Introduction
Endotoxins, also referred to as lipopolysaccharides
(LPS), are considered as the most powerful stimulators of the
immune system and measurement of their levels is considered to be a
useful specific indicator of infection by Gram-negative bacteria in
diverse eukaryotes, ranging from insects to humans (1). LPS are present in many liquids or
many biomaterials, even if the material is sterile (2), and can elicit a biological effect
even at extremely dilute concentrations (3). High levels of endotoxins in the
blood can cause various disease status, including systemic
inflammatory response syndrome, sepsis, severe shock, multiple
organ dysfunction syndrome, multiple organ failure and even death
(4). Despite the recognized
potential for causing harm, there is currently no effective method
available for treating LPS-induced diseases.
LPS and other microbial products are recognized by
the innate immune system of the body through the action of
Toll-like receptors (TLRs), leading to activation of the downstream
signal transduction pathways and stimulation of a range of immune
responses (5,6). TLR4 is the first TLR discovered in
humans (7,8) and is capable of identifying
bacterial LPS. LPS binding to TLR4 triggers myeloid differentiation
through primary response gene-88 (MyD88)-independent pathways
(9), leading to subsequent
activation of nuclear transcription factor nuclear factor-κB
(NF-κB). Activated NF-κB translocates to the nucleus and mediates
the transcription of a number of genes (10). Studies have shown that NF-κB can
effectively induce the expression of genes encoding inflammatory
mediators and stimulate their release from cells (11). Therefore, treatments aimed at
inhibiting the TLR4/MyD88/NF-κB signaling pathway may have
potential therapeutic advantages over currently available
approaches for managing the effects of LPS. However, the inhibition
of the TLR4/MyD88/NF-κB signaling pathway may also damage the
anti-microbial immunity of the host.
Upon encountering pathogens and environmental
insults, recruitment and activation of effector T and B cells and,
importantly, regulatory T cells (Tregs) contribute to the
maintenance of immune homeostasis, prevention of autoimmunity and
moderation of the inflammatory response (12). Tregs are a functionally mature
subpopulation of T cells, characterized as
CD4+/CD25+/Foxp3+. Tregs are
generated in the thymus or by differentiation from peripheral
CD4+CD25− native T cells (13). Tregs play key roles in the
maintenance of immunologic self-tolerance and negative control of a
number of physiological and pathological immune responses, with
growing recognition of the clinical importance of Tregs. Since TLR4
is expressed on Tregs, LPS may affect their function through
stimulation of TLR4. Foxp3 protein is considered to be the most
reliable molecular marker of mature Tregs and is involved in the
development and function of Tregs. Several mechanisms of
Treg-mediated suppression of the immune response have been
proposed, including secretion of immunosuppressive cytokines,
cell-contact-dependent suppression and functional modification or
killing of antigen-presenting cells (12,14), among others. Tregs are
unresponsive to T cell receptor (TCR) stimulation, express
transforming growth factor-β (TGF-β) and interleukin-10 (IL-10),
inhibit normal T cell proliferation, and suppress CD4+
expansion in vivo.
Many researchers are engaged in identifying novel
active compounds from plants for the treatment of human diseases.
Qinghaosu (artemisinin) used as an antimalarial drug is well known
(15). Compound Danshen dropping
pill has been widely used for cardiovascular disease in China and
some Asia countries (16).
Moreover, numerous traditional medicines have anti-endotoxin
effects such as andrographolide, resveratrol, praeruptorin and
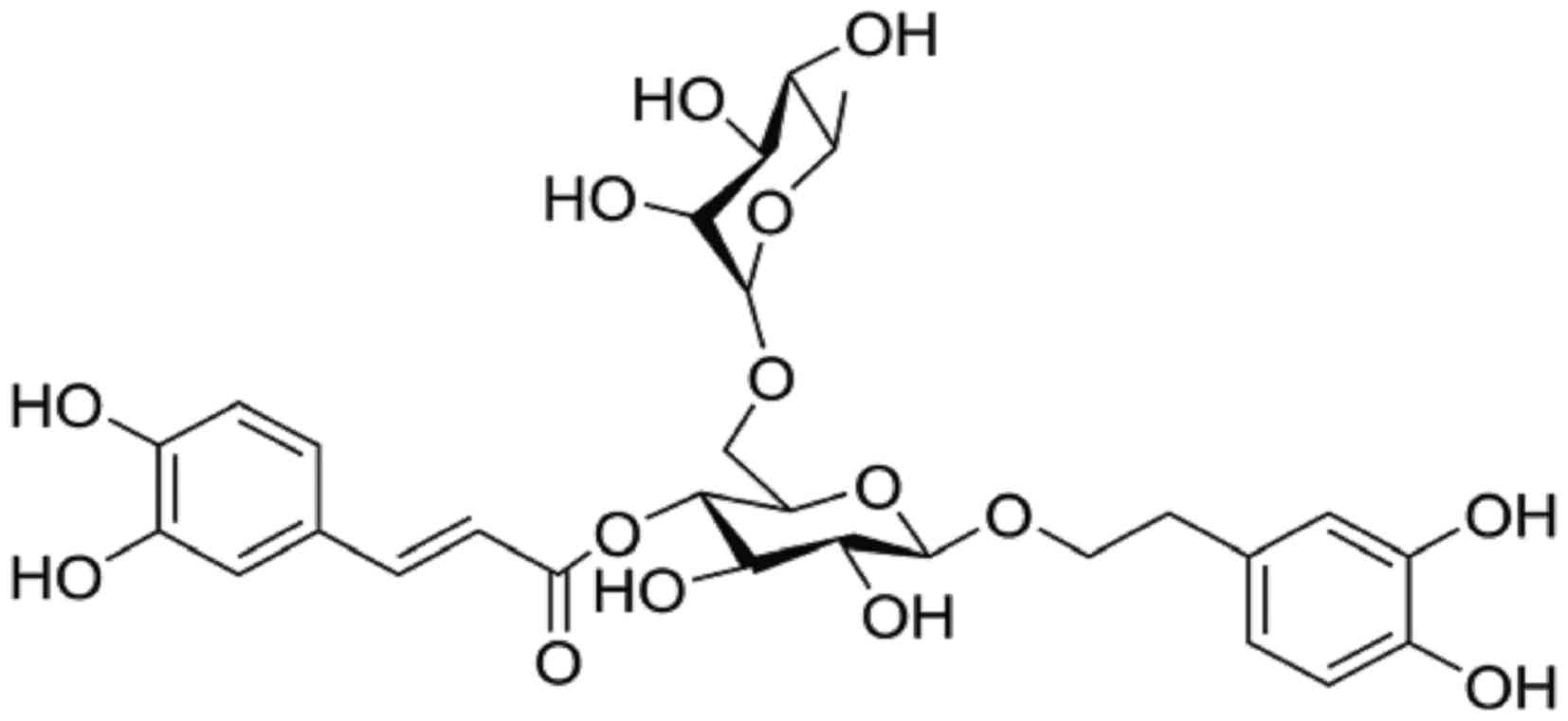
osthole (17–20). Forsythoside A (FTA) (Fig. 1) is a pharmacologically active
monomer of benzene glycoside extract from the Forsythia
suspensa plant. It was reported to possess anti-bacterial,
anti-viral, anti-oxidant, and immunomodulatory properties (21). Recent studies have shown that
forsythoside can significantly enhance macrophage phagocytosis and
reduce tumor necrosis factor-α (TNF-α) secretion in LPS-stimulated
RAW264.7 cells, thereby suggesting that it can elicit various
anti-LPS effects (22). However,
the precise anti-endotoxin effects of FTA have not been elucidated
to date.
