxLDL to induce the inflammatory response.
Pro-inflammatory cytokines, chemokines and related molecule
expression such as interleukin-6 (IL-6), C-C motif chemokine ligand
2 (CCL-2), intercellular adhesion molecule-1 (ICAM-1) and VCAM-1
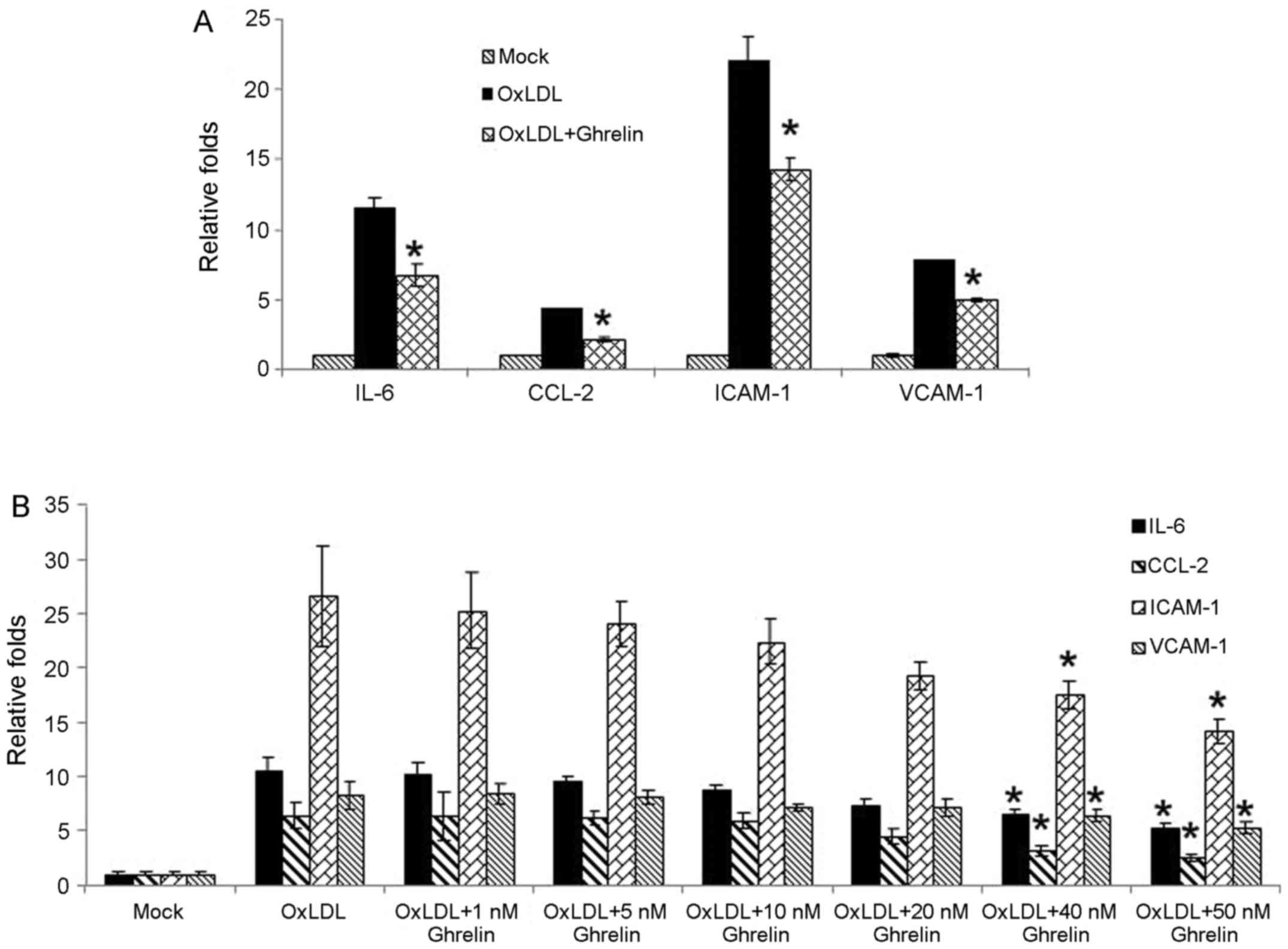
were examined by RT-qPCR. Based on our results, stimulation with
oxLDL alone led to the significant upregulation in the expression
levels of these molecules (Fig.
