Introduction
microRNAs (miRNAs or miRs) are a family of small
non-coding RNAs which modulate gene expression by binding to
complementary sequences of target mRNAs in the coding or non-coding
region such as the 3′ untranslated region (3′UTR) and 5′UTR
(1). The mature miRNAs cause post
transcriptional gene repression by increasing mRNA degradation or
by inhibiting translation (2). In
the human body, miRNAs play important roles in the responses to
injury or adaptation to chronic stress. More and more studies have
revealed that specific miRNAs can serve as biomarkers during
disease progression and development, such as disorders of the lung,
by regulating cell proliferation and differentiation (3–6).
Chronic obstructive pulmonary disease (COPD) is
considered as a type of airway disorder and respiratory disease,
which is associated with persistent inflammation (7). It may become the third leading cause
of death by 2020 globally (8).
Usually this type of chronic condition is influenced by a
combination of environmental, genetic and epigenetic components and
physiological changes. Different signaling pathways and important
molecular biomarkers involved in the progression of chronic
inflammation in lung disorders have been presented in miRNA studies
(9,10). miRNAs, such as miR-218 and
miR-128b, are regulators of smoking-induced gene expression
alterations in human airway epithelium (11). Scientists have also found that
cigarette smoke condensate increases the expression of miR-31 in
airway cells (12), and let-7d is
involved in idiopathic pulmonary fibrosis (13). Recently the expression of let-7c
and miR-125b in sputum samples from patients with COPD was
demonstrated to be much lower than levels in healthy controls
(14). In lung tissue samples
from patients with COPD, high expression of miR-199a-5p and miR-34a
is associated with downregulation of HIF-1α protein expression
which is important during the progression of COPD (15). We believe that numerous functional
miRNAs associated with COPD are still unknown.
Thus, a completed profile of alternative miRNAs in
lung samples from COPD patients and healthy donors was detected by
microarray analysis in this study. According to the results, 15
miRNAs with high expression and 15 with low expression were
selected and validated in vitro using real-time PCR.
Finally, miR-483-5p was selected as a study candidate and,
importantly, miR-483-5p transfection significantly inhibited the
transforming growth factor-β (TGF-β)-mediated decrease in cell
proliferation, and α-smooth muscle actin (α-SMA) and fibronectin
expression in BEAS-2B and HFL1 cells in vitro. Our results
suggest that miR-483-5p plays an important and protective role in
patients with COPD.
Materials and methods
Patient characteristics, clinical
features and serum harvest
This study was approved by the Third Xiangya
Hospital, Central South University (Hunan, China); and an informed
consent form (ICF) was provided by each participant. All of the
patients gave informed consent to have their tissues banked.
Lung tissue samples from 20 patients were included
in this study and were divided into two groups according to the
Global Initiative for Chronic Obstructive Lung Disease (GOLD)
classification. Ten samples were collected from patients with
normal lung function (no COPD, n=10); the rest of the samples were
from patients with diagnosed COPD (n=10; stage I/II/III, GOLD
classification). Table I
summarizes the patient characteristics and clinical features. The
diagnosis of emphysema was made by a pathologist based on
histological examination, and all of the lung tissue samples
collected from patients with COPD had centrilobular emphysema.
 | Table IDemographic, clinical and biological
data of the COPD patients and healthy controls in the miRNA screen
study. |
Table I
Demographic, clinical and biological
data of the COPD patients and healthy controls in the miRNA screen
study.
| COPD (N=10) | Healthy controls
(N=10) |
|---|
| Gender (male), n
(%) | 8 (80) | 1 (10) |
| Age (years) | 70.43 (±16.25) | 60.25 (±15.89) |
| FEV1/FVC, % | 55.70 (±7.28) | 81.35 (±5.92) |
| FEV1, %
predicted | 59.76 (±9.95) | 92.35 (±5.14) |
| BDR, % | 6.30 (±1.90) | 2.35 (±1.92) |
| Smoking,
pack-years | 41.00 (±32.09) | 0 |
| Current smoker,
n | 0 | 0 |
| Medication, n | | |
| Oral
corticosteroid | 1 | 0 |
| Inhaled
corticosteroid | 0 | 0 |
| Lung cancer
diagnosis, n | 9 | 2 |
All lung tissue samples were maintained at −80°C
until the processing of total RNA isolation.
Chemicals
TGF-β was purchased from Sigma (St. Louis, MO, USA).
Other chemicals were commercially available and purchased as
reagent grade from Sinopharm (Shanghai, China).
Cell culture and treatment
Human normal pulmonary epithelial BEAS-2B cells
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA) were cultured in growth media containing Roswell Park
Memorial Institute-1640 (RMPI-1640) medium, supplemented with no
serum and 1% penicillin-streptomycin (Mediatech, Herndon, VA, USA)
at 37°C in a humidified atmosphere of 5% CO2 in air.
Within the same culture condition, human normal lung fibroblast
HFL1 cells (ATCC) were cultured in growth media containing Ham's
F12K medium (F12K).
Before being diluted into single-cell suspensions
and seeded in 12-well plates (1×105 cells/ml), the cells
were treated with or without TGF-β (1 mg/ml) for 24 h, and then
transfected with or without different miRNA mimics. Finally, the
cells were harvested for total protein isolation. The cells
receiving no treatment served as the negative control group, and
cells with TGF-β only treatment served as the positive control
group.