In the present study, we investigated the ability of
FTA to suppress the effects of LPS in vitro. Furthermore, we
evaluated whether the effects involve the LPS/TLR4/MyD88/NF-κB
signaling pathway and inhibition of Tregs.
Materials and methods
Reagents
Bacterial LPS (Escherichia coli: 055:B5, used
at 100 ng/ml) and polymyxin B (PMB, used at 10 μg/ml) were
obtained from Sigma (St. Louis, MO, USA). FTA solution (>98%
HPLC purity; cat. no. L28-110506; Jiangxi Tiangong Herbal
Technology Co., Ltd.) was prepared at a concentration of 1 mg/ml in
sterile water. The materials under study were endotoxin-free.
Animals
Male BALB/c mice (18±2 g, 6–8 weeks old) were
purchased from Hunan Slack King Laboratory Animal Co., Ltd. The
mice were housed in a room maintained at 24±1°C with 40–80%
humidity. All animals received food and water ad libitum.
Mice were housed for 2–3 days to adapt to the environment before
the experiments. The experimental procedures were approved by the
Commission of Nanchang University for Ethics of Experiments on
Animals and were conducted in accordance with international
standards. All surgery was performed under chloral hydrate
anesthesia, and all efforts were made to minimize animal
suffering.
Leukocyte isolation
Two to four spleens were converted into single-cell
suspensions by squeezing through a 74-μm nylon net with the
rough end of a 5-ml syringe plunger. The single-cell suspensions
were filtered using a 37-μm nylon net. Then, the cells were
spun down in a 15-ml Falcon tube (10 min, 1,500 rpm) and the
supernatant was discarded. The pellets were resuspended in 1 ml of
warm RPMI-1640 medium, layered above 2 ml of lymphocyte separation
medium (LSM; Solarbio, Beijing, China) and centrifuged for 20 min
at 20°C at 2,000 rpm. The top layer of clear plasma was aspirated
to within 2–3 mm above the lymphocyte layer and discarded. Next,
the lymphocyte layer were aspirated and diluted with warm RPMI-1640
medium into a new centrifuge tube and centrifuged for 10 min at
1,000 rpm twice. Finally, the cells were resuspended in warm
RPMI-1640 medium.
Cell culture and cell groups
RAW264.7 cells (Department of Gastroenterology,
Jiangxi, China) were maintained in Dulbecco's modified Eagle's
medium (DMEM) containing 10% heat-inactivated fetal bovine serum
(FBS) and antibiotics (100 U/ml of penicillin, 100 mg/ml of
streptomycin). RAW264.7 cells were divided into 6 groups: i)
control group, incubated without any treatment; ii) LPS group,
incubated with LPS (100 ng/ml) for 12 h; (iii-v) FTA groups:
preconditioned by incubation with FTA (20, 80 or 320 μg/ml)
for 2 h before the addition of LPS (100 ng/ml) and incubation for
12 h; vi) PMB group, preconditioned with PMB (10 μg/ml)
before the addition of LPS (100 ng/ml) and incubation for 12 h.
Isolated mouse lymphocytes were suspended in 10% FBS
and RPMI-1640 medium at a concentration of 1×106
cells/ml and plated in 6-well multiplates prior to the addition of
ConA (final concentration, 5 μg/ml) and IL-2 (final
concentration, 100 ng/ml) (both from Sigma). Cell cultures were
maintained at 37°C in a humidified atmosphere of 5% CO2
and 95% air. Lymphocytes were divided into 6 groups and treated as
described above for RAW264.7 cells, except with longer (48 h) LPS
stimulation.
Cell viability assay
Cell viability was assessed using the MTT assay. All
cells were cultured in 96-well plates in an incubator at 37°C and
5% CO2, with RAW264.7 cells cultured at a density of
105 cells/well for 12 h and lymphocytes cultured at
106 cells/well for 2 h. Cells were washed with fresh
medium prior to incubation with a range of FTA concentrations (20,
80 or 320 μg/ml) or PMB (10 μg/ml) for 2 h. The
medium was discarded and LPS (100 ng/ml) was added to the
incubation mixture. RAW264.7 cells and lymphocytes were incubated
with LPS for 12 and 48 h, respectively. Cells were washed and 20
μl of MTT (5 mg/ml) was added, followed by incubation for 4
h. Finally, DMSO (150 μl) was added to solubilize the
formazan salt formed and the amount of formazan salt was determined
by measuring the absorbance at 570 nm using a microplate reader
(Bio-Rad, Hercules, CA, USA). Data are expressed as means ± SD from
at least 3 independent experiments.
ELISA for quantification of TNF-α, IL-10
and TGF-β1 levels
Expression of TNF-α in RAW264.7 cell culture
supernatants was quantified using a commercially available ELISA
kit, according to the manufacturer's instructions (eBioscience, San
Diego, CA, USA). IL-10 and TGF-β1 levels were determined in
supernatants obtained from the lymphocyte culture using ELISA kits
(Westang Co., Ltd., Shanghai, China). The absorbance was read at
450 nm using a microplate reader (Bio-Rad). The levels of cytokines
were calculated using standard curves prepared by analyzing a range
of concentrations of purified recombinant TNF-α, IL-10 and
TGF-β1.
Flow cytometric analysis
Primary lymphocytes in each group was collected and
washed twice with phosphate-buffered saline (PBS) and further
divided into the isotype control group and experimental group.
Cells in the isotype control group were stained with Armenian
hamster IgG isotype control PE-cyanine5, rat IgG1 isotype control
PE, and rat IgG2a isotype control FITC (all from eBioscience),
while the experimental group was stained with anti-mouse CD3e
PE-Cy5, anti-mouse CD4 FITC, and anti-mouse CD25 PE (eBioscience)
at the concentrations recommended by the manufacturer for 15 min in
the dark at room temperature. Red blood cell lysis buffer (1 ml)
was added to each sample of collected primary lymphocytes and
incubated for 10 min in the dark at room temperature. Finally, the
cells were washed with PBS and fixed with RPMI-1640 medium prior to
analysis in a FACSCalibur flow cytometer using CellQuest software
(BD Biosciences, San Jose, CA, USA).
