Introduction
Since their discovery by Asahara in 1997,
endothelial progenitor cells (EPCs) are believed to play important
roles in endothelial repair and postnatal angiogenesis (1,2).
The development of some ischemic diseases, including coronary
artery ischemia, diabetic ulcers and myocardial infarction, is
always related to the dysfunction of EPCs in the patients (3–5).
Therefore, the allogeneic transplantation of healthy EPCs have
currently become a focus of regenerative treatment for ischemic
diseases.
EPCs can be obtained from human peripheral blood
(PB) (1,6), bone marrow (BM) and umbilical cord
blood (UCB) (7), and have been
proven to improve tissue ischemia; however, UCB-derived EPCs
(UCB-EPCs) may exhibit distinctive advantages over other sources.
Circulating PB-derived EPCs (PB-EPCs) have been reported to
contribute to neovascularization in adults (8,9).
Human BM-derived EPCs (BM-EPCs) have been proven to increase the
capillary density and the rate of limb salvage in a murine model of
hind limb ischemia (10–12). However, a critical limitation for
the therapeutic application of adult EPCs is their low number in
circulation (13). More
importantly, the numbers and functional activity of the adult EPC
population have been found to decrease with age (14), and body disease conditions,
including type II diabetes (15)
and heart failure (16–18). These causes severely limit their
clinical application. Human UCB-EPCs have also been found to
promote neovascularization (19).
In contrast to adult BM- or PB-EPCs, UCB-EPCs contains a
significantly higher frequency of EPCs (20), and have distinctive proliferative
advantages, including a greater number of colonies, a longer
telomere and a higher cell-cycle rate (19,21). Moreover, UCB transplants have been
shown to be associated with a lower incidence of and less severe
graft-versus-host disease than BM and PB transplants in allogeneic
transplantation (22–24). The immediate availability of cells
and the absence of risk to the donor are the additional benefits of
UCB-derived cells in clinical transplantation. These findings
collectively indicate that human UCB is a more valuable source of
EPCs for future clinical application (25,26).
The improvements of transplanted UCB-EPCs have been
reported in various animal models of ischemic diseases. Using a
mouse model of hind limb ischemia, Yang et al reported that
expanded EPCs transplanted via the tail vein incorporated into
capillary networks, augmented neovascularization and improved
ischemic limb salvage (27).
Another study demonstrated that the expanded UCB-EPCs significantly
improved left ventricular ejection fraction in a rat model of
myocardial infarction (28).
Additionally, human UCB-EPCs have been shown to exert protective
effects on experimental acute kidney injury (29). However, these studies do not
provide uniform rules for cell passage selection in the treatment
of ischemia. More importantly, there is no evaluation of the
angiogenic properties of UCB-EPCs in the process of in vitro
expansion. The changes of cell quality and functional activity
induced by the in vitro expansion and subculture will
essentially influence the therapeutic effects of cytotherapy, and
the underlying mechanisms are also unknown.
As an important angiogenesis-related receptor,
PDGFR-β plays important roles in the angiogenic behavior of EPCs.
In previous studies, Guo et al found that bFGF triggered
PDGFR-β to promote the proliferation and migration of EPCs
(30). PDGF-BB and PDGFR-β have
been shown to influence EPC-mediated angiogenesis in differentiated
endothelial cells (31). As a
downstream target of PDGFR-β, studies have revealed that the
phosphoinositide 3-kinase (PI3K)/Akt pathway is involved in cell
proliferation, migration, differentiation and angiogenesis
(32). In particular, the
PI3K/Akt pathway has been found to participate in PDGF-BB-induced
proliferation and migration, and in the angiogenesis of EPCs
through PDGFR-β (33).
Accordingly, it is reasonable to explore the role of
PDGFR-β/PI3K/Akt in the angiogenic property changes of in
vitro expanded EPCs. In this study, we isolated EPCs from human
UCB. In the process of in vitro expansion, we examined the
changes of cellular properties at passage 2, 4, 6, and 8, including
the proliferative ability, the apoptotic rate, the telomere length
and the expression of surface markers. Additionally, the angiogenic
potential of EPCs at different passages was evaluated by vascular
formation assay in vitro. The therapeutic effects of EPCs at
different passages were then examined and analyzed in a mouse model
of hind limb ischemia. For further investigation of the mechanisms
involved, the expression of angiogenic-related factors,
particularly angiogenesis-related receptors, was measured by qPCR
and western blot analysis. Finally, the involvement of the PI3K/Akt
signaling pathway in the decreased angiogenic properties of EPCs
was verified. These findings may enhance our understanding of the
mechanisms of EPC characteristic changes in the process of in
vitro expansion, and may aid in pre-determining which passage
of EPCs will be of value for cell-based clinical therapies for
ischemic disease.
Materials and methods
Ethics statement
The study protocol was approved by the Central South
University Institutional Review Board. All methods used in this
study were carried out in accordance with the approved Ethical
Guidelines of Central South University. Informed consent was
obtained from all subjects prior to the study.
Isolation and culture of EPCs
Cord blood (CB) was obtained from 10 normal
full-term deliveries in the Women and Child Health Hospital of
Hunan Province. UCB-EPCs were isolated and cultured as previously
described (34). Briefly, CB was
diluted 1:1 with Dulbecco's phosphate-buffered saline (DPBS; Gibco,
Grand Island, NY, USA), and then overlaid onto 1.077 g/ml
Ficoll-Paque™ Premium (GE Healthcare, Logan, UT, USA). The liquid
was centrifuged for 30 min at 400 × g. Monocytes were collected and
washed with DPBS. The cells were seeded on tissue culture plates
coated with fibronectin (Millipore, Billerica, MA, USA) in EGM-2
(Lonza, Rockland, ME, USA) at 37°C, 5% CO2 humidified
incubator. The culture medium was changed every other day until the
EPC colonies appeared. The cells were harvested for expansion and
freezing after they reached 80–90% confluence.
Isolation and culture of mesenchymal stem
cells (MSCs)
Human adipose tissues were obtained from Xiangya
Hospital of Central South University (Changsha, China) and digested
with 2 mg/ml collagenase I, 2 U/ml dispase and 2 mg/ml
hyaluronidase (all purchased from Sigma-Aldrich, St. Louis, MO,
USA) for 90 min at 37°C. The digested tissues were centrifuged
(1,000 rpm for 10 min) and the stromal vascular fraction (SVF) was
washed with DPBS. SVF was then cultured in Dulbecco's modified
Eagle's medium-F12 (DMEM/F-12) containing 10 ng/ml basic fibroblast
growth factor (bFGF; Gibco) and 10% fetal bovine serum (FBS). The
medium was changed every 2 days. The cells were harvested for
expansion and freezing when the cells reach 80–90% confluence. The
cells at passage 4 were used for the following experiments.
Flow cytometric analysis
The EPC single-cell suspension was generated into
the concentration of 1×107 cells/ml. The cells were then
incubated respectively with anti-human CD31-FITC (eBioscience, San
Diego, CA, USA), vascular endothelial growth factor receptor
(VEGFR2)/KDR-PE (R&D Systems, Minneapolis, MN, USA), CD144-FITC
(Abcam, Cambridge, UK), CD34-PE, CD45-FITC (both from Biolegend,
San Diego, CA, USA), CD14-FITC (eBioscience), CD29-PE, CD90-PE and
SSEA4-PE (all from Biolegend). Briefly, 100 µl cell
suspension was incubated with 5 µl antibody solution at 4°C
for 30 min in the dark. After washing twice with phosphate buffer
saline (PBS), cells were resuspended in 400 µl PBS and
analyzed with a FACSAria I (Becton-Dickinson, San Jose, CA, USA)
and Becton-Dickinson CellQuest software.
Apoptosis assay
The Alexa Fluor 488 Annexin V and prop-idium iodide
kit (Invitrogen, Carlsbad, CA, USA) was used for the analysis of
apoptosis. Briefly, 1×105 cells were harvested and
washed twice with cold PBS, then resuspended in 100 µl
binding buffer. Subsequently, 5 µl Annexin V-FITC and 1
µl propidium iodide were added to the solution. Following 15
min of incubation, 400 µl binding buffer were added to the
solution, and the cells were analyzed using a flow cytometer (BD
Accuri™ C6 Flow Cytometer; BD Biosciences San Jose, CA, USA).
Terminal
deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL)
assay
The TUNEL apoptosis detection kit (Beyotime,
Shanghai, China) was also used for the analysis of cell apoptosis.
Briefly, the EPCs were fixed with 4% paraform/PBS, followed by
permeabilization with 0.1% Triton X-100 for 2 min on ice. The cells
then underwent TUNEL staining in the dark for 1 h at 37°C. After
washing twice with PBS, the suspension was analyzed by flow
cytometry (BD Accuri™ C6 Flow Cytometer; BD Biosciences).
EPC proliferation assay
EPCs at passage 2, 3, 4, 5, 6, 7 and 8 were seeded
at 1×105 cells/well for 4 wells in 6-well plates,
respectively. Following 3 days of incubation in EGM-2, the cells
were digested with TrypLE Express (Gibco) and resuspended into
single-cell suspension, followed by counting under a light
microscope (IX71; Olympus, Tokyo, Japan). The proliferation index
was calculated as follows: proliferation index = total number at
day 3/1×105.
EPC migration assay
In order to measure the migration of the EPCs,
1.5×105 cells at passage 4, 6 and 8 with or without
pre-treatment with tyrphostin AG1295 (Sigma-Aldrich) at 20
µM for 1 h were seeded in the upper Transwell chamber (BD
Biosciences) in serum-free medium, with 500 µl DMEM with 10%
FBS in the lower chamber. After 24 h, cells that did not migrate
through the pores were carefully wiped out with a cotton-tipped
swab. The filters were fixed in 90% alcohol, followed by staining
with 0.1% crystal violet (Meryer, Shanghai, China). After washing
with PBS 3 times, the filters were observed under an inverted
microscope (Olympus).