Total RNA isolation and reverse
transcription
Total RNA from the lung sample short RNAs (<200
bp) was harvested and extracted using an RNA Mini Elute kit
(Qiagen, Venlo, The Netherlands) according to the manufacturer's
instructions. RNA quality was ascertained using Agilent 2100
bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). One
microgram of total RNA was reverse-transcribed and the product (11
μl) was pre-amplified using Megaplex PreAmp Primers and DBI
Bestar® qPCR RT kit (Applied Biosystems, Foster City,
CA, USA) in a 20-μl PCR reaction. The pre-amplification
cycling conditions were 37°C for 60 min and 98°C for 10 min. The
pre-amplified cDNA was diluted with 0.1X TE (pH 8.0) to 10
μl and then 1 μl diluted cDNA was used in each plate
for real-time PCR reactions.
miRNA microarray labeling and
hybridization
Extracted RNA was quantitated using a NanoDrop
ND-1000 spectrophotometer (NanoDrop, Wilmington, DE, USA) and
monitored by agarose gel electrophoresis. Then, the samples were
labeled and hybridized on Affymetrix GeneChip miRNA arrays 3.0
(Affymetrix, Santa Clara, CA, USA) according to the manufacturer's
protocol. Samples were denatured at 99°C for 5 min followed by 45°C
for another 5 min, injected into the array chips and hybridization
was allowed for 17 h at 48°C in an Affymetrix Hybridization Oven
645 in constant movement at 60 rpm. The raw intensity of the image
was read using GenePix Pro V6.0. The intensity of the green signal
was calculated after background subtraction, and four replicated
spots for each probe on the same slide were averaged. The median
normalization method was used to obtain ʻnormalized data'
[Normalized data = (foreground − background)/median]. The median
was defined as the 50% quantile of miRNA intensity that was >50
in all samples after background correc tion. The statistical
significance of the differentially expressed miRNAs was analyzed
using the Student's t-test.
Quantitative RT-PCR of mature miRNAs
The solution contained 1 μl of RT product, 5
μl of 2X SYBR®-Green Mix, 0.5 μl of each
primer and 3 μl nuclease-free water. The reactions were
performed in a 96-well optical plate at 94°C for 2 min, followed by
40 cycles of 94°C for 20 sec, 58°C for 20 sec and 72°C for 20 sec,
and the fluorescence signal was collected by ABI PRISM®
7900HT system (Applied Biosystems). All reactions were run in
triplicate. All primers used are listed in Table II.
 | Table IISequence of the primers used for
validation of selected miRNAs. |
Table II
Sequence of the primers used for
validation of selected miRNAs.
| miRNA | Primers
(5′-3′) |
|---|
| hsa-miR-24-3p | F:
ACACTCCAGCTGGGTGGCTCAGTTCAGC |
| hsa-miR-101-3p | F:
ACACTCCAGCTGGGTACAGTACTGTGAT |
|
hsa-miR-125a-5p | F:
ACACTCCAGCTGGGTCCCTGAGACCCTTTA |
| hsa-miR-30c-5p | F:
ACACTCCAGCTGGGTGTAAACATCCTACAC |
| hsa-let-7b-5p | F:
ACACTCCAGCTGGGTGAGGTAGTAGGTTG |
|
hsa-miR-193a-3p | F:
ACACTCCAGCTGGGAACTGGCCTACAAAG |
|
hsa-miR-200c-3p | F:
ACACTCCAGCTGGGTTAATACTGCCGGGTA |
| hsa-miR-140-3 | F:
ACACTCCAGCTGGGTACCACAGGGTAGAAC |
| hsa-miR-22-3p | F:
ACACTCCAGCTGGAAGCTGCCAGTTGAAG |
| hsa-miR-195-5p | F:
ACACTCCAGCTGGGTAGCAGCACAGAAAT |
| hsa-miR-4328 | F:
ACACTCCAGCTGGGCCAGTTTTCCCAG |
| hsa-miR-16-5p | F:
ACACTCCAGCTGGGTAGCAGCACGTAAA |
| hsa-miR-141-3p | F:
ACACTCCAGCTGGGTAACACTGTCTGGTAA |
|
hsa-miR-146b-5p | F:
ACACTCCAGCTGGGTGAGAACTGAATTCC |
| hsa-miR-191-5p | F:
ACACTCCAGCTGGGCAACGGAATCCCAAAAG |
| hsa-miR-4451 | F:
ACACTCCAGCTGGGTGAGAACTGAATTCC |
| hsa-miR-204-3p | F:
ACACTCCAGCTGGGGCTGGGAAGGCA |
| hsa-miR-340-5p | F:
ACACTCCAGCTGGGTTATAAAGCAATGAG |
| hsa-miR-3611 | F:
ACACTCCAGCTGGGTTGTGAAGAAAGAA |
| hsa-miR-665 | F:
ACACTCCAGCTGGGACCAGGAGGCTGAGG |
| hsa-miR-483-5p | F:
ACACTCCAGCTGGGAAGACGGGAGGAA |
| hsa-miR-4644 | F:
ACACTCCAGCTGGGTGGAGAGAGAAAAGAG |
| hsa-miR-485-3p | F:
ACACTCCAGCTGGGGTCATACACGGCTCTC |
| hsa-miR-4698 | F:
ACACTCCAGCTGGGTCAAAATGTAGAGG |
| hsa-miR-185-5p | F:
ACACTCCAGCTGGGTGGAGAGAAAGGCAG |
| hsa-miR-659-3p | F:
ACACTCCAGCTGGGCTTGGTTCAGGGAGGG |
|
hsa-miR-378a-3p | F:
ACACTCCAGCTGGGACTGGACTTGGAG |
|
hsa-miR-3653-3p | F:
ACACTCCAGCTGGGCTAAGAAGTTGAC |
| hsa-miR-4421 | F:
ACACTCCAGCTGGGACCTGTCTGTGGAAAG |
| hsa-miR-663a | F:
ACACTCCAGCTGGGAGGCGGGGCGCCGCG |
| U6 | F:
CTCGCTTCGGCAGCACA |
| U6 | R:
AACGCTTCACGAATTTGCGT |
| All | R:
CTCAACTGGTGTCGTGGA |
Cell proliferation detection
After TGF-β treatment for 24 h, miR-483-5p mimics
and miRNA mimic negative control (NC) (1 μg; GenePharma,
Shanghai, China) were transfected into BEAS-2B and HFL1 cells using
Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA). Then, after
transfection for 24, 48 and 72 h, 100 μl Cell Counting Kit-8
(CCK-8) (Dojindo, Kumamoto, Japan) solution was added into each
well and the plates were incubated in a incubator for 1 h. The
absorbance was measured at a wavelength of 450 nm using a
microplate reader.