Western blotting for TLR4, MyD88, NF-κB
and Foxp3
RAW264.7 cells and lymphocytes were plated onto 6
60-mm plastic dishes and incubated as described above. The
expression levels of TLR4, NF-κB and MyD88 in RAW264.7 cells and
Foxp3 in lymphocytes were determined by western blot analysis,
using β-actin expression as a reference. Briefly, after drug
treatment, the cells were lysed with ice-cold RIPA buffer
(Solarbio), and the protein content of the lysates was measured
using the bicinchoninic acid (BCA) method. Equal amounts of
cellular proteins were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to a nitrocellulose filter membrane. After blocking
with 5% non-fat milk powder, the membranes were incubated with
respective rabbit anti-mouse polyclonal antibodies [TLR4 (ab13556),
MyD88 (ab2068), Foxp3 (ab54501) or NF-κB (ab16502)] and murine
monoclonal antibodies [β-actin (ab8226)] (both from Abcam,
Cambridge, MA, USA) at 4°C overnight. After washing in TBST (3
washes of 10 min each), the membranes were incubated with
peroxidase-conjugated Affinipure goat anti-rabbit (ZB-2301) or
mouse (ZB-2305) immunoglobulin G antibodies (ZSGB-BIO, Beijing,
China) for 1 h at room temperature and visualized with enhanced
chemiluminescence reagents (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) following exposure to X-ray film. The relative
band intensity was quantified by Quantity One software v4.62
(Bio-Rad) to determine the protein levels.
Quantitative (real-time) PCR
Using EZN Total RNA Kit II (Omega Bio-Teck,
Doraville, GA, USA), the total RNA was extracted from RAW264.7
cells and lymphocytes, according to the maufacturer's instructions.
cDNA was produced using PrimeScript RT reagent kit with gDNA Eraser
(Takara Biotechnology, Dalian, China). Real-time polymerase chain
reaction was performed on an ABI-Prism StepOne using SYBR Premix
Ex Taq II (Takara Biotechnology). The primers used were
5′-TTTATTCAGAGCCGTTGG-3′ and 5′-AGTTGC CGTTTCTTGTTG-3′ for mouse
TLR4; 5′-ACTCGCAGTTT GTTGGATG-3′ and 5′-ACTCGCAGTTTGTTGGATG-3′ for
mouse MyD88; 5′-CTCATGATAGTGCCTGTGTCCTCAA-3′ and
5′-AGGGCCAGCATAGGTGCAAG-3′ for mouse Foxp3;
5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and 5′-ATGGA GCCACCGATCCACA-3′ for
mouse β-actin. The PCR amplification profiles consisted of
denaturation at 95°C for 30 sec, followed by 40 cycles of
denaturation at 95°C for 5 sec and annealing at 62°C for 34 sec.
All amplification reactions for each sample were carried out in
triplicate, and the relative expression values were normalized to
the expression value of mouse β-actin.
Data processing
Statistical analysis was performed with IBM SPSS
Statistics software (version 19.0; SPSS, Inc., Chicago, IL, USA).
Data are expressed as mean ± SEM. The differences between the data
sets were assesses by one-way analysis of variance (ANOVA) and
Student-Newman-Keuls (SNK)-q test. P-value <0.05 was considered
to indicate a statisical significant result.
Results
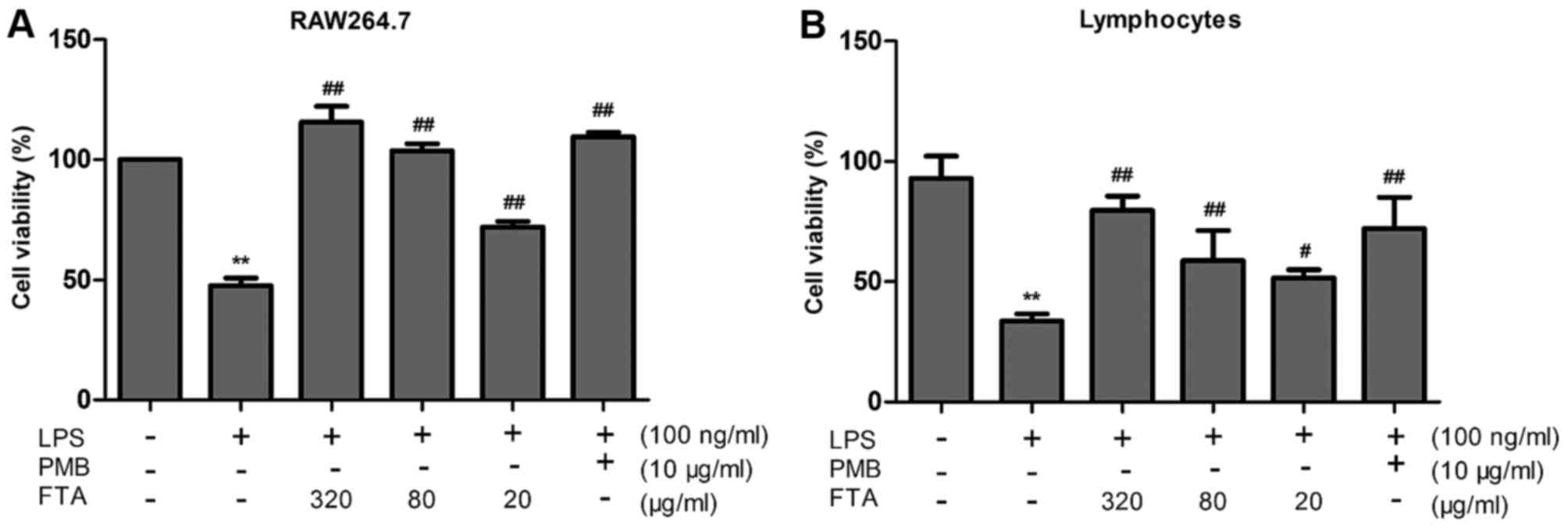
FTA increases the viability of
LPS-treated RAW264.7 cells and lymphocytes
RAW264.7 cells and lymphocytes treated with LPS
alone exhibited severe cell damage and reduced cell viability
compared to the untreated control cells (P<0.01) (Fig. 2). Treatment with FTA
dose-dependently reduced LPS-induced damage in the RAW264.7 cells
and lymphocytes (Fig. 2).
