Introduction
Oral squamous cell carcinoma (OSCC) is currently a
highly prevalent disease worldwide (1). More than half of patients die of
this disease or the associated complications within 5 years even
under available therapies (2).
The prognosis of OSCC remains dismal (2). The low median survival rate is
associated with chemotherapeutic resistance (3,4).
Presently, there is limited information regarding the regulatory
mechanisms of chemoresistance in oral cancer.
Epithelial to mesenchymal transition (EMT) is an
essential process for driving plasticity during development and in
the context of different morphogenetic events; however it is also
an unintentional behavior of cells during malignant transformation
(5–6). During this process, the cells lose
their epithelial characteristics, including their polarity and
specialized cell-cell contacts, and acquire a migratory behavior,
allowing them to move away from their epithelial cell community and
to integrate into surrounding tissue, even at remote locations. EMT
illustrates the differentiation plasticity during development and
is complemented by another process, called mesenchymal to
epithelial transition (MET) (8).
Emerging evidence suggests that there is a strong link between
therapeutic resistance and the induction of EMT in cancer (9). Identifying the mechanisms that
promote EMT and the development of drug resistance could be a key
approach for the development of novel therapeutic targets.
p21 (RAC1) activated kinase 1 (PAK1) lies within the
11q13 region. Aberrant expression/activation of PAK1 has been
described in OSCC as well as in several other types of cancers
including breast, brain, pancreatic, colon, bladder, ovarian,
hepatocellular, urinary tract, renal cell carcinoma and thyroid
cancers (10). Stimulating OSCC
cells with serum growth factors was found to lead to PAK1
re-localization and caused profound cytoskeletal remodeling
(11). PAK1 was also found to be
involved in the invasion, migration and cytoskeletal remodelling
for OSCC cells (11). In this
study, we showed that PAK1 could be a potential therapeutic target
for OSCC.
Materials and methods
Human OSCC cell lines, SCC25 and
SCC25-res (cisplatin-resistant cells)
SCC25 cells were purchased from the American Type
Culture Collection (ATCC, Manassas, VA, USA). To obtain
cisplatin-resistant tongue cancer cells, we treated SCC25 cells
with escalating concentrations of cisplatin from 107 to
105 M. The established SCC25-res (cisplatin-resistant
SCC25) cells grew at a similar rate in the presence or absence of
105 M cisplatin for 3 days (data not shown). The
IC50 is the cisplatin concentration that reduces
proliferating cells by 50%. The IC50 of SCC25-res cells
increased by 12-fold, respectively, as compared with the SCC25
cells (data not shown). All cancerous cell lines were grown in
RPMI-1640 medium (Thermo Scientific, Waltham, MA, USA) supplemented
with 10% fetal bovine serum (FBS) (HyClone, Rockford, IL, USA) and
100 U/ml penicillin and streptomycin.
MTT assay
Cell proliferation was assessed by
3-(4,5–dimethylthiazol-2–yl)-2,5-diphenyltetrazolium (MTT) assay
(Sigma, St. Louis, MO, USA). MTT assay was performed as previously
described (12–14). In brief, the cells were plated in
96-well plates in Dulbecco's modified Eagle's medium containing 10%
fetal bovine serum at a density of 8×103 cells per well
at 37°C in a 5% CO2 incubator for 12 h. The cells were
treated as indicated in each figure for 12 h. MTT solution (5
mg/ml) was then added to the wells (20 µl per well). The
plates were incubated in a cell incubator for 4 h, and the
supernatant was then removed and 150 µl of dimethyl
sulfoxide were added to each well. Following incubation for 10 min,
the absorbance of each well was measured using a Synergy™ 4 (BioTek
Instruments, Winooski, VT, USA) at a wavelength of 570 nm, with the
reference wavelength set at 630 nm. Absorbance was directly
proportional to the number of surviving cells. The viability of the
control group (cells transfected with pcDNA3.1) was considered to
be 100%.
BrdU incorporation assay
Cell proliferation was also assessed using a
colorimetric BrdU proliferation kit by following the manufacturer's
instructions (Cat. no. 11647229001; Roche; Basel, Switzerland).
Briefly, the cells treated with the peptides were labeled with BrdU
for 3 to 4 h. The genomic DNA was then fixed and denatured,
followed by incubation with peroxidase-conjugated anti-BrdU
antibody for 90 min. The substrate of the conjugated peroxidase was
then added and the reaction product was measured by determining the
absorbance (A370–A492 nm). The results were then normalized by the
number of total viable cells, which was determined by a
side-by-side cell viability assay as described above.
PAK1-expressing plasmids/empty vectors
and transfection experiments
PAK1-expressing plasmids and empty vectors
(pcDNA3.1) were obtained from Tiangen Biotech (Beijing, China). The
cells were seeded at a density of 1.5×105 per well in
6-well plates or 60-mm dishes in 2 ml of complete medium containing
10% FCS for 24 h. Transfections were performed using Lipofectamine
2000 transfection reagent (Invitrogen, Carlsbad, CA, USA) following
the manufacturer's instructions.
Western blot analysis
The total proteins in cells were extracted with
protein lysis solution (Tiangen Biotech). The protein concentration
was measured with a bicinchoninic acid kit (Tiangen Biotech).
Protein extracts were resolved through 8% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred
to polyvinylidene difluoride membranes (Bio-Rad, Berkeley, CA,
USA), probed with anti-bodies against human ERCC1 (1:500;
ab129267), YAP (1:500; ab52771), PAK1 (1:500; ab40795), vimentin
(1:500; ab92547), E-cadherin (1:500; ab40772) or β-actin (1:500;
ab8227) (all from Abcam, Cambridge, MA, USA) and then with
IRDye™-800 conjugated anti-rabbit secondary antibodies (ab218695;
Abcam) for 30 min at room temperature. The specific proteins were
visualized by Odyssey™ Infrared Imaging System (Gene Company,
Lincoln, NE, USA).