Protein isolation and western blot
analysis
To evaluate the target gene expression change in
vitro affected by miR-483-5p, the protein extracted from the
cells was lysed using RIPA buffer (150 mM NaCl, 1% Nonidet P-40,
0.1% sodium dodecyl sulfate (SDS), 50 mM Tris-HCl pH 7.4, 1 mM
EDTA, 1 mM PMSF, 1X Roche complete mini protease inhibitor
cocktail, Roche PhosSTOP phosphatase inhibitor cocktail) and then
determined using BCA kit (Pierce, Rockford, IL, USA) and 20
μg protein lysates were separated on 10% SDS-PAGE gels
followed by transfer to nitrocellulose membranes. Western blot
analysis was performed as previously described (16), and the signal was visualized using
the Odyssey Imaging system (LI-COR Bioscience, Lincoln, NE, USA).
Antibodies used in this study included anti-human α-SMA (1:4,000),
anti-human fibronectin (1:2,000) and anti-human glyceraldehyde
3-phosphate dehydrogenase (GAPDH) (1:10,000) (all from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA).
Data analysis
The miRNA microarray data used the total gene
signal, which was proportional to the total number of targets bound
by the probes targeting each miRNA. Differentially expressed
signals were determined by one-way ANOVA with P<0.01. To compare
qPCR-array and microarray assays, the log2 of microarray
signals was used.
Real-time PCR assay was used to determine the
changes in the expression of the target miRNAs in cells or lung
tissue samples. The change in amplification was normalized to the
expression of U6 RNA. The fold-change in expression was calculated
for each sample using 2−ΔΔCt. 2−ΔΔCt >1.5
or <0.67 was indicative of miRNAs that were differentially
expressed.
Results
Differentially expressed miRNAs in COPD
patients
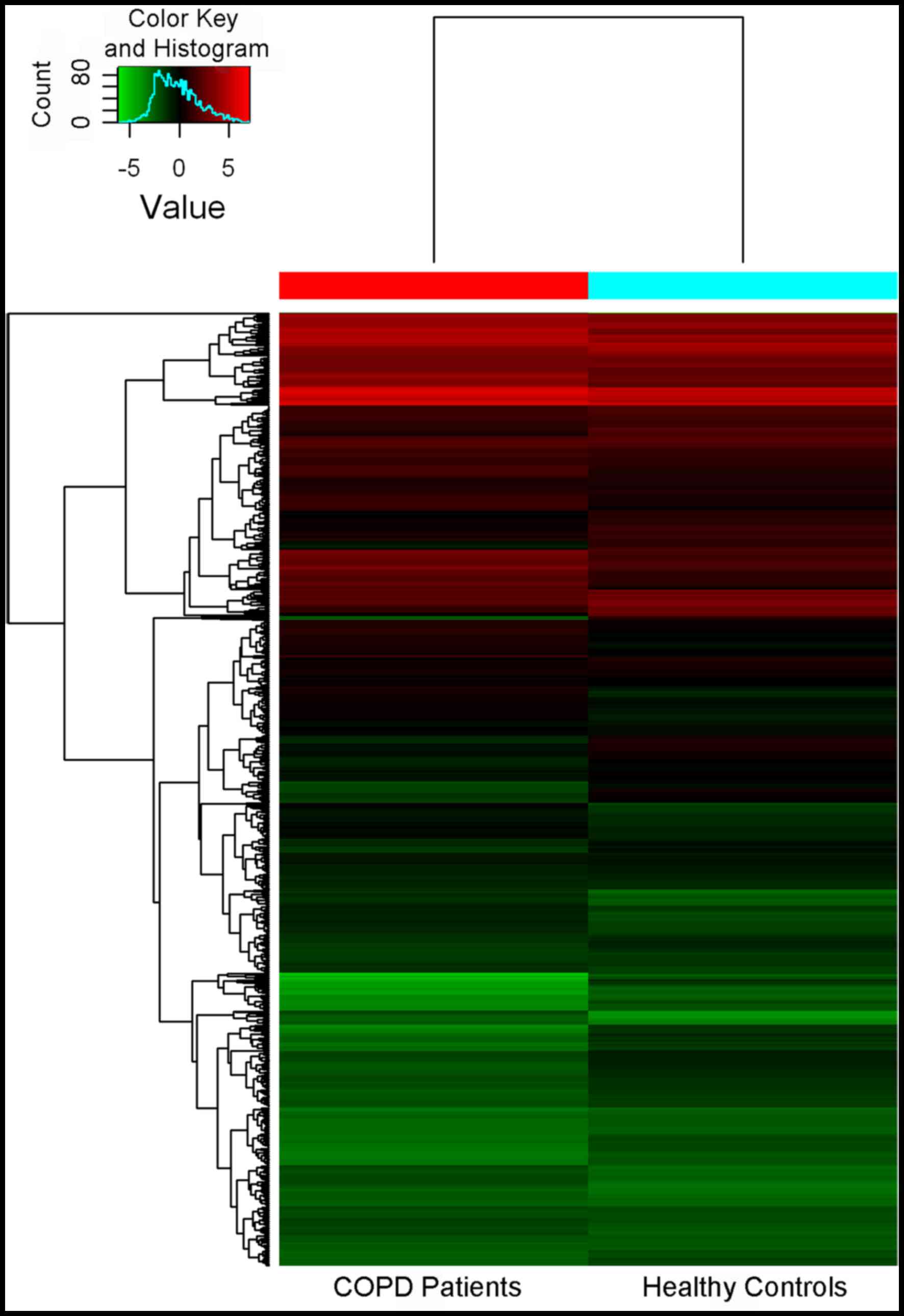
A total of 3,000 miRNAs were identified. Among
these, 138 miRNAs were differentially expressed with a
>2-fold-change in the lung tissues between COPD patients and
healthy donors, and 203 miRNAs had significantly downregulated
expression (Fig. 1).
Validation of miRNA microarray results in
clinical samples by real-time PCR
In order to confirm the results obtained from the
miRNA microarray, 15 high-expression candidates and 15
low-expression candidates with at least a 2-fold difference and
P<0.05 were randomly selected from the result identified by the
microarray, and the expression of these miRNAs was analyzed by
real-time PCR. As shown in Fig.