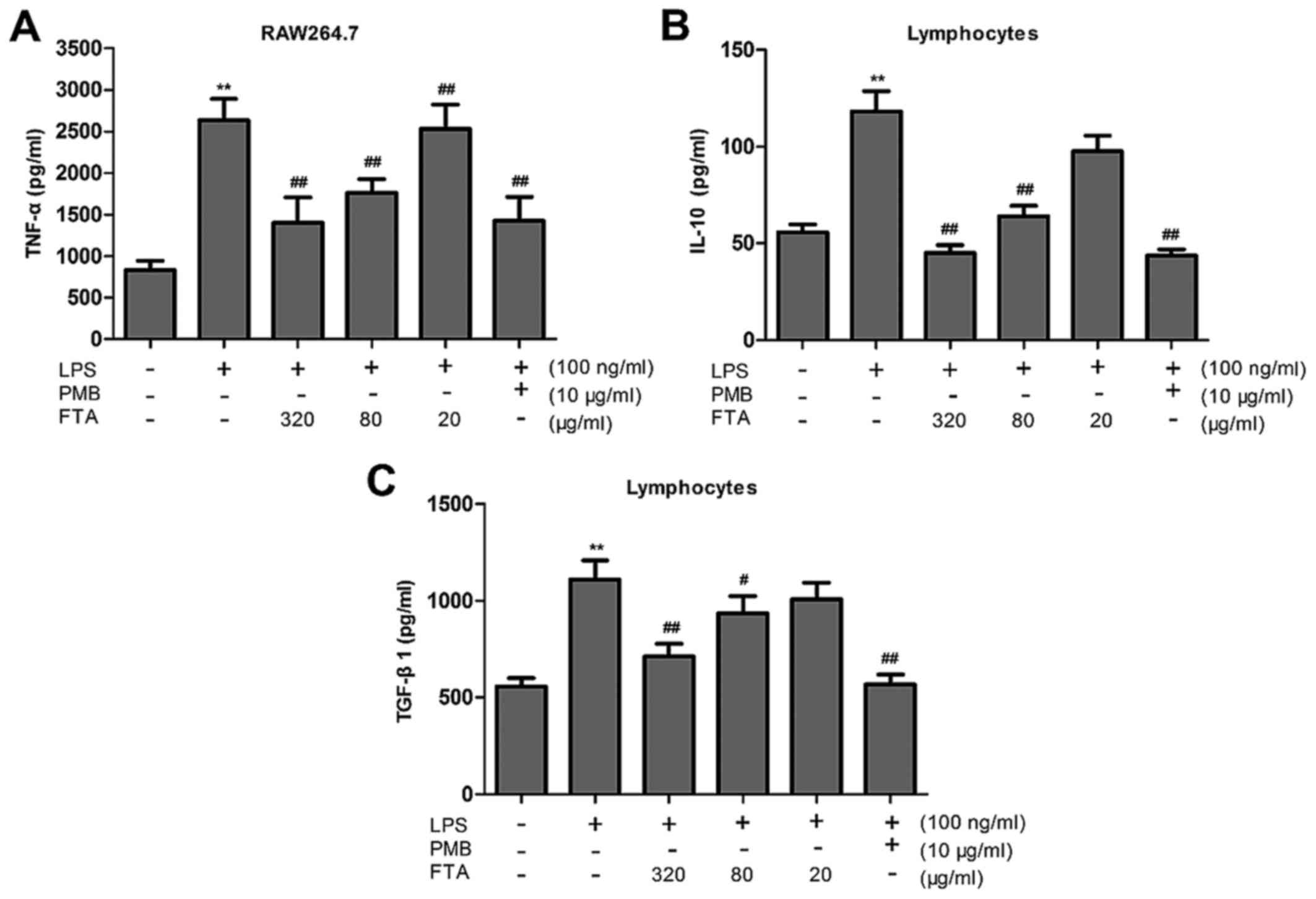
FTA reduces cytokine production
TNF-α
The accumulation of TNF-α was measured to assess the
effect of FTA treatment on this proinflammatory cytokine.
LPS-treated RAW264.7 cells exhibited markedly increased cytokine
production compared to the control group, while FTA treatment
significantly inhibited the LPS-induced increase in TNF-α
concentration in a dose-dependent manner (Fig. 3A).
IL-10 and TGF-β1
We examined the effect of FTA on the production of
proinflammatory cytokines IL-10 and TGF-β1. Lymphocytes were
pretreated with FTA for 2 h and levels of IL-10 and TGF-β1 were
measured in culture media by ELISA. IL-10 and TGF-β1 were found to
be upregulated in the LPS-stimulated lymphocytes, with the
pretreatment with FTA eliciting suppression of the LPS-induced
increase (Fig. 3B and C). These
results suggest that the immunomodulatory effect of FTA may be
mediated through the inhibition of IL-10 and TGF-β1 production.
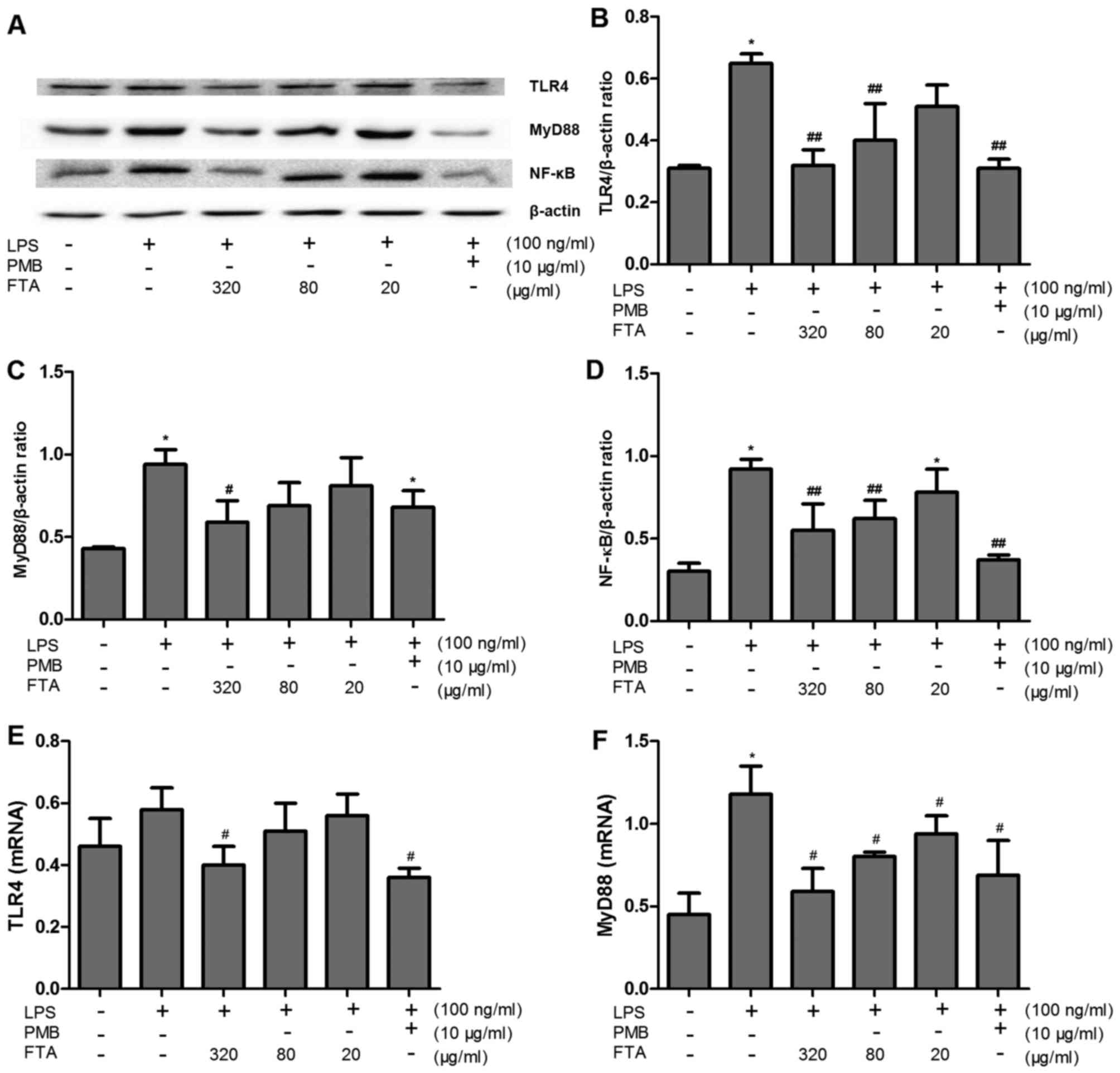
FTA inhibits LPS-induced activation of
TLR4/MyD88/NF-κB signaling
To elucidate the mechanisms underlying the
inhibition of LPS-induced production of proinflammatory cytokines,
we investigated the expression levels of TLR4, MyD88 and NF-κB
protein, and TLR4 and MyD88 mRNA in RAW264.7 cells. LPS binds TLR4,
leading to the activation of MyD88-dependent and MyD88-independent
signaling pathways. NF-κB is activated within the MyD88-dependent
pathway and has been implicated in the regulation of the TNF-α
promoter (23). The expression of
proteins involved in the TLR4 signaling pathway was increased in
the RAW264.7 cells after LPS administration (Fig. 4). Incubation with FTA
significantly inhibited the effect of LPS in a concentration
dependent-manner. These results suggest that FTA inhibits the TLR4
signaling pathway and thereby protects macrophages from LPS
stimulation.