Real-time PCR for microRNAs
Total RNA from the cultured cells, with efficient
recovery of small RNAs, was isolated using the mirVana miRNA
isolation kit (Ambion, Austin, TX, USA). Detection of the mature
form of miRNAs was performed using the mirVana qRT-PCR miRNA
detection kit and qRT-PCR primer sets, according to the
manufacturer's instructions (Ambion). The sequences of the primers
were as follows: miR-485-5p forward,
5′-CCAAGCTTCACCCATTCCTAACAGGAC-3′ and reverse,
5′-CGGGATCCGTAGGTCAGTTACATGCATC-3′. The U6 small nuclear RNA was
used as an internal control.
Immunofluorescence staining
Cells were stained for immuno-fluorescence on
coverslips. After fixation and permeabilization, the cells were
incubated with primary antibodies against PAK1, E-cadherin or
vimentin (Abcam) and then incubated with FITC-conjugated secondary
antibodies (Invitrogen). The coverslips were counterstained with
4′,6-diamidino-2-phenylindole (DAPI) and imaged under a confocal
microscope TCS SP5 (Lecia, Solms, Germany).
Reverse transcription-PCR and real-time
PCR for mRNA
PCR was performed as previously described (12,15). The PCR primer sequences are as
follows, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward,
5′-ATTCAACGGCACAGTCAAGG-3′ and reverse, 5′-GCAGAAGGGGCGGAGATGA-3′;
E-cadherin forward, 5′-TCAACGATCCTGACCAGCAGTTCG-3′ and reverse,
5′-GGTGAACCATCATCTGTGG CGATG-3′; vimentin forward,
5′-GACAATGCGTCTCTGGCACGTCTT-3′ and reverse,
5′-TCCTCCGCCTCCTGCAGGTTCTT-3′.
Methods of bioinformatics
The analysis of potential microRNA target sites was
performed using the commonly used prediction algorithm, miRanda
(http://www.microrna.org/).
Migration and invasion assays
Migration and invasion assays were performed as
previously described (16). The
migration and invasion assays were performed using 24 well
Transwell chambers (8 µm; Corning Inc., Corning, NY, USA).
For the migration assay, 1×105 cells suspended in 200
µl serum-free RPMI-1640 medium were seeded into the upper
chamber of the Transwell invasion system, and 500 µl
RPMI-1640 medium containing 10% FBS was added to the lower chamber.
Following a 24-h incubation, cells on the upper surface of the
membrane were scrubbed off, and the migrated cells were fixed with
95% ethanol and stained with 0.1% crystal violet for 10 min. The
number of migrated cells was determined by counting 5 random fields
on each membrane. The invasion assay protocol was similar to that
of the migration assay, with the exception that the upper chambers
were first covered with 1 mg/ml Matrigel, as previously described
(12,14,15,17).
Statistical analysis
Data are presented as the means ± SEM. Student's
t-test (two-tailed) was used to compare two groups (P<0.05 was
considered significant).
Results
PAK1 does not affect the proliferation of
oral squamous cell carcinoma SCC25 cells
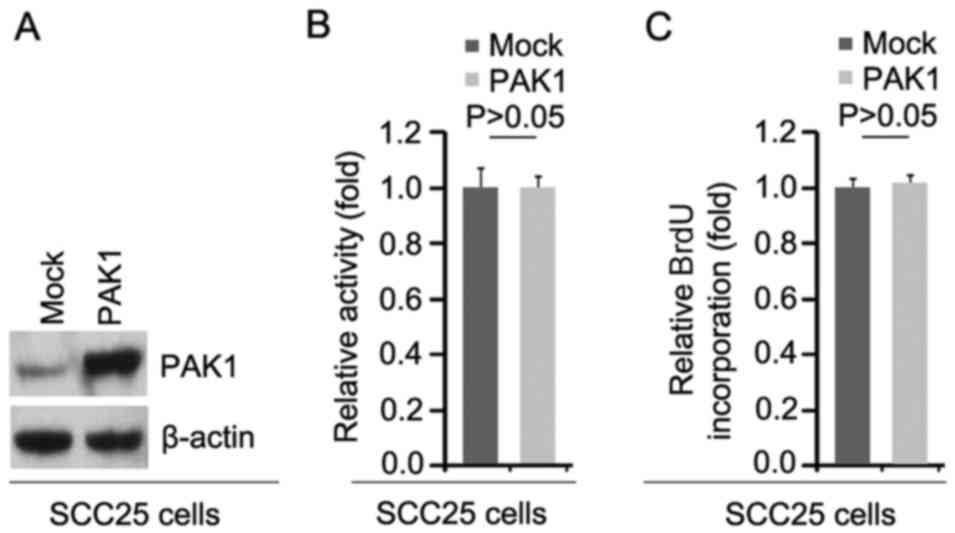
To investigate whether PAK1 can affect the
proliferation of SCC25 cells, firstly using western blot analysis,
we tested whether PAK1-expressing plasmids could cause stable
expression of PAK1 protein in the SCC25 cells. The results showed
that PAK1 protein was significantly increased by PAK1-expressing
plasmids in the cells (Fig. 1A).
In addition, we performed MTT assay to detect the proliferation of
SCC25 cells transfected with the PAK1-expressing plasmids. The
results showed that PAK1 did not affect the proliferation of the
SCC25 cells after 48 h of transfection (Fig. 1B). To further show the effects of
PAK1 on proliferation, we performed BrdU incorporation assay to
detect DNA synthesis in the cells. The results confirmed that PAK1
did not affect DNA synthesis in the cells (Fig. 1C).