2A, hsa-miR-24-3p, hsa-miR-101-3p, hsa-miR-125a-5p,
hsa-miR-30c-5p, hsa-let-7b-5p, hsa-miR-146-5p,hsa-miR-193a-3p,
hsa-miR-200c-3p, hsa-miR-140-3p, hsa-miR-22-3p, hsa-miR-195-5p,
hsa-miR-16-5p, hsa-miR-141-3p, hsa-miR-4328 and hsa-miR-191-5p were
upregulated and hsa-miR-4451, hsa-miR-204-3p, hsa-miR-340-5p,
hsa-miR-3611, hsa-miR-665, hsa-miR-483-5p, hsa-miR-4644,
hsa-miR-485-3p, hsa-miR-4698, hsa-miR-185-5p, hsa-miR-659-3p,
hsa-miR-378a-3p, hsa-miR-3653-3p, hsa-miR-4421, and hsa-miR-663a
were downregulated in each lung sample (Fig. 2A), which was consistent with the
results from the miRNA microarray (Fig. 2B and C). Among these candidates,
we chose miR-483-5p as our target for the following detections.
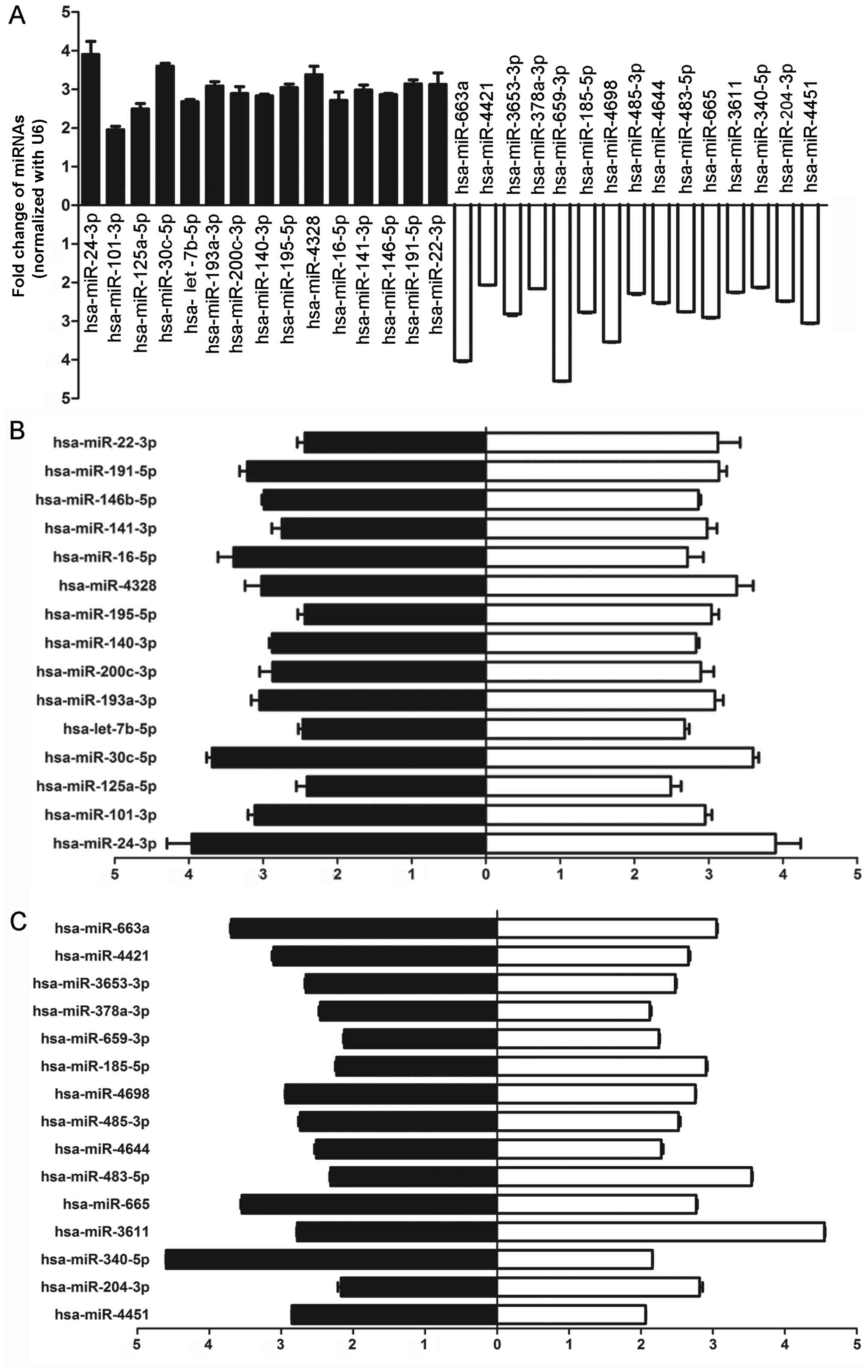 | Figure 2Thirty differentially expressed
hsa-miRNAs related to disease sensitivity between cohort chronic
obstructive pulmonary disease (COPD) patients and healthy controls
were screened and identified by real-time PCR. (A) Validation of 30
hsa-miRNAs using real-time PCR showed that hsa-miR-24-3p,
hsa-miR-101-3p, hsa-miR-125a-5p, hsa-miR-30c-5p, hsa-miR-30d-5p,
hsa-let-7b-5p, hsa-miR-193a-3p, hsa-miR-200c-3p, hsa-miR-140-3p,
hsa-miR-22-3p, hsa-miR-195-5p, hsa-miR-16-5p, hsa-miR-141-3p,
hsa-miR-30b-5p and hsa-miR-191-5p were upregulated and
hsa-miR-4451, hsa-miR-204-3p, hsa-miR-3611, hsa-miR-665,
hsa-miR-483-5p, hsa-miR-4644, hsa-miR-485-3p, has-miR-185-5p,
hsa-miR-4698, hsa-miR-185-5p, hsa-miR-659-3p, hsa-miR-378a-3p,
hsa-miR-3653-3p, hsa-miR-4421 and hsa-miR-663a were downregulated
in each lung sample from COPD patients. Black, relative change in
COPD patients normalized with the control group; white, relative
change in healthy donors normalized to COPD patients. (B) High
level of 15 hsa-miRNAs randomly selected for the validation of
expression level in COPD patients by real-time RT-PCR was
consistent with the results from the miRNA microarray. Black,
relative change in COPD patients normalized with the control group
as detected by real-time PCR; white, relative change in COPD
patients normalized to the control group as detected by microarray.