FTA decreases the Treg percentage and the
expression levels of Foxp3 protein and mRNA
FTA decreases Treg percentage
LPS induced a marked increase in the relative
percentage of CD4+CD25+/CD4+ T
lymphocytes (P<0.01), which was significantly attenuated by
treatment with FTA (80 and 320 μg/ml) (Fig. 5G). FTA treatment decreased
LPS-induced changes in the relative percentage of Tregs in a dose
dependent manner. These findings are in agreement with the results
of flow cytometry (Fig. 5A–F).
The in vitro results, therefore, suggest that FTA elicits
immunomodulatory effects.
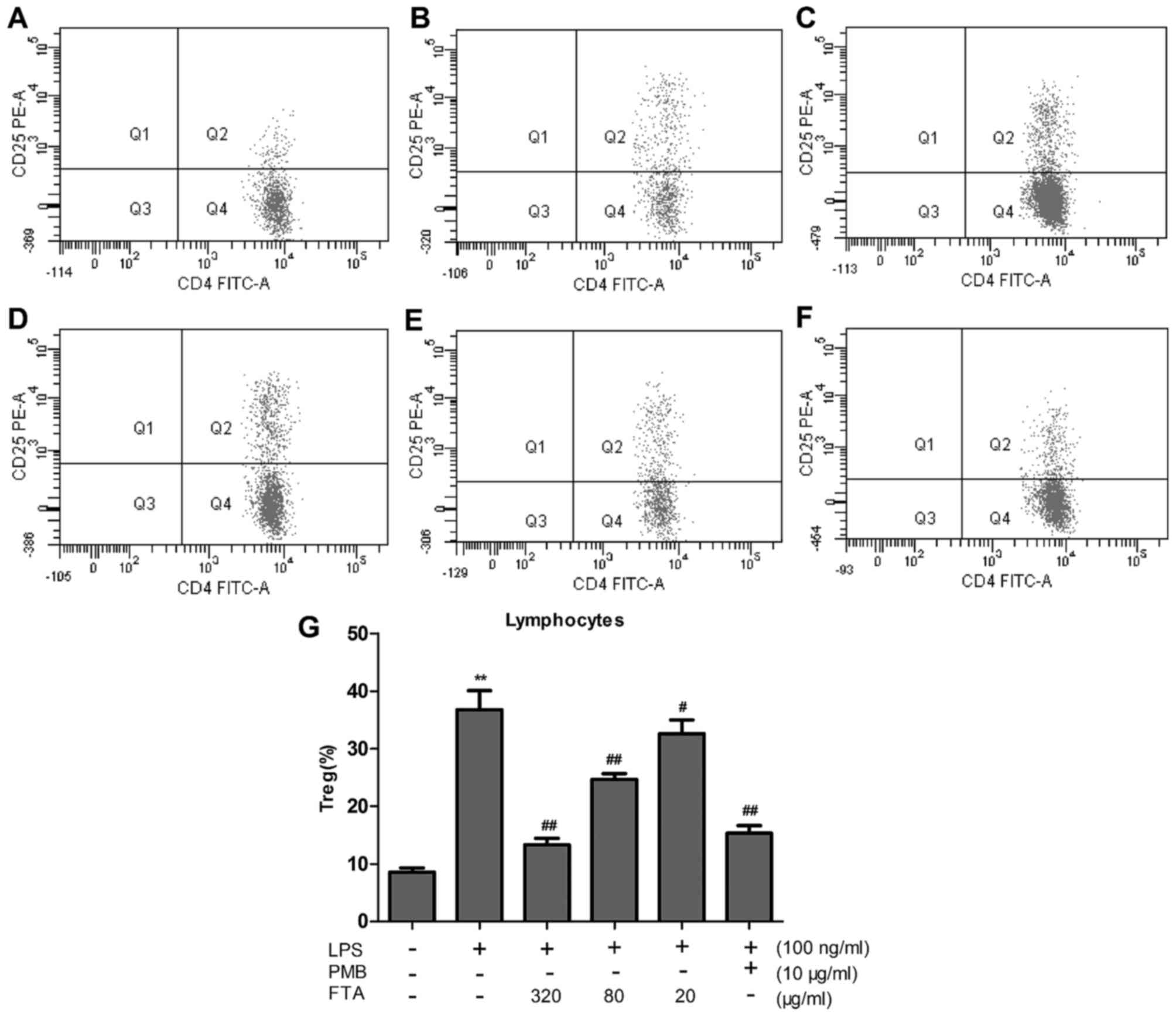 | Figure 5Forsythoside A (FTA) decreases the
Treg percentage in a dose-dependent manner. Lymphocytes were
stimulated with lipopolysaccharides (LPS) (100 ng/ml, 48 h) in the
absence or presence of pretreatment with polymyxin B (PMB, 10
μg/ml, 2 h) or FTA (320, 80 and 20 μg/ml, 2 h). The
Treg percentage in total lymphocytes was quantified by flow
cytometric analysis of (A) control-treated lymphocytes, (B)
lymphocytes treated with LPS alone, (C) lymphocytes treated with
LPS with FTA pretreatment (320 μg/ml), (D) lymphocytes
treated with LPS with FTA pretreatment (80 μg/ml), and (E)
lymphocytes treated with LPS with FTA pretreatment (20
μg/ml), (F) lymphocytes treated with LPS with pretreatment
with PMB (10 μg/ml). (G) Histogram showing flow cytometry
results. Values are expressed as the mean ± SEM of three
independent experiments. **P<0.01 vs. the control
group; ##P<0.01 and #P<0.05 vs. the
LPS-treated group. |
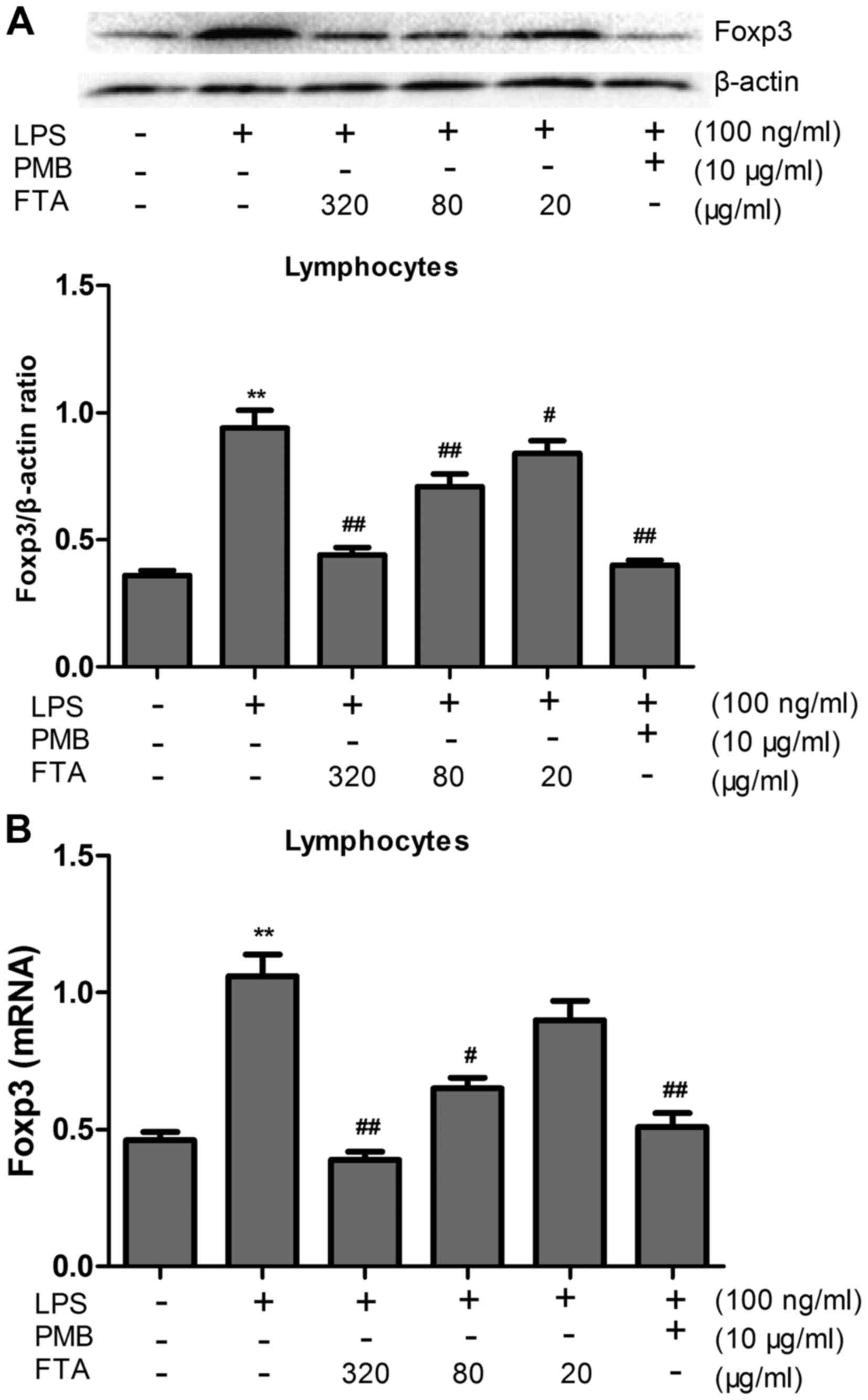
FTA decreases the expression levels of
Foxp3 protein and mRNA
The expression levels of Foxp3 protein and mRNA in
the LPS-treated cells were markedly increased compared to these
levels in the control group (P<0.01) (Fig. 6). However, FTA treatment
significantly decreased levels of Foxp3 protein and mRNA in the
LPS-treated cells in a dose-dependent manner (P<0.01 or
P<0.05).
Discussion
Plants used in traditional medicine are rich in
physiologically active ingredients. There is a trend in recent
pharmacological research to seek novel therapeutic agents on the
basis of traditional Chinese medicine. Of the 80% of
pharmaceuticals that are derived from plants, few are currently
used as antimicrobial agents (24). LPS are ubiquitous and act as
powerful stimulators of the immune system. Recently, a number of
research groups have sought to develop treatments that are
effective against LPS-mediated toxic effects by investigating the
ingredients of traditional medicinal plants. The decoction of
Forsythia was reported to elicit anti-inflammatory effects
and, importantly, to ameliorate the adverse effects of LPS
(25). In the present study, we
demonstrated that FTA, the main antimicrobial constituent in
Forsythia (26), exerts
significant anti-endotoxin activity in LPS-treated mice and
LPS-stimulated cells. The effect appears to be associated with the
inhibition of the LPS-TLR4 signaling pathway and decreased number
of Tregs.
PMB, a compound that exhibits strong affinity for