1A). However, in the cells pre-treated with ghrelin, the
expression of these cytokines and related molecules was decreased
(Fig. 1A), which suggested an
inhibitory effect of ghrelin on the oxLDL-induced inflammatory
response. Moreover, to confirm our data, treatment of the HUVECs
with a gradient dose of ghrelin was also conducted. As shown in
Fig. 1B, the increase in the
expression levels of inflammatory cytokines and related molecules
induced by oxLDL was suppressed as the concentration of ghrelin
increased, which was consistent with our above-mentioned
observation.
Ghrelin upregulates UCP2 to suppress ROS
generation
Studies have demonstrated that the oxLDL-induced
inflammatory response always correlates with the generation of ROS
(21,22). Therefore, in this study, we
examined whether the oxLDL-induced generation of ROS can be
inhibited by ghrelin. By utilizing DHE fluorescence-based ROS
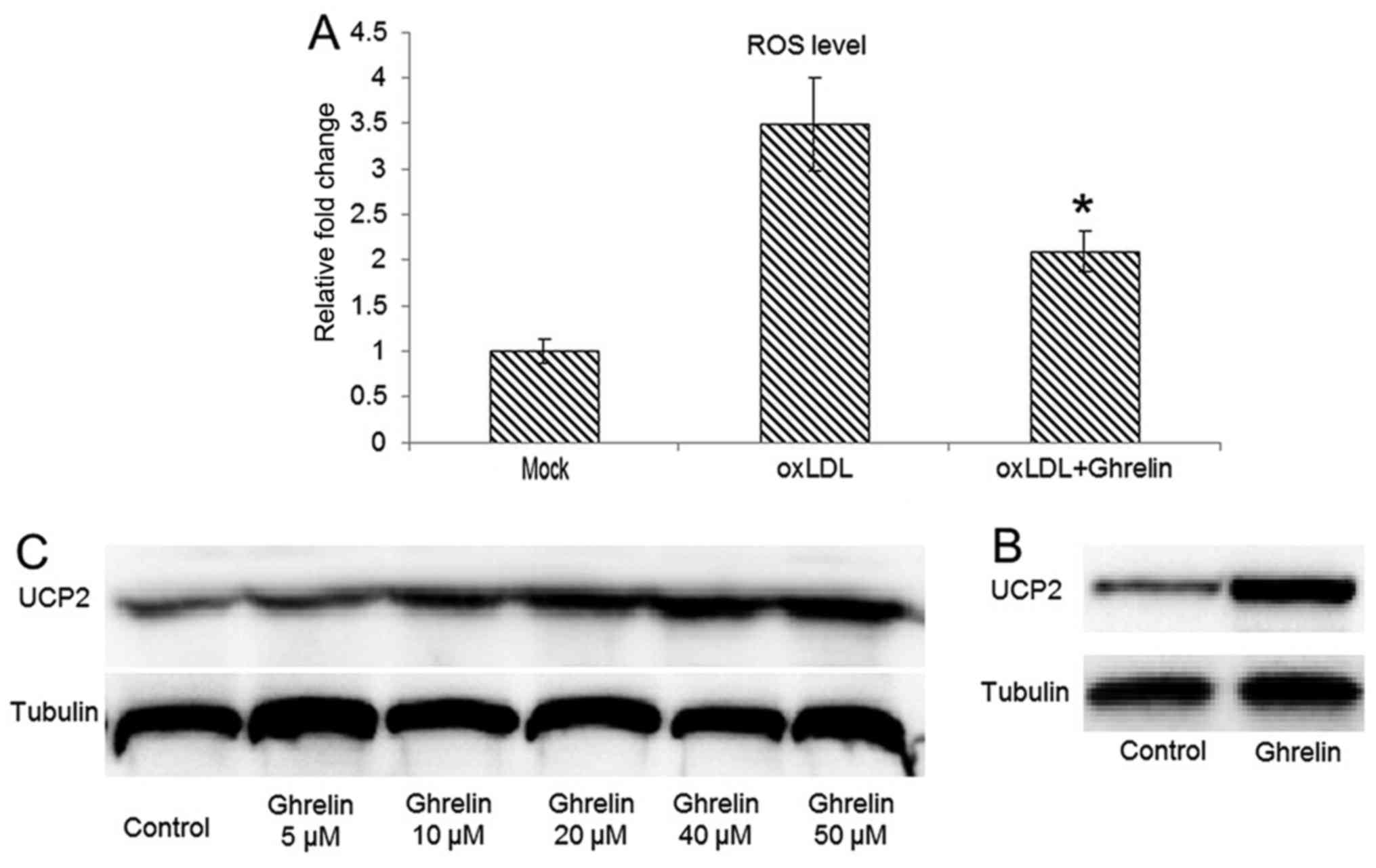
assay, our data demonstrated that in the HUVECs pre-treated with
ghrelin, oxLDL-induced ROS generation was inhibited (Fig. 2A), which fulfilled our
expectations. As a natural byproduct of the normal oxygen
metabolism in physiological conditions, ROS are involved in many
cell signaling processes, such as cell proliferation, inflammation,
apoptosis and phagocytosis (23).