(C) Low level of 15 hsa-miRNAs randomly selected for the validation
of expression level in COPD patients by real-time RT-PCR was
consistent with the results from miRNA microarray. Black, relative
change in healthy donors normalized to COPD patients as detected by
real-time PCR; white, relative change in healthy donors normalized
to the COPD patients as detected by the microarray. |
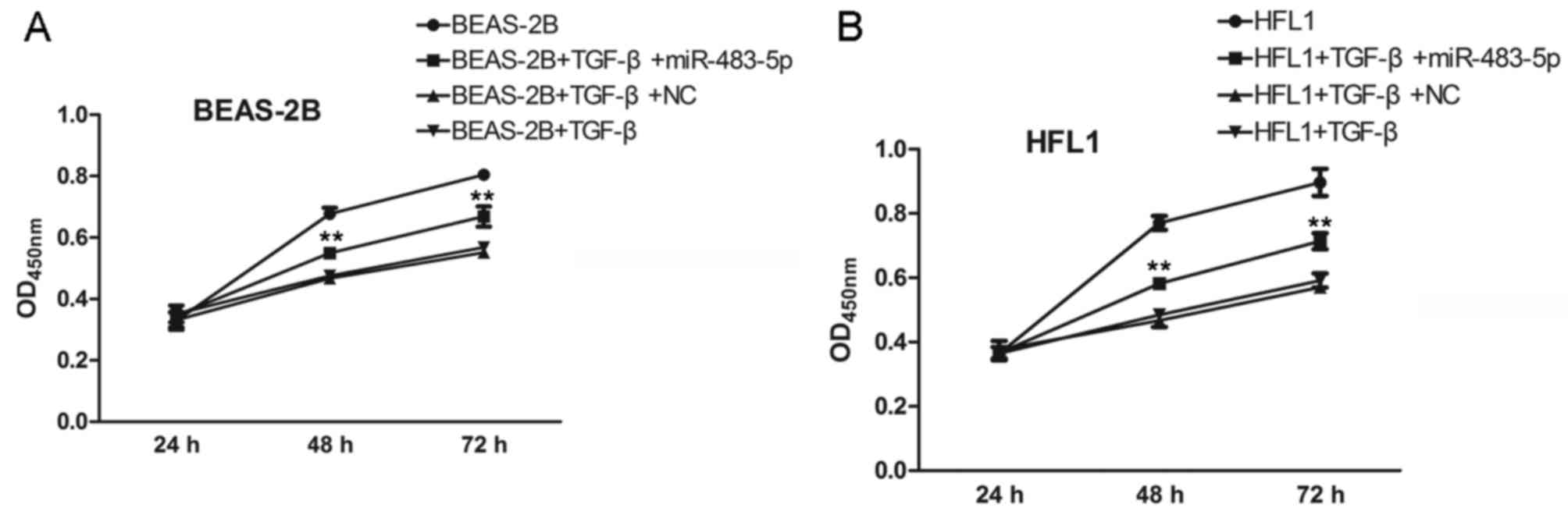
Effect of miR-483-5p on cell
proliferation in vitro
Furthermore, we examined the effects of miR-483-5p
on TGF-β-treated BEAS-2B and HFL1 cell proliferation after
transfection for 24, 48 and 72 h. As shown in Fig. 3A, miR-483-5p, which was
significantly downregulated in COPD samples, exhibited an
inhibitory effect against the TGF-β-induced decrease in cell
proliferation compared to the negative control group in the BEAS-2B
and HFL1 cells (Fig. 3B).
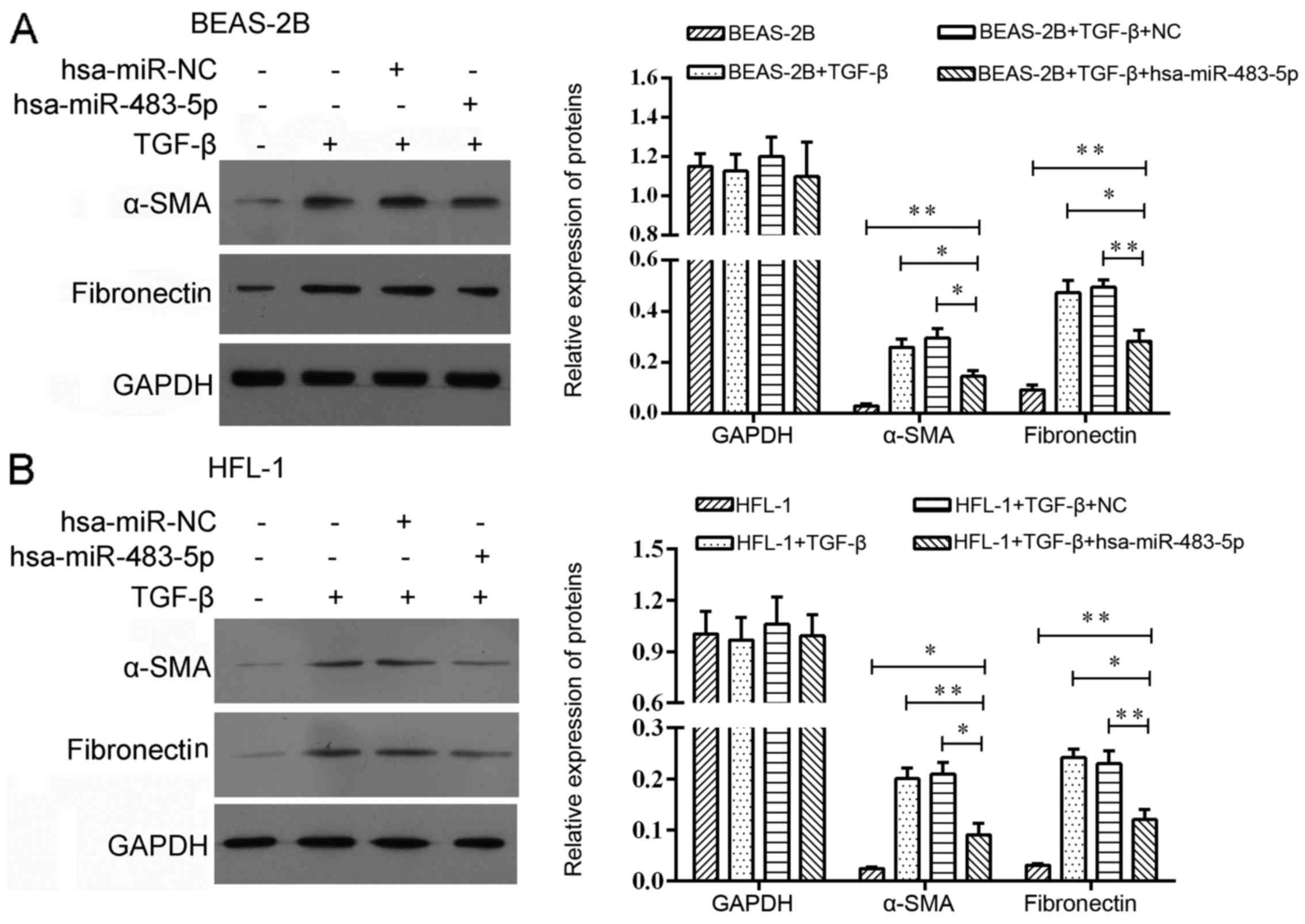
Effect of miR-483-5p on expression of
COPD-related proteins
In order to explore the effect of miR-483-5p in
COPD, we harvested BEAS-2B and HFL1 cells after TGF-β treatment for
24 h followed by miR-483-5p mimic transfection. As a result, we
found that the protein levels of α-SMA and fibronectin were
decreased 48 h post-transfection (Fig. 4).
Discussion
COPD is a debilitating lung disease that generally
affects older individuals, owing to the duration of smoking
(17). miRNAs may be quiescent
while lung homeo stasis is maintained after development, but may
become perturbed in early states of COPD involving cell
differentiation and inflammation (18). In this study, we report that COPD,
rather than smoking, has a significant impact on the miRNA
expression profile based on miRNA microarray analysis using lung
samples from patients with and without COPD. Consistent with the
microarray results, expression levels of certain miRNAs were
validated in vitro by real-time PCR.
Recently, miR-483-3p has been reported to be
involved in the occurrence of many diseases, such as esophageal
squamous cell carcinoma (19),
cardiomyocyte apoptosis (20) and
gastric cancer (21). It has also
been shown to be dysregulated and associated with poorer
disease-specific survival in various cancers (22–26). Although Song et al
(24) reported that miR-483-5p
can serve as a negative regulator of lung cancer metastasis
suppressors RhoGDI1 and ALCAM, the role of miR-483 in lung disease
particularly COPD and the molecular mechanisms by which miR-483
regulates such diseases are still not clear. Meanwhile, Soeda et
al (27) also found that
miR-483-5p was significantly downregulated in the plasma from COPD
patients when compared with normal smokers by TaqMan low-density
array screening. Therefore, in order to clarify the role of
miR-483-5p in COPD, miR-483-5p was selected as the target in this