LPS, is generally perceived to be an endotoxin antagonist. A low
dose of PMB (50,000 U/day) was reported to decrease plasma
endotoxin levels without eliciting any significant side effects
(27). PMB-immobilized fiber
column hemoperfusion has been used for treating septic shock,
despite its potential to induce severe nephrotoxicity and
neurotoxicity (28). Due to its
anti-LPS efficacy, PMB was used as a positive control in our
present study.
TLR, a receptor family closely related to the innate
immunity, can recognize pathogen-associated molecular patterns
(PAMP) and regulate innate and acquired immunity. TLR4 is the first
TLR identified in humans, and is considered to be the most
important receptor involved in the effects of LPS. Tissue damage
and infection lead to the recognition of bacterial lipoprotein and
bacterial LPS by TLR4, initiating the signal transduction cascade
and inducing the release of endogenous mediators, thereby
stimulating the inflammatory response. The amplification cascade of
inflammatory factors can aggravate infection and cause further
tissue damage (29). Cell
viability was significantly decreased compared to the
vehicle-treated control group (P<0.05). Our findings, therefore,
confirm the presence of adverse effects of LPS on cellular
function. However, therapeutic agents have been shown to
significantly reduce LPS-induced pathological changes in a
dose-dependent manner. In our present study, we confirmed that FTA
can ameliorate the effect of LPS on cell proliferation at the
cellular level. Further study showed that levels of TLR4, MyD88,
NF-κB and TNF-α protein in the FTA groups to be significantly and
dose-dependently reduced (P<0.05), compared to those measured in
the LPS groups. Through the effect involving several intracellular
signaling molecules, LPS stimulates TLR4 signaling pathway
downstream of the receptor, affecting both MyD88-dependent and
MyD88-independent (TRIF-dependent) pathways (30). Within the MyD88-dependent pathway,
infection and cell damage activate signaling cascades involving
inflammatory cytokines. Regardless of the signaling pathway,
intracellular factors such as NF-κB and IRF3 are activated. NF-κB
is an important inducible transcription factor which can regulate
the expression of cytokines such as TNF-α, IL-6 and IL-8, along
with other cytokines involved in the inflammatory and immune
response, cell proliferation, tissue differentiation and apoptosis
(31,32). While TNF-α is important for the
normal inflammatory response to infection, inappropriate or
excessive production can be harmful (33). TNF-α acts through the TNF-α
receptor I or TNF-α receptor II to induce apoptosis, regulate cell
survival, or modulate inflammation (34). The successful use of TNF blockade
in the management of chronic inflammatory diseases highlights the
physiological role of TNF in sepsis (33). FTA may, therefore, inhibit
inflammatory factors TNF-α and NF-κB through blockade of the
LPS/TLR4 signaling pathway to elicit anti-endotoxin effects.