However, the cellular ROS level can be markedly increased during
cell responses to stress and increases in ROS levels cause
oxidative stress, which results in damage to the cells (24).
On the other hand, the UCP2 family is involved in
the downregulation of the oxidative phosphorylation by increasing
membrane proton conductance (25). Therefore, the function of UCPs is
considered a defense mechanism with which to attenuate ROS-induced
damage. Among the members of the UCP family, UCP2 can be identified
in a variety of tissues (26),
while UCP1 and UCP3 have a more tissue-specific expression in brown
adipose tissue and skeletal muscle, respectively (26–28). Thus, we then examined whether UCP2
expression is upregulated in ghrelin-treated cells, leading to a
reduced of oxLDL induced ROS level in HUVECs. Our results revealed
that the UCP2 protein level was significantly upregulated in the
ghrelin-treated cells (Fig. 2B).
Moreover, a concentration-dependent upregulation of UCP2 expression
by ghrelin treatment was also observed, which suggested that the
inhibitory effects of ghrelin on ROS generation were due to the
upregulation of UCP2.
siRNA-mediated knockdown of UCP2
expression antagonizes the inhibitory effects of ghrelin on ROS
generation
To further understand the role that UCP2 plays
during the ghrelin-mediated inhibition of ROS generation and the
inflammatory response, siRNA transfection was employed for the
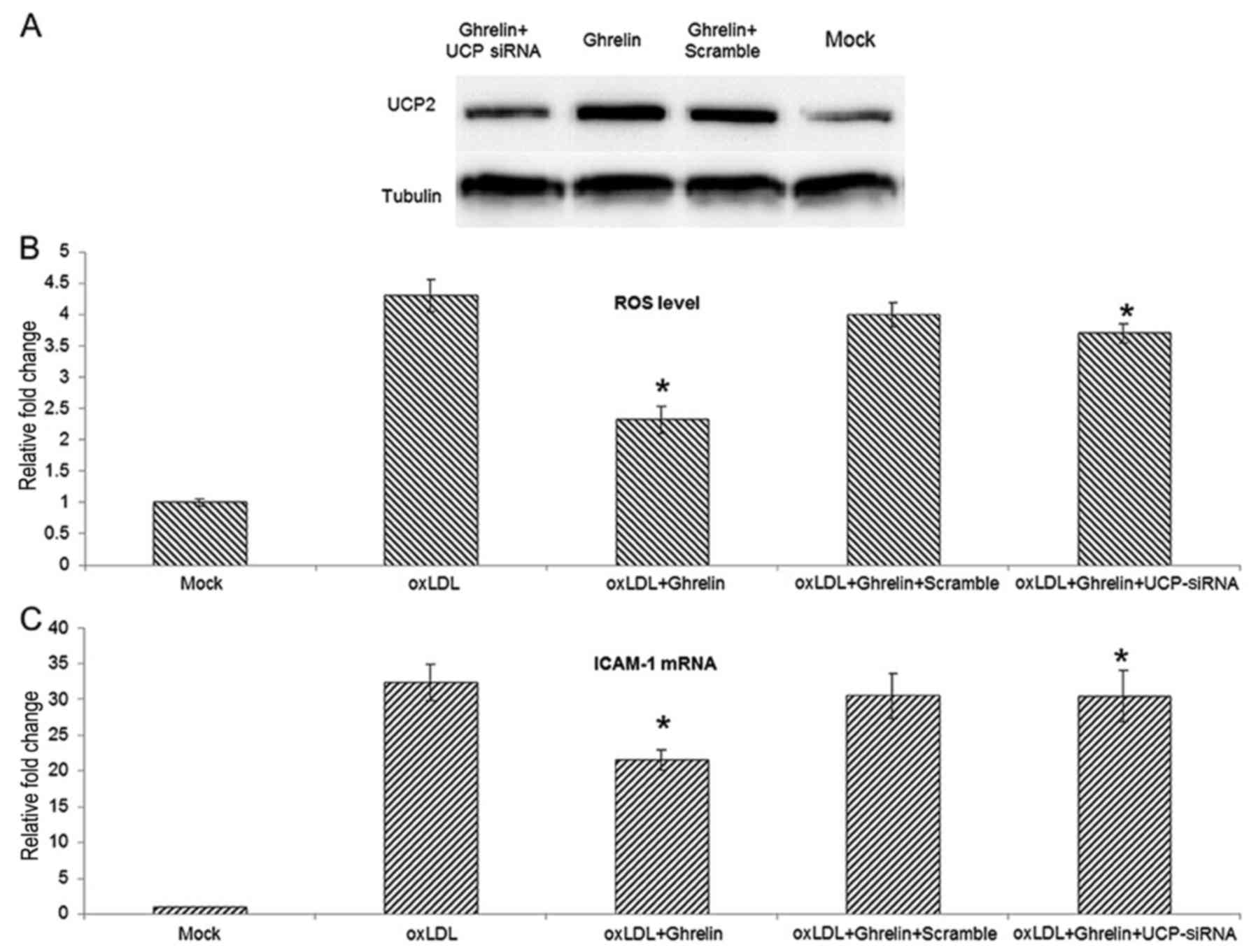
knockdown of UCP2 expression in HUVECs. The knockdown efficiencty