study. Based on the results of microarray and RT-PCR, the
miR-483-5p expression was significantly decreased (~2.5-fold
reduction) in COPD compared to the healthy controls.
Cigarette smoke or other inhaled irritants activate
epithelial cells to release growth factors, such as TGF-β and
fibroblast growth factor (FGF) which induce fibroblast
proliferation, resulting in small-airway inflammation and fibrosis
(28,29). Studies have shown that
myofibroblasts can be transdifferentiated from fibroblasts in
vitro by their exposure to the fibrogenic cytokine TGF-β
(30-32). Furthermore, others have shown that
TGF-β is a potent stimulus for myofibroblast differentiation and
induction of pulmonary fibrosis in vivo (33,34). In addition, Burgess et al
(35) used TGF-β-treated human
lung fibroblasts to study pulmonary myofibroblast differentiation.
Therefore, in our study, in order to imitate COPD in vitro,
TGF-β treatment was used to induce a similar condition of COPD in
BEAS-2B and HFL1 cells. Our results showed that miR-483-5p
transfection significantly abrogated the TGF-β-mediated decrease in
cell proliferation, and α-SMA and fibronectin expression in BEAS-2B
and HFL1 cells. This indicates that the abrogation of
TGF-β-decreased cell proliferation by miR-483-5p may be associated
with the expression of α-SMA and fibronectin.
α-SMA and fibronectin play important roles during
COPD progression. A high molecular weight glycoprotein,
fibronectin, is present in the human body as two major isoforms: an
insoluble extracellular matrix isomer and a soluble form in the
blood (36). The primary function
of blood fibronectin is to heal wounds by inducing the
reticulo-endothelial system and by mediating cellular adhesion,
motility, differentiation, apoptosis and hemostasis (37). This raises the possibility that
fibronectin may play an important role in predicting the clinical
outcomes in a cohort of patients with mild-to-moderate COPD.
Meanwhile, although smooth muscle and endothelial cells also
express this marker, α-SMA is still the most commonly used but not
a specific marker for myofibroblasts (38-41), whose expression is mainly
intracellular also in spindle-shaped cells (42). α-SMA-positive cells are increased
in the airways of COPD patients (43), which suggests that most
α-SMA-positive cells reveal typical expression profile of
myofibroblasts being positive for α-SMA, vimentin and negative for
desmin (44). Based on our
findings, we hypothesis that, during COPD progression, low
expression of miR-483-5p may upregulate the expression of these two
important proteins by decreasing the chance of binding them
directly. In further research, the molecular mechanism through
which miR-483-5p regulates α-SMA and fibronectin needs to be
clarified before miR-483-5p can be developed as a potential
molecular biomarker for COPD patients.