Western blot analysis
To examine protein expression in PDGF-BB-stimulated
cells, the EPCs were harvested and lysed. Proteins were subjected
to sodium dodecyl sulfate-poly-acrylamide gel electrophoresis
(SDS-PAGE) and transferred onto PVDF membranes. The membranes were
incubated at 4°C with primary antibodies overnight
[anti-platelet-derived growth factor receptor-β (PDGFR-β; ab32570),
anti-phospho-PDGFR-β (ab16868), anti-PI3K (ab86714),
anti-phospho-PI3K (ab182651), anti-Akt (ab8805), anti-phospho-Akt
(ab38449), or anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
ab9485); all from Abcam], and then stained with horseradish
peroxidase-coupled secondary antibodies (ab131366; Abcam). Finally,
the bands were visualized by chemiluminescence (Amersham Pharmacia
Biotech, Amersham, UK).
PDGF-BB stimulation and inhibitor
pre-treatment
To examine the effects of PDGFR-β, EPCs at passage
4, 6 and 8 were pre-treated with PDGF-BB (PeproTech, Rocky Hill,
NJ, USA) at 40 ng/ml for 24 h. The cells were then used in the
subsequent experiments. To examine whether the PDGFR-β/PI3K
signaling pathway is involved in the PDGF-BB-induced biological
function changes of EPCs, EPCs were treated with 20 mM tyrphostin
AG1295 (Sigma-Aldrich) for 1 h, followed by PDGF-BB stimulation as
mentioned above. The cells were then used in the subsequent
experiments.
Analysis of telomere length by qPCR
Chromosomal DNA was extracted using Qiagen DNeasy
Blood and Tissue kit according to the manufacturer's instructions.
DNA from human embryonic stem cells was used as control or
reference DNA. DNA was used as templates in SYBR-Green qPCR with
specific primers. The primer sequences for telomere (tel) and 36B4
(single copy gene) genes were as follows: tel (tel1b, CGG TTT GTT
TGG GTT TGG GTT TGG GTT TGG GTT TGG GTT; tel2b, GGC TTG CCT TAC CCT
TAC CCT TAC CCT TAC CCT TAC CCT); 36B4 (36B4u, CAG CAA GTG GGA AGG
TGT AAT CC; 36B4d, CCC ATT CTA TCA TCA ACG GGT ACA A). Two PCR runs
were performed for each sample: one to determine the cycle
threshold (Ct) value for telomere; the other to determine the Ct
value for the amplification of 36B4. PCR was performed in a total
volume of 20 µl, including 10 µl of SYBR-Green qPCR
mix, 1 µl of each forward and reverse primer (final
concentration: 400 nM for telomere; 300 nM for 36B4), 1 µl
each DNA sample and 7 µl H2O. Amplifications were
carried out in triplicate in 96-well microtiter plates. The thermal
cycling conditions for telomere PCR were as follows: 95°C for 10
min (stage 1), followed by 35 cycles of 95°C for 5 sec, 56°C for 10
sec, and 72°C for 1 min (stage 2), and finally followed by 95°C for
5 sec, and 60°C for 10 sec. For 36B4: 95°C for 10 min (stage 1),
followed by 40 cycles of 95°C for 5 sec, 58°C for 10 sec, 72°C for
40 sec.
Tube formation assay on Matrigel
A 96-well plate was covered with Matrigel (BD
Biosciences). The EPCs (4×103) were suspended in 50
µl EGM-2 and seeded on Matrigel. The plate was incubated at
37°C. Images of tubules were captured after 2 h using a Camera
Nikon TE2000-U (Nikon, Tokyo, Japan).
Angiogenesis co-culture model of
tubulogenesis
The MSCs were seeded onto 24-well plates at
3×104 cells/ml and incubated until 80% confluency. The
EPCs at passage 4, 6 and 8 were then seeded on the MSC monolayer at
2×104 cells/ml and incubated in EGM-2. After 6 days of
co-culture, UEA-I (Vector Laboratories, Burlingame, CA, USA) was
used to perform the staining of EPCs. Images were acquired using a
fluorescence microscope (ELX800; BioTek Instruments, Inc.,
Winooski, VT, USA) and a Nikon photographic system (Nikon Eclipse
Ti-S; Nikon). Quantification analysis was carried out using ImageJ
software (National Institutes of Health, Bethesda, MD, USA).
qPCR
Total RNA was extracted from the cells using TRIzol
reagent (Life Technologies, Shanghai, China) according to the
manufacturer's instructions. cDNA was synthesized using the
Transcriptor First Strand cDNA synthesis kit (Roche, Basel,
Switzerland). qPCR was performed using a Lightcycler 480 SYBR-Green
I Master system (Roche) according to the manufacturer's
instructions. GAPDH were used as an internal control. The sequences
of the human primers were as follows: VEGF-A sense, AGG GCA GAA TCA
TCA CGA AGT and antisense, AGG GTC TCG ATT GGA TGG CA; transforming
growth factor-β1 (TGF-β1) sense, CTA ATG GTG GAA ACC CAC AAC G and
antisense, TAT CGC CAG GAA TTG TTG CTG; PDGF-B sense, CTC GAT CCG
CTC CTT TGA TGA and antisense, CGT TGG TGC GGT CTA TGA G; ANG-1
sense, GCC TGA TCT TAC ACG GTG CTG and antisense, GCA TCA AAC CAC
CAT CCT CC; PDGFR-β sense, GGA GAG GGC AGT AAG GAG GA and
antisense, ATG GTG TCC TTG CTG CTG AT; TIE-2 sense, TGT GCT GTT CCT
TCT TGC CT and antisense, GCA CCT TCC ACA GTT CCA GA; VEGFR2 sense,
GCA GAA CAG TAA GCG AAA GAG and antisense, TGA GGC AAG AAC CAT ACC
ACT; interferon gamma receptor (IFNGR)1 sense, TAA ATG GAG ACG AGC
AGG AAG and antisense, TGA ATA CCA GGC TAA GCA CTA; IFNGR2 sense,
TTT AGA GTC GGG CAT TTA AGC A and antisense, TCA GGA CCA GGA AGA
AAC AGG; fibro-nectin 1 (FN1) sense, ACA AAC ACT AAT GTT AAT TGC
CCA and antisense, AAC TCC CAG GGT GAT GCT TG; laminin subunit
alpha 2 (LAMA2) sense, CTG TTG CTG ATA ACC TCC TCT T and antisense,
AGT TCT TGA TGC TAC GAT ACG G; integrin subunit beta 1 (ITGB1)
sense, CCT ACT TCT GCA CGA TGT GAT G and antisense, CCT TTG CTA CGG
TTG GTT ACA TT; integrin subunit alpha 1 (ITGA1) sense, GTG CTT ATT
GGT TCT CCG TTA GT and antisense, CAC AAG CCA GAA ATC CTC CAT;
collagen type IV alpha 1 chain (COL4A1) sense, CCA GGG GTC GGA GAG
AAA G and antisense: GGT CCT GTG CCT ATA ACA ATT CC; GAPDH sense,
AGA AGC CCA GCC AGT CGC CAT CA and antisense, AGC AAA GCC CGC CTT
ACA GAG CC. PCR was performed in a total volume of 20 µl,
including 10 µl of SYBR-Green qPCR Mix, 1 µl of each
forward and reverse primer (10 µmol/l), 1 µl each
cDNA sample, and 7 µl H2O. Amplifications were
carried out in triplicate in 96-well microtiter plates. Thermal
cycling conditions were as follows: 95°C for 5 min, followed by 45
cycles of 95°C for 10 sec, 60°C for 10 sec, and 72°C for 10 sec,
and finally followed by 95°C for 5 sec, and 60°C for 10 sec.
Establishment of mouse model of hind limb
ischemia and cell transplantation
Procedures involving animals and their care were
conducted in conformity with NIH guidelines (NIH Publication no.
85-23, revised 1996) and was approved by the Animal Care and Use
Committee of the Central South University. A total of 30 male
BALB/C nude mice, weighing 20–25 g were anesthetized with 4%
chloral hydrate by intraperitoneal injection. The right femoral
artery and vein were coagulated and then cut out to induce critical
ischemia at day 0. Twenty-four hours later (day 1), the mice were
randomly divided into 3 groups and received cell transplantations
by tail vein injection: EPCs at passage 4, EPCs at passage 6 and
EPCs at passage 8 (n=10).
Laser doppler perfusion imaging
The mice were anesthetized using 4% chloral hydrate,
and then examined using a Laser Doppler Perfusion Imager (LDPI;
Moor Instruments, Devon, UK) on days 0, 7, 14, 21 and 28. The
animal was placed on a 37°C heating pad for 2–5 min to allow
acclimation to the ambient conditions before measurements were
taken. The results are reported as the perfusion ratio of the
ischemic limb relative to the contralateral untreated hind
limb.
Histological analysis
Mice were euthanized at day 28. The quadriceps
femoris muscles of the mouse hindlimbs were isolated and fixed in
10% buffered formalin, dehydrated in 30% sucrose solution and
embedded in paraffin. Sections (7 µm-thick) were cut and
mounted on slides. The samples were used for hematoxylin and eosin
(H&E) staining (Beyotime) and Masson's trichrome staining (Baso
Diagnostics Inc., Zhuhai, China). The sections were observed and
captured using a microscope (Sunny CX40; Sunny, Guangdong, China)
and the corresponding SmartV350Dc software.