of the UCP2 siRNA was examined by western blot analysis (Fig. 3A). With the knockdown of UCP2
expression, the inhibitory effects of ghrelin on ROS generation
were markedly suppressed, and the ROS level induced by oxLDL in the
cells treated with ghrelin and transfected with UCP siRNA was
similar to the level in the cells treated with oxLDL alone
(Fig. 3B). Moreover, using ICAM-1
as an indicator of the oxLDL-induced inflammatory response, the
knockdown of UCP2 also antagonized the ghrelin-mediated inhibition
of ICAM-1 expression. Taken together, these data suggest that
ghrelin upregulates UCP2, thus inhibiting the oxLDL-induced
inflammatory response.
Ghrelin extends the protein half-life of
UCP2 without affecting its mRNA level
Since our data suggested that UCP2 was be
upregulated by ghrelin treatment, we then examined whether the
accumulation of UCP2 was the result of the upregulation of its mRNA
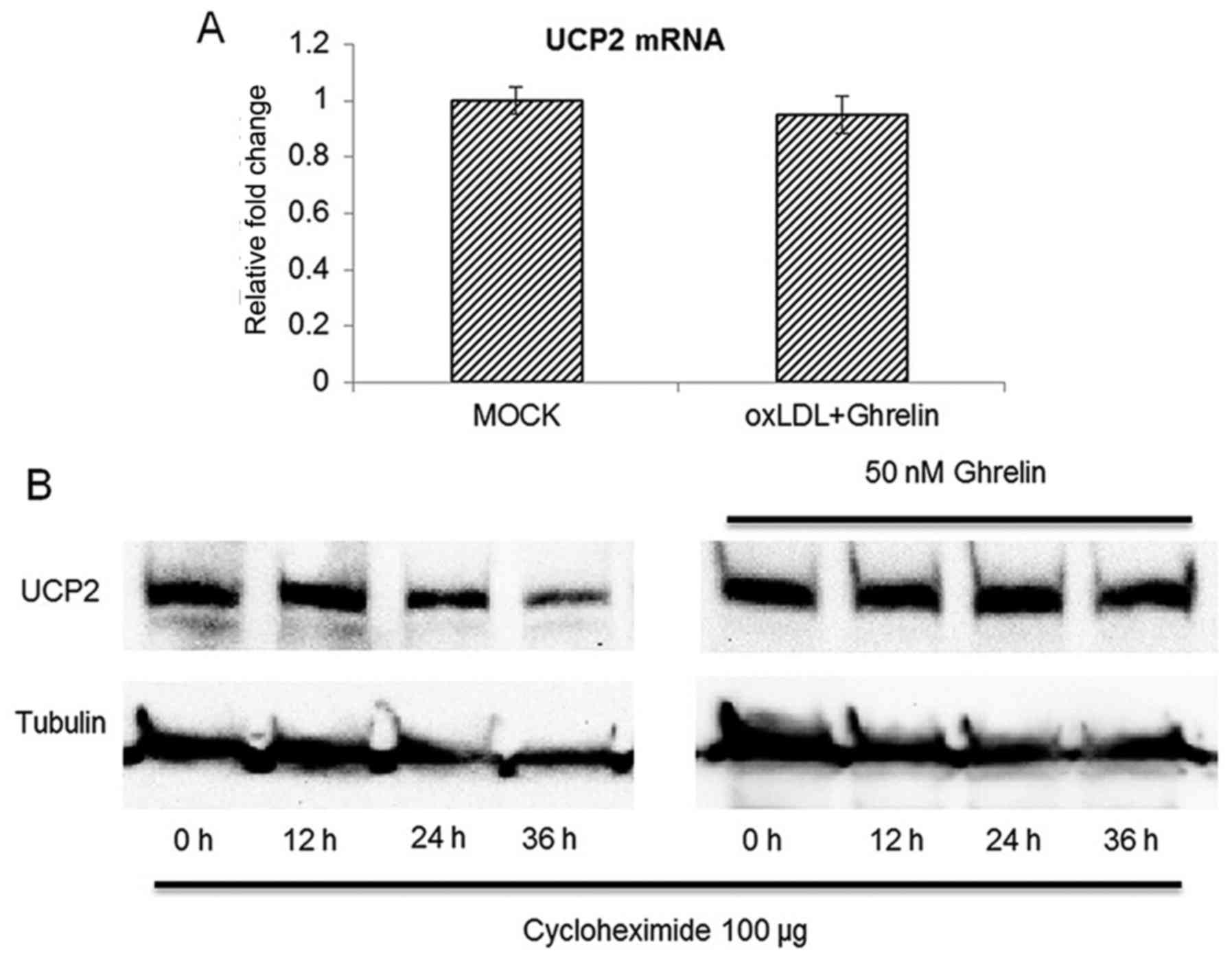
expression. However, to our surprise, with 24 h of treatment of the
HUVECs with ghrelin, the UCP2 mRNA level between the treatment
groups and the control cells was similar (Fig. 4A), which suggested that ghrelin
treatment did not cause the upregulation of UCP2 gene expression
and implied that UCP2 functions at the post-translational level of
UCP2 mRNA. To further confirm our findings, we examined whether the
protein half-life of UCP2 was extended by ghrelin. A protein
translation inhibitor, cycloheximide was added to the HUVECs to
inhibit mRNA translation as previously described (20). The cells were then harvested at