In conclusion, we found a new miRNA named miR-483-5p
with relatively low expression in COPD patients, which may protect
human lung cells by promoting cell growth and activating important
proteins such as α-SMA and fibronectin. Our findings can provide
clues for future functional studies aimed at determining the role
of miR-483-5p suppression, as observed in COPD patients, in
modulating the adaptive immune balance.
References
|
1
|
Felice KM, Salzman DW, Shubert-Coleman J,
Jensen KP and Furneaux HM: The 5′ terminal uracil of let-7a is
critical for the recruitment of mRNA to Argonaute2. Biochem J.
422:329–341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jonas S and Izaurralde E: Towards a
molecular understanding of microRNA-mediated gene silencing. Nat
Rev Genet. 16:421–433. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iorio MV and Croce CM: microRNA
involvement in human cancer. Carcinogenesis. 33:1126–1133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Corvalan AH and Maturana MJ: Recent
patents of DNA methylation biomarkers in gastrointestinal oncology.
Recent Pat DNA Gene Seq. 4:202–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Felicetti F, Errico MC, Segnalini P,
Mattia G and Carè A: MicroRNA-221 and -222 pathway controls
melanoma progression. Expert Rev Anticancer Ther. 8:1759–1765.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang YW and Chen LA: microRNAs as tumor
inhibitors, oncogenes, biomarkers for drug efficacy and outcome
predictors in lung cancer (Review). Mol Med Rep. 5:890–894.
2012.PubMed/NCBI
|
|
7
|
Reid DJ and Pham NT: Emerging Therapeutic
Options for the Management of COPD. Clin Med Insights Circ Respir
Pulm Med. 7:7–15. 2013.PubMed/NCBI
|
|
8
|
Lococo F, Cesario A, Del Bufalo A,
Ciarrocchi A, Prinzi G, Mina M, Bonassi S and Russo P: Novel
therapeutic strategy in the management of COPD: A systems medicine
approach. Curr Med Chem. 22:3655–3675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang M, Huang Y, Liang Z, Liu D, Lu Y, Dai
Y, Feng G and Wang C: Plasma miRNAs may be promising biomarkers of
chronic obstructive pulmonary disease. Clin Respir J. 10:104–111.
2014. View Article : Google Scholar
|
|
10
|
Navratilova Z, Kolek V and Petrek M:
Matrix metalloproteinases and their inhibitors in chronic
obstructive pulmonary disease. Arch Immunol Ther Exp (Warsz).
64:177–193. 2015. View Article : Google Scholar
|
|
11
|
Zanette DL, Rivadavia F, Molfetta GA,
Barbuzano FG, Proto-Siqueira R, Silva-Jr WA, Falcão RP and Zago MA:
miRNA expression profiles in chronic lymphocytic and acute
lymphocytic leukemia. Braz J Med Biol Res. 40:1435–1440. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xi S, Yang M, Tao Y, Xu H, Shan J,
Inchauste S, Zhang M, Mercedes L, Hong JA, Rao M, et al: Cigarette
smoke induces C/EBP-β-mediated activation of miR-31 in normal human
respiratory epithelia and lung cancer cells. PLoS One.
5:e137642010. View Article : Google Scholar
|
|
13
|
Huleihel L, Ben-Yehudah A, Milosevic J, Yu
G, Pandit K, Sakamoto K, Yousef H, LeJeune M, Coon TA, Redinger CJ,
et al: Let-7d microRNA affects mesenchymal phenotypic properties of
lung fibroblasts. Am J Physiol Lung Cell Mol Physiol.
306:L534–L542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van Pottelberge GR, Mestdagh P, Bracke KR,
Thas O, van Durme YM, Joos GF, Vandesompele J and Brusselle GG:
MicroRNA expression in induced sputum of smokers and patients with
chronic obstructive pulmonary disease. Am J Respir Crit Care Med.
183:898–906. 2011. View Article : Google Scholar
|
|
15
|
Mizuno S, Bogaard HJ, Gomez-Arroyo J,
Alhussaini A, Kraskauskas D, Cool CD and Voelkel NF:
MicroRNA-199a-5p is associated with hypoxia-inducible factor-1α
expression in lungs from patients with COPD. Chest. 142:663–672.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu DD, Tang RN, Lv LL, Wen Y, Liu H,
Zhang XL, Ma KL and Liu BC: Interleukin-1β mediates high glucose
induced phenotypic transition in human aortic endothelial cells.
Cardiovasc Diabetol. 15:422016. View Article : Google Scholar
|
|
17
|
Liu Y, Pleasants RA, Croft JB, Wheaton AG,
Heidari K, Malarcher AM, Ohar JA, Kraft M, Mannino DM and Strange
C: Smoking duration, respiratory symptoms, and COPD in adults aged
≥45 years with a smoking history. Int J Chron Obstruct Pulmon Dis.
10:1409–1416. 2015. View Article : Google Scholar :
|
|
18
|
Johar D, Siragam V, Mahood TH and Keijzer
R: New insights into lung development and diseases: The role of
microRNAs. Biochem Cell Biol. 93:139–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma J, Hong L, Xu G, Hao J, Wang R, Guo H,
Liu J, Zhang Y, Nie Y and Fan D: miR-483-3p plays an oncogenic role
in esophageal squamous cell carcinoma by targeting tumor suppressor
EI24. Cell Biol Int. 40:448–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiao Y, Zhao Y, Liu Y, Ma N, Wang C, Zou
J, Liu Z, Zhou Z, Han D, He J, et al: miR-483-3p regulates
hyperglycaemia-induced cardiomyocyte apoptosis in transgenic mice.