Statistical analysis
All experiments were repeated at least 3 times
independently. Data are expressed as the means ± standard deviation
(SD). Comparisons between groups were performed by one-way ANOVA
using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). For animal exterior
recovery study, Kruskal-Wallis was used. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of human UCB-EPCs at
different passages
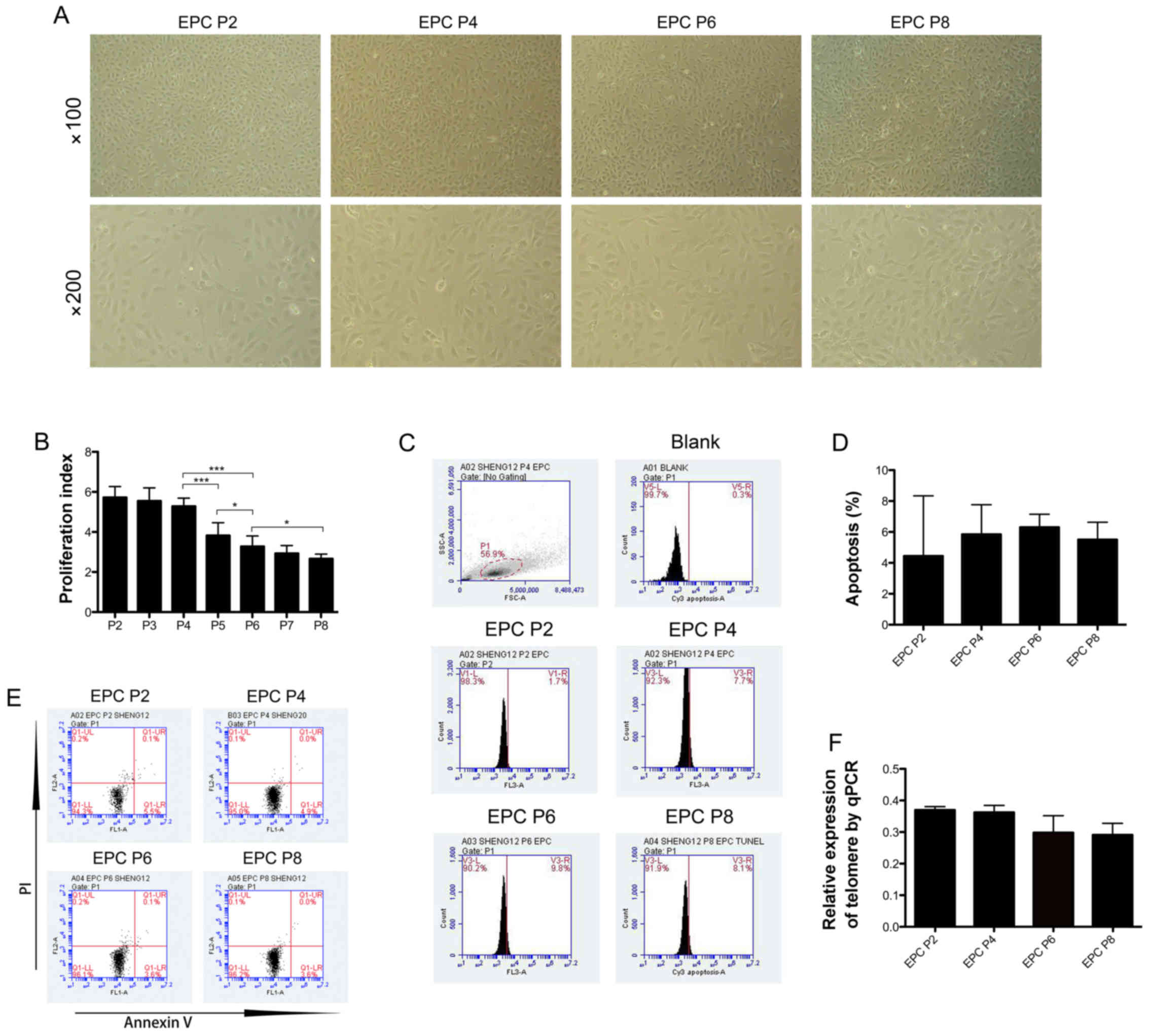
EPCs were isolated from human UCB. In serial
subculture, the EPCs exhibited a uniform cobblestone morphology,
with no observed differences among the cells at different passages
(Fig. 1A). In order to measure
the proliferative ability of the EPCs, cells at different passages
were seeded quantitatively in culture plates, and the total cell
number after 3 days of culture was calculated to reflect the
proliferation index. The proliferative ability of the EPCs was
significantly decreased with the increase number of passages in
culture (Fig. 1B). In subsequent
experiments, we used EPCs at passage (P)2, P4, P6 and P8 for the
examination of cellular properties. The cell apoptotic rate was
then analyzed by TUNEL assay (Fig.
1C). No significant difference was observed in the apoptotic
rate of the EPCs at different passages, and the average apoptotic
rate was 4.45±2.75% at P2, 5.85±1.35% at P4, 6.3±0.6% at P6 and
5.5±0.8% at P8 (Fig. 1D). This
trend was then confirmed by Annexin V/PI staining (Fig. 1E). In addition, the quantification
of telomere length was measured by qPCR in order to determine the
senescence of EPCs at different passages. The results revealed that
although no significant changes were observed in telomere length
among the EPCs at P2, P4, P6 and P8, telomere length exhibited a
decreasing trend as the passage number increased in culture
(Fig. 1F).
Changes in surface marker expression in
human UCB-EPCs in subculture
The expression of surface markers was analyzed
(Fig. 2A). In the expansion
process from passage 2 to 8, all EPCs homogeneously exhibited
positive expression for the endothelial marker, CD31 (>90%), and
the mesenchymal marker, CD29 (>95%), and expressed low levels of
monocyte differentiation antigen CD14 (<7%),
hematopoietic-related antigen CD45 (<6%) and SSEA4 (<4%)
(Fig. 2B). The expression of CD34
was maintained at approximately 40%. of note, the expression of
CD90 (from 2.79±2.12% at P2 to 1.3±0.44% at P8, P<0.05) and that
of the endothelial markers, CD144 (VE-Cadherin) (from 50.18±23.75%
at P2 to 15.86±8.77% at P8, P<0.01) and KDR (VEGFR2) (from
53.32±14.63% at P2 to 30.28±18.48% at P8, P<0.01), was
downregulated with increasing number of passages (Fig. 2B). This indicated that the EPC
phenotype was partly altered during the process of in vitro
expansion.
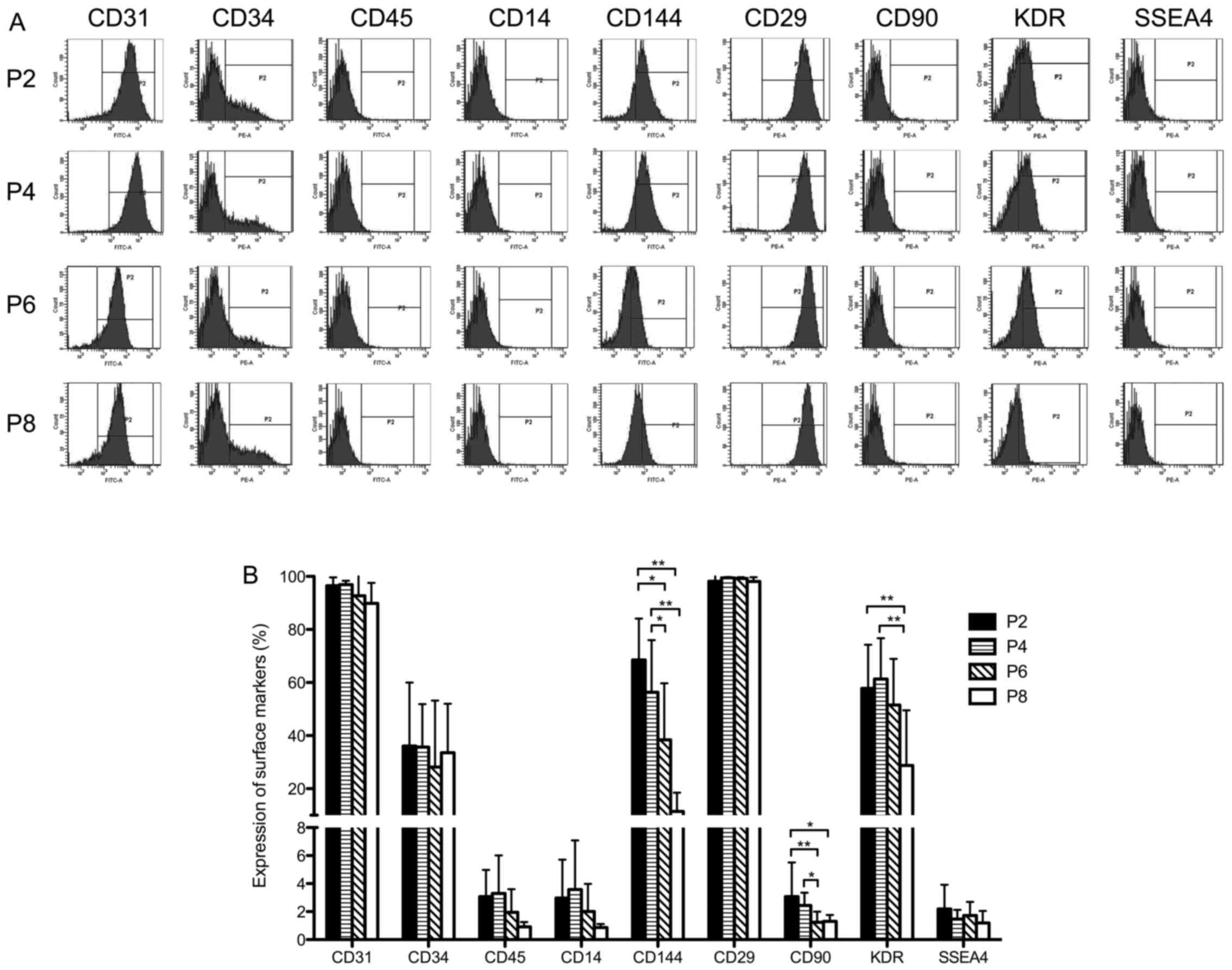 | Figure 2Surface marker detection of human
umbilical cord blood-derived endothelial progenitor cells
(UCB-EPCs) at passage 2, 4, 6 and 8. (A) Representative images of
cytometric analysis of human UCB-EPCs. Cells at passage 2, 4, 6 and
8 were labeled with antibodies against CD31, CD34, CD45, CD14,
CD144, CD29, CD90, KDR and SSEA4. The 'P2' region marked in each
plot stands for the range of positive expression. (B) Statistical
analysis of surface marker expression on EPCs. Bars represent the
mean values ± SD of 10 independent experiments.
**P<0.01; *P<0.05. |
EPCs at P4 and P6 exhibit better
angiogenic properties in vitro
Since there was no difference observed in cellular
properties between the EPCs at P2 and the EPCs at P4, including
proliferative ability, apoptotic rate, telomere length and surface
marker expression, and the total cell number obtained at P4 is
greater than that at P2, we selected the EPCs at P4, P6 and P8 for
use in further experiments.