different time points to examine the protein level of UCP2. As
shown in Fig. 4B, without ghrelin
treatment, the UCP2 protein level began to decrease at 24 h after
cycloheximide treatment. However, in the ghrelin-treated cells, the
protein level of UCP2 was not significantly altered until 36 h
after cycloheximide treatment. Taken together, these data
demonstrated that ghrelin treatment extended the protein half-life
of UCP2 rather than promoting the transcription of UCP2 mRNA.
Ghrelin prevents UCP2 degradation by
inhibiting UCP2 ubiquitination
Protein ubiquitination is considered as the major
pathway for cellular protein degradation (29). Although UCP2 is located in the
inner membrane of the mitochondria, it has been demonstrated that
mitochondrial proteins, including UCP2 can be degraded by the
ubiquitin-proteasome system as well (30). As our data demonstrated that
ghrelin extended the protein half-life of UCP2, we further examined
the ubiquitination status of UCP2 to explain the reason leading to
the accumulation of UCP2 in ghrelin-treated cells. As shown in
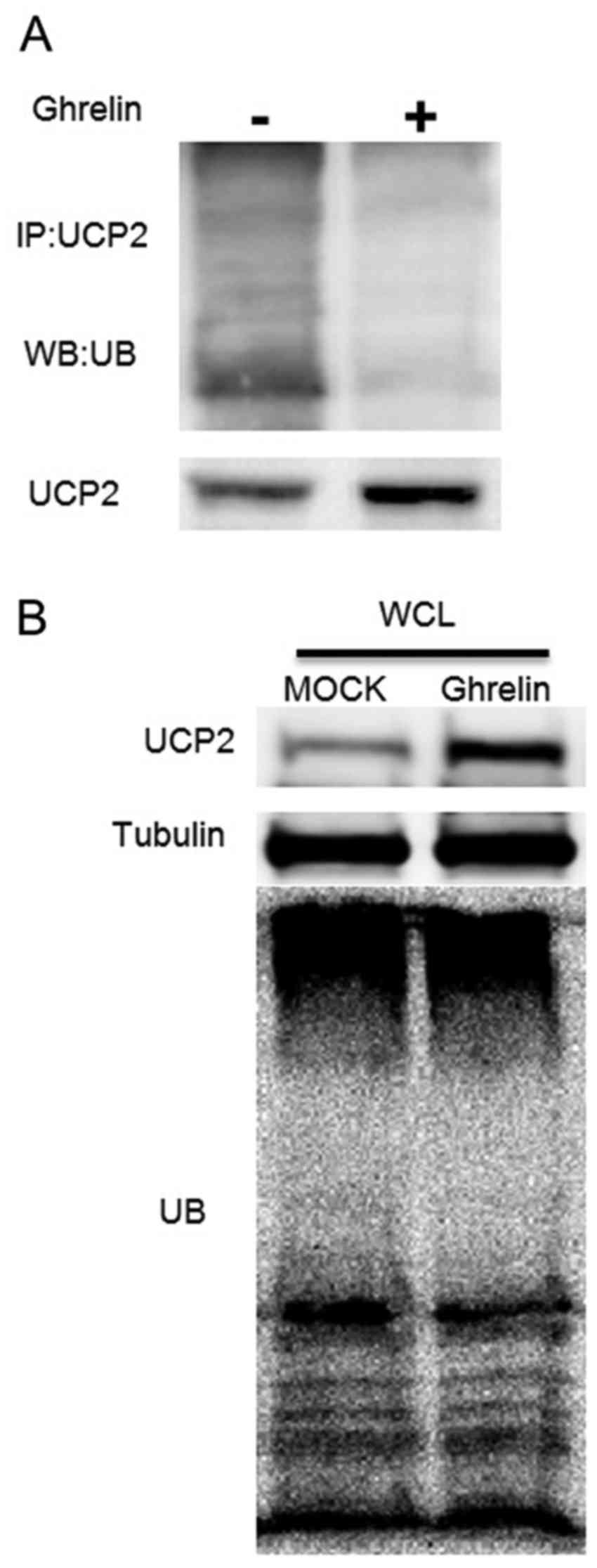
Fig. 5A, treatment of the HUVECs
with ghrelin significantly inhibited the ubiquitination level of
UCP2 when compared with the controls. Moreover, the ubiquitination
level of the whole cell lysate demonstrated that ghrelin did not
alter the ubiquitination status of the whole cell, which suggested
that the decreased ubiquitination level of UCP2 appeared to be
specific (Fig. 5B). These
observations suggested that ghrelin specifically extended the UCP2