PAK1 promotes EMT, migration and invasion
in SCC25 cells
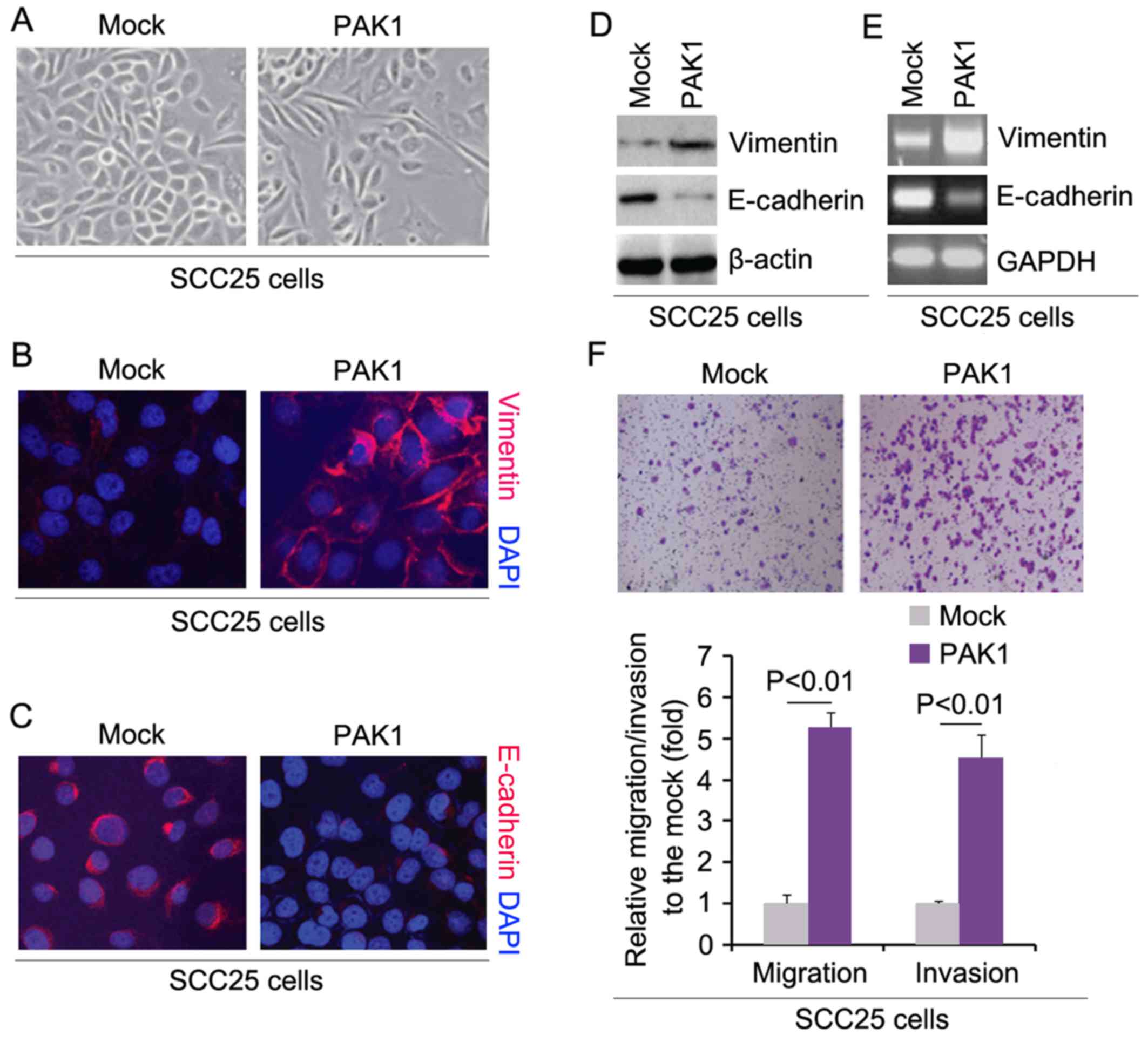
In order to assess the role of PAK1 in the EMT of
SCC25 cells, we transfected SCC25 cells with PAK1-expressing
plasmids and then we found that its overexpression caused
significant changes in the cell morphology (EMT, phenotype from a
cobblestone-like to a spindle-like morphology) (Fig. 2A). To further verify that the
changes in cell morphology were caused by EMT, we performed
immunofluorescence to detect expression of epithelial marker,
E-cadherin, and mesenchymal marker, vimentin, in the SCC25 cells
transfected with the PAK1-expressing plasmids and the cells
transfected with the empty vectors. The results demonstrated that
PAK1 promoted vimentin protein expression (Fig. 2B) and inhibited E-cadherin protein
expression (Fig. 2C). To further
verify the results of the immunofluorescence, we performed western
blot analysis to detect the expression of vimentin and E-cadherin
in the SCC25 cells transfected with the PAK1-expressing plasmids
and the cells transfected with the empty vectors. The results
revealed that vimentin protein was increased and E-cadherin protein
was decreased by PAK1 (Fig. 2D).
We also performed RT-PCR to detect vimentin and E-cadherin mRNA in
the cells transfected with PAK1. Consistent with the western blot
analysis, we found that vimentin mRNA was upregulated and
E-cadherin mRNA was downregulated by PAK1 (Fig. 2E). We next sought to determine
whether PAK1 has any impact on migration and invasion in the cells.
The migration and invasion assays showed that overexpression of
PAK1 promoted the migration and invasion of the PAK1-overexpressing
SCC25 cells (Fig. 2F).