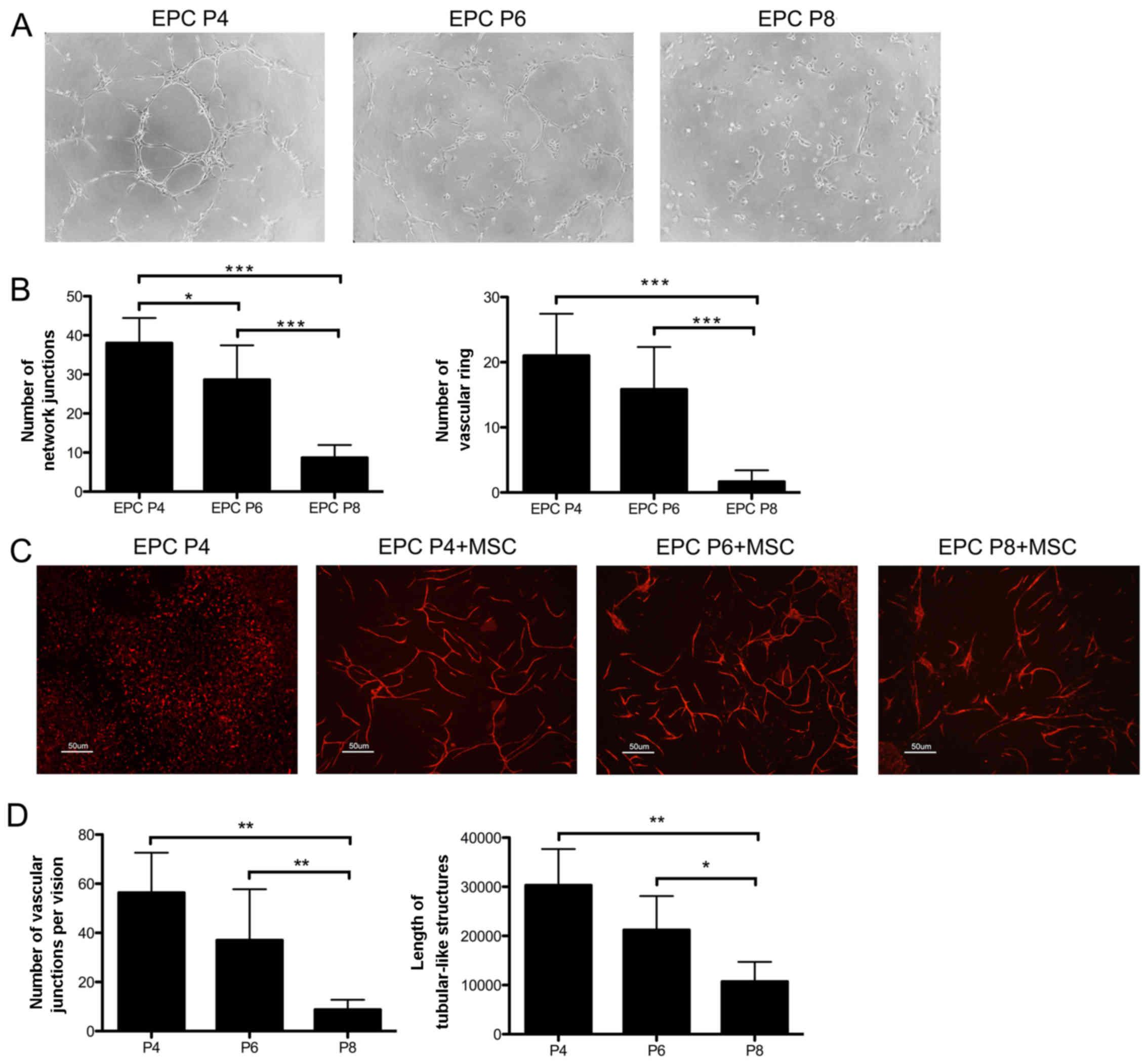
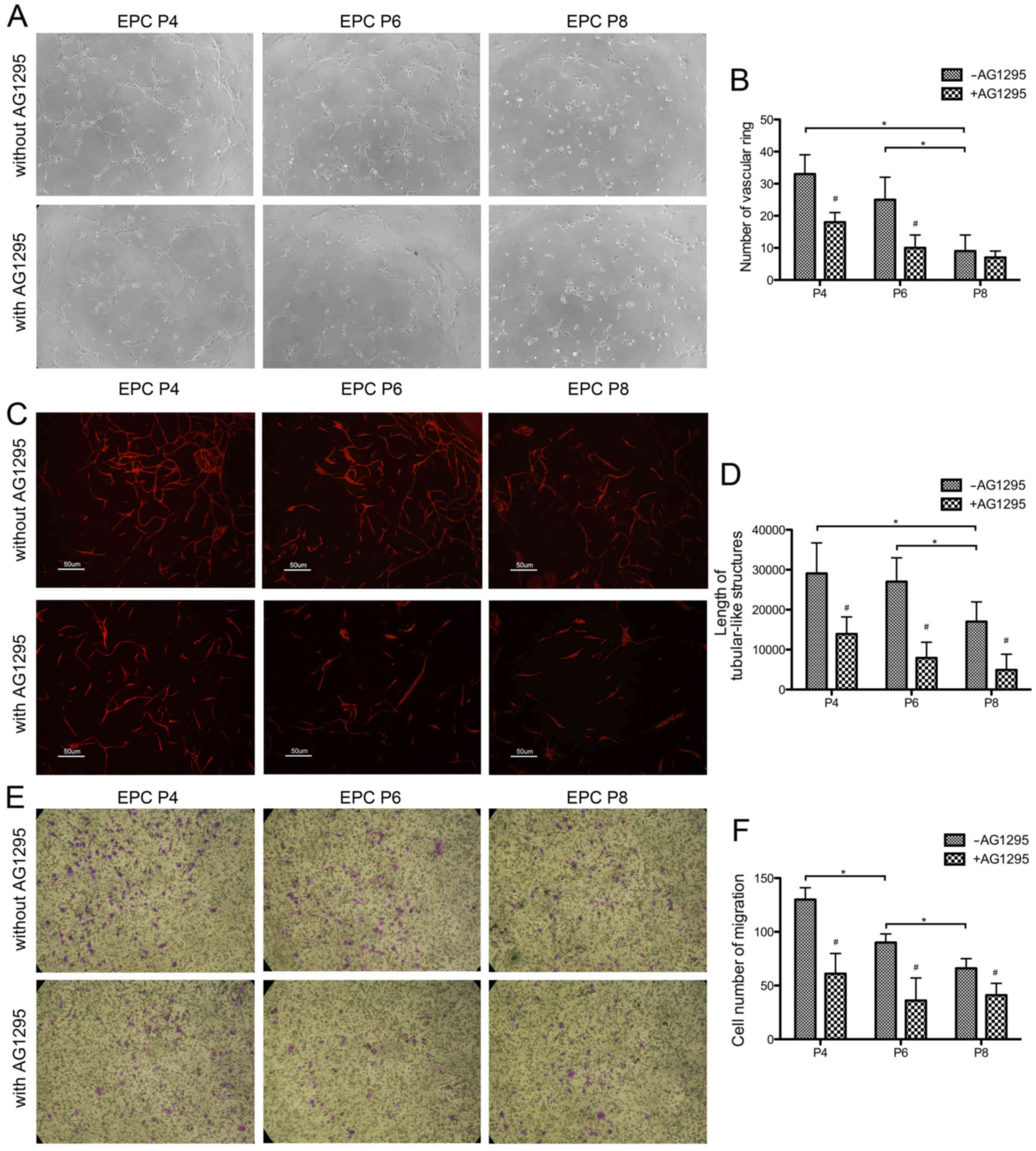
To compare the angiogenic ability of the EPCs at
different passages in vitro, the EPCs was seeded on
Matrigel. As shown in Fig. 3A,
the EPCs at P4, P6 and P8 all assembled into tubular-like
structures, but the more integrated network formed by the EPCs at
P4 was not observed in the EPCs at P6 and at P8 in particular. In
addition, the EPCs at P4 and P6 formed more network junctions and
vascular rings than those at P8 (P<0.001) (Fig. 3B).
The EPCs were subsequently seeded on the monolayer
of MSCs. After 6 days of culture, EPCs without feeders only
exhibited a scattered distribution, while the EPCs seeded on MSCs
formed capillary-like networks (Fig.
3C). Notably, the EPCs at P4 and P6 assembled into more
organized and integrated networks than the EPCs at P8, which was
quantified by an increased number of vascular junctions (P4,
P<0.01, P6, P<0.01, compared with P8) and longer tubular-like
structures (P4, P<0.01, P6, P<0.05) (Fig. 3D). Thus, the EPCs at P4 and P6
exhibited an enhanced angiogenic ability in vitro. These
findings collectively incidated that the expanded EPCs at different
passages exhibited inequable angiogenic properties in
vitro.
Transplantation of EPCs at P4 and P6
exerts more enhanced therapeutic effects by promoting
neovascularization in a mouse model of hind limb ischemia
The angiogenic properties of the EPCs were further
examined in a mouse model of hind limb ischemia. After 24 h of
right femoral artery ligation and excision surgery, the EPCs at P4,
P6 and P8 were transplanted into mice by tail intravenous
injection. Blood perfusion was detected using a LDPI on days 0, 7,
14, 21 and 28 (Fig. 4A). The
statistical analysis of the blood perfusion rate in the leg
revealed no significances among the different mouse groups
transplanted with EPCs at different passages, although the
transplation of of EPCs at P4 on day 21 showed a certain advantage
(Fig. 4B). However, the perfusion
condition in the paw revealed the statistical superiority of EPCs
at P4 and P6. The transplantation of EPCs at P6 (0.46±0.25) more
efficiently improved blood flow in the paw of the ischemic limb on
day 7 compared with the transplantion of EPCs at P8 (0.22±0.11;
P=0.017). On day 14, the transplantion of EPCs at P4 (0.56±0.16,
P=0.002) and P6 (0.51±0.18, P=0.014) led to a relatively higher
perfusion rate than the transplantion of EPCs at P8 (0.32±0.11). No
statistically significant difference was observed among the 3
groups on days 21 and 28 (Fig.