half-life by inhibiting UCP2 ubiquitination, which leads to UCP2
degradation. On the whole, our data demonstrated that ghrelin
prevented UCP2 degradation, thus reducing the cellular ROS level
induced by oxLDL, and inhibiting the oxLDL-induced inflammatory
response in HUVECs.
Discussion
In the present study, we demonstrated that
stimulation of HUVECs with oxLDL induced the expression of
pro-inflammatory cytokines and adhesion molecules, and increased
ROS generation. However, pro-treatment of the HUVECs with ghrelin
prevented the oxLDL-induced inflammatory response via the
upregulation of UCP2. Further analysis demonstrated that the
ubiquitination of UCP2 was inhibited by ghrelin, therefore
preventing the degradation of UCP2, while the mRNA level of UCP2
was not altered.
The UCP family is a sub-category of the
mitochondrial anion-carrier proteins superfamily, which can be both
found in animal and plant species (27). The genome of the mammalian species
encodes 5 UCP homologues (UCP1 to 5), with UCP1 to 3 demonstrating
highest sequence similarity (27,31,32). Generally, UCPs locate in the inner
membrane of the mitochondria to regulate the thermogenic proton
leak (27). Compared with UCP1
and UCP3, which have a more tissue-specific expression in brown
adipose tissue and skeletal muscle (26–28), the expression of UCP2 is more
universal (26). The biochemical
function of UCPs is to downregulate oxidative phosphorylation by
increasing membrane proton conductance (25). The uncoupling function increases
respiration and leads in the decreased generation of superoxide
(25,33).