Biochem Biophys Res Commun. 477:541–547. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu K, Ma L and Zhu J: miR-483-5p promotes
growth, invasion and self-renewal of gastric cancer stem cells by
Wnt/β-catenin signaling. Mol Med Rep. 14:3421–3428. 2016.PubMed/NCBI
|
|
22
|
Xu H, Yang Y, Zhao H, Yang X, Luo Y, Ren
Y, Liu W and Li N: Serum miR-483-5p: A novel diagnostic and
prognostic biomarker for patients with oral squamous cell
carcinoma. Tumour Biol. 37:447–453. 2016. View Article : Google Scholar
|
|
23
|
Qu X, Zhao M, Wu S, Yu W, Xu J, Xu J, Li J
and Chen L: Circulating microRNA 483-5p as a novel biomarker for
diagnosis survival prediction in multiple myeloma. Med Oncol.
31:2192014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song Q, Xu Y, Yang C, Chen Z, Jia C, Chen
J, Zhang Y, Lai P, Fan X, Zhou X, et al: miR-483-5p promotes
invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1
and ALCAM. Cancer Res. 74:3031–3042. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patel D, Boufraqech M, Jain M, Zhang L, He
M, Gesuwan K, Gulati N, Nilubol N, Fojo T and Kebebew E: MiR-34a
and miR-483-5p are candidate serum biomarkers for adrenocortical
tumors. Surgery. 154:1224–1229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen J, Wang A, Wang Q, Gurvich I, Siegel
AB, Remotti H and Santella RM: Exploration of genome-wide
circulating microRNA in hepatocellular carcinoma: MiR-483-5p as a
potential biomarker. Cancer Epidemiol Biomarkers Prev.
22:2364–2373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soeda S, Ohyashiki JH, Ohtsuki K, Umezu T,
Setoguchi Y and Ohyashiki K: Clinical relevance of plasma miR-106b
levels in patients with chronic obstructive pulmonary disease. Int
J Mol Med. 31:533–539. 2013.PubMed/NCBI
|
|
28
|
Retamales I, Elliott WM, Meshi B, Coxson
HO, Pare PD, Sciurba FC, Rogers RM, Hayashi S and Hogg JC:
Amplification of inflammation in emphysema and its association with
latent adenoviral infection. Am J Respir Crit Care Med.
164:469–473. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barnes PJ: The cytokine network in asthma
and chronic obstructive pulmonary disease. J Clin Invest.
118:3546–3556. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grunstein M: Histone acetylation in
chromatin structure and transcription. Nature. 389:349–352. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Laurent GJ: Regulation of lung collagen
production during wound healing. Chest. 99(Suppl 3): 67S–69S. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Oliveira MV, Silva PL and Rocco PR:
Animal models of chronic obstructive pulmonary disease. J
Biomedical Sci. 5:12016. View Article : Google Scholar
|
|
33
|
Jaiswal AK: Nrf2 signaling in coordinated
activation of antioxidant gene expression. Free Radic Biol Med.
36:1199–1207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rangasamy T, Cho CY, Thimmulappa RK, Zhen
L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM and
Biswal S: Genetic ablation of Nrf2 enhances susceptibility to
cigarette smoke-induced emphysema in mice. J Clin Invest.
114:1248–1259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burgess HA, Daugherty LE, Thatcher TH,
Lakatos HF, Ray DM, Redonnet M, Phipps RP and Sime PJ: PPARgamma
agonists inhibit TGF-β induced pulmonary myofibroblast
differentiation and collagen production: Implications for therapy
of lung fibrosis. Am J Physiol Lung Cell Mol Physiol.
288:L1146–L1153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rotundo RF, Vincent PA, McKeown-Longo PJ,
Blumenstock FA and Saba TM: Hepatic fibronectin matrix turnover in
rats: Involvement of the asialoglycoprotein receptor. Am J Physiol.
277:G1189–G1199. 1999.PubMed/NCBI
|
|
37
|
Chou CW, Zhuo YL, Jiang ZY and Liu YW: The
hemodynamically-regulated vascular microenvironment promotes
migration of the steroidogenic tissue during its interaction with
chromaffin cells in the zebrafish embryo. PLoS One. 9:e1079972014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma X, Yang F, Yang S, Rasul A, Li T, Liu
L, Kong M, Guo D and Ma T: Number and distribution of
myofibroblasts and α-smooth muscle actin expression levels in fetal
membranes with and without gestational complications. Mol Med Rep.
12:2784–2792. 2015.PubMed/NCBI
|
|
39
|
le Rolle AF, Chiu TK, Fara M, Shia J, Zeng
Z, Weiser MR, Paty PB and Chiu VK: The prognostic significance of
CXCL1 hypersecretion by human colorectal cancer epithelia and
myofibroblasts. J Transl Med. 13:1992015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rossi FW, Napolitano F, Pesapane A,
Mascolo M, Staibano S, Matucci-Cerinic M, Guiducci S, Ragno P, di
Spigna G, Postiglione L, et al: Upregulation of the N-formyl
Peptide receptors in scleroderma fibroblasts fosters the switch to
myofibroblasts. J Immunol. 194:5161–5173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiao X, Huang C, Zhao C, Gou X,
Senavirathna LK, Hinsdale M, Lloyd P and Liu L: Regulation of
myofibroblast differentiation by miR-424 during
epithelial-to-mesenchymal transition. Arch Biochem Biophys.
566:49–57. 2015. View Article : Google Scholar :
|
|
42
|
Dey NB, Foley KF, Lincoln TM and Dostmann
WR: Inhibition of cGMP-dependent protein kinase reverses phenotypic
modulation of vascular smooth muscle cells. J Cardiovasc Pharmacol.
45:404–413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Milara J, Serrano A, Peiró T, Gavaldà A,
Miralpeix M, Morcillo EJ and Cortijo J: Aclidinium inhibits human
lung fibroblast to myofibroblast transition. Thorax. 67:229–237.
2012. View Article : Google Scholar :
|
|
44
|
Löfdahl M, Kaarteenaho R, Lappi-Blanco E,
Tornling G and Sköld MC: Tenascin-C and alpha-smooth muscle actin
positive cells are increased in the large airways in patients with
COPD. Respir Res. 12:482011. View Article : Google Scholar : PubMed/NCBI
|