4C).
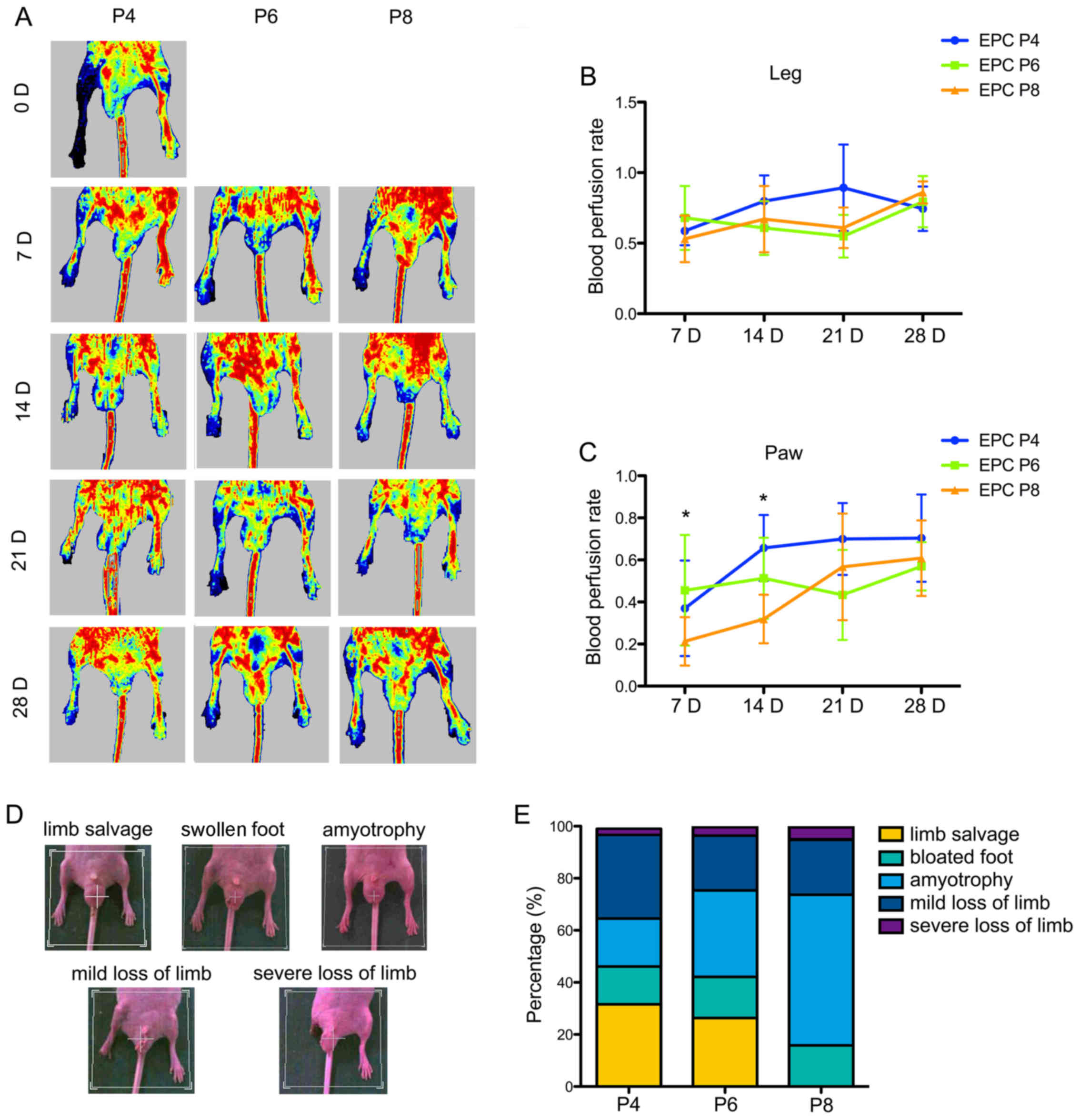 | Figure 4Evaluation of the therapeutic
efficacyof umbilical cord blood-derived endothelial progenitor
cells (UCB-EPCs) in a murine model of hind limb ischemia. (A)
Representative images of perfusion heatmaps in different cell
groups on days 0, 7, 14, 21 and 28. EPCs at passage 4, 6, and 8
were transplanted by tail intravenous injection 24 h after right
femoral and saphenous artery ligation. Laser Doppler perfusion
imaging (LDPI) was used to visualize the dynamic changes in hind
limb perfusion at the indicated time points. (B) Statistical
analysis of blood perfusion rate in the mouse leg. Blood perfusion
was quantified using the perfusion rate, i.e., the rate of average
LDPI index of ischemic limb (left) to non-ischemic hind limb
(right). Bars represent the mean perfusion rate ± SD of 10 mice in
each group. (C) Statistical analysis of blood perfusion rate in the
mouse paw. Bars represent the mean perfusion rate ± SD of 10 mice
in each group. *P<0.05. (D) Representative images of
5 progressive exterior morphological recovery levels of ischemic
mice on day 28, including limb salvage, bloated foot, amyotrophy,
mild loss of limb and severe loss of limb. (E) Percentage bar chart
of exterior recovery statistics in different cytotherapy
groups. |
We defined hind limb recovery after 28 days as five
progressive levels, including limb salvage, swollen foot,
amyotrophy, mild loss of limb and severe loss of limb (Fig. 4D). The proportion of limb salvage
in the groups transplanted with EPCs at P4 and P6 was approximately
30%, whereas the injection of EPCs at P8 resulted in almost no
final limb salvage (Fig. 4E). The
transplantation of EPCs at P8 caused approximately 60% amyotrophy.
Notably, the rate of limb loss (including mild and severe loss of
limb) in the 3 groups was >20%, and was even >30% in the
group injected with EPCs at P4.
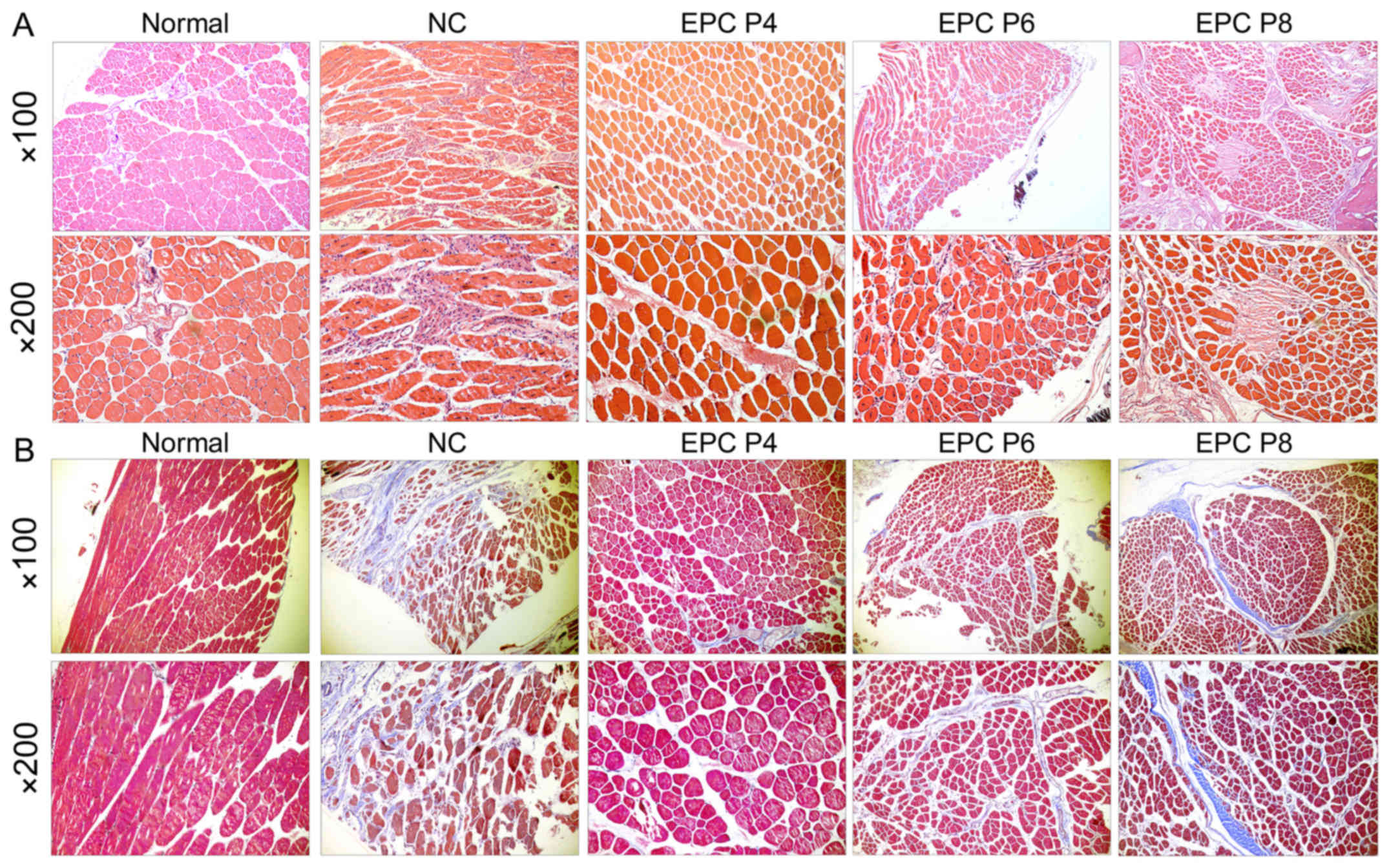
We further evaluated the therapeutic effects of EPCs
at different passages on muscle degradation of the ischemic hind
limb by histological examination. H&E staining revealed that
following the transplantation of EPCs at P4, the muscle fibers were
arranged neatly in a regular round shape, with small gaps among the
muscle bundles (Fig. 5A). The
nucleus located on the edge of the muscle fibers. These were very
similar to the normal control group. However, in the groups
transplanted with EPCs at P6 and P8, the muscle fibers became
smaller and wizened, with the nucleus located in the center of the
muscle fibers. The gap among the muscle bundles became larger, and
was filled with the infiltrated cells and hyperplastic connective
tissue. Fibrous morphology was even observed in the group
transplanted with EPCs at P8. These features were similar to those
of the negative control (NC) group, although to a lesser extent.