Overexpression of the PAK1 promotes
cisplatin resistance in SCC25 cells
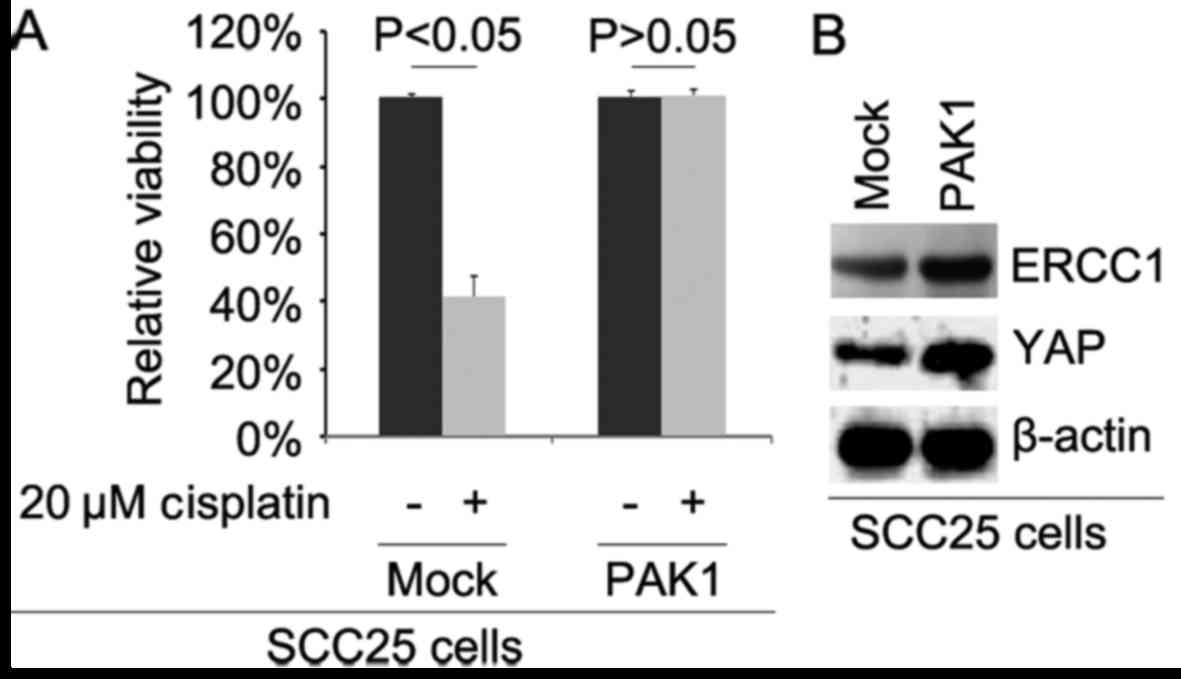
To further identify whether PAK1 affects cisplatin
efficacy in SCC25 cells, we transfected the cells with
PAK1-expressing plasmids. We then performed MTT assay in the SCC25
cells treated as indicated. The results showed that overexpression
of PAK1 could transform SCC25 cells to SCC25-CR cells (Fig. 3A), suggesting that its
overexpression promoted cisplatin resistance. We also performed
western blot analysis to detect ERCC1 and YAP protein expression in
SCC25 cells transfected with the PAK1-expressing plasmids and empty
vectors. The results demonstrated that the levels of ERCC1 and YAP
protein were increased by PAK1 (Fig.
3B).
miR-485-5p inhibits PAK1 protein
expression in SCC25 cells
Having demonstrated that overexpression of PAK1
promoted EMT, migration and invasion in SCC25 cells, we next
studied the mechanisms regulating PAK1 expression in SCC25 cells.
MicroRNAs (miRs) are a class of small noncoding RNAs (~22
nucleotides) that negatively regulate protein-coding gene
expression by targeting mRNA degradation or translation inhibition
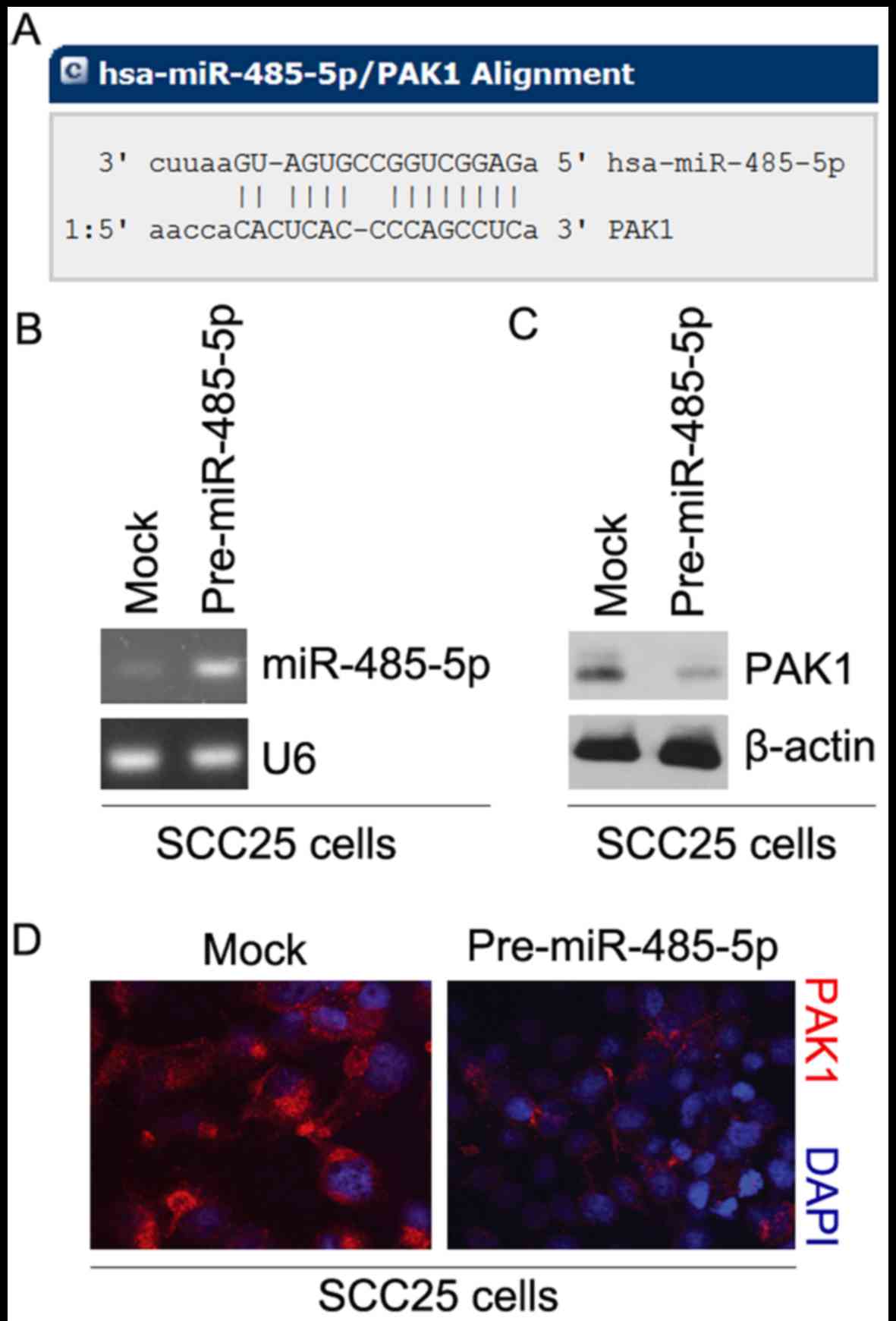
(18–20). To further confirm whether PAK1 is
regulated by microRNAs, we used the commonly used prediction
algorithm, miRanda (http://www.microrna.org/microrna/home.do), to analyze
the 3′ untranslated region (3′UTR) of PAK1. A number of microRNAs
were found by the algorithm. But we were interested in miR-485-5p,
as it has been reported that miR-485-5p is a tumor-suppression gene