Caramalho et al previously reported that
Tregs interact with LPS since they selectively express TLR4,
thereby supporting their survival and proliferation, while also
enhancing their immunosuppressive function (35). The present study showed that FTA
suppresses LPS-mediated induction of the TLR4 pathway. Therefore,
we propose that the protective effect of FTA may be elicited by
regulation of Tregs.
Tregs elicit immunomodulatory effects and play a
pivotal role in maintenance of the immune balance.
CD4+CD25+ Tregs inhibit CD4+ T
cell proliferation (36). In
infection, Tregs mediate the responses of T cells to pathogens and
their activation of inflammatory response to tissue damage
(37). Our data showed a
significantly lower survival rate of LPS-stimulated lymphocytes,
compared to the control group, while the relative presence of Tregs
in culture was significantly higher (P<0.01). These findings
indicate that the immunosuppressive ability of LPS-stimulated
lymphocytes is inhibited, and confirm that LPS adversely affects
Treg function. The effects of LPS may be related to the excessive
activation of Tregs, resulting in inhibition of
CD4+CD25− cell proliferation. Experiments
evaluating the effects of FTA intervention showed that FTA can
significantly increase the survival rate of LPS-stimulated
lymphocytes and decrease the relative presence of Tregs in a
dose-dependent manner (P<0.05). In comparing cells treated with
a high dose of FTA with the control group, no obvious difference
was found (P>0.05), suggesting that the cytoprotective effect of
FTA may involve the inhibition of Treg activation to correct the
LPS-induced inhibition of cell immunity. Foxp3 is widely recognized
to act as a master switch and transcription factor for Treg
development and function (38),
and is the most specific biomarker for Treg activation (39). Deletion of the Foxp3 gene was
found to eliminate the immunosuppressive activity of
CD4+CD25+ Tregs, while the ectopic expression
of Foxp3 in CD25− Tregs conferred immunosuppressive
activity to the cells (40).
Human Foxp3 gene mutation has been linked with immunological
dysfunction, inflammatory bowel disease (IBD), X-linked syndrome
and allergic dermatitis (41). In
light of the significance of Foxp3, we evaluated the expression of
this protein in our experiments. In LPS-stimulated groups, Foxp3
expression was markedly increased, with this increase being
significantly suppressed in FTA groups. These findings indicate
that FTA may inhibit Treg activity by inhibiting Foxp3. IL-10 and
TGF-β1 are involved in the regulation of Foxp3 expression and the
secretion of IL-10 and TGF-β1 is one of the ways in which Tregs
suppress antigen-driven response of CD4+CD25−
cells (42–45). The two aspects contribute to the
immunosuppressive activity of CD4+CD25+
Tregs. In this study, FTA intervention could significantly inhibit
IL-10 and TGF-β1, which may be one of the mechanisms underlying the
observed downregulation of Foxp3.
In conclusion, we validated the therapeutic
potential of FTA for endotoxin-induced diseases. Furthermore, we
identified the blockade of the LPS/TLR4 signaling pathway and
inhibition of Tregs as putative mechanisms underlying the
protective action of FTA. While this study demonstrates the
potential for clinical efficacy of FTA, it should be noted that the
clinical effects may differ from our experimental results due to
the involvement of bacteria other than the LPS-producing species in
the clinical setting. Further studies are, therefore, warranted to
extend our experimental findings into the clinical setting.
Acknowledgments
The study was supported by the National Natural
Science Foundation, China (no. 81060354) and a grant from the
Education Department of Jiangxi Province of China (no. GJJ11338).
This manuscript has been edited and proofread by Editage.
Glossary
Abbreviations
Abbreviations:
|
ALI
|
acute lung injury
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FTA
|
forsythoside A
|
|
FBS
|
fetal bovine serum
|
|
IL
|
interleukin
|
|
LPS
|
lipopolysaccharides
|
|
PAMP
|
pathogen-associated molecular
patterns
|
|
NF-κB
|
nuclear factor-κB
|
|
PAMP
|
pathogen-associated molecular
patterns
|
|
PMB
|
polymyxin B
|
|
TCR
|
T-cell receptor
|
|
TLR
|
Toll-like receptors
|
|
TNF
|
tumor necrosis factor
|
|
Tregs
|
regulatory T cells
|
References
|
1
|
Alexander C and Rietschel ET: Bacterial
lipopolysaccharides and innate immunity. J Endotoxin Res.
7:167–202. 2001.PubMed/NCBI
|
|
2
|
Unger RE, Peters K, Sartoris A, Freese C
and Kirkpatrick CJ: Human endothelial cell-based assay for
endotoxin as sensitive as the conventional Limulus Amebocyte Lysate
assay. Biomaterials. 35:3180–3187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beutler B and Rietschel ET: Innate immune
sensing and its roots: The story of endotoxin. Nat Rev Immunol.
3:169–176. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bone RC, Balk RA, Cerra FB, Dellinger RP,
Fein AM, Knaus WA, Schein RM and Sibbald WJ; The ACCP/SCCM
Consensus Conference Committee. American College of Chest
Physicians/Society of Critical Care Medicine: Definitions for
sepsis and organ failure and guidelines for the use of innovative
therapies in sepsis. Chest. 101:1644–1655. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto M, Sato S, Hemmi H, Sanjo H,
Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T,
et al: Essential role for TIRAP in activation of the signalling
cascade shared by TLR2 and TLR4. Nature. 420:324–329. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X and Qin J: Modulation of
Toll-interleukin 1 receptor mediated signaling. J Mol Med (Berl).
83:258–266. 2005. View Article : Google Scholar
|
|
7
|
Miyake K: Endotoxin recognition molecules,
Toll-like receptor 4-MD-2. Semin Immunol. 16:11–16. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Medzhitov R, Preston-Hurlburt P and
Janeway CA Jr: A human homologue of the Drosophila Toll protein
signals activation of adaptive immunity. Nature. 388:394–397. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moynagh PN: TLR signalling and activation
of IRFs: Revisiting old friends from the NF-kappaB pathway. Trends
Immunol. 26:469–476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huo M, Cui X, Xue J, Chi G, Gao R, Deng X,
Guan S, Wei J, Soromou LW, Feng H, et al: Anti-inflammatory effects
of linalool in RAW264.7 macrophages and lipopolysaccharide-induced
lung injury model. J Surg Res. 180:e47–e54. 2013. View Article : Google Scholar
|
|
11
|
Craig R, Larkin A, Mingo AM, Thuerauf DJ,
Andrews C, McDonough PM and Glembotski CC: p38 MAPK and NF-kappaB
collaborate to induce interleukin-6 gene expression and release.
Evidence for a cytoprotective autocrine signaling pathway in a
cardiac myocyte model system. J Biol Chem. 275:23814–23824. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen W, Jin W, Hardegen N, Lei KJ, Li L,
Marinos N, McGrady G and Wahl SM: Conversion of peripheral
CD4+CD25− naive T cells to
CD4+CD25+ regulatory T cells by TGF-beta
induction of transcription factor Foxp3. J Exp Med. 198:1875–1886.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shevach EM: Mechanisms of
foxp3+ T regulatory cell-mediated suppression. Immunity.