Furthermore, Masson's trichrome staining revealed that fibrosis was
markedly attenuated following the transplantation of EPCs, compared
with the negative control (Fig.
5B). The EPCs at P4 and P6 exerted more positive effects.
Phosphorylation levels of PDGFR-β and
PI3K/Akt are downregulated in EPCs at P8 compared with EPCs at
P4
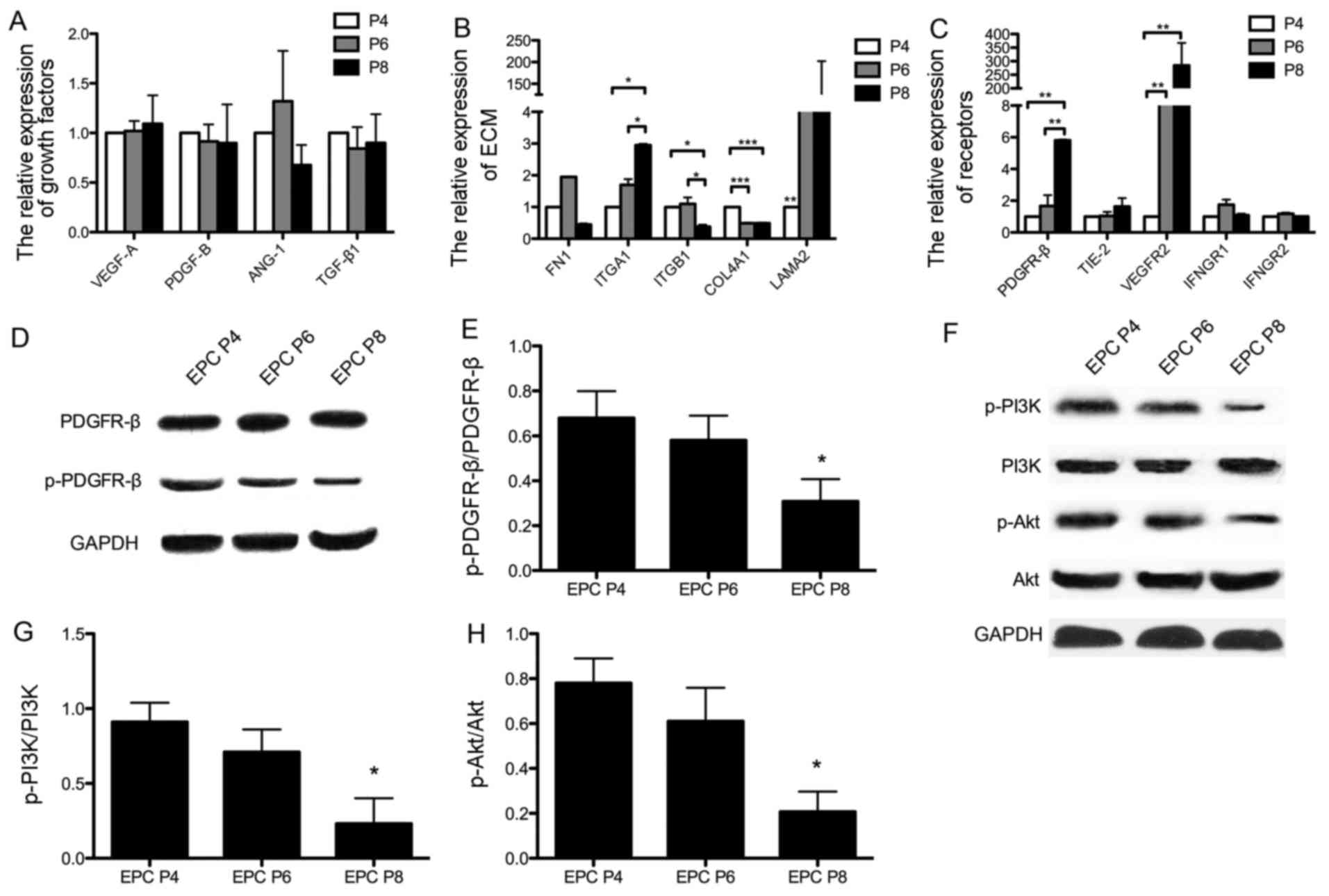
For further analysis, we detected the expression of
angiogenic-related factors in the EPCs at different passages by
qPCR. Although there were no statistically significant differences
observed in the expression of angiogenic cytokines (such as VEGF-A,
PDGF-B, ANG-1 and TGF-β1) among the EPCs at different passages
(Fig. 6A), significant changes
were observed in the expression of some extracellular matrix (ECM)
components (Fig. 6B). The
expression levels of ITGA1 and LAMA2 in the EPCs at P8 were higher
when compared with those in the EPCs at P4, and the expression
levels of ITGB1 and COL4A1 were significantly decreased in the EPCs
at later passages.
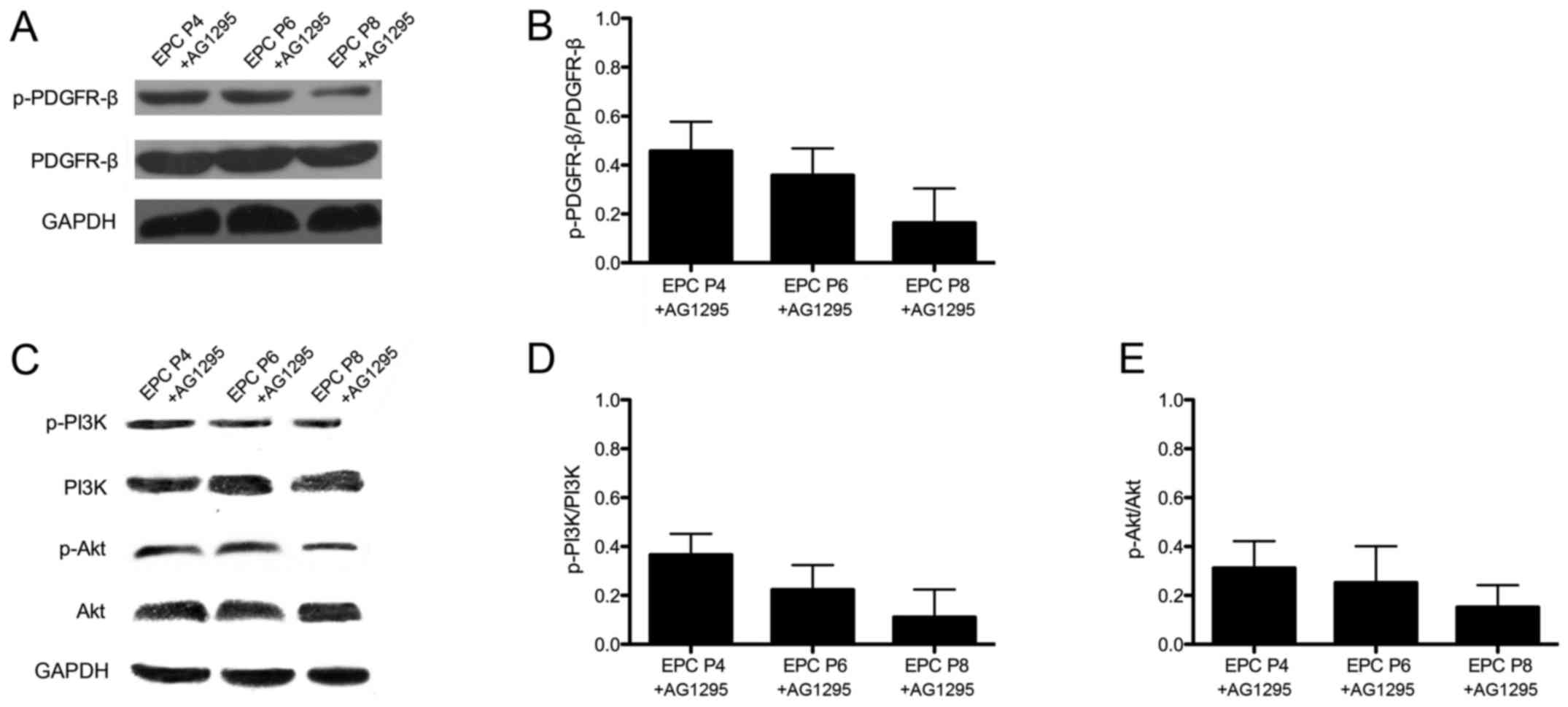
In addition, angiogenic-related receptors on EPCs
were measured following PDGF-BB stimulation. As shown in Fig. 6C, both PDGFR-β and VEGFR2 were
significantly highly expressed in the EPCs at P8. However, when the
receptor expression was measured by western blot analysis (Fig. 6D), the level of phosphorylated
PDGFR-β was found to be significantly decreased in the EPCs at P8
compared to those at P4 (P<0.05; Fig. 6E). Since the binding of PI3K to
PDGFR-β has been shown to be important for cell behavior (32), we further examined whether the
PDGFR-β/PI3K/Akt signaling pathway is involved in EPCs by examining
the phosphorylation levels of PI3K and Akt by western blot analysis
(Fig. 6F). The results of
statistical analysis indicated that the phosphorylation levels of
PI3K and Akt were both significantly decreased in the EPCs at P8
compared with those at P4 (P<0.05 and P<0.05; Fig. 6G and H).
Effects of PDGFR inhibitor on
tubulogenesis and migration of EPCs at different passages
We further examined whether PDGFR-β plays a role in
the changes of angiogenesis and migration ability among the EPCs at
different passages. The selective inhibitor of PDGFR, tyrphostin
AG1295, was used to inhibit the activation of PDGFR-β. Treatment
with tyrphostin AG1295 led to less interconnected vascular network
being formed by the EPCs at passage 4, 6 and 8 (Fig. 7A). No significant difference was
observed among the groups of EPCs at different passages (Fig. 7B). In addition, when seeded on the
monolayer of MSCs, the EPCs at different passages pretreated with
tyrphostin AG1295 formed a smaller number of tubular-like
structures compared with the cells not treated with tyrphostin
AG1295 (Fig. 7C), and there was
no significant difference observed among the tyrphostin
AG1295-treated groups (Fig. 7D).