that inhibits oncogene expression (21–24). Thus, we reasoned that miR-485-5p
could downregulate PAK1 protein expression by targeting its 3′UTR
in SCC25 cells. The target sites on the 3′UTR of PAK1 are shown in
Fig. 4A.
To identify the role of miR-485-5p in regulating
PAK1 expression in SCC25 cells, we transfected SCC25 cells with
pre-miR-485-5p and control miR. After transfection, miR-485-5p
expression was detected by real-time PCR and the results showed
that miR-485-5p was significantly increased by pre-miR-485-5p in
the cells (Fig. 4B). To confirm
that miR-485-5p can regulate PAK1 expression, we performed western
blot analysis to detect PAK1 protein expression in the SCC25 cells
transfected with pre-miR-485-5p and control miR. The results showed
that PAK1 protein was significantly inhibited by miR-485-5p
(Fig. 4C). We next performed
immunofluorescence analyses in SCC25 cells transfected with
pre-miR-485-5p and control miR. The results showed that PAK1
protein was evidently inhibited in the cells transfected with
pre-miR-485-5p (Fig. 4D).
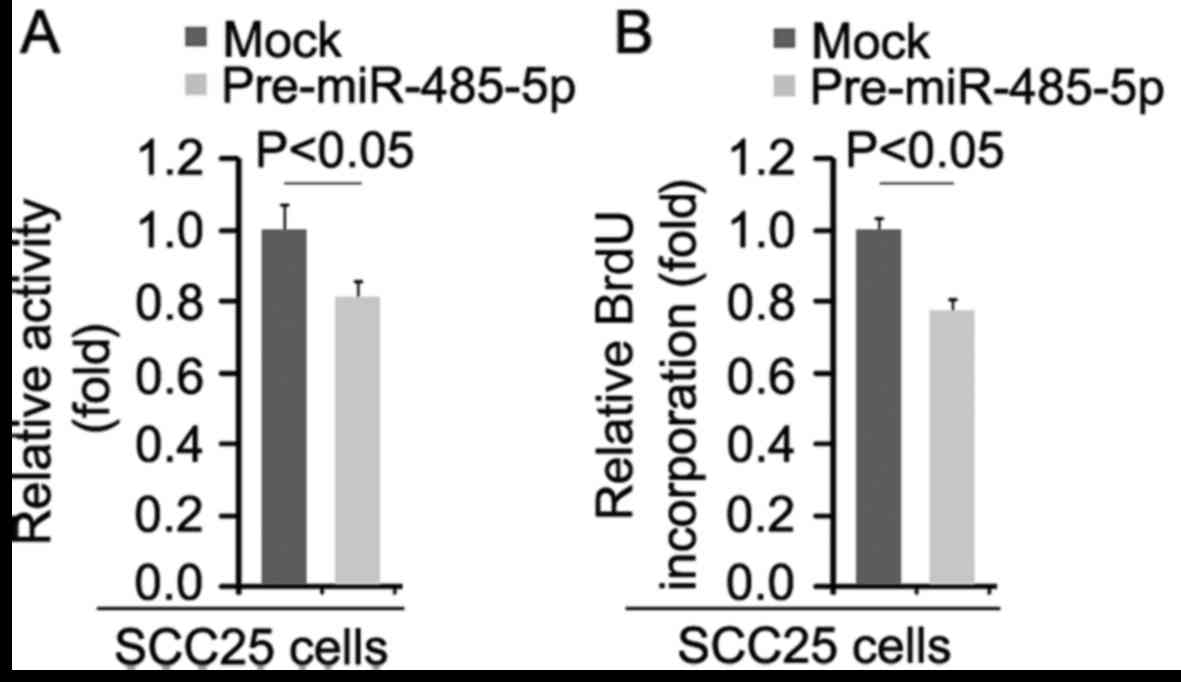
miR-485-5p inhibits the proliferation of
SCC25 cells
We performed MTT assay to detect the proliferation
rate of the SCC25 cells transfected with miR-485-5p and control
miR. The results demonstrated that miR-485-5p inhibited the
proliferation of the SCC25 cells after 48 h of transfection
(Fig. 5A). To further show the
effects of miR-485-5p on proliferation, we performed BrdU
incorporation assay to detect DNA synthesis in the cells. The
results confirmed that DNA synthesis of the cells was significantly
inhibited (Fig. 5B).