To date, while UCP1 is still a key regulator of
adaptive thermogenesis, UCP2 and UCP3 also have important
functions, decreasing ROS which are produced by electron transport
(34,35). With the function of regulating
ROS, accumulating evidence suggests that UCP2 is involved in many
diseases, such as neurodegenerative diseases, cardiovascular
diseases, type 2 diabetes and cancer (36). A previous study demonstrated that
the rapid degradation of UCP2 was mediated by the
ubiquitin-proteasome system (30). However, as a protein located in
the inner membrane of the mitochondria and no mitochondrial protein
export machinery has been identified (30), the mechanisms through which UCP2
can be transported to the cytoplasm for ubiquitin-mediated
degradation are still unclear, since the ubiquitin-proteasome
system is a cytosolic degradation machinery. Moreover, the
E3-ligease of ubiquitin mediated degradation for UCP2 is still
unclear. Therefore, the mechanisms through which ghrelin prevents
UCP2 from ligating with poly-ubiquitin chains warrant further
investigation. There may be other cellular proteins involved in
this process as well.
In conclusion, our data suggest a novel role of
ghrelin in preventing the oxLDL-induced inflammatory response in
HUVECs. Our findings may aid in the understanding of the
pathogenesis of atherosclerosis.
References
|
1
|
Ross R: The pathogenesis of
atherosclerosis: A perspective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perez-Ruiz F and Becker MA: Inflammation:
A possible mechanism for a causative role of hyperuricemia/gout in
cardiovascular disease. Curr Med Res Opin. 31(Suppl 2): 9–14. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Packard RR and Libby P: Inflammation in
atherosclerosis: From vascular biology to biomarker discovery and
risk prediction. Clin Chem. 54:24–38. 2008. View Article : Google Scholar
|
|
4
|
Funk SD, Yurdagul A Jr and Orr AW:
Hyperglycemia and endothelial dysfunction in atherosclerosis:
Lessons from type 1 diabetes. Int J Vasc Med.
2012:5696542012.PubMed/NCBI
|
|
5
|
Schanberg LE and Sandborg C:
Dyslipoproteinemia and premature atherosclerosis in pediatric
systemic lupus erythe-matosus. Curr Rheumatol Rep. 6:425–433. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller YI, Chang MK, Binder CJ, Shaw PX
and Witztum JL: Oxidized low density lipoprotein and innate immune
receptors. Curr Opin Lipidol. 14:437–445. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cybulsky MI and Gimbrone MA Jr:
Endothelial expression of a mononuclear leukocyte adhesion molecule
during atherogenesis. Science. 251:788–791. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Cybulsky MI, Gimbrone MA Jr and
Libby P: An atherogenic diet rapidly induces VCAM-1, a
cytokine-regulatable mononuclear leukocyte adhesion molecule, in
rabbit aortic endothelium. Arterioscler Thromb. 13:197–204. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cybulsky MI, Iiyama K, Li H, Zhu S, Chen
M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW and Milstone
DS: A major role for VCAM-1, but not ICAM-1, in early
atherosclerosis. J Clin Invest. 107:1255–1262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farmer JA and Torre-Amione G:
Atherosclerosis and inflammation. Curr Atheroscler Re. 4:92–98.
2002. View Article : Google Scholar
|
|
11
|
Dickson SL, Egecioglu E, Landgren S,
Skibicka KP, Engel JA and Jerlhag E: The role of the central
ghrelin system in reward from food and chemical drugs. Mol Cell
Endocrinol. 340:80–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baatar D, Patel K and Taub DD: The effects
of ghrelin on inflammation and the immune system. Mol Cell
Endocrinol. 340:44–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kadoglou NP, Sailer N, Moumtzouoglou A,
Kapelouzou A, Tsanikidis H, Vitta I, Karkos C, Karayannacos PE,
Gerasimidis T and Liapis CD: Visfatin (nampt) and ghrelin as novel
markers of carotid atherosclerosis in patients with type 2
diabetes. Exp Clin Endocrinol Diabetes. 118:75–80. 2010. View Article : Google Scholar
|
|
14
|
Li Y, Hai J, Li L, Chen X, Peng H, Cao M
and Zhang Q: Administration of ghrelin improves inflammation,
oxidative stress, and apoptosis during and after non-alcoholic
fatty liver disease development. Endocrine. 43:376–386. 2013.