Furthermore, the cell migration ability was examined by Transwell
assay (Fig. 7E). The EPC
migration ability decreased with the in vitro expansion
process without pre-treatment with tyrphostin AG1295 (P<0.05;
Fig. 7F). Treatment with
tyrphostin AG1295 led to significant decrease in migration in the
EPCs at each passage (P<0.05). However, following treatment with
tyrphostin AG1295, no significant difference was observed in
migration ability among the EPCs at different passages. These
results indicated that following treatment with the PDGFR
inhibitor, tyrphostin AG1295, the differences in angiogenesis and
migration ability of the EPCs at different passages were no longer
observed.
Treatment with PDGFR inhibitor leads to
similar phosphorylation levels of PDGFR-β and PI3K/Akt in EPCs at
different passages
As demonstrated above (Fig. 6D and E), the levels of
phosphorylated PDGFR-β were decreased in the EPCs with the
increasing passage number. In particular, the levels of
phosphorylated PDGFR-β were significantly decreased in the EPCs at
passage 8 compared to those at passage 4. Subsequently, in order to
confirm the effect of PDGFR inhibitor, the expression of PDGFR-β
was measured in the EPCs by western blotting (Fig. 8A) following treatment with the
PDGFR inhibitor, tyrphostin AG1295. Following pre-treatment with
the inhibitor, the EPCs at different passages exhibited no
significant difference in the levels of phosphorylated PDGFR-β when
stimulated with PDGF-BB (Fig.
8B). Moreover, we also measured the phosphorylation levels of
PI3K/Akt by western blot analysis in the EPCs pre-treated with the
inhibitor (Fig. 8C); no
significant difference was observed in the phosphorylation level of
PI3K/Akt among the EPCs at different passages (Fig. 8D and E). These findings indicated
that following pre-treatment with the PDGFR inhibitor, tyrphostin
AG1295, no significant differences were observed in the levels of
phosphorylated PDGFR-β and PI3K/Akt expression among the EPCs at
different passages.
Discussion
EPCs have the potential to differentiate into mature
endothelial cells and secreting cytokines (35,36), and they thus play a role in
endothelial repair and post-natal angiogenesis (1,2).
Due to the higher cell frequency (20) and the stronger cell proliferative
ability (19,21), human UCB has been defined as a
more ideal source of EPCs. UCB-EPCs have been a focus of
regenerative treatment for ischemic diseases, and a number of
studies have examined their application in ischemic diseases in
various animal models; however, some researchers have used EPCs at
ununiformed passages, such as passage 2–5 (37), passage 3–4 (29), passage 5 (38), or in some case, have not stated
the specific cell passage used (39,40). Thus, further supportive evidence
for cell passage selection in ischemic treatment is still needed.
In this study, we compared the cellular properties and angiogenic
potential of EPCs expanded at different passages, in order to
provide reference data to aid the selection of cells at the best
passage therapeutic effects on ischemia.
We first examined the cellular properties of the
EPCs at different passages, and found that the proliferation index
was decreased. Since telomere shortening has been shown to be
related to the proliferative ability of cells (41), we further detected the relative
telomere length of EPCs, which confirmed that the proliferative
ability of the EPCs at later passages decreased. This is in
accordance with the results of another study on in vitro
expanded human MSCs (41). In
addition, the CD markers exhibited an altered expression on the
EPCs. CD144, also known as VE-Cadherin, is an important adherent
junction (AJ) protein that is specifically responsible for
endothelial cell-cell AJ assembly and barrier architecture
(42–44). It has been proven that VE-cadherin
gene knockout leads to severe angiogenic defects, attributed to
endothelial apoptosis and abnormal VEGF signaling (45,46). Additionally, interfering with
VE-cadherin in embryos and adult mice has been shown to affect
vascular integrity (47,48). Furthermore, KDR, also known as
human VEGFR2, is largely restricted to vascular endothelial cells
(49). After being activated, KDR
triggers multiple downstream pathways to regulate endothelial
functions, such as cell migration, endothelium-dependent relaxation
and angiogenesis (49). It has
been reported that in Flk-1 (the counterpart of human KDR in mice)
knockout mice, endothelial cells fail to develop (50). In this study, as EPCs underwent
repeated passaging, the expression of VE-Cadherin and KDR
decreased, and this may diminish their angiogenic abilities by
influencing the normal endothelial function. This is also in
accordance with our results of angiogenesis assay in vitro
in this study, which revealed the decreased angiogenic ability of
the EPCs at P8. Additionally, CD90 is always used as a marker for a
variety of stem cells. It has been shown to be expressed by
endothelial cells in human tumors (51). Its downregulated expression in the
expansion process of our EPCs may indicate the declined stemness of
the cells. In this study, as there was no difference in cellular
properties between the EPCs at P2 and those at P4, and the total
cell number obtained after expansion at P4 was much greater than
that at P2, we selected EPCs at P4 as ideal candidates.
In an aim to evaluate the therapeutic effects of
EPCs at different passages on ischemia, a mouse model of hind limb
ischemia mouse was used for further research. Mice injected with
EPCs at different passages exhibited no statistically significant
differences in blood flow patterns, as shown by LDPI. However,
there is a limitation to this method as LDPI measurements cannot
accurately differentiate between skin perfusion and deeper muscular
perfusion. To partially overcome this limitation, we then analyzed
blood perfusion in the paws of mice, which we believe is more
likely to represent the actual perfusion of blood flow restoration.
The injection of EPCs at P4 led to a higher blood perfusion rate in
the paws on days 7 and 14, which was supported by the final higher
limb salvage rate and the better histomorphological performance in
the group injected with EPCs at P4.
In this study, we observed an interesting event.
Following stimulation with PDGF-BB, the expression of
angiogenic-related receptors was inconsistently detected, as shown
by qPCR and western blot analysis. The increased expression of
PDGFR-β in the EPCs at P8 at the mRNA level was not confirmed at
the protein level by western blot analysis. The detected levels of
phosphorylated PDGFR-β were even found to be downregulated in the
EPCs at P8 compared to those at P4. This may be attributed to the
epigenetic regulatory mechanism in the process of translation. The
specific details in this regulatort process warrant further
investigation.
In conclusion, in this study, we demonstrated that
EPCs in the process of in vitro expansion exhibit changes in
cellular properties, and EPCs at passage 4 are more efficient
promoting in tube formation and attenuating hind limb ischemia.
Therefore, the 4th passage of the in vitro expanded EPCs may
be the most ideal cell for the clinical treatment of ischemic
disease. These data may aid in the more effective sselection of
EPCs for the treatment of ischemic disease.
Acknowledgments
This study was supported by the National High
Technology Research and Development Program (863 program) of China
(grant no. 2011AA020113), the Science Project of the Department of
Science and Technology of Hunan Province, China (grant no.
2013SK5070), and the Fundamental Research Funds for the Central
Universities of Central South University (grant no. 2012zzts133).
We are grateful to the staff of the Women and Child Health Hospital
of Hunan Province, Reproductive and Genetic Hospital of
CITIC-XIANGYA, Xiangya Hospital of Central South University, and
the Third Xiangya Hospital of Central South University for
collecting cord blood and human body tissue samples. We are also
grateful to Dr Chen Yan for providing technological support for the
histological analysis in this study.
References
|
1
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krenning G, van Luyn MJ and Harmsen MC:
Endothelial progenitor cell-based neovascularization: Implications
for therapy. Trends Mol Med. 15:180–189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nathan DM, Cleary PA, Backlund JY, Genuth
SM, Lachin JM, Orchard TJ, Raskin P, Zinman B and Diabetes Control;
Diabetes Control and Complications Trial/Epidemiology of Diabetes
Interventions and Complications (DCCT/EDIC) Study Research Group:
Intensive diabetes treatment and cardiovascular disease in patients
with type 1 diabetes. N Engl J Med. 353:2643–2653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adeghate E: Molecular and cellular basis
of the aetiology and management of diabetic cardiomyopathy: A short
review. Mol Cell Biochem. 261:187–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Federman DG, Bravata DM and Kirsner RS:
Peripheral arterial disease. A systemic disease extending beyond
the affected extremity. Geriatrics. 59:2629–30, 32 passim.
2004.PubMed/NCBI
|
|
6
|
Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C,
Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, et al: Evidence
for circulating bone marrow-derived endothelial cells. Blood.
92:362–367. 1998.PubMed/NCBI
|
|
7
|
Hristov M, Erl W and Weber PC: Endothelial
progenitor cells: Mobilization, differentiation, and homing.
Arterioscler Thromb Vasc Biol. 23:1185–1189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Pastore C, Silver M, Kearne M, Magner M and Isner JM: Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculo-genesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rafii S and Lyden D: Therapeutic stem and
progenitor cell transplantation for organ vascularization and
regeneration. Nat Med. 9:702–712. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chavakis E, Aicher A, Heeschen C, Sasaki
K, Kaiser R, El Makhfi N, Urbich C, Peters T, Scharffetter-Kochanek
K, Zeiher AM, et al: Role of beta2-integrins for homing and
neovascularization capacity of endothelial progenitor cells. J Exp
Med. 201:63–72. 2005. View Article : Google Scholar
|
|
11
|
Kalka C, Masuda H, Takahashi T, Kalka-Moll
WM, Silver M, Kearney M, Li T, Isner JM and Asahara T:
Transplantation of ex vivo expanded endothelial progenitor cells
for therapeutic neovascularization. Proc Natl Acad Sci USA.
97:3422–3427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murasawa S and Asahara T: Endothelial
progenitor cells for vasculogenesis. Physiology (Bethesda).