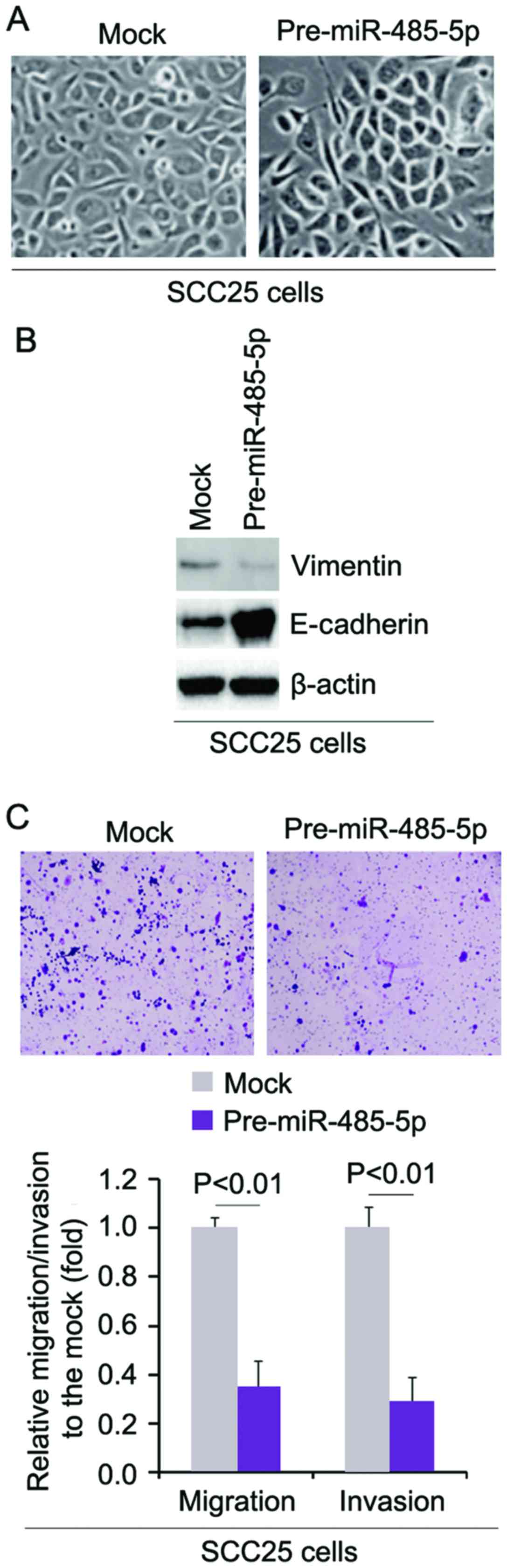
miR-485-5p reverses EMT and inhibits the
migration and invasion of SCC25 cells
In order to assess the role of miR-485-5p in EMT of
SCC25 cells, we transfected SCC25 cells with pre-miR-485-5p and
then found that its overexpression caused significant changes in
the cell morphology (MET, phenotype from a spindle-like morphology
to a cobblestone-like) (Fig. 6A).
To further verify that the changes in cell morphology were caused
by MET, we performed western blot analysis to detect the expression
of epithelial marker, E-cadherin, and mesenchymal marker, vimentin,
in the SCC25 cells transfected with pre-miR-485-5p and the cells
transfected with control miR. The results demonstrated that
miR-485-5p inhibited vimentin protein expression and promoted
E-cadherin protein expression (Fig.
6B). We next sought to determine whether miR-485-5p has any
impact on migration and invasion in the cells. The migration and
invasion assays showed that overexpression of miR-485-5p inhibited
the migration and invasion of cells transfected with pre-miR-485-5p
(Fig. 6C).
