Introduction
Diabetic individuals are susceptible to ischemic
heart disease and sustain a more unfavorable prognosis for survival
than non-diabetic individuals, despite advancements in surgical
techniques and pharmacological therapies (1). Hyperglycemia stimulates reactive
oxygen species (ROS) production and induces oxidative stress
(2), which is greater in the
presence of diabetes mellitus following reperfusion injury, and
contributes to the exacerbation of myocardial ischemia/reperfusion
(I/R) injury (3). Furthermore,
this condition has been evidenced to cause myocardial fibrosis and
impair cardiac function in the left ventricle (LV) of diabetic rats
(2). Therefore, the scavenging of
ROS may effectively prevent the initiation or progression of
diabetic myocardial I/R injury and heart failure (2,4).
A recent finding suggests that there is a link
between silent information regulator 1 (SIRT1) and the levels of
ROS (5). As a type of histone
deacetylase, SIRT1 plays a key role in a number of cell signaling
pathways and widely regulates diverse biological processes, such as
cell survival, apoptosis oxidative stress response and aging
(6,7). The activation of SIRT1 has been
shown to significantly decrease ROS levels and promote cell
survival (8). Furthermore,
studies have shown that SIRT1 is an important cytoprotective and
defensive cytokine against oxidative insults and the
cardiac-specific overexpression of SIRT1 protects rats against
myocardial I/R injury (9).
(−)-Epigallocatechin-3-gallate (EGCG) is a major
bioactive polyphenol derived from green tea that has been found to
possess potent antioxidant and free radical scavenging properties
(10–12). It has been well documented that
EGCG exerts multiple beneficial effects on cardiovascular
performance, including reducing myocardial I/R injury and
alleviating post-ischemic myocardial dysfunction in vitro
and in vivo (11,12). Recent studies have indicated that
some polyphenols reduce oxidative stress through the activation of
SIRT1, thus resulting in the improvement of cardiac function in
diabetic cardiomyopathy (13,14). As an important poly-phenol, EGCG
may inhibit hyperglycemia-induced myocardial I/R injury in diabetic
hearts by a mechanism relative to the altered expression of SIRT1
and its downstream target genes.
Therefore, the present study aimed to evaluate the
effects of EGCG treatment on cardiac function, myocardial apoptosis
and oxidative stress, as well as its preventive role in the
progression of cardiomyocyte injury under hyperglycemic conditions,
as well as the effects of EGCG in diabetic rats subjected to
myocardial I/R injury. In addition, we aimed to elucidate the
possible mechanisms involving the altered expression of SIRT1 and
its downstream target genes.
Materials and methods
Experimental animals and the induction of
diabetes
All experimental protocols used in this study
conformed to the guidelines for the Care and Use of Laboratory
Animals published by the US National Institutes of Health (NIH
Publication, revised 1996) and was approved by the Renmin Hospital
of Wuhan University, Wuhan, China. Adult male Sprague-Dawley rats
(weighing 270±10 g) were purchased from Beijing HFK Bioscience Co.
(Beijing, China), as previously reported (15). The rats were allowed to
acclimatize to a purified American Institute of Nutrition (AIN-93G)
diet and distilled water ad libitum in the specific
pathogen-free experimental animal facility of the Renmin Hospital
for 5 days. Following acclimatization, the rats were randomly
divided into the control group and the diabetic group. Type 1
diabetes was induced in the rats in the diabetic group by a single
intraperitoneal injection of a freshly prepared streptozotocin
(STZ) solution (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.1
M citrate buffer (pH 4.5) at a dose of 65 mg/kg body weight, as
previously described (15). Rats
in the control group received a single intraperitoneal injection of
citrate buffer alone as a control. Three days post-STZ injection,
tail vein blood glucose levels were measured using a One Touch
Ultra Glucose meter (LifeScan, Milpitas, CA, USA) and rats with
fasting blood glucose levels >16.7 mM were considered as
diabetic (15). Body weight and
serum glucose levels of the rats were recorded at the beginning of
the experiment, and at 3 days, 6 and 8 weeks after the
STZ-injection.
Animal experimental protocols
The animals were divided into 5 subgroups (n=18 for
each subgroup) as follows: i) control rats (control); ii) diabetic
rats (DM); iii) diabetic rats treated with EGCG (DM + EGCG); iv)
diabetic rats treated with EGCG and the SIRT1 inhibitor, EX527
(Tocris Bioscience, Bristol, UK) (DM + EGCG + EX); and v) diabetic
rats treated with EX527 (DM + EX). EGCG (≥98% purity) was provided
by Zhejiang Yixin Pharmaceutical Co., Ltd. (Zhejiang, China) (Cat
no. 989-51-5). EGCG was dissolved in sterile-distilled deionized
water as a stock solution of 10 mM at −80°C until dilution prior to
use, as previously described (16,17). EGCG was intragastrically
administered to the rats for 14 consecutive days at a dose of 100
mg/kg body weight prior to the onset of myocardial I/R (17). EX527 was first dissolved in
dimethyl sulfoxide (DMSO) and then diluted in sterile saline (final
DMSO concentration <2%). EX527 at a dose of 5 mg/kg was
intraperitoneally injected every 2 days for 7 times prior to
myocardial I/R operation. Rats in the control group were treated
with sterile-distilled water by oral gavage (vehicle control for
EGCG). Rats in the control, DM and DM + EGCG groups received a
single injection of sterile saline with the final DMSO
concentration <2% respectively (vehicle control for EX527). The
doses and time course of the experiments used for EGCG and EX527 in
this study were based on published studies using the same animal
species (12,17–19). After the treatment period, the
diabetic and control rats were subjected to myocardial I/R
operation. In the second set of experiments, 5 similar experimental
groups of rats (n=7 in each group) were subjected to the same
experimental procedures and sacrificed at 14 days after I/R to
assess myocardial fibrosis.
In vivo model of myocardial I/R
injury
The animals were anesthetized by an intraperitoneal
injection of sodium pentobarbital (65 mg/kg body weight) and then
placed on a controlled heating pad to maintain rectal temperature
at 37°C, incubated and ventilated. The procedure for creating the
myocardial I/R model was similar to that previously described
(20). Briefly, a left
thoracotomy was performed and the left anterior descending coronary
artery (LAD) was ligated with 6-0 silk suture. The artery was
occluded for 30 min by tightening the ligature. Following 30 min of
ischemia, the ligature was loosened to allow reperfusion for 2
h.
Hemodynamic detection
Invasive hemodynamic measurements were performed to
evaluate I/R-induced cardiac dysfunction. Myocardial function was
intermittently monitored at the baseline and after I/R insult.
After the right common carotid artery (CCA) was separated, a
polyethylene catheter was then advanced into the left ventricle via
the incision on the right CCA. The catheter was connected to a
pressure transducer (DPT-248; Yixinda, Shenzhen, China) and cardiac
functional variables were recorded using LabChart 7 software (AD
Instruments, Colorado Springs, CO, USA). Left ventricular systolic
pressure (LVSP), maximum speed of pressure development of LV
(+dp/dtmax) and pressure decline (−dp/dtmax) were continuously
monitored using an electro-physiolograph (MH150; BioPAC, Goleta,
CA, USA) at the time of 10 min prior to ischemia (baseline) and 2 h
after reperfusion.
Assessment of myocardial injury
To assess the levels of lactate dehydrogenase (LDH),
arterial blood samples were collected at the end of reperfusion and
centrifuged at 2,000 × g for 10 min to collect serum. Levels of
serum LDH were detected using a commercial enzyme-linked
immunosorbent assay (ELISA) kit (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) according to the manufacturer's
instructions.
Determination of apoptosis and myocardial
infarction
At the end of the 2 h of reperfusion, the hearts
were rapidly removed and rinsed in ice-cold saline and then
embedded in optimal cutting temperature compound, followed by being
frozen at −80°C for cryosectioning. Myocardial apoptosis was
analyzed by terminal deoxynucleotidyl transferase dUTP nick-end
labeling (TUNEL) reaction using an in situ cell death
detection kit (Roche Diagnostics GmbH, Mannheim, Germany) as
previously described (20). For
each heart sample, a total of 15 random microscopy fields were
selected and the index of apoptosis was calculated as a percentage
of apoptotic myocytes to the total number of myocytes. The infarct
size (IS) and area at risk (AAR) were measured using Evans blue dye
(2% EB; Sigma-Aldrich) and 2,3,5-triphenylte trazolium chloride (1%
TTC; Sigma-Aldrich) staining. At the end of reperfusion, the
ligature around the coronary artery was retired again and 1 ml of
2% EB was injected into the aorta. The presence of EB was used to
identify the area that was not subjected to ischemia. The rats were
euthanized and the hearts were rapidly excised and frozen at −20°C,
and then sliced into 2-mm-thick sections parallel to the
atrioventricular groove using a heart slice chamber. The slices
were incubated in 1% TTC in buffer (pH 7.4) for 15 min at 37°C. The
viable tissue was stained red by TTC, while the infarct portion not
taking up TTC stain remained pale. Morphometric measurements of the
area at AAR and IS in each slice were performed as previously
described (20). The percentage
of ratios of AAR vs. LV (AAR/LV) and IS vs. AAR (IS/AAR) were
calculated.
Masson's trichrome staining
To objectively quantify the amount of tissue
fibrosis, another subset of rats (=35) were euthanized 14 days
after I/R. Their hearts were rapidly removed and incubated with 10%
formalin overnight at room temperature overnight, and embedded with
paraffin. The hearts were sliced horizontally to the long axis, and
stained with hematoxylin and eosin, and Masson's trichrome stain
for light microscopy examinations. Masson's trichrome staining was
performed using a Masson Stain kit (D026; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). Digital images were
obtained at 400 magnification by microscopy (Olympus, Tokyo,
Japan). Fifteen randomly selected microscopic fields from Masson's
trichrome-stained sections were analyzed. The percentage of
fibrosis was determined using ImageJ software (NIH, Bethesda, MD,
USA) to quantify blue (fibrotic) vs. non-blue (non-fibrotic)
areas.
Determination of free
15-F2t-isoprostane (15-F2t-IsoP) and
malonaldehyde content
Myocardial tissue from the left ventricular ischemic
region was homogenized immediately after reperfusion. As a specific
indicator of oxidative stress, free 15-F2t-IsoP in the
homogenized heart tissue was determined using commercially
available kits (Cayman Chemical Co., Ann Arbor, MI, USA). The level
of malonaldehyde (MDA) from the homogenized heart tissue and
culture mediaum were measured using respective commercial kits
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China)
according to the manufacturer's instructions. The results were
expressed as mg/g protein for 15-F2t-IsoP and nmol/mg
protein for MDA.
Cell culture and establishment of a
hypoxia/reoxygenation (H/R) model
Rat myocardium-derived H9c2 cells were obtained from
the American Type Culture Collection (ATCC, Manassas, VA, USA) and
maintained in Dulbecco's modified Eagle's medium (DMEM) containing
10% fetal bovine serum (FBS), 100 U/ml penicillin and 100
μg/ml streptomycin at 37°C in a humidified atmosphere with
5% CO2 and 95% air. DMEM containing 30 mM glucose has
been applied to simulate chronic hyperglycemia in cell-based
studies (15). The H9c2 cells in
our study were cultured in DMEM containing 5.5 mM glucose [normal
glucose (NG)] or 30 mM glucose [high glucose (HG)]. Following
exposure to normal glucose or high glucose medium for 48 h, the
cells were randomly assigned to 5 groups as follows: i) normal
glucose (NG) medium + H/R; ii) high glucose HG medium + H/R; iii)
HG + EGCG + H/R; iv) HG + EGCG + SIRT1 small interfering RNA
(siRNA) + H/R; v) HG + EGCG + scramble siRNA + H/R. The cells were
treated with EGCG and/or siRNA 24 h prior to the H/R challenge. For
the induction of H/R injury, hypoxic conditions were obtained by a
three gas incubator containing 94% N2 and 5%
CO2. Cardiomyocytes were subjected to H/R by hypoxia for
2 h followed by 4 h of reoxygenation in all groups. EGCG was
freshly prepared as a stock solution of 10 mM by dissolving the
compound in deionized water (12)
and added to culture medium at a concentration of 20 μM
prior to H/R. Scramble siRNA or SIRT1 siRNA (sc-108043; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) were transfected into the
cells 24 h prior to H/R challenge according to the manufacturer's
instructions.
Measurement of cell viability and LDH
activity
After the treatments, cell viability was assessed by
using 3-[4, 5-dimeth-ylthiazol-2-yl]-2,5-diphenyltetrazolium
bromide (MTT; Beyotime Biotechnology Institution, Jiangsu, China)
according to the manufacturer's instructions. Cardiomyocyte injury
was assessed by measuring LDH release into the culture medium with
an LDH activity assay kit (Beyotime, Haimen, China). The LDH
concentration was measured in the medium using a spectrophotometer
set at 450 nm.
Assessment of cell apoptosis by flow
cytometric analysis
After the completion of the various treatments, the
cells were collected and resuspended in binding buffer. Following
the additioin of fluorescein isothiocyanate (FITC)-conjugated
Annexin V and propidium iodide (PI), cellular fluorescence was
measured on a FACSCalibur and data obtained was analyzed with
CellQuest Pro software (BD Biosciences, Franklin Lakes, NJ,
USA).
Western blot analysis
Equal amount of proteins from rat hearts and H9c2
cell homogenate were separated by electrophoresis and transferred
to polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). Each membrane was incubated overnight at 4°C with the
following specific primary antibodies: SIRT1 (Rab. sc-15404, 1:500
in blocking buffer in TBS) and MnSOD (Rab. sc-137254, 1:500 in
blocking buffer in TBS) (all from Santa Cruz Biotechnology, Inc.).
The membranes were then incubated with secondary antibody
(1:10,000; IRDye 800CW; LI-COR Corporate, Lincoln, NE, USA) for 1 h
at room temperature. Immune complexes were visualized by
fluorescence imaging scanner (LI-COR Corporate). Band densities
were quantified and calculated by Odyssey Image Analysis software
(LI-COR Corporate) and then normalized to glyceraldehyde
3-phosphate dehydrogenase (GAPDH). The protein expression amounts
were represented relative to those of the control.
Statistical analysis
All data were expressed as the means ± SEM and
analyzed using GraphPad Prism 6 statistic software (GraphPad
Software, Inc., La Jolla, CA, USA). Differences between the groups
were analyzed by using a one-way ANOVA with Tukey's test. Unpaired
two-tailed Student's t-test was applied for the comparison between
two different groups. The changes in body weight, serum glucose
levels and systemic hemodynamics over time were analyzed via a
two-way ANOVA with Bonferroni's test. The values at P-value
<0.05 were considered statistically significant.
Results
A total of 125 rats were used in our experiments: 7
rats died during the experimental intervention of ischemia for 30
min (1 in the control group, 2 in the DM group, 1 in the DM + EGCG
group, 2 in the DM + EGCG + EX group, and 1 in the DM + EX group)
and 7 rats died during the experimental intervention of reperfusion
for 2 h (1 in the control group, 2 in the DM group, 1 in the DM +
EGCG group, 1 in the DM + EGCG + EX group and 2 in the DM + EX
group). Right after I/R intervention, the rats from each group were
sacrificed for the experiments of hemodynamic detection, apoptosis,
infarct size, MDA and western blot analysis. Additionally, 6 rats
died during 14 days after I/R administration (2 in the DM group,
one in the DM + EGCG group, one in the DM + EGCG + EX group and 2
in the DM + EX group). Fourteen days after I/R administration, in
order to assess myocardial fibrosis, 5 surviving rats from each
group were adopted and then sacrificed for Masson's trichrome
staining (29 rats). Data were reported on the remaining 105
rats.
Characteristics of control and diabetic
rats
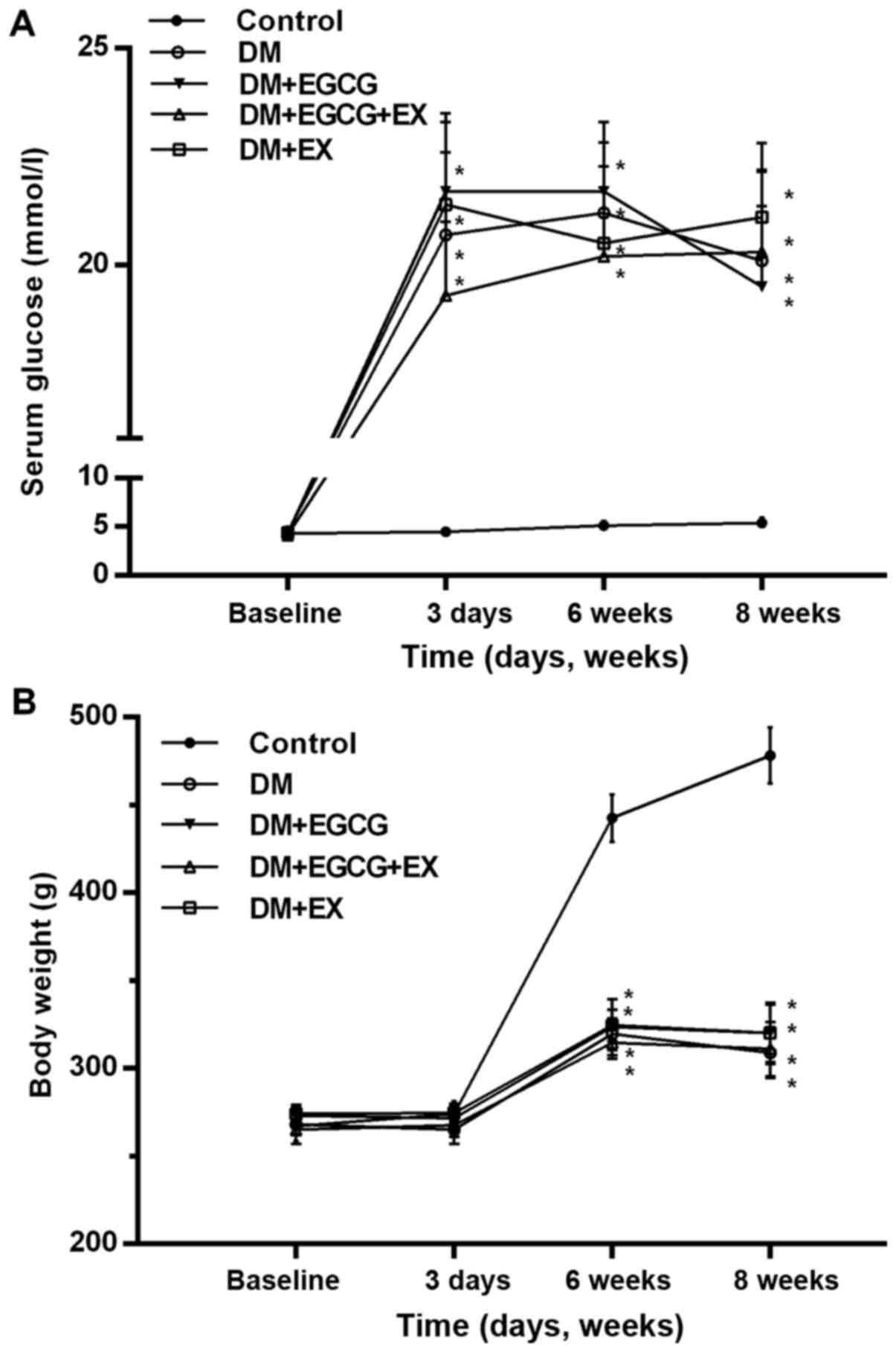
As shown in Fig.
1, initial body weight and serum glucose levels were similar
between all groups (P>0.05). The diabetic rats exhibited a
marked increase in serum glucose levels and a decrease in body
weight following the induction of diabetes (P<0.001 vs.
control). EGCG and EX treatment did significantly alter these
levels in the diabetic rats, as compared to the vehicle-treated
diabetics (P>0.05).
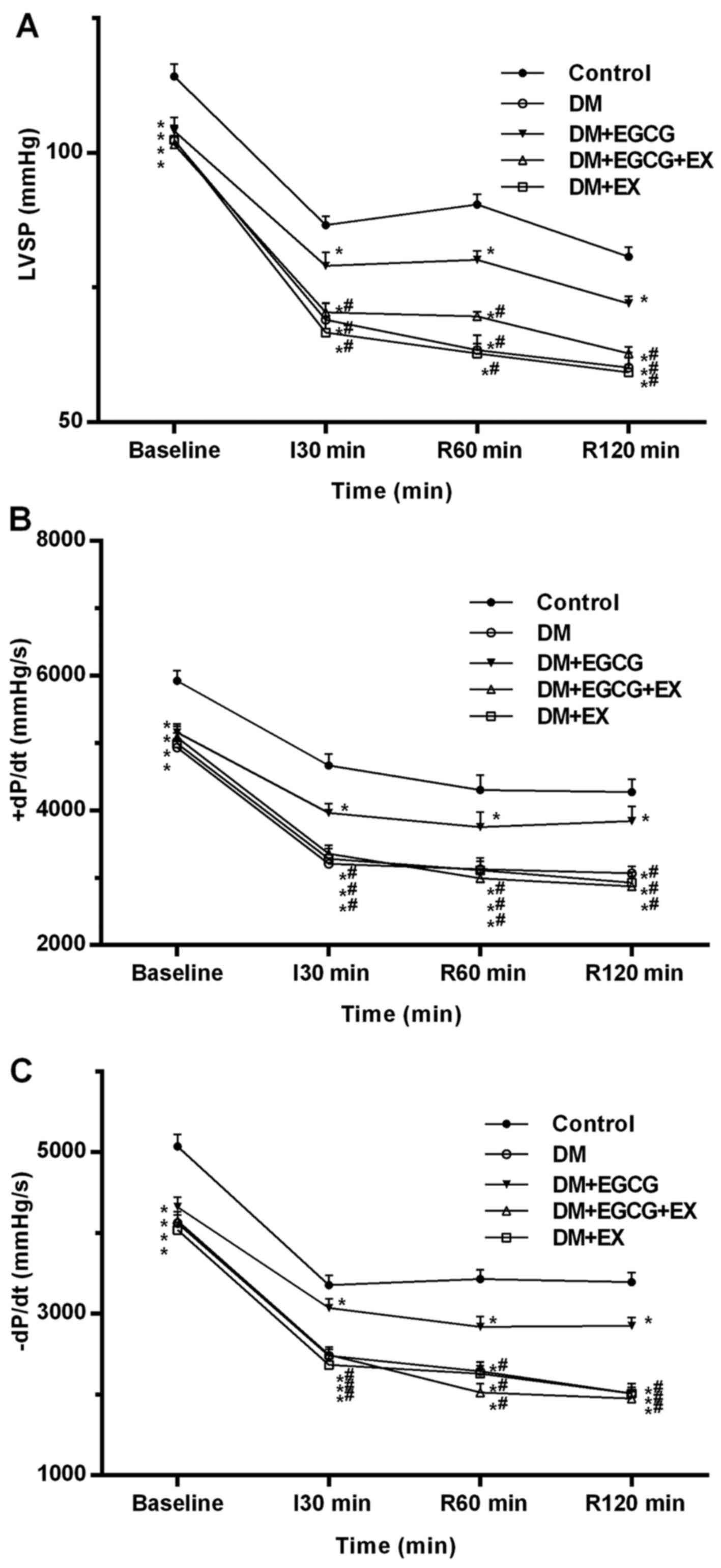
Status of systemic hemodynamics in
control and diabetic rats
The serial changes in LV hemodynamic parameters,
including LVSP, +dp/dtmax and −dp/dtmax during the experiments, are
shown in Fig. 2. At baseline, the
LVSP, +dP/dtmax and −dP/dtmax in all diabetic groups were lower
than those in the control group (all P<0.05) (Fig. 2). There were no LV function
differences between the diabetic groups at baseline (P>0.05). At
the time point of I30, R60 min and R120 min, a significant increase
in LVSP, +dP/dtmax and −dP/dtmax was observed in the DM + EGCG
group (P<0.05), as compared to the DM group, although the
respective values were still lower than those in the control group
(P<0.05). However, the improvements in LV function induced by
EGCG were not observed in the DM + EGCG + EX group (P<0.05).
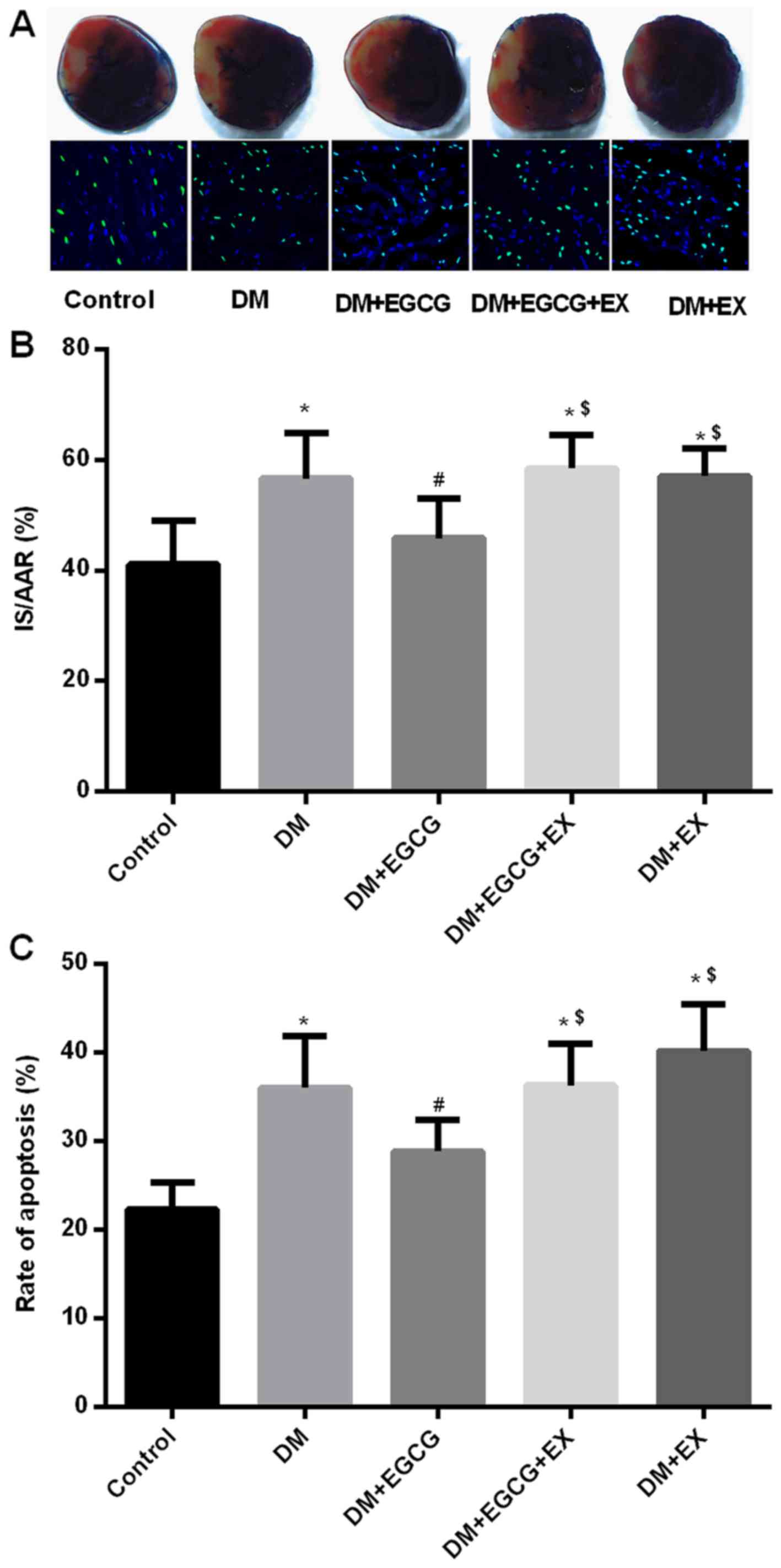
Myocardial infarct size, cardiomyocyte
apoptosis and serum LDH level in control and diabetic rats
Compared with the control group, the infarct size,
cardiomyocyte apoptosis and serum LDH levels significantly
increased in the DM group (all P<0.05) (Figs. 3 and 4), whereas the EGCG administration
significantly decreased the infarct size (Fig. 3A and B), cardiomyocyte apoptosis
(Fig. 3C) and serum LDH levels
(Fig. 4A) by 17.8, 22.7 and
27.7%, respectively (all P<0.05). However, the protective
effects of EGCG were abolished by concomitant treatment with EGCG
and EX527, with the infarct size, rate of apoptosis and serum LDH
levels increasing by 25.6, 30.5 and 38.9%, respectively (all
P<0.05), compared to the diabetic group treated with EGCG alone
(Figs. 3 and 4).
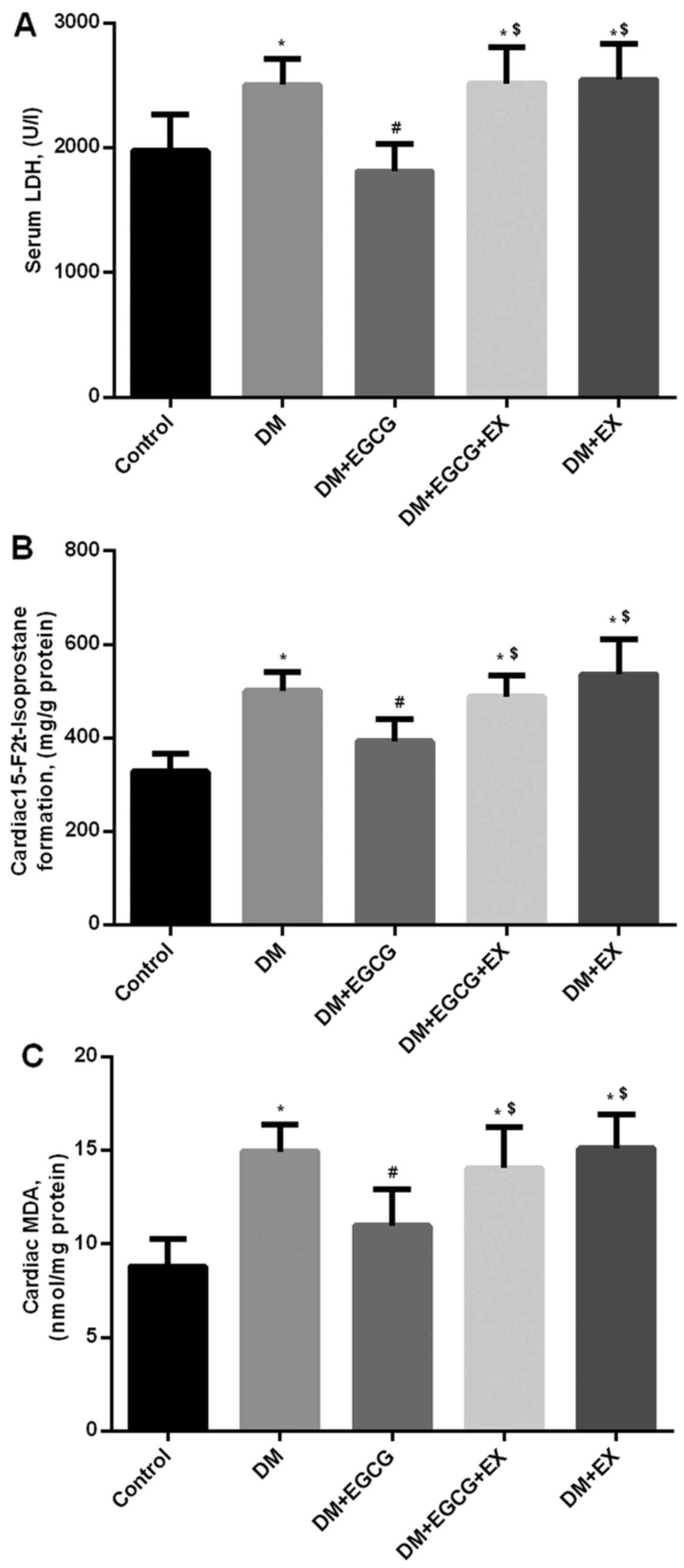
Myocardial 15-F2t formation
and MDA levels in control and diabetic rats
Following 2 h of reperfusion, 15-F2t
formation and MDA levels in the diabetic rats were higher than
those in the control group (152.6 and 165.8%, respectively, both
P<0.05) (Fig. 4B and C).
However, EGCG treatment reduced 15-F2t and MDA formation
by 21.4 and 24.9% in the diabetic rats following myocardial I/R
injury, compared to those in the DM group (P<0.05); these
effects were abrogated by the administration of EX527 (Fig. 4B and C).
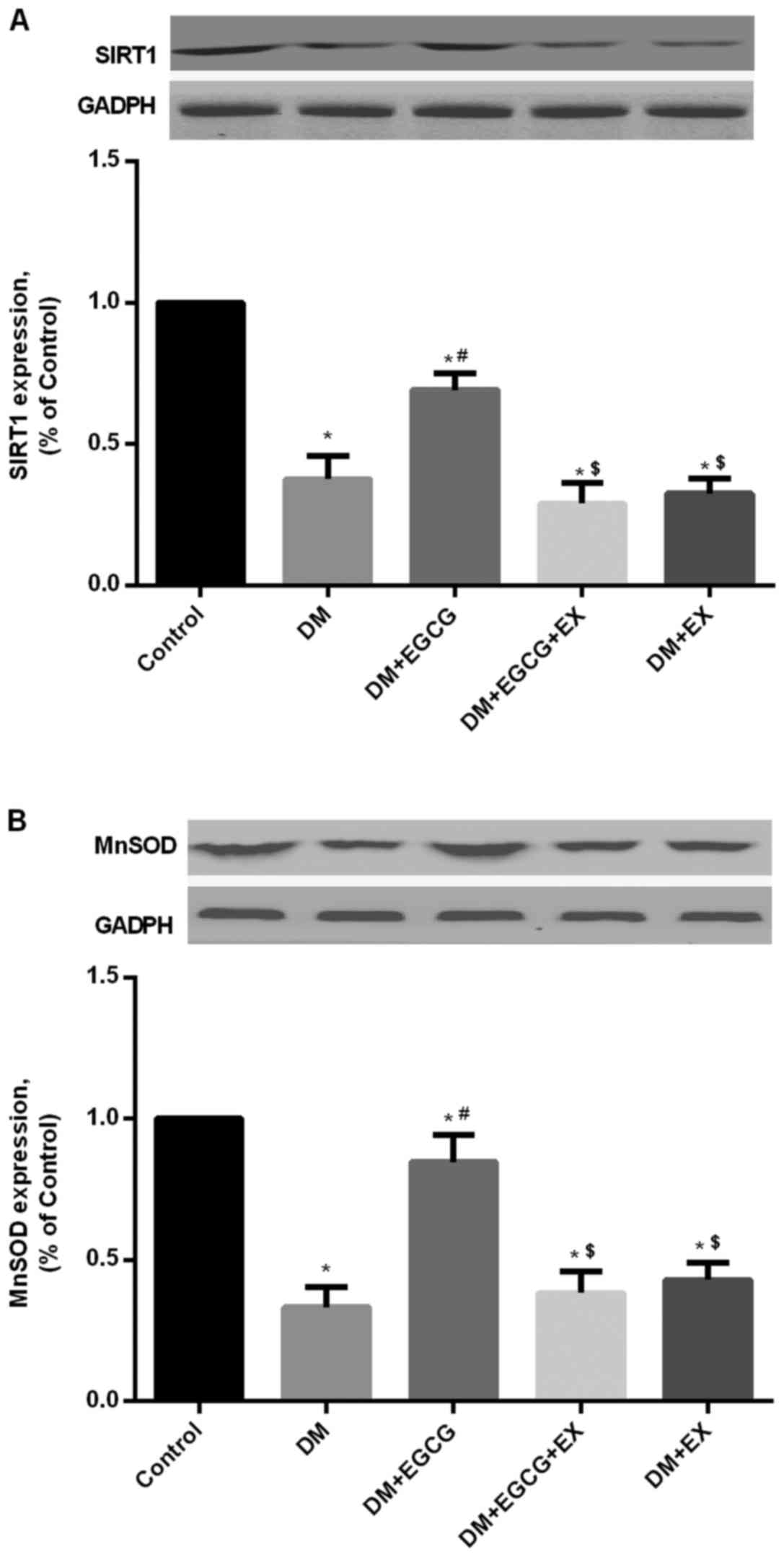
Protein expression of SIRT1 and MnSOD in
control and diabetic rats
As shown in Fig.
5, STZ-induced diabetes reduced the expression of SIRT1 protein
in the DM group (P<0.05), compared with the age-matched control
group following I/R injury, whereas EGCG pre-treatment
significantly increased SIRT1 expression compared to the DM group
(P<0.05) (Fig. 5A). EGCG did
not promote the increased expression of SIRT1 in the diabetic rats
in the presence of EX527 pre-treatment (P>0.05), compared with
the DM + EGCG group (Fig. 5A).
Additionally, a higher level of MnSOD expression was also found in
the EGCG pre-treatment group, but the increment was abolished by
EX527 intervention (P<0.05), compared to the DM + EGCG group
(Fig. 5).
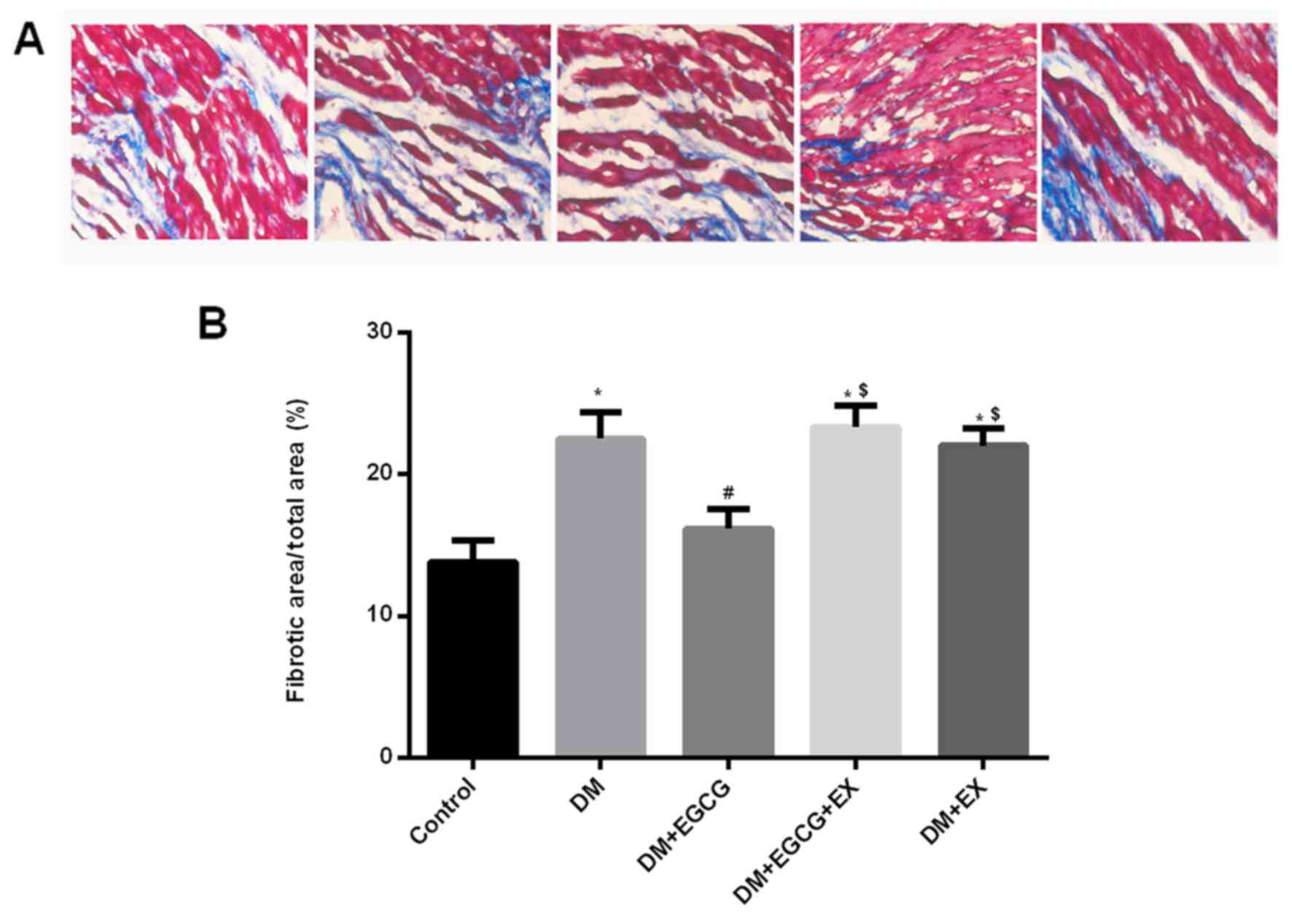
Effect of EGCG on cardiac fibrosis in
diabetic rats
Quantitative analysis of the fibrotic region of the
LV myocardium indicated an increased level of interstitial fibrosis
in the hearts of the rats with STZ-induced diabetes after I/R
injury compared to the control (P<0.05) (Fig. 6). However, EGCG treatment
significantly reduced the extent of cardiac fibrosis compared with
the diabetic rats (P<0.05). Additionally, the EGCG-induced
reduction of cardiac fibrosis was abolished by co-treatment with
EX527 in the diabetic rats (P<0.05).
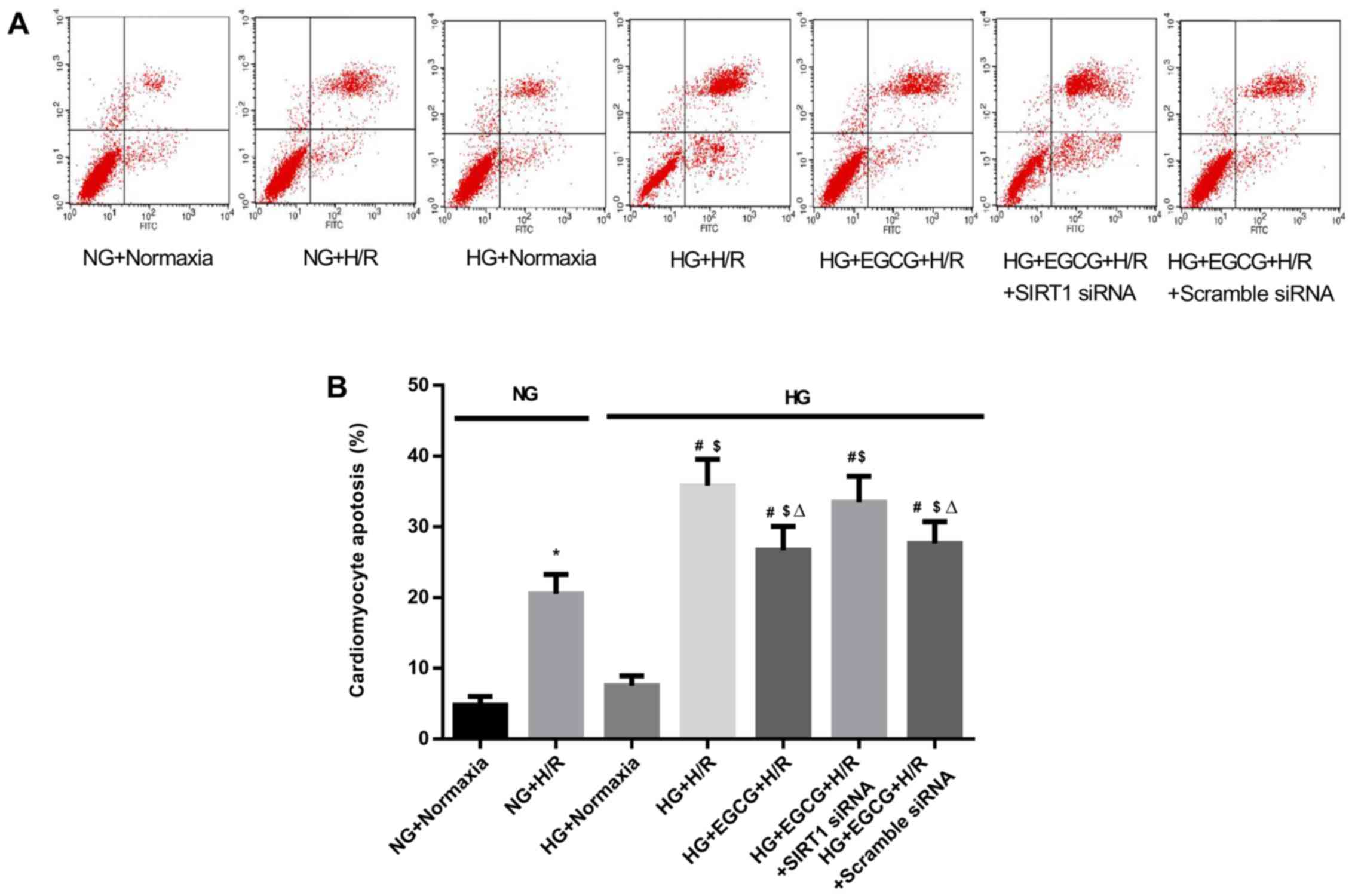
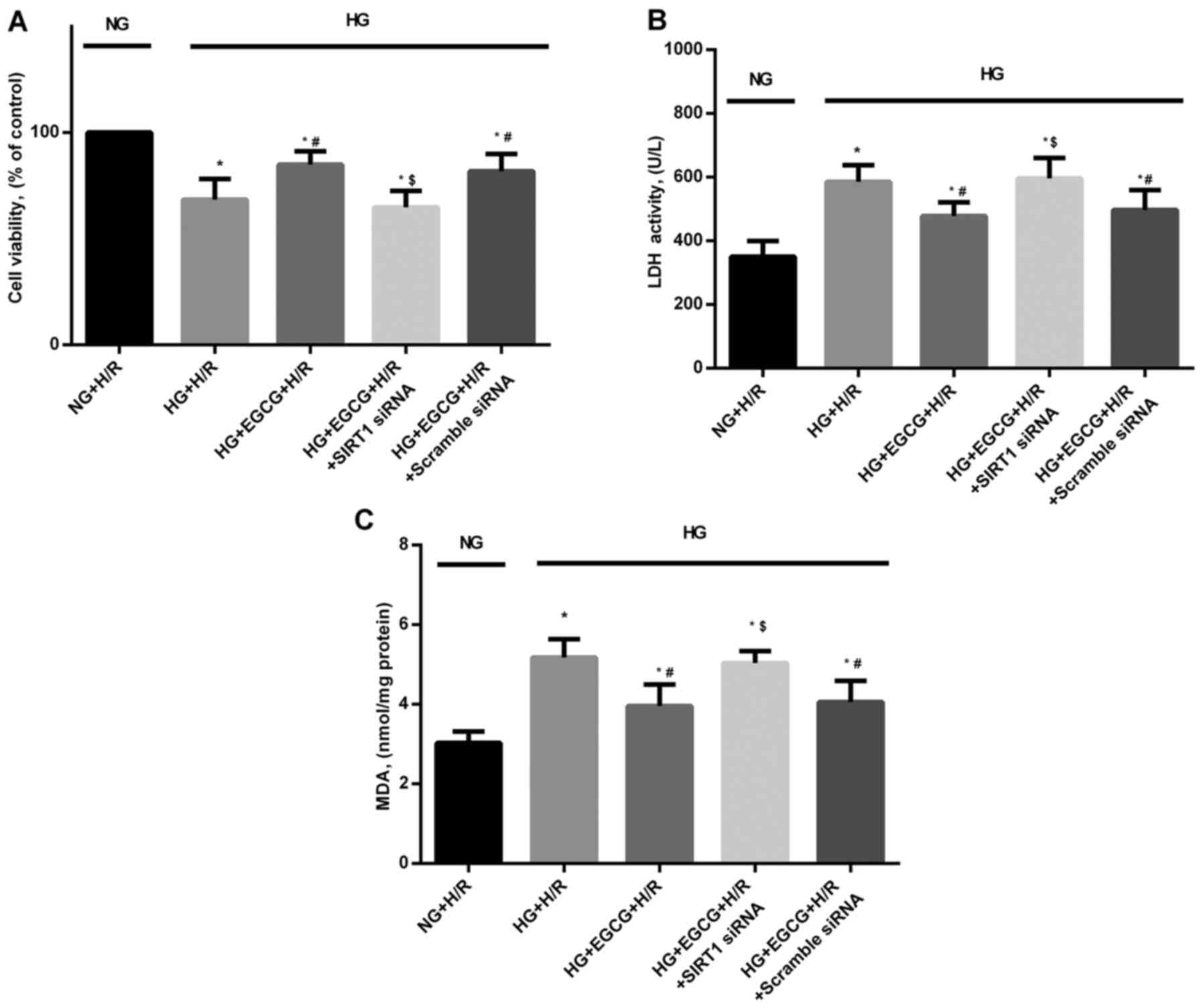
Cellular apotosis, cell viability, LDH
activity and MDA levels in H/R-injured H9c2 cardiomyocytes
H/R caused a marked increase in the apoptosis of the
H9C2 cells compared to the normoxia groups (P<0.05) (Fig. 7). Exposure to HG + H/R induced an
increase in the apoptotic index (P<0.05, HG + H/R vs. NG + H/R
group) and the administration of EGCG attenuated these changes (HG
+ EGCG vs. HG + H/R group). HG sensitized the cardiomyocytes to H/R
injury, which was manifested as reduced cardiomyocyte viability
(Fig. 8A), and increased LDH
(Fig. 8B) and MDA leels (Fig. 8C) (all P<0.05 vs. NG + H/R
group).
To confirm the role of SIRT1 in EGCG-mediated
cellular protection and to explore the signaling pathway involved,
we employed SIRT1 siRNA to specifically knockdown SIRT1 expression
in H9c2 cells. Pre-treatment with EGCG markedly decreased the
apoptosis to 25.5% and increased cell viability by 24.1% following
H/R (P<0.05), compared with the HG + H/R group (Fig. 7). In addition, EGCG pre-treatment
significantly reduced LDH activity and MDA formation by 16.7 and
23.6%, respectively in the H/R-injured H9c2 cardiomyocytes
(P<0.05), compared to the HG + H/R group (Fig. 8B and C). However, the protective
effects of EGCG pre-treatment were abrogated by transfection with
SIRT1 siRNA (P<0.05), compared to EGCG treatment alone (Fig. 8).
Protein expression of SIRT1 and MnSOD in
H9c2 cardiomyocytes subjected to H/R injury
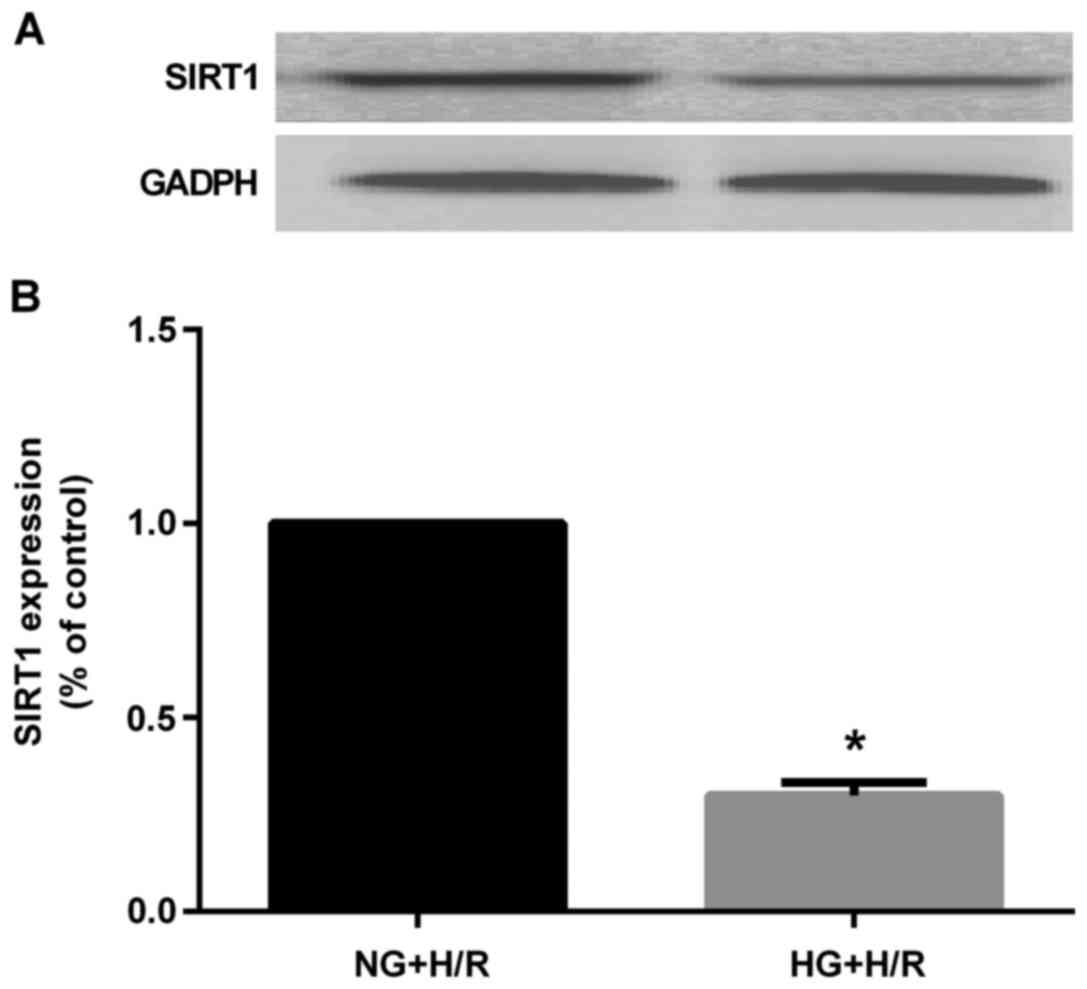
HG markedly decreased SIRT1 protein expression
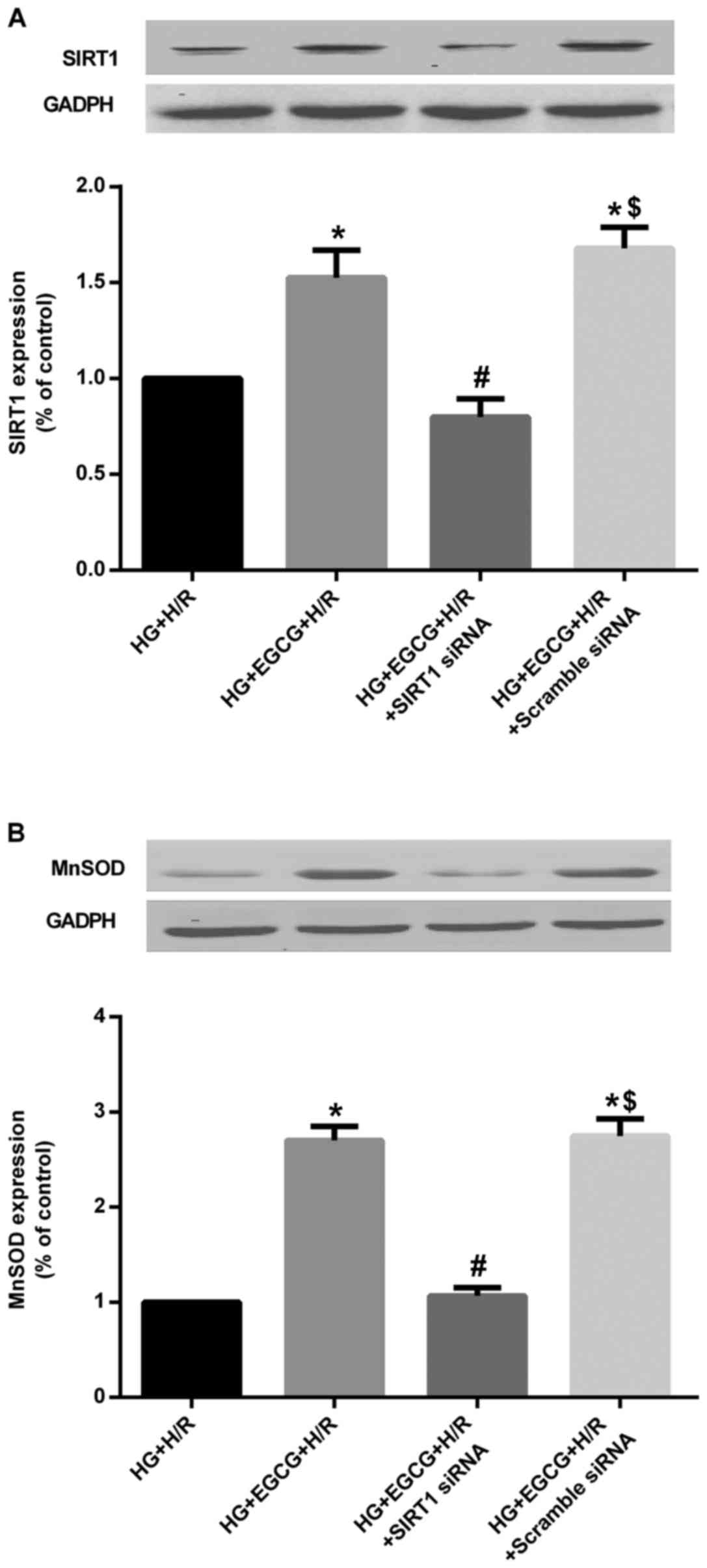
compared to that in the NG + H/R group (P<0.05) (Fig. 9). However, EGCG pre-treatment
significantly increased the protein expression of SIRT1 and MnSOD
(all P<0.05), compared to the HG group (Fig. 10). Moreover, the effect of EGCG
pre-treatment on the expression of these proteins was abolished by
transfection with SIRT1 siRNA (all P<0.05), compared with EGCG
pre-treatment alone (Fig.
10).
Discussion
This study provided evidence that rats with
STZ-induced diabetes exhibited enhanced cardiac dysfunction, an
increased myocardial infarct size, myocardial apoptosis and
elevated oxidative stress, as well as cardiac fibrosis when they
were subjected to myocardial I/R injury. However, these unfavorable
outcomes were attenuated by EGCG administration. Additionally, HG
aggravated cardiomyocyte apoptosis, reduced cellular viability and
increased the oxidative stress induced by H/R injury in H9c2 cells,
whereas EGCG protected the cardiomyocytes against H/R
impairment.
EGCG, as a polyphenol, possesses various
pharmacological and biological properties and potentially exerts
protective effects on the cardiovascular system (21). Previous studies have illustrated
that EGCG alleviates myocardical I/R injury in non-diabetic rat
hearts (22–25). However, these researchers have
stated that further studies need to be performed using animal
models of human disease such as diabetes, hypertension and aging
(22–26). Thus, the present study was
conducted to further investigate the effect of EGCG on diabetic
cardiomyopathy during myocardial I/R injury.
In the present study, all rats were fed with the
AIN-93 diet without phytochemicals. The dosage of EGCG was
determined based on a human dietary survey and animal studies 5
mg/(kg body weight/day) of EGCG administration in rats has been
extrapolated from a human study based on the average daily EGCG
intake (27). However, most
studies adopted EGCG to investigate its preventive and therapeutic
effects on cardiovascular diseases in animal models ranging from 25
to 460 mg/kg body weight/day (17,19,28–30). The hepatotoxic effects of EGCG
were observed when the daily oral dose was 1,500 mg/kg in mice
(31), but there are no reports
showing that daily EGCG intake at the dose less than 460 mg/kg body
weight/day causes toxicity in rats. Based on the above evidence and
our previous studies, we used 100 mg/kg body weight/day of EGCG in
the present study.
There is substantial evidence to indicate that H9c2
cardiomyocytes cultured under HG can be used to explore the
pathophysiology of diabetes (32,33). The dose of EGCG that previous
studies commonly adopted in H9c2 cells ranged from 5 to 50
μM (12,36,40). Although EGCG is poorly absorbed
and its concentration in animal blood is lower than the dose (20
μM) we adopted in the present study in vitro, Lambert
et al observed that EGCG treatment at doses ranging from 20
to 600 μM resulted in a linear increase in the cytosolic
concentration of EGCG in human colon cancer cells (16). In fact, the beneficial effects of
EGCG depend on the long-term cumulative action in the body
(16). The protective effects of
EGCG shown in the present study may not be observed in vitro
experiments for such a short period of pre-treatment (24 h) if we
use the subnanomolar concentration which is detectable in animal
blood after absorption.
It has previously been demonstrated that the
myocardial protective effects of EGCG are primary related to its
antioxidant properties (36). The
intragastrical administration of EGCG was found to inhibit
oxidative stress in rodent hearts (37). Hsieh et al also found that
EGCG pre-treatment for 30 min protected H9c2 cells from
H2O2-induced oxidative stress (12). Similarly, we found that EGCG
treatment reduced oxidative stress either in the hearts of the
diabetic rats or in cardiomyocytes under hyperglycemic conditions
after I/R injury.
It has been established that experimental diabetes
aggravates LV dysfunction under conditions of I/R injury, as
usually observed under clinical conditions. Hyperglycemia worsens
cardiac performance and cell survival following myocardial I/R
injury via increased oxidative stress (36). Our data also indicated that the
levels of oxidative damage indicators (15-F2t and MDA)
were increased and cardiac dysfunction was manifested as a decrease
in LVSP, +dP/dtmax and −dP/dtmax in I/R-injured diabetic rats,
which is similar to the finding by Xu et al that rats with
STZ-induced diabetes at at 8 weeks exhibited progressive abnormal
cardiac systolic and diastolic function (2). Additionally, we also observed that
EGCG improved cardiac performance, which was manifested as
augmented LVSP, +dP/dtmax and −dP/dtmax.
Oxidative stress injury induced by the accumulation
of ROS in diabetic hearts plays an important role in cardiac
fibrosis (38). Our data
demonstrated that oxidative damage indicators (15-F2t
and MDA) and fibrosis were increased in diabetic rats after
myocardial I/R injury, which was also in agreement with the
findings of previous studies indicating that diabetes was
associated with enhanced cardiac fibrosis after myocardial I/R
injury (34,35,39), which led to increased LV stiffness
and decreased ventricular wall compliance, resulting in both
systolic and diastolic dysfunction (34,35). However, in the present study, EGCG
pre-treatment reduced I/R injury-induced fibrosis in diabetic rats
and attenuated adverse LV remodeling.
It has been reported that as a member of histone
deacetylases III, SIRT1 plays a key role in cardioprotective
effects during myocardial I/R injury (9,33).
These effects are mediated via the deacetylation of transcription
factors and thye upregulation of antioxidant enzymes and other
downstream gene targets (9,29).
Diabetic hearts are resistant to the myocardial infarct-limiting
effects of ischemic preconditioning, accompanied with a marked
inhibition of SIRT1 activity (7).
These pathological alterations may diminish the ability of the
heart to resist I/R injury. In the present study, we also observed
that SIRT1 expression in the I/R-injured diabetic rats was m
markedly decreased compared to the normal rats, which paralleled
with increased myocardial apoptosis, elevated oxidative stress and
myocardial dysfunction in diabetic rats. Moreover, the decrement of
SIRT1 triggered ROS generation and the impaired ROS clearance may
thus aggravate diabetic myocardial I/R injury (41).
Although EGCG improves age-associated inflammation
and oxidative stress in the liver by activating SIRT1 in healthy
rats (42), few studies have
examined the role of SIRT1 in regulating the effects of EGCG in
ameliorating cardiac dysfunction, attenuating cardiomyocyte
apoptosis and alleviating oxidative stress after myocardial I/R
injury in diabetes. In the present study, we found that SIRT1
expression was significantly reduced in the hearts of diabetic rats
and in H9c2 cells under hyperglycemic conditions subjected to I/R
injury. Moreover, EGCG upregulated the protein expression of SIRT1,
and it improved cardiac dysfunction, ameliorated cardiomyocyte
apoptosis and attenuated oxidative stress injury in cardiomyocytes
of diabetic rats and in H9c2 cells after I/R injury under
hyperglycemic conditions.
It has been demonstrated that SIRT1 can mediate
protective effects against oxidative stress through the regulation
of antioxidant genes, such as MnSOD (43). Likewise, we found that the
EGCG-induced upregulation of SIRT1 protein expression also
paralleled with an increase in MnSOD protein expression in diabetic
cardiomyocytes in the setting of I/R injury. Moreover, the effects
of EGCG on the protein expression of MnSOD were abolished by SIRT1
inhibitor, EX527, in vivo and SIRT1 siRNA in vitro.
Therefore, EGCG may exert protective effects on diabetic hearts
subjected to myocardial I/R injury by stimulating the SIRT1 signal.
However, the precise role of SIRT1 in the EGCG-conferred
cardioprotective effects under a diabetic state needs to be
confirmed by further studies.
In conclusion, the present study demonstrated that
EGCG attenuated cardiac dysfunction, reduced myocardial infarct
size and cardiac fibrosis, and decreased myocardial apoptosis and
oxidative stress by stimulating the SIRT1 signaling pathway in
diabetic rats and in H9c2 cells under hyperglycemic conditions when
they were subjected to myocardial I/R injury. This suggests that
EGCG may be a promising dietary supplementation for the attenuation
or prevention of diabetic cardiomyopathy.
Abbreviations:
|
AAR
|
area at risk
|
|
FoxO
|
Forkhead box O
|
|
CCA
|
common carotid artery
|
|
CON
|
control group
|
|
DM
|
streptozotocin-induced diabetic
rats
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
+dp/dtmax
|
maximum speed of pressure development
of left ventricle
|
|
−dp/dtmax
|
minimum speed of pressure decline of
left ventricle
|
|
EGCG
|
(−)-epigallocatechin-3-gallate
|
|
EX
|
EX527
|
|
FBS
|
fetal bovine serum
|
|
H/R
|
hypoxia/re-oxygenation
|
|
HG
|
high glucose
|
|
I/R
|
ischemia/reperfusion
|
|
IS
|
infarct size
|
|
LAD
|
left anterior descending coronary
artery
|
|
LDH
|
lactate dehydrogenase
|
|
LV
|
left ventricle
|
|
LVSP
|
left ventricular systolic pressure
|
|
MDA
|
malonaldehyde
|
|
MnSOD
|
manganese superoxide dismutase
|
|
NG
|
normal glucose
|
|
N
|
nomoxia
|
|
ROS
|
reactive oxygen species
|
|
STZ
|
streptozotocin
|
|
siRNA
|
small interfering RNA
|
|
SIRT1
|
silent information regulator 1
|
|
TTC
|
2,3,5-triphenyl tetrazolium
chloride
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
dUTP nick-end labeling
|
|
15-F2t-IsoP
|
15-F2t-isoprostane
|
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (nos. 81201458, 81471844 and
81170768).
References
|
1
|
Donahoe SM, Stewart GC, McCabe CH,
Mohanavelu S, Murphy SA, Cannon CP and Antman EM: Diabetes and
mortality following acute coronary syndromes. JAMA. 298:765–775.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu J, Li H, Irwin MG, Xia ZY, Mao X, Lei
S, Wong GT, Hung V, Cheung CW, Fang X, et al: Propofol ameliorates
hyperglycemia-induced cardiac hypertrophy and dysfunction via heme
oxygenase-1/signal transducer and activator of transcription 3
signaling pathway in rats. Crit Care Med. 42:e583–e594. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu X, Wei J, Peng DH, Layne MD and Yet
SF: Absence of heme oxygenase-1 exacerbates myocardial
ischemia/reperfusion injury in diabetic mice. Diabetes. 54:778–784.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koka S, Das A, Salloum FN and Kukreja RC:
Phosphodiesterase-5 inhibitor tadalafil attenuates oxidative stress
and protects against myocardial ischemia/reperfusion injury in type
2 diabetic mice. Free Radic Biol Med. 60:80–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng Z, Chen H, Li J, Li T, Zheng B,
Zheng Y, Jin H, He Y, Gu Q and Xu X: Sirtuin 1-mediated cellular
metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and
therapeutic effects of metformin. Diabetes. 61:217–228. 2012.
View Article : Google Scholar
|
|
6
|
Nadtochiy SM, Yao H, McBurney MW, Gu W,
Guarente L, Rahman I and Brookes PS: SIRT1-mediated acute
cardioprotection. Am J Physiol Heart Circ Physiol. 301:H1506–H1512.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pillai VB, Sundaresan NR and Gupta MP:
Regulation of Akt signaling by sirtuins: Its implication in cardiac
hypertrophy and aging. Circ Res. 114:368–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hori YS, Kuno A, Hosoda R and Horio Y:
Regulation of FOXOs and p53 by SIRT1 modulators under oxidative
stress. PLoS One. 8:e738752013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Y, Duan W, Li Y, Jin Z, Yan J, Yu S
and Yi D: Novel role of silent information regulator 1 in
myocardial ischemia. Circulation. 128:2232–2240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Menegazzi M, Tedeschi E, Dussin D, De
Prati AC, Cavalieri E, Mariotto S and Suzuki H: Anti-interferon
gamma action of epigallocatechin-3-gallate mediated by specific
inhibition of STAT1 activation. FASEB J. 15:1309–1311.
2001.PubMed/NCBI
|
|
11
|
Townsend PA, Scarabelli TM, Pasini E,
Gitti G, Menegazzi M, Suzuki H, Knight RA, Latchman DS and
Stephanou A: Epigallocatechin-3-gallate inhibits STAT-1 activation
and protects cardiac myocytes from ischemia/reperfusion-induced
apoptosis. FASEB J. 18:1621–1623. 2004.PubMed/NCBI
|
|
12
|
Hsieh SR, Hsu CS, Lu CH, Chen WC, Chiu CH
and Liou YM: Epigallocatechin-3-gallate-mediated cardioprotection
by Akt/GSK-3β/caveolin signalling in H9c2 rat cardiomyoblasts. J
Biomed Sci. 20:862013. View Article : Google Scholar
|
|
13
|
Sulaiman M, Matta MJ, Sunderesan NR, Gupta
MP, Periasamy M and Gupta M: Resveratrol, an activator of SIRT1,
upregulates sarcoplasmic calcium ATPase and improves cardiac
function in diabetic cardiomyopathy. Am J Physiol Heart Circ
Physiol. 298:H833–H843. 2010. View Article : Google Scholar :
|
|
14
|
Queen BL and Tollefsbol TO: Polyphenols
and aging. Curr Aging Sci. 3:34–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Yao W, Irwin MG, Wang T, Wang S,
Zhang L and Xia Z: Adiponectin ameliorates hyperglycemia-induced
cardiac hypertrophy and dysfunction by concomitantly activating
Nrf2 and Brg1. Free Radic Biol Med. 84:311–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lambert JD, Lee MJ, Diamond L, Ju J, Hong
J, Bose M, Newmark HL and Yang CS: Dose-dependent levels of
epigal-locatechin-3-gallate in human colon cancer cells and mouse
plasma and tissues. Drug Metab Dispos. 34:8–11. 2006. View Article : Google Scholar
|
|
17
|
Hsieh SR, Tsai DC, Chen JY, Tsai SW and
Liou YM: Green tea extract protects rats against myocardial
infarction associated with left anterior descending coronary artery
ligation. Pflugers Arch. 458:631–642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu L, Sun Y, Cheng L, Jin Z, Yang Y, Zhai
M, Pei H, Wang X, Zhang H, Meng Q, et al: Melatonin
receptor-mediated protection against myocardial
ischemia/reperfusion injury: Role of SIRT1. J Pineal Res.
57:228–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan J, Feng Z and Liu J, Shen W, Wang Y,
Wertz K, Weber P, Long J and Liu J: Enhanced autophagy plays a
cardinal role in mitochondrial dysfunction in type 2 diabetic
Goto-Kakizaki (GK) rats: Ameliorating effects of
(−)-epigallocatechin-3-gallate. J Nutr Biochem. 23:716–724. 2012.
View Article : Google Scholar
|
|
20
|
Wu Y, Xia ZY, Dou J, Zhang L, Xu JJ, Zhao
B, Lei S and Liu HM: Protective effect of ginsenoside Rb1 against
myocardial ischemia/reperfusion injury in streptozotocin-induced
diabetic rats. Mol Biol Rep. 38:4327–4335. 2011. View Article : Google Scholar
|
|
21
|
Yamazaki KG, Romero-Perez D,
Barraza-Hidalgo M, Cruz M, Rivas M, Cortez-Gomez B, Ceballos G and
Villarreal F: Short- and long-term effects of (−)-epicatechin on
myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ
Physiol. 295:H761–H767. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yanagi S, Matsumura K, Marui A, Morishima
M, Hyon SH, Ikeda T and Sakata R: Oral pretreatment with a green
tea poly-phenol for cardioprotection against ischemia-reperfusion
injury in an isolated rat heart model. J Thorac Cardiovasc Surg.
141:511–517. 2011. View Article : Google Scholar
|
|
23
|
Kim CJ, Kim JM, Lee SR, Jang YH, Kim JH
and Chun KJ: Polyphenol (−)-epigallocatechin gallate targeting
myocardial reperfusion limits infarct size and improves cardiac
function. Korean J Anesthesiol. 58:169–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piao CS, Kim DS, Ha KC, Kim HR, Chae HJ
and Chae SW: The protective effect of epigallocatechin-3 gallate on
ischemia/reperfusion injury in isolated rat hearts: An ex vivo
approach. Korean. J Physiol Pharmacol. 15:259–266. 2011. View Article : Google Scholar
|
|
25
|
Kim SJ, Li M, Jeong CW, Bae HB, Kwak SH,
Lee SH, Lee HJ, Heo BH, Yook KB and Yoo KY:
Epigallocatechin-3-gallate, a green tea catechin, protects the
heart against regional ischemia-reperfusion injuries through
activation of RISK survival pathways in rats. Arch Pharm Res.
37:1079–1085. 2014. View Article : Google Scholar
|
|
26
|
Lejay A, Fang F, John R, Van JA, Barr M,
Thaveau F, Chakfe N, Geny B and Scholey JW: Ischemia reperfusion
injury, ischemic conditioning and diabetes mellitus. J Mol Cell
Cardiol. 91:11–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arts IC, Hollman PC, Feskens EJ, Bueno de
Mesquita HB and Kromhout D: Catechin intake and associated dietary
and lifestyle factors in a representative sample of Dutch men and
women. Eur J Clin Nutr. 55:76–81. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu TT, Liang NS, Li Y, Yang F, Lu Y, Meng
ZQ and Zhang LS: Effects of long-term tea polyphenols consumption
on hepatic microsomal drug-metabolizing enzymes and liver function
in Wistar rats. World J Gastroenterol. 9:2742–2744. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi Z, Fu F, Yu L, Xing W, Su F, Liang X,
Tie R, Ji L, Zhu M, Yu J, et al: Vasonatrin peptide attenuates
myocardial ischemia-reperfusion injury in diabetic rats and
underlying mechanisms. Am J Physiol Heart Circ Physiol.
308:H281–H290. 2015. View Article : Google Scholar
|
|
30
|
Niu Y, Na L, Feng R, Gong L, Zhao Y, Li Q,
Li Y and Sun C: The phytochemical, EGCG, extends lifespan by
reducing liver and kidney function damage and improving
age-associated inflammation and oxidative stress in healthy rats.
Aging Cell. 12:1041–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lambert JD, Kennett MJ, Sang S, Reuhl KR,
Ju J and Yang CS: Hepatotoxicity of high oral dose
(−)-epigallocatechin-3-gallate in mice. Food Chem Toxicol.
48:409–416. 2010. View Article : Google Scholar
|
|
32
|
Xue R, Lei S, Xia ZY, Wu Y, Meng Q, Zhan
L, Su W, Liu H, Xu J, Liu Z, et al: Selective inhibition of PTEN
preserves ischaemic post-conditioning cardioprotection in
STZ-induced type 1 diabetic rats: Role of the PI3K/Akt and
JAK2/STAT3 pathways. Clin Sci (Lond). 130:377–392. 2016. View Article : Google Scholar
|
|
33
|
Yu L, Liang H, Dong X, Zhao G, Jin Z, Zhai
M, Yang Y, Chen W, Liu J, Yi W, et al: Reduced silent information
regulator 1 signaling exacerbates myocardial ischemia-reperfusion
injury in type 2 diabetic rats and the protective effect of
melatonin. J Pineal Res. 59:376–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu R, Smeele KM, Wyatt E, Ichikawa Y,
Eerbeek O, Sun L, Chawla K, Hollmann MW, et al: Reduction in
hexokinase II levels results in decreased cardiac function and
altered remodeling after ischemia/reperfusion injury. Circ Res.
108:60–69. 2011. View Article : Google Scholar
|
|
35
|
Toldo S, Breckenridge DG, Mezzaroma E, Van
Tassell BW, Shryock J, Kannan H, Phan D, Budas G, Farkas D,
Lesnefsky E, et al: Inhibition of apoptosis signal-regulating
kinase 1 reduces myocardial ischemia-reperfusion injury in the
mouse. J Am Heart Assoc. 1:e0023602012. View Article : Google Scholar
|
|
36
|
Chen WC, Hsieh SR, Chiu CH, Hsu BD and
Liou YM: Molecular identification for
epigallocatechin-3-gallate-mediated antioxidant intervention on the
H2O2-induced oxidative stress in H9c2 rat
cardiomyoblasts. J Biomed Sci. 21:562014. View Article : Google Scholar
|
|
37
|
Li H, Bian Y, Zhang N, Guo J, Wang C, Lau
WB and Xiao C: Intermedin protects against myocardial
ischemia-reperfusion injury in diabetic rats. Cardiovasc Diabetol.
12:912013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang B, Yang Q, Bai WW, Xing YF, Lu XT,
Sun YY and Zhao YX: Tongxinluo protects against pressure
overload-induced heart failure in mice involving VEGF/Akt/eNOS
pathway activation. PLoS One. 9:e980472014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eguchi M, Kim YH, Kang KW, Shim CY, Jang
Y, Dorval T, Kim KJ and Sweeney G: Ischemia-reperfusion injury
leads to distinct temporal cardiac remodeling in normal versus
diabetic mice. PLoS One. 7:e304502012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu J, Tang Y, Feng Z, Hou C, Wang H, Yan
J, Liu J, Shen W, Zang W, Liu J, et al: Acetylated FoxO1 mediates
high-glucose induced autophagy in H9c2 cardiomyoblasts: Regulation
by a polyphenol -(−)-epigallocatechin-3-gallate. Metabolism.
63:1314–1323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alcendor RR, Gao S, Zhai P, Zablocki D,
Holle E, Yu X, Tian B, Wagner T, Vatner SF and Sadoshima J: Sirt1
regulates aging and resistance to oxidative stress in the heart.
Circ Res. 100:1512–1521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Puthanveetil P, Wan A and Rodrigues B:
FoxO1 is crucial for sustaining cardiomyocyte metabolism and cell
survival. Cardiovasc Res. 97:393–403. 2013. View Article : Google Scholar
|
|
43
|
Salminen A, Kaarniranta K and Kauppinen A:
Crosstalk between oxidative stress and SIRT1: Impact on the aging
process. Int J Mol Sci. 14:3834–3859. 2013. View Article : Google Scholar : PubMed/NCBI
|