20:36–42. 2005. View Article : Google Scholar
|
|
13
|
Iwami Y, Masuda H and Asahara T:
Endothelial progenitor cells: Past, state of the art, and future. J
Cell Mol Med. 8:488–497. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scheubel RJ, Zorn H, Silber RE, Kuss O,
Morawietz H, Holtz J and Simm A: Age-dependent depression in
circulating endothelial progenitor cells in patients undergoing
coronary artery bypass grafting. J Am Coll Cardiol. 42:2073–2080.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tepper OM, Galiano RD, Capla JM, Kalka C,
Gagne PJ, Jacobowitz GR, Levine JP and Gurtner GC: Human
endothelial progenitor cells from type II diabetics exhibit
impaired proliferation, adhesion, and incorporation into vascular
structures. Circulation. 106:2781–2786. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hill JM, Zalos G, Halcox JP, Schenke WH,
Waclawiw MA, Quyyumi AA and Finkel T: Circulating endothelial
progenitor cells, vascular function, and cardiovascular risk. N
Engl J Med. 348:593–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vasa M, Fichtlscherer S, Aicher A, Adler
K, Urbich C, Martin H, Zeiher AM and Dimmeler S: Number and
migratory activity of circulating endothelial progenitor cells
inversely correlate with risk factors for coronary artery disease.
Circ Res. 89:E1–E7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valgimigli M, Rigolin GM, Fucili A, Porta
MD, Soukhomovskaia O, Malagutti P, Bugli AM, Bragotti LZ,
Francolini G, Mauro E, et al: CD34+ and endothelial
progenitor cells in patients with various degrees of congestive
heart failure. Circulation. 110:1209–1212. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murohara T, Ikeda H, Duan J, Shintani S,
Sasaki K, Eguchi H, Onitsuka I, Matsui K and Imaizumi T:
Transplanted cord blood-derived endothelial precursor cells augment
postnatal neovascularization. J Clin Invest. 105:1527–1536. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Madlambayan G and Rogers I: Umbilical
cord-derived stem cells for tissue therapy: Current and future
uses. Regen Med. 1:777–787. 2006. View Article : Google Scholar
|
|
21
|
Mayani H and Lansdorp PM: Thy-1 expression
is linked to functional properties of primitive hematopoietic
progenitor cells from human umbilical cord blood. Blood.
83:2410–2417. 1994.PubMed/NCBI
|
|
22
|
Cohen Y and Nagler A: Umbilical cord blood
transplantation -how, when and for whom? Blood Rev. 18:167–179.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rocha V, Wagner JE Jr, Sobocinski KA,
Klein JP, Zhang MJ, Horowitz MM and Gluckman E; Eurocord and
International Bone Marrow Transplant Registry Working Committee on
Alternative Donor and Stem Cell Sources: Graft-versus-host disease
in children who have received a cord-blood or bone marrow
transplant from an HLA-identical sibling. N Engl J Med.
342:1846–1854. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu E, Law HK and Lau YL: Tolerance
associated with cord blood transplantation may depend on the state
of host dendritic cells. Br J Haematol. 126:517–526. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de La Selle V, Gluckman E and
Bruley-Rosset M: Newborn blood can engraft adult mice without
inducing graft-versus-host disease across non H-2 antigens. Blood.
87:3977–3983. 1996.PubMed/NCBI
|
|
26
|
Murohara T: Therapeutic vasculogenesis
using human cord blood-derived endothelial progenitors. Trends
Cardiovasc Med. 11:303–307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang C, Zhang ZH, Li ZJ, Yang RC, Qian GQ
and Han ZC: Enhancement of neovascularization with cord blood
CD133+ cell-derived endothelial progenitor cell
transplantation. Thromb Haemost. 91:1202–1212. 2004.PubMed/NCBI
|
|
28
|
Senegaglia AC, Barboza LA, Dallagiovanna
B, Aita CA, Hansen P, Rebelatto CL, Aguiar AM, Miyague NI, Shigunov
P, Barchiki F, et al: Are purified or expanded cord blood-derived
CD133+ cells better at improving cardiac function? Exp
Biol Med (Maywood). 235:119–129. 2010. View Article : Google Scholar
|
|
29
|
Burger D, Viñas JL, Akbari S, Dehak H,
Knoll W, Gutsol A, Carter A, Touyz RM, Allan DS and Burns KD: Human
endothelial colony-forming cells protect against acute kidney
injury: Role of exosomes. Am J Pathol. 185:2309–2323. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo S, Yu L, Cheng Y, Li C, Zhang J, An J,
Wang H, Yan B, Zhan T, Cao Y, et al: PDGFRβ triggered by bFGF
promotes the proliferation and migration of endothelial progenitor
cells via p-ERK signalling. Cell Biol Int. 36:945–950. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wyler von Ballmoos M, Yang Z, Völzmann J,
Baumgartner I, Kalka C and Di Santo S: Endothelial progenitor cells
induce a phenotype shift in differentiated endothelial cells
towards PDGF/PDGFRβ axis-mediated angiogenesis. PloS One.
5:e141072010. View Article : Google Scholar
|
|
32
|
Zhang H, Bajraszewski N, Wu E, Wang H,
Moseman AP, Dabora SL, Griffin JD and Kwiatkowski DJ: PDGFRs are
critical for I3K/Akt activation and negatively regulated by mTOR. J
Clin Invest. 117:730–738. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Wang YC, Hu XB, Zhang BF, Dou GR,
He F, Gao F, Feng F, Liang YM, Dou KF and Han H: Notch-RBP-J
signaling regulates the mobilization and function of endothelial
progenitor cells by dynamic modulation of CXCR4 expression in mice.
PloS One. 4:e75722009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoder MC, Mead LE, Prater D, Krier TR,
Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT and Ingram DA:
Redefining endothelial progenitor cells via clonal analysis and
hematopoietic stem/progenitor cell principals. Blood.
109:1801–1809. 2007. View Article : Google Scholar
|
|
35
|
Lavergne M, Vanneaux V, Delmau C, Gluckman
E, Rodde-Astier I, Larghero J and Uzan G: Cord blood-circulating
endothelial progenitors for treatment of vascular diseases. Cell
Prolif. 44(Suppl 1): 44–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moubarik C, Guillet B, Youssef B,
Codaccioni JL, Piercecchi MD, Sabatier F, Lionel P, Dou L,
Foucault-Bertaud A, Velly L, et al: Transplanted late outgrowth
endothelial progenitor cells as cell therapy product for stroke.
Stem Cell Rev. 7:208–220. 2011. View Article : Google Scholar
|
|
37
|
Kim J, Jeon YJ, Kim HE, Shin JM, Chung HM
and Chae JI: Comparative proteomic analysis of endothelial cells
progenitor cells derived from cord blood and peripheral blood for
cell therapy. Biomaterials. 34:1669–1685. 2013. View Article : Google Scholar
|
|
38
|
Kim SW, Jin HL, Kang SM, Kim S, Yoo KJ,
Jang Y, Kim HO and Yoon YS: Therapeutic effects of late outgrowth
endothelial progenitor cells or mesenchymal stem cells derived from
human umbilical cord blood on infarct repair. Int J Cardiol.
203:498–507. 2016. View Article : Google Scholar
|
|
39
|
Zhang Y, Li Y, Wang S, Han Z, Huang X, Li
S, Chen F, Niu R, Dong JF, Jiang R, et al: Transplantation of
expanded endothelial colony-forming cells improved outcomes of
traumatic brain injury in a mouse model. J Surg Res. 185:441–449.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liang CJ, Shen WC, Chang FB, Wu VC, Wang
SH, Young GH, Tsai JS, Tseng YC, Peng YS and Chen YL: Endothelial
progenitor cells derived from Wharton's Jelly of human umbilical
cord attenuate ischemic acute kidney injury by increasing
vascularization and decreasing apoptosis, inflammation, and
fibrosis. Cell Transplant. 24:1363–1377. 2015. View Article : Google Scholar
|
|
41
|
Samsonraj RM, Raghunath M, Hui JH, Ling L,
Nurcombe V and Cool SM: Telomere length analysis of human
mesenchymal stem cells by quantitative PCR. Gene. 519:348–355.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gavard J and Gutkind JS: VEGF controls
endothelial-cell permeability by promoting the
beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol.
8:1223–1234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Taddei A, Giampietro C, Conti A, Orsenigo
F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S and
Dejana E: Endothelial adherens junctions control tight junctions by
VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol.
10:923–934. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Heupel WM, Efthymiadis A, Schlegel N,
Müller T, Baumer Y, Baumgartner W, Drenckhahn D and Waschke J:
Endothelial barrier stabilization by a cyclic tandem peptide
targeting VE-cadherin transinteraction in vitro and in vivo. J Cell
Sci. 122:1616–1625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Carmeliet P, Lampugnani MG, Moons L,
Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R,
Oosthuyse B, Dewerchin M, et al: Targeted deficiency or cytosolic
truncation of the VE-cadherin gene in mice impairs VEGF-mediated
endothelial survival and angiogenesis. Cell. 98:147–157. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vittet D, Buchou T, Schweitzer A, Dejana E
and Huber P: Targeted null-mutation in the vascular
endothelial-cadherin gene impairs the organization of vascular-like
structures in embryoid bodies. Proc Natl Acad Sci USA.
94:6273–6278. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Crosby CV, Fleming PA, Argraves WS, Corada
M, Zanetta L, Dejana E and Drake CJ: VE-cadherin is not required
for the formation of nascent blood vessels but acts to prevent
their disassembly. Blood. 105:2771–2776. 2005. View Article : Google Scholar
|
|
48
|
Corada M, Mariotti M, Thurston G, Smith K,
Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro
A, Ruco L, et al: Vascular endothelial-cadherin is an important
determinant of microvascular integrity in vivo. Proc Natl Acad Sci
USA. 96:9815–9820. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Edirisinghe I and Rahman I: Cigarette
smoke-mediated oxidative stress, shear stress, and endothelial
dysfunction: Role of VEGFR2. Ann NY Acad Sci. 1203:66–72. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shalaby F, Rossant J, Yamaguchi TP,
Gertsenstein M, Wu XF, Breitman ML and Schuh AC: Failure of
blood-island formation and vasculogenesis in Flk-1-deficient mice.
Nature. 376:62–66. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Inoue A, Tanaka J, Takahashi H, Kohno S,
Ohue S, Umakoshi A, Gotoh K and Ohnishi T: Blood vessels expressing
CD90 in human and rat brain tumors. Neuropathology. 36:168–180.
2016. View Article : Google Scholar
|