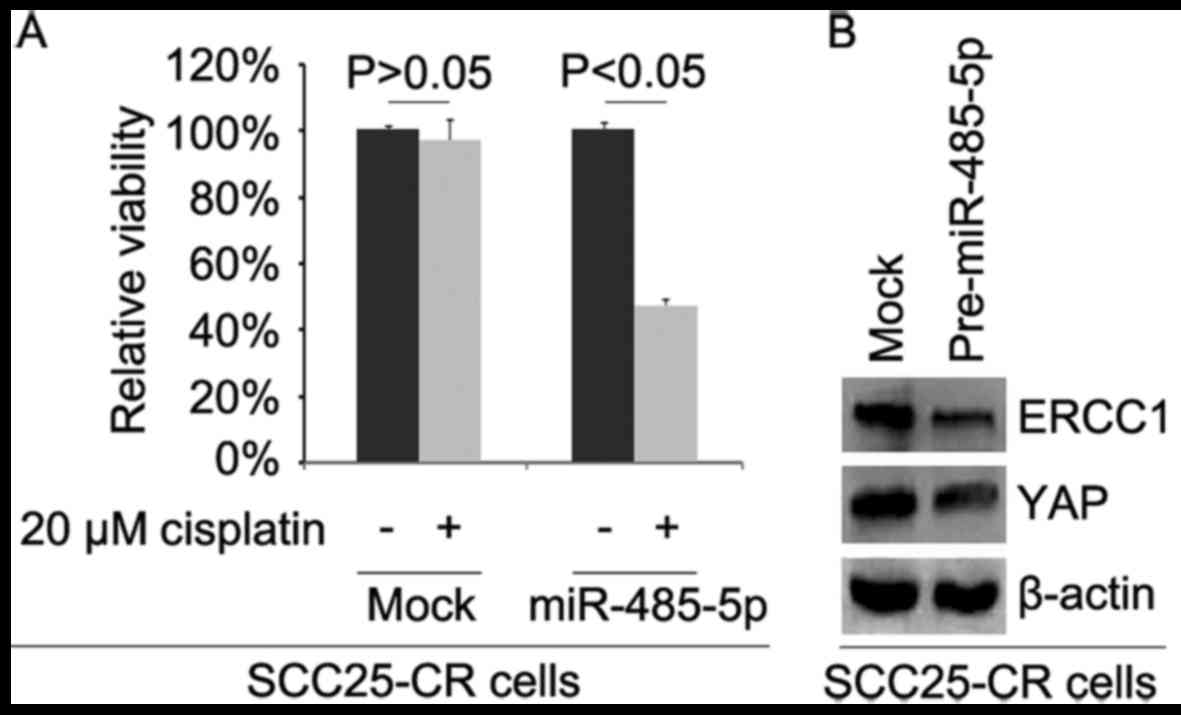
Overexpression of miR-485-5p reverses
cisplatin resistance in SCC25-CR (cisplatin-resistant cells)
cells
To further identify whether miR-485-5p affects
cisplatin efficacy in SCC25-CR cells, we transfected the cells with
pre-miR-485-5p. We then performed MTT assay in the SCC25-CR cells
treated as indicated (Fig. 7A).
The results showed that overexpression of miR-485-5p transformed
SCC25-CR cells to SCC25 cells (Fig.
7A), suggesting that its overexpression reversed cisplatin
resistance. We also performed western blot analysis to detect ERCC1
and YAP protein expression in the SCC25-CR cells transfected with
pre-miR-485-5p and control miR. The results demonstrated that
levels of ERCC1 and YAP protein were decreased by miR-485-5p
(Fig. 7B).
Discussion
Most OSCC-associated deaths are in part due to the
spread of tumor cells resistant to conventional therapies (25). Aggressive cells acquire genetic
and epigenetic changes that promote formation of their
metastasis-associated abilities such as acquisition of increased
motility and invasiveness (25).
Increased abilities of motility and invasiveness are linked to
enhanced local growth of tumor cells, decreased cell-cell adhesion
as well as degradation of basement membranes and stroma, referred
to as EMT (26,27), a frequently observed phenotypic
change usually caused by oncogenes (28–30), and by degradation of the basement
membranes initiated by increased matrix metalloproteinases and
collagenases (31). In this
study, we found that PAK1 induced EMT and promoted migration and
invasion in SCC25 cells. Emerging evidence associates
chemoresistance with acquisition of EMT in cancer (32). Consistent with the study, we
showed that overexpression of PAK1 promoted cisplatin resistance in
SCC25 cells. High expression of ERCC1 and YAP are associated with
cispantin resistance in locally advanced squamous cell carcinoma of
the head and neck (18,33). Our results demonstrated that
overexpression of PAK1 promoted ERCC1 and YAP protein expression in
the SCC25 cells.
miR-485-5p has been reported as a potential tumor
suppressor in gastric cancer, breast cancer, hepatocellular
carcinoma and ovarian cancer, but its expression, cellular function
and clinical features in OSCC are not known (21–24). Consistent with the roles of
miR-485-5p in other cancers, we showed that its overexpression
inhibited the proliferation of SCC25 cells. PAK1 is a target gene
of miR-485-5p in SCC25. But we found that PAK1 did not inhibit the
proliferation of SCC25 cells, implying that miR-485-5p inhibits
proliferation by regulating other genes. It has been reported that
sensitivity to anticancer drugs is confined to the 'epithelial'
subset, and the sensitivity to anticancer drugs could be
re-established by microRNA-mediated molecular reversal of EMT
(34). Consistent with this
report, we found that miR-485-5p reversed EMT and
cisplatin-resistance in SCC25-CR cells. Thus, an
miR-485-5p-mediated molecular mechanism is responsible for
cisplatin resistance. It can block the oncogenic role of PAK1 and
hence restoration of miR-485-5p has therapeutic potential in
PAK1-positive OSCC.
References
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar
|
|
2
|
Lo WL, Kao SY, Chi LY, Wong YK and Chang
RC: Outcomes of oral squamous cell carcinoma in Taiwan after
surgical therapy: Factors affecting survival. J Oral Maxillofac
Surg. 61:751–758. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pérez-Sayáns M, Somoza-Martín JM,
Barros-Angueira F, Diz PG, Rey JM and García-García A: Multidrug
resistance in oral squamous cell carcinoma: the role of vacuolar
ATPases. Cancer Lett. 295:135–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Q, Shi S, Yen Y, Brown J, Ta JQ and
Le AD: A subpopulation of CD133(+) cancer stem-like cells
characterized in human oral squamous cell carcinoma confer
resistance to chemotherapy. Cancer Lett. 289:151–160. 2010.
View Article : Google Scholar
|
|
5
|
Nieto MA: The ins and outs of the
epithelial to mesenchymal transition in health and disease. Annu
Rev Cell Dev Biol. 27:347–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Savagner P, Yamada KM and Thiery JP: The
zinc-finger protein slug causes desmosome dissociation, an initial
and necessary step for growth factor-induced epithelial-mesenchymal
transition. J Cell Biol. 137:1403–1419. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar R, Gururaj AE and Barnes CJ:
21-activated kinases in cancer. Nat Rev Cancer. 6:459–471. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parvathy M, Sreeja S, Kumar R and Pillai
MR: Potential role of p21 activated kinase 1 (PAK1) in the invasion
and motility of oral cancer cells. BMC Cancer. 16(Suppl 1):
2932016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liao XH, Lu DL, Wang N, Liu LY, Wang Y, Li
YQ, Yan TB, Sun XG, Hu P and Zhang TC: Estrogen receptor α mediates
proliferation of breast cancer MCF-7 cells via a
p21/PCNA/E2F1-dependent pathway. FEBS J. 281:927–942. 2014.
View Article : Google Scholar
|
|
13
|
Li H, Xiang Y, Fan LJ, Zhang XY, Li JP, Yu
CX, Bao LY, Cao DS, Xing WB, Liao XH and Zhang TC: Myocardin
inhibited the gap protein connexin 43 via promoted miR-206 to
regulate vascular smooth muscle cell phenotypic switch. Gene.
616:22–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao XH, Xiang Y, Yu CX, Li JP, Li H, Nie
Q, Hu P, Zhou J and Zhang TC: STAT3 is required for
MiR-17-5p-mediated sensitization to chemotherapy-induced apoptosis
in breast cancer cells. Oncotarget. 8:15763–15774. 2017.PubMed/NCBI
|
|
15
|
Xiang Y, Lu DL, Li JP, Yu CX, Zheng DL,
Huang X, Wang ZY, Hu P, Liao XH and Zhang TC: Myocardin inhibits
estrogen receptor alpha-mediated proliferation of human breast
cancer MCF-7 cells via regulating MicroRNA expression. IUBMB Life.
68:477–487. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Y, Chopp M, Zheng X, Katakowski M,
Buller B and Jiang F: MiR-145 reduces ADAM17 expression and
inhibits in vitro migration and invasion of glioma cells. Oncol
Rep. 29:67–72. 2013.
|
|
17
|
Liao XH, Zheng L, He HP, Zheng DL, Wei ZQ,
Wang N, Dong J, Ma WJ and Zhang TC: STAT3 regulated ATR via
microRNA-383 to control DNA damage to affect apoptosis in A431
cells. Cell Signal. 27:2285–2295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshikawa K, Noguchi K, Nakano Y, Yamamura
M, Takaoka K, Hashimoto-Tamaoki T and Kishimoto H: The Hippo
pathway transcriptional co-activator, YAP, confers resistance to
cisplatin in human oral squamous cell carcinoma. Int J Oncol.
46:2364–2370. 2015.PubMed/NCBI
|
|
19
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pasquinelli AE, Reinhart BJ, Slack F,
Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B,
Müller P, et al: Conservation of the sequence and temporal
expression of let-7 heterochronic regulatory RNA. Nature.
408:86–89. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang M, Ren MP, Zhao L, Li CP and Deng MM:
miR-485-5p acts as a negative regulator in gastric cancer
progression by targeting flotillin-1. Am J Transl Res. 7:2212–2222.
2015.
|
|
22
|
Jing LL and Mo XM: Reduced miR-485-5p
expression predicts poor prognosis in patients with gastric cancer.
Eur Rev Med Pharmacol Sci. 20:1516–1520. 2016.PubMed/NCBI
|
|
23
|
Kim TH, Kim YK, Kwon Y, Heo JH, Kang H,
Kim G and An HJ: Deregulation of miR-519a, 153, and 485-5p and its
clinicopathological relevance in ovarian epithelial tumours.
Histopathology. 57:734–743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lou C, Xiao M, Cheng S, Lu X, Jia S, Ren Y
and Li Z: MiR-485-3p and miR-485-5p suppress breast cancer cell
metastasis by inhibiting PGC-1α expression. Cell Death Dis.
7:e21592016. View Article : Google Scholar
|
|
25
|
De Vita V Jr, Hellman S, Rosenberg S and
Markoe AM: Cancer: Principles and practice of oncology. Am J Clin
Oncol. 9:901986. View Article : Google Scholar
|
|
26
|
Birchmeier W, Hulsken J and Behrens J:
E-cadherin as an invasion suppressor. Ciba Found Symp. 189:124–141.
174–176. 1995.PubMed/NCBI
|
|
27
|
Vleminckx K, Vakaet L Jr, Mareel M, Fiers
W and van Roy F: Genetic manipulation of E-cadherin expression by
epithelial tumor cells reveals an invasion suppressor role. Cell.
66:107–119. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Comijn J, Berx G, Vermassen P, Verschueren
K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D and van Roy
F: The two-handed E box binding zinc finger protein SIP1
downregulates E-cadherin and induces invasion. Mol Cell.
7:1267–1278. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Woodhouse EC, Chuaqui RF and Liotta LA:
General mechanisms of metastasis. Cancer. 80(Suppl 8): 1529–1537.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang W, Feng M, Zheng G, Chen Y, Wang X,
Pen B, Yin J, Yu Y and He Z: Chemoresistance to 5-fluorouracil
induces epithelial-mesenchymal transition via up-regulation of
Snail in MCF7 human breast cancer cells. Biochem Biophys Res
Commun. 417:679–685. 2012. View Article : Google Scholar
|
|
33
|
Jun HJ, Ahn MJ, Kim HS, Yi SY, Han J, Lee
SK, Ahn YC, Jeong HS, Son YI, Baek JH, et al: ERCC1 expression as a
predictive marker of squamous cell carcinoma of the head and neck
treated with cisplatin-based concurrent chemoradiation. Br J
Cancer. 99:167–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McConkey DJ, Choi W, Marquis L, Martin F,
Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al: Role
of epithelial-to-mesenchymal transition (EMT) in drug sensitivity
and metastasis in bladder cancer. Cancer Metastasis Rev.
28:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|