30:636–645. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klayman DL: Qinghaosu (artemisinin): An
antimalarial drug from China. Science. 228:1049–1055. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo J, Xu H and Chen K: Systematic review
of compound danshen dropping pill: a chinese patent medicine for
acute myocardial infarction. Evid Based Complement Alternat Med.
2013:8080762013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu T, Wang DX, Zhang W, Liao XQ, Guan X,
Bo H, Sun JY, Huang NW, He J, Zhang YK, et al: Andrographolide
protects against LPS-induced acute lung injury by inactivation of
NF-κB. PLoS One. 8:e564072013. View Article : Google Scholar
|
|
18
|
Yu PJ, Li JR, Zhu ZG, Kong HY, Jin H,
Zhang JY, Tian YX, Li ZH, Wu XY, Zhang JJ, et al: Praeruptorin D
and E attenuate lipopolysaccharide/hydrochloric acid induced acute
lung injury in mice. Eur J Pharmacol. 710:39–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li T, Zhang J, Feng J, Li Q, Wu L, Ye Q,
Sun J, Lin Y, Zhang M, Huang R, et al: Resveratrol reduces acute
lung injury in a LPS induced sepsis mouse model via activation of
Sirt1. Mol Med Rep. 7:1889–1895. 2013.PubMed/NCBI
|
|
20
|
Shi Y, Zhang B, Chen XJ, Xu DQ, Wang YX,
Dong HY, Ma SR, Sun RH, Hui YP and Li ZC: Osthole protects
lipopolysaccharide-induced acute lung injury in mice by preventing
downregulation of angiotensin-converting enzyme 2. Eur J Pharm Sci.
48:819–824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu WB, Li DP, Zhang GL, Sun L and Zhang
NS: Study progress of the pharmacological activity of forsythoside
A. Chin Anim Husbandry Vet Med. 38:236–238. 2011.In Chinese.
|
|
22
|
Lu S, Chen SN, Guan JY and Shen H: Effects
of forsythoside on cell functions of raw264.7 stimulated by LPS.
Zhongguo Nongxue Tongbao. 28:58–62. 2012.In Chinese.
|
|
23
|
Liu H, Sidiropoulos P, Song G, Pagliari
LJ, Birrer MJ, Stein B, Anrather J and Pope RM: TNF-alpha gene
expression in macrophages: Regulation by NF-kappa B is independent
of c-Jun or C/EBP beta. J Immunol. 164:4277–4285. 2000. View Article : Google Scholar
|
|
24
|
Perumal Samy R and Gopalakrishnakone P:
Therapeutic potential of plants as anti-microbials for drug
discovery. Evid Based Complement Alternat Med. 7:283–294. 2010.
View Article : Google Scholar :
|
|
25
|
Hu JY, Lei L, Yu Y and Deng WL: Studies on
the anti-inflammatory and antipyretic effect of forsythia.
Pharmocol Clin Chin Mater Med. 23:51–52. 2007.
|
|
26
|
Qin Z, Xu J and Zhang LJ: Studies on the
anti-bacteria effect of fructus forsythia and folium forsythia in
vitro. Food Eng. 2:49–52. 2013.
|
|
27
|
Shao Y, Wang X, Cai SX, Gao W and Wu X:
Hemodynamic effect of polymyxin B sulfate on rats with endotoxemia.
Chongqing Univ (Natur Sci Ed). 25:84–86. 2002.
|
|
28
|
Mitaka C and Tomita M: Polymyxin
B-immobilized fiber column hemoperfusion therapy for septic shock.
Shock. 36:332–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Figueiredo RT, Bittencourt VCB, Lopes LCL,
Sassaki G and Barreto-Bergter E: Toll-like receptors (TLR2 and
TLR4) recognize polysaccharides of Pseudallescheria boydii cell
wall. Carbohydr Res. 356:260–264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JK, Kim SY, Kim YS, Lee WH, Hwang DH
and Lee JY: Suppression of the TRIF-dependent signaling pathway of
Toll-like receptors by luteolin. Biochem Pharmacol. 77:1391–1400.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Słotwiński R, Słotwińska S, Kędziora S and
Bałan BJ: Innate immunity signaling pathways: Links between
immunonutrition and responses to sepsis. Arch Immunol Ther Exp
(Warsz). 59:139–150. 2011. View Article : Google Scholar
|
|
33
|
Bradley JR: TNF-mediated inflammatory
disease. J Pathol. 214:149–160. 2008. View Article : Google Scholar
|
|
34
|
Wu Y and Zhou BP:
TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and
invasion. Br J Cancer. 102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Caramalho I, Lopes-Carvalho T, Ostler D,
Zelenay S, Haury M and Demengeot J: Regulatory T cells selectively
express toll-like receptors and are activated by
lipopolysaccharide. J Exp Med. 197:403–411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kosmaczewska A, Ciszak L, Potoczek S and
Frydecka I: The significance of Treg cells in defective tumor
immunity. Arch Immunol Ther Exp (Warsz). 56:181–191. 2008.
View Article : Google Scholar
|
|
37
|
Nakamura K, Kitani A and Strober W: Cell
contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T
cells is mediated by cell surface-bound transforming growth factor
beta. J Exp Med. 194:629–644. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fontenot JD, Gavin MA and Rudensky AY:
Foxp3 programs the development and function of
CD4+CD25+ regulatory T cells. Nat Immunol.
4:330–336. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lal G, Zhang N, van der Touw W, Ding Y, Ju
W, Bottinger EP, Reid SP, Levy DE and Bromberg JS: Epigenetic
regulation of Foxp3 expression in regulatory T cells by DNA
methylation. J Immunol. 182:259–273. 2009. View Article : Google Scholar
|
|
40
|
Fontenot JD and Rudensky AY: A well
adapted regulatory contrivance: Regulatory T cell development and
the forkhead family transcription factor Foxp3. Nat Immunol.
6:331–337. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wildin RS and Freitas A: IPEX and FOXP3:
Clinical and research perspectives. J Autoimmun. 25(Suppl): 56–62.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Murai M, Turovskaya O, Kim G, Madan R,
Karp CL, Cheroutre H and Kronenberg M: Interleukin 10 acts on
regulatory T cells to maintain expression of the transcription
factor Foxp3 and suppressive function in mice with colitis. Nat
Immunol. 10:1178–1184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kawamoto K, Pahuja A, Hering BJ and
Bansal-Pakala P: Transforming growth factor beta 1 (TGF-beta1) and
rapamycin synergize to effectively suppress human T cell responses
via upregulation of FoxP3+ Tregs. Transpl Immunol.
23:28–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yan B and Da WM:
CD4+CD25+ regulatory T cells and their
function in graft-versus-host disease - review. Zhongguo Shi Yan
Xue Ye Xue Za Zhi. 14:408–412. 2006.In Chinese. PubMed/NCBI
|
|
45
|
Khazaie K and von Boehmer H: The impact of
CD4+CD25+ Treg on tumor specific
CD8+ T cell cytotoxicity and cancer. Semin Cancer Biol.
16:124–136. 2006. View Article : Google Scholar : PubMed/NCBI
|