View Article : Google Scholar
|
|
15
|
Patel D, Opriessnig T, Stein DA, Halbur
PG, Meng XJ, Iversen PL and Zhang YJ: Peptide-conjugated morpholino
oligomers inhibit porcine reproductive and respiratory syndrome
virus replication. Antiviral Res. 77:95–107. 2008. View Article : Google Scholar
|
|
16
|
Patel D, Nan Y, Shen M, Ritthipichai K,
Zhu X and Zhang YJ: Porcine reproductive and respiratory syndrome
virus inhibits type I interferon signaling by blocking STAT1/STAT2
nuclear translocation. J Virol. 84:11045–11055. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Peshavariya HM, Dusting GJ and Selemidis
S: Analysis of dihydroethidium fluorescence for the detection of
intracellular and extracellular superoxide produced by NADPH
oxidase. Free Radic Res. 41:699–712. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nan Y, Wang R, Shen M, Faaberg KS, Samal
SK and Zhang YJ: Induction of type I interferons by a novel porcine
reproductive and respiratory syndrome virus isolate. Virology.
432:261–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nan Y, Ma Z, Wang R, Yu Y, Kannan H,
Fredericksen B and Zhang YJ: Enhancement of interferon induction by
ORF3 product of hepatitis E virus. J Virol. 88:8696–8705. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu W, Yin Y, Zhou Z, He M and Dai Y:
OxLDL-induced IL-1 beta secretion promoting foam cells formation
was mainly via CD36 mediated ROS production leading to NLRP3
inflam-masome activation. Inflamm Res. 63:33–43. 2014. View Article : Google Scholar
|
|
22
|
Magwenzi S, Woodward C, Wraith KS, Aburima
A, Raslan Z, Jones H, McNeil C, Wheatcroft S, Yuldasheva N,
Febbriao M, et al: Oxidized LDL activates blood platelets through
CD36/NOX2-mediated inhibition of the cGMP/protein kinase G
signaling cascade. Blood. 125:2693–2703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salganik RI: The benefits and hazards of
antioxidants: controlling apoptosis and other protective mechanisms
in cancer patients and the human population. J Am Coll Nutr.
20(Suppl 5): 464S–475S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lobo V, Patil A, Phatak A and Chandra N:
Free radicals, antioxidants and functional foods: Impact on human
health. Pharmacogn Rev. 4:118–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Echtay KS: Mitochondrial uncoupling
proteins - what is their physiological role? Free Radic Biol Med.
43:1351–1371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Donadelli M, Dando I, Fiorini C and
Palmieri M: UCP2, a mitochondrial protein regulated at multiple
levels. Cell Mol Life Sci. 71:1171–1190. 2014. View Article : Google Scholar
|
|
27
|
Krauss S, Zhang CY and Lowell BB: The
mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol.
6:248–261. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin CS and Klingenberg M: Characteristics
of the isolated purine nucleotide binding protein from brown fat
mitochondria. Biochemistry. 21:2950–2956. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu L and Qu Z: Roles of protein
ubiquitination and degradation kinetics in biological oscillations.
PLoS One. 7:e346162012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Azzu V and Brand MD: Degradation of an
intramitochondrial protein by the cytosolic proteasome. J Cell Sci.
123:578–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fleury C, Neverova M, Collins S, Raimbault
S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS,
Ricquier D and Warden CH: Uncoupling protein-2: A novel gene linked
to obesity and hyperinsulinemia. Nat Genet. 15:269–272. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boss O, Samec S, Paoloni-Giacobino A,
Rossier C, Dulloo A, Seydoux J, Muzzin P and Giacobino JP:
Uncoupling protein-3: A new member of the mitochondrial carrier
family with tissue-specific expression. FEBS Lett. 408:39–42. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Azzu V and Brand MD: The on-off switches
of the mitochondrial uncoupling proteins. Trends Biochem Sci.
35:298–307. 2010. View Article : Google Scholar
|
|
34
|
Richard D and Picard F: Brown fat biology
and thermogenesis. Front Biosci (Landmark Ed). 16:1233–1260. 2011.
View Article : Google Scholar
|
|
35
|
Garlid KD, Jabůrek M, Jezek P and Varecha
M: How do uncoupling proteins uncouple? Biochim Biophys Acta.
1459:383–389. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mattiasson G and Sullivan PG: The emerging
functions of UCP2 in health, disease, and therapeutics. Antioxid
Redox Signal. 8:1–38. 2006. View Article : Google Scholar : PubMed/NCBI
|