Introduction
Betulinic acid (BA) (Fig. 1A) is a naturally occurring
lupane-type triterpene found in the bark of white birch trees, and
it is one of the most promising lead compounds for new cancer
therapeutics (1). BA has been
introduced as a potential anticancer compound against a wide
variety of cancer cells such as leukemia, prostate, ovarian,
breast, lung and hepatoblastoma (2–5).
This anticancer activity has been linked to its ability to directly
trigger mitochondrial membrane permeabilization independent of p53,
a central event in the apoptotic process in many cancer cells
(6–8). However, the mechanism by which BA
induces apoptosis in human cervical cancer has yet to be fully
elucidated.
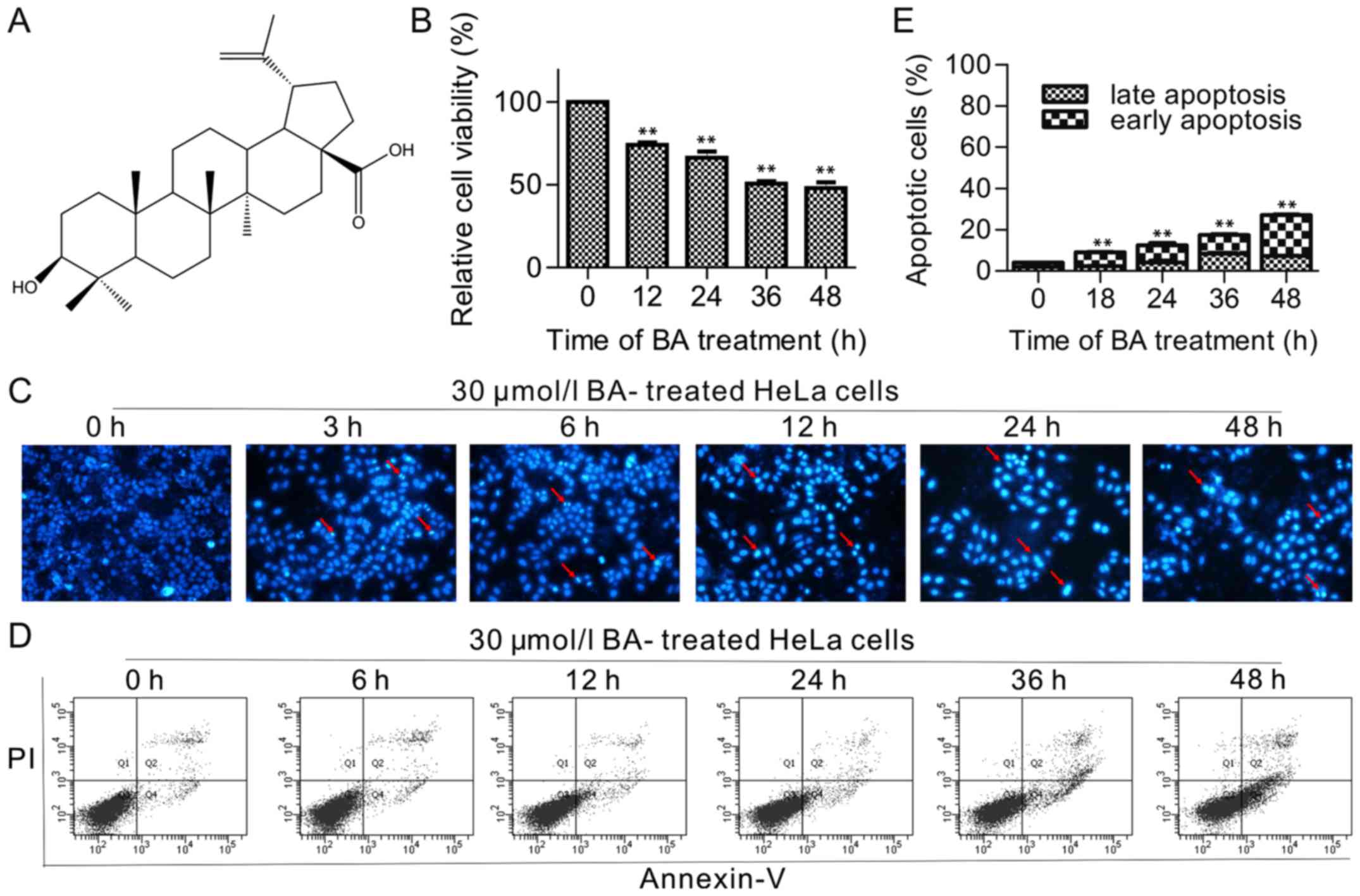 | Figure 1Effects of BA on the proliferation
and apoptosis of HeLa cells. (A) Chemical structure of BA. (B) Cell
viability was determined by the MTT assay. Cells were treated with
indicated time of BA (12, 24, 36 and 48 h). The values for each BA
concentration tested represented the mean of three experiments,
data were presented as mean ± standard deviation. (C) Hoechst
33258-staining of HeLa cells treated with 30 µmol/l BA at 3,
6, 12, 24 and 48 h under a fluorescence microscope at ×200
magnification. Red arrows indicate several apoptotic cells with
typical condensation of chromatin. (D) Cells were treated with 30
µmol/l BA at different time (6, 12, 24, 36 and 48 h). (E)
Flow cytometric histograms. Columns showed mean values of three
experiments, mean ± standard deviation. **P<0.01 vs. control
group BA, betulinic acid. |
Triterpenoids have been reported to induce cell
cycle arrest and apoptosis via modulation of the
phosphatidylinositol 3-kinase (PI3K)/Akt pathway (9), which is an intracellular signaling
pathway important in regulating several cellular processes.
Activation of the PI3K/Akt pathway has been shown to promote
cellular survival in a vast of cancer cells, because it can inhibit
the cell cycle progression by repressing downstream factors:
p27Kip and p21Waf1/Cip1 belong to
cyclin-dependent kinase (10,11). At the same time, it also can
modulate apoptosis by phosphorylating and inactivating several
targets, like Bad and caspase-9, that have important roles in
mitochondrial apoptosis pathways (12). Apoptosis induced by BA involves
activation of caspases, was suggested to depend on the
mitochondrial pathway, therefore, the authors hypothesized that the
PI3K/Akt pathway may involve in the apoptosis process by BA
induction (13).
In a previous study, it was demonstrated that BA can
induce apoptosis activity in HeLa by proteomic (14), but the molecular mechanisms behind
its function are still not fully understood. In the present study,
the authors attempted to evaluate the mechanistic role of BA in a
cervical cancer cell line (HeLa) by exploring its effects on
apoptosis and the cell cycle. In addition, they attempted to
investigate the important signaling network, such as the PI3K/Akt
signaling pathway and the mitochondrial pathway involved in BA
treatment in HeLa cells. The aim of the present study was to better
understand the apoptosis mechanism induced by BA.
Materials and methods
Antibodies and reagents
BA powder was purchased from Shanghai Boyle Chemical
Co., Ltd. (Shanghai, China) and dissolved in dimethylformamide.
Primary antibodies were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA): anti-PI3K p85 (cat. no. 4257), anti-PI3K
p110a (cat. no. 4249), anti-Akt (cat. no. 4691), anti-phospho-Akt
(Thr308) (cat. no. 2965), anti-phospho-Akt (Ser473) (cat. no.
4060), anti-Bcl-xL (cat. no. 2764), anti-Bad (cat. no. 9292),
anti-caspase-9 (cat. no. 9501), anti-p21Waf1/Cip1 (cat.
no. 2947), anti-p27Kip (cat. no. 2552) and anti-β-actin
(cat. no. 4970). Peroxidase-conjugated goat anti-rabbit IgG (H+L)
was from Santa Cruz Biotechnology, Inc. (Dallas, Texas, USA) and
SuperSignal West Femto Maximum Sensitivity Substrate from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). Wortmannin and reduced
L-glutathione (GSH) was purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany).
Cell culture
The human cancer cell line HeLa was purchased from
the Tumor Center (Beijing Yinzijing Biological Company, Beijing,
China). Cells were cultured in RPMI-1640 medium (HyClone; GE
Healthcare Life Sciences, Chalfont, UK) with 10% fetal bovine serum
(FBS; GE Healthcare Life Sciences), 100 U/ml penicillin and 100
µg/ml streptomycin (HyClone; GE Healthcare Life Sciences).
Cells were seeded in 6-, 12- and 96-well microplates and then
incubated at 37°C in 5% CO2. The control group was HeLa
cells with a corresponding concentration of DMF, such as 30
µmol/l BA corresponding control group is 0.15% DMF in
medium. The concentration of probe drug is 20 mmol/l.
Cell proliferation assay (MTT assay)
Cells (2×104/ml) were seeded in 96-well
microplates and then incubated at 37°C in 5% CO2. At 24
h, the medium was removed and replaced with fresh medium containing
various concentrations of BA for various periods of time. Next, 30
µl of 3 mg/ml MTT (Amresco, Inc., Framingham, MA, USA) in
phosphate-buffered saline (PBS) was added to each well, and then
the plate was further incubated for 4 h. The remaining supernatant
was removed, and 150 µl DMSO was added to each well and
mixed thoroughly to dissolve the formazan crystals that formed.
After 10 min incubation, the absorbance of each well was read at
490 nm using a BioTek-ELISA plate reader (BioTek Instruments, Inc.,
Winooski, VT, USA).
Detection of morphological changes
To detect morphological changes that occurred during
apoptosis process, nuclear staining was performed using a 5
µg/ml Hoechst 33258 (Sigma-Aldrich; Merck KGaA) stain, and
samples were visualized using a Nikon fluorescence microscope
(Eclipse Ti-S; Nikon Corp., Tokyo, Japan).
Flow cytometry analysis of cell
apoptosis
BA-induced apoptosis was observed by Annexin
V-fluorescein isothiocyanate (FITC)/propicium iodide (PI) staining
(Beyotime Institute of Biotechnology, Haimen, China) according to
the manufacturer's instructions. Flow cytometry (BD FACSCanto; BD
Biosciences, Franklin Lakes, NJ, USA) was used to analyze
differences in apoptosis between control and BA-treated for various
periods of time cells at 48 h post treatment. Cells were collected
and analyzed by counting normal cells, early-stage apoptotic cells
and late-stage apoptotic/necrotic cells in three fields of view of
the microscope. The data acquisition and analysis were performed
using BD FACSDiva software (version A, BD FACSCanto; BD
Biosciences, Franklin Lakes, NJ, USA).
Western blot assay
HeLa cells (1.7×105) were seeded in
6-well microplates before treatment with BA or wortmannin (a
specific inhibitor of PI3K) at the concentration of 30
µmol/l for 6 h time period. Wortmannin was used as a
positive control here. Cells were collected and lysed in RIPA
buffer (Beyotime Institute of Biotechnology) with PhosSTOP
phosphatase inhibitor cocktail tablets (Roche Diagnostics,
Indianapolis, IN, USA). Equal amounts of protein (30
µg/lane) by Bradford were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to a polyvinyli-dene difluoride membrane (EMD
Millipore, Billerica, MA, USA). After blocking in 5% non-fat milk
and washing, the blots were incubated overnight at 4°C with
specific primary antibodies diluted to 1:1,000 following the CST
protocol. The membrane was washed three times with Tris-buffered
saline (containing 1% Tween-20), and then incubated with secondary
antibodies (peroxidase-conjugated goat anti-rabbit IgG (H+L)
diluted to 1:8,000 for 1 h, followed with the same washing steps as
above. Then, the immunoreactive proteins were visualized using the
enhanced chemiluminescence reagent. Image Lab software (version
3.0; Bio-Rad, Hercules, CA, USA) was used to quantify of proteins
using β-actin as a loading control. Changed fold was represented by
the protein expression ratio [(target protein/β-actin)/the control
group].
Measurement of oxidative stress
The levels of intracellular ROS were determined
using a ROS assay kit (Beyotime Institute of Biotechnology)
following the manufacturer's protocol. Cells were harvested after
various periods of time (0–48 h) of 30 µmol/l BA treatment
and then washed twice with PBS and incubated with DCFH-DA (10
µmol/l) at 37°C for 40 min in a darkroom for final analysis
by flow cytometry.
Measurement of mitochondrial membrane
potential (MMP)
MMP was determined using JC-1 probe (Beyotime
Institute of Biotechnology) as described previously (15). Briefly after treating the cells
with 30 µmol/l BA for various periods of time (0–48 h),
cells were stained with 0.5 mg/ml of the fluorescence probe JC-1
for 20 min at 37°C. After washing, cells were analyzed for the
decrease in red/green fluorescence by flow cytometry. The
mitochondrial membrane potential is indicated by a decrease in
red/green fluorescence intensity ratio.
Cell cycle analysis
HeLa cells were harvested via trypsin after 30
µmol/l BA for different time treatment, washed with PBS and
fixed with 70% ethanol at 4°C for overnight. After washing twice
with PBS, cells were stained with cell cycle analysis kit (Beyotime
Institute of Biotechnology) containing propidium iodide (PI) and
RNase A for 30 min in the dark at room temperature, as described
previously (16). The DNA
contents for cell cycle phase distribution were analyzed by Modfit
LT 3.2 software (Verity Software House, Topsham, ME, USA).
ROS inhibitor treatment
In order to investigate the contribution of ROS in
BA induced apoptosis, GSH, a ROS scavenger, was used as an ROS
inhibitor. HeLa cells were seeded in 6-well plates
(1.7×105 cells/well) for 24 h, then pre-incubation GSH
for 1 h before treatment with 30 µmol/l BA for 6 h, the
changes and the percentage of apoptotic cells were examined by flow
cytometry, as described above. In parallel, western blotting of
proteins in PI3K/Akt pathway experiments were accomplished.
Statistical analysis
The data were presented as mean ± standard deviation
and the results were taken from at least three independent
experiments. The statistically significant differences were
determined using one-way analysis of variance by SPSS software
(version 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
BA inhibited proliferation and induced
apoptosis in HeLa cells
The antiproliferative activity induced by BA in HeLa
cells was evaluated by an MTT assay. To identify the beginning of
apoptosis and morphological changes for further experiment, the
authors used flow cytometry and fluorescence microscopy to detect
the HeLa cells after incubation with BA (30 µmol/l) in a
series of time ranging up to 48 h.
The cell viability, as indicated by an MTT assay,
displayed a general decline with increasing duration (12, 24, 36
and 48 h) of treatment with 30 µmol/l BA (Fig. 1B), suggesting that BA inhibited
the cell viability of HeLa in a dose-dependent manner; and the
IC50 was 66.75±1.73 µmol/l, 39.75±2.16
µmol/l and 30.42±2.39 µmol/l when HeLa cells were
treated with BA for 24, 36 and 48 h, respectively (the
IC50 was >100 µmol/l for a 12 h BA treatment).
In order to examine the effect of BA-induced apoptosis in HeLa
cells, the typical morphology of apoptosis was detected in
BA-treated HeLa cells in more time points, (3, 6, 12, 24 and 48 h),
as presented in Fig. 1C, the cell
counts significantly declined following 12 h. To confirm that the
reduction in the cell numbers was reflective of cell death, flow
cytometric detection was performed using labeled Annexin V-FITC/PI,
did not show a significant change until 12 h of incubation (little
change at 6 h). The total percentage of apoptotic cells was
9.53±1.46% (7.2±1.14% of cells in early apoptosis and 2.33±0.25% of
cells in late apoptosis), 13.10±1.45% (9.03±1.22% of cells in early
apoptosis and 4.07±1.76% of cells in late apoptosis), 18.97±2.25%
(9.90±1.30% of cells in early apoptosis and 9.10±1.00% of cells in
late apoptosis) and 25.38±3.42% (18.01±3.38% of cells in early
apoptosis and 7.36±0.21% of cells in late apoptosis) for cells
treated with 30 µmol/l BA for 12, 24, 36 and 48 h,
respectively (Fig. 1D and E).
Effect of BA on the expression of PI3K
and phosphorylated Akt
To investigate the involvement of the PI3K/Akt
pathway which plays an important role for cell survival and
apoptosis, the authors performed a time-dependence study in which
HeLa cells were incubated with BA for times ranging from 0–6 h. To
select the treatment time for the western blot experiments, the
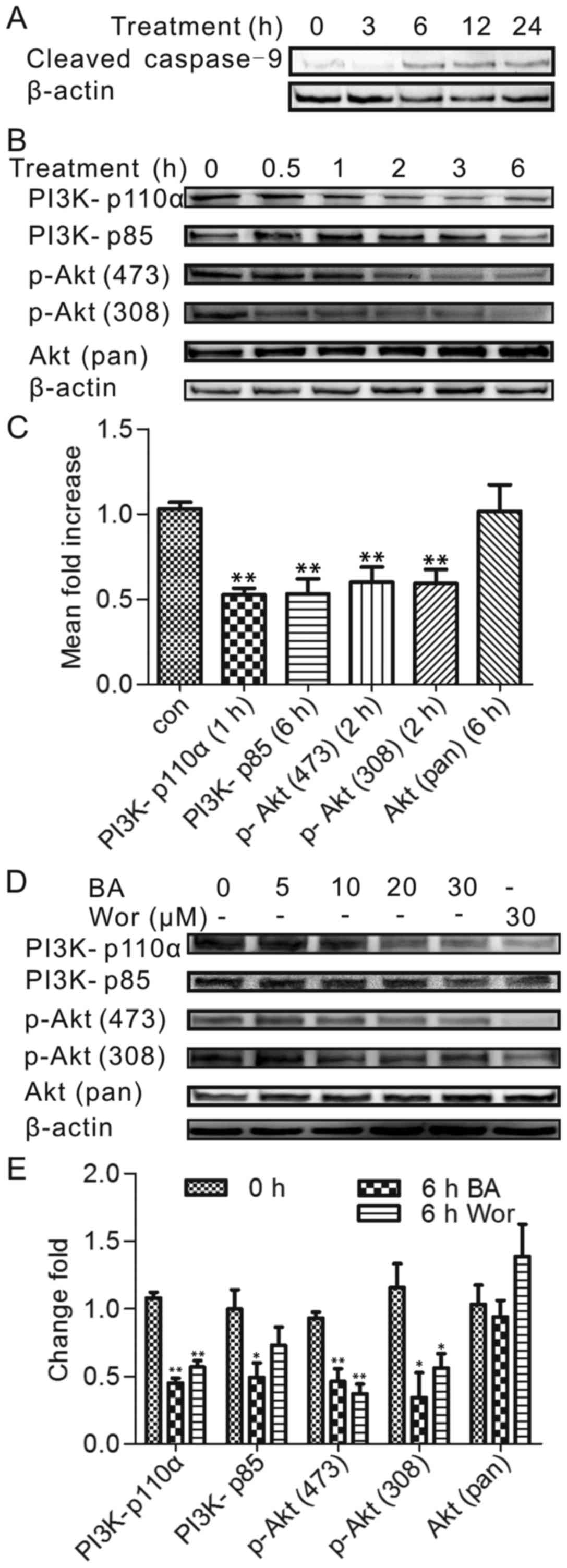
caspase-9 expression was tested firstly, which is a
well-established role in the process of mitochondrial apoptosis
(16). The expression level of
caspase-9 was identified by western blot analysis at a series of
time points (0, 1, 3, 6, 12 and 24 h), as Fig. 2A shown, after stimulation with 30
µmol/l BA for 6 h, the cleaved caspase-9 expression was
prominently increased. Therefore, before 6 h treatment time
including 6 h was chosen to figure out the PI3K/Akt factors in HeLa
incubated with BA because the caspase family activation is relative
late in apoptosis process.
The expression level of PI3K (p110a), PI3K (p85),
phospho-Akt (Ser473) and phospho-Akt (Thr308) was detected by
western blotting after BA treatment at 0, 0.5, 1, 2, 3 and 6 h. As
presented in Fig. 2B and C, PI3K
(p110a) protein level was reduced significantly in BA-treated HeLa
cells after 1 h of incubation (P<0.05) and the extent of protein
activation decreased over time. Consistently, the phospho-Akt
(Ser473) and phospho-Akt (Thr308) levels after 2 h and the PI3K
(p85) level after 6 h significantly changed, indicating that PI3K
(p110a) was an upstream regulatory factor that regulated other
proteins. The above sequence of BA-induced inhibition was PI3K
(p110a) 1 h, phospho-Akt (Ser473) 2 h, phospho-Akt (Thr308) 2 h,
PI3K (p85) 6 h; interestingly, the phosphorylation of p85 was
repressed by BA later than phospho-Akt (Ser473), phospho-Akt
(Thr308). However, the expression of PI3K (p110a) was still blocked
by BA in the beginning, which also can influence the downstream
factors: Phospho-Akt (Ser473), phospho-Akt (Thr308) in BA
treatment.
To further evaluate the dose-dependent study, a
separate set of experiments was performed to determine the above
protein activation effected by various concentrations (0, 5, 10, 20
and 30 µmol/l) of BA for 6 h incubation. As shown in
Fig. 2D and E, although some
proteins showed a fluctuating trend when treated with a small dose,
there still had a significant change at 30 µmol/l BA
treatment. In addition, the authors compared the potency of BA with
wortmannin, the inhibitor of PI3K. The observation was the similar
to the effect by BA on HeLa cells, suggesting that the PI3K/Akt
pathway was involved in BA- induced HeLa apoptosis.
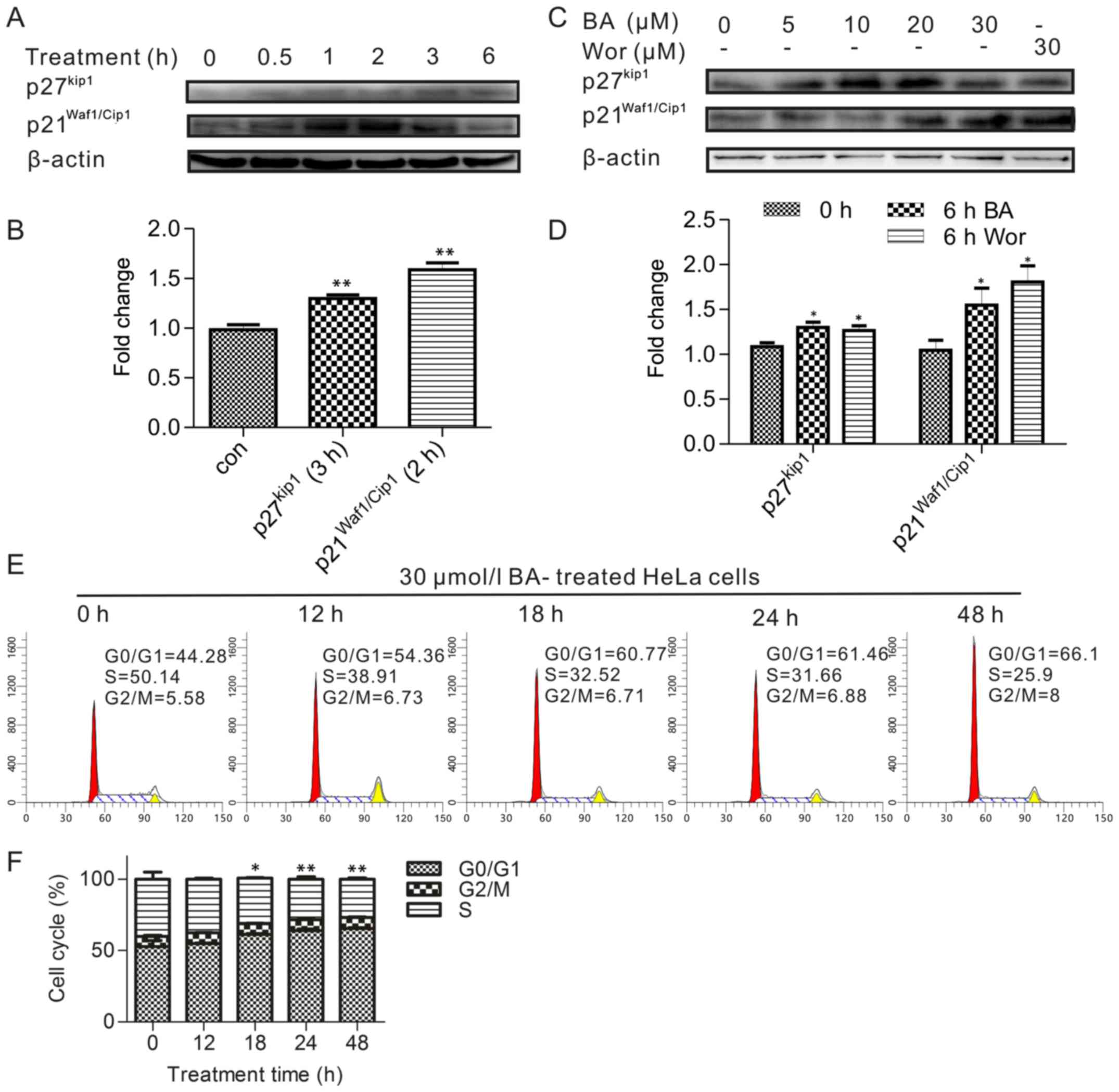
Upregulation of p21 and p27 and the
inhibition of the cell cycle in BA-treated HeLa cells
The above results indicated the PI3K/Akt pathway
involved in BA induced apoptosis, and the cell cycle is one of the
most important processes regulated by the PI3K/Akt signaling
pathway for cancer cell proliferation. Therefore, this part was to
detect the cell cycle whether involved in BA induction. Treatments
of durations were also applied for measuring the expression of
p21Waf1/Cip1 and p27Kip proteins, which are
important role in inhibition of cell cycle and regulated by Akt. As
presented in Fig. 3A, the
expression of p27Kip and p21Waf1/Cip1 has an
increasing trend, and significantly respective went up after 3 and
2 h in duration results (Fig.
3B), whereas the level of p21Waf1/Cip1
phosphorylation reached a plateau and began to decrease after 2 h,
indicated a transient phosphorylation pattern in this process. On
the other hand, the level of p27Kip did not change until
3 h treatment by BA. The result was consistent with the fact that
p21Waf1/Cip1 and p27Kip were substrates of
the PI3K/Akt pathway because the activation time of
p21Waf1/Cip1 and p27Kip was later than pAkt.
Conversely, BA treatment of HeLa cells caused a drastic
upregulation in the expression of p27Kip at 10, 20
µmol/l BA for 6 h treatment (Fig. 3C and D).
At the same time, the cell cycle was then analyzed
by flow cytometry after the cells were treated with 30
µmol/l BA for various periods ranging up to 48 h. The
authors observed that the number of cells in the S and G2/M phases
decreased, and 18–48 h treatment time caused a dramatically
increased G0/G1 phase population compared with in the control group
(Fig. 3E and F). Therefore, this
result suggested that BA arrested HeLa cells in the G0/G1 phase
after the inhibition of the PI3K/Akt pathway depending on the
response time.
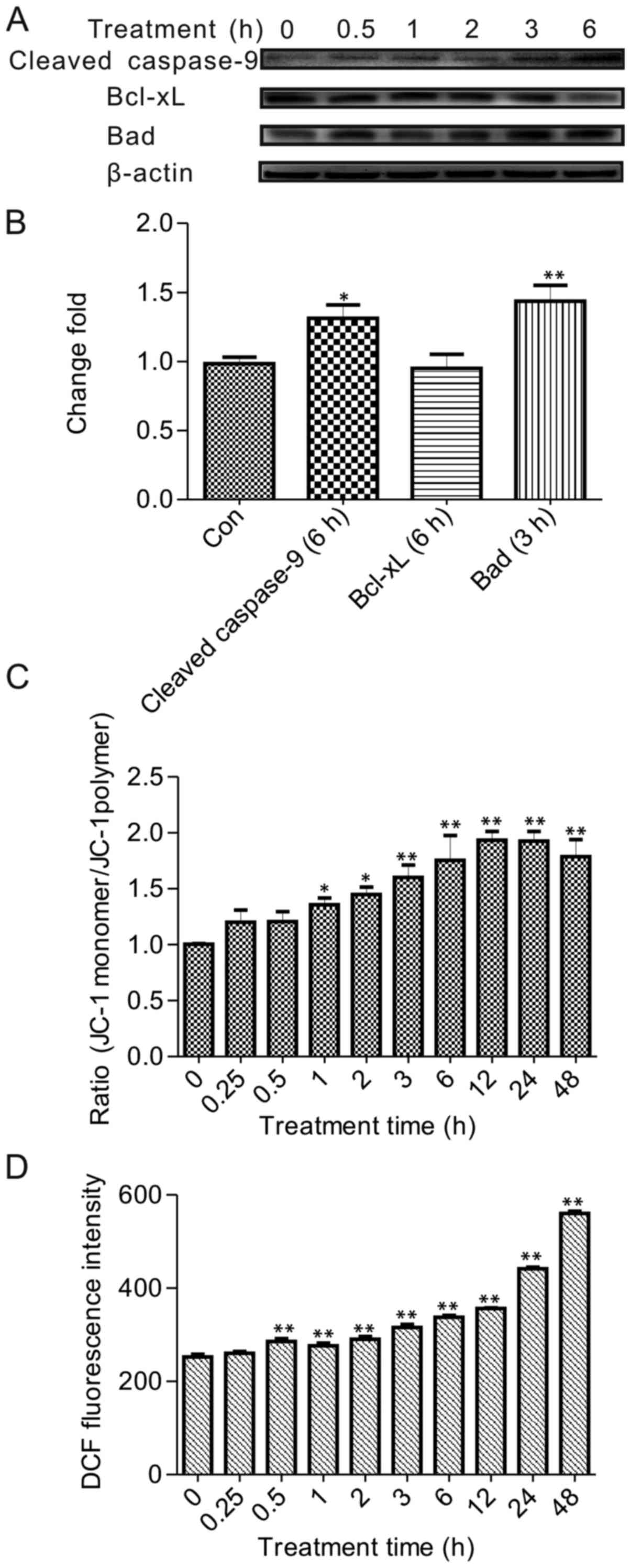
BA induced mitochondrial-dependent
apoptosis and disrupted the mitochondrial membrane potential (MMP)
in HeLa cells
Subsequently, we explored whether this effect of BA
was associated with an activation of mitochondrial pathway, which
was modulated though PI3K/Akt regulated the process of
pro-apoptotic factors, such as the Bcl-2 family and caspase-9.
To investigate the relationship between
mitochondrial pathway and the PI3K/Akt pathway, as well as the
effect of MMP influenced by BA, we examined the expression level of
landmark target caspase-9 and Bad, Bcl-xL which were regulated by
Akt. The results demonstrated that 30 µmol/l BA treatment
promoted the expression of Bad after 3 h. Unexpectedly, there were
no differences in the level of Bcl-xL, but the ratio of Bcl-xL/Bad
protein expression was reduced by BA treatment. This result may
have simply been a transposition and needs further investigation
(16). Consistently for Bcl-xL,
no alterations in expression levels have been reported upon
exposure to BA in other cancer cells (17). Detectable cleavage products of
caspase-9 clearly increased after 3 h in a time treatment trail
(Fig. 4A and B). Therefore, this
finding indicated that mitochondrial pathway took the initiative to
regulate the apoptosis by BA treatment.
The role of the mitochondrial permeability
transition (PT) pore was essential in apoptosis signaling. Opening
of the PT pore resulted in membrane depolarization and finally lead
to cell apoptosis. In the present study, the HeLa cells were
treated with 30 µmol/l BA and subsequently stained with JC-1
to test the effect of PT depolarization by BA. JC-1 predominantly
existed in monomeric form in cells with depolarized mitochondria
and displayed green fluorescence. If with polarized mitochondria,
JC-1 primarily formed aggregates in cells and showed reddish-orange
fluorescence. The green and the red fluorescence gradually changed,
with the green fluorescence significantly increasing during the
treatment (data not shown). Changes in the ratio of JC-1 forms
(monomeric form/aggregate form) were analyzed and graphically
documented to demonstrate significant changes at 1 h with 30
µmol/l BA treatment (Fig.
4C). Strikingly, in HeLa cell, it was clearly observed that BA
triggered the PT pore in a time-dependent way.
BA induced intracellular ROS
generation
These observations indicated ROS scavenging probably
involved in apoptosis induced by BA because the mitochondrial
depolarization was always affected by ROS. To clarify whether ROS
was related to the mitochondrial pathway, the ROS generation was
detected using an oxidation-sensitive fluorescent dye, DCFH-DA, to
determine the starting ROS generation time. As demonstrated in
Fig. 4D, ROS generation was
enhanced in a time-dependent manner with BA treatment, and the
initiation of ROS production had a significant 1.2-fold increase
compared to the control at 30 min. Furthermore, the trend was
rising at the treatment time and arrived 1.5-fold compared to the
control group at 48 h. Combined with above results, ROS generation
was initiated earlier than MMP decrease, which suggested that ROS
was upstream to regulate the apoptosis by BA, at least in HeLa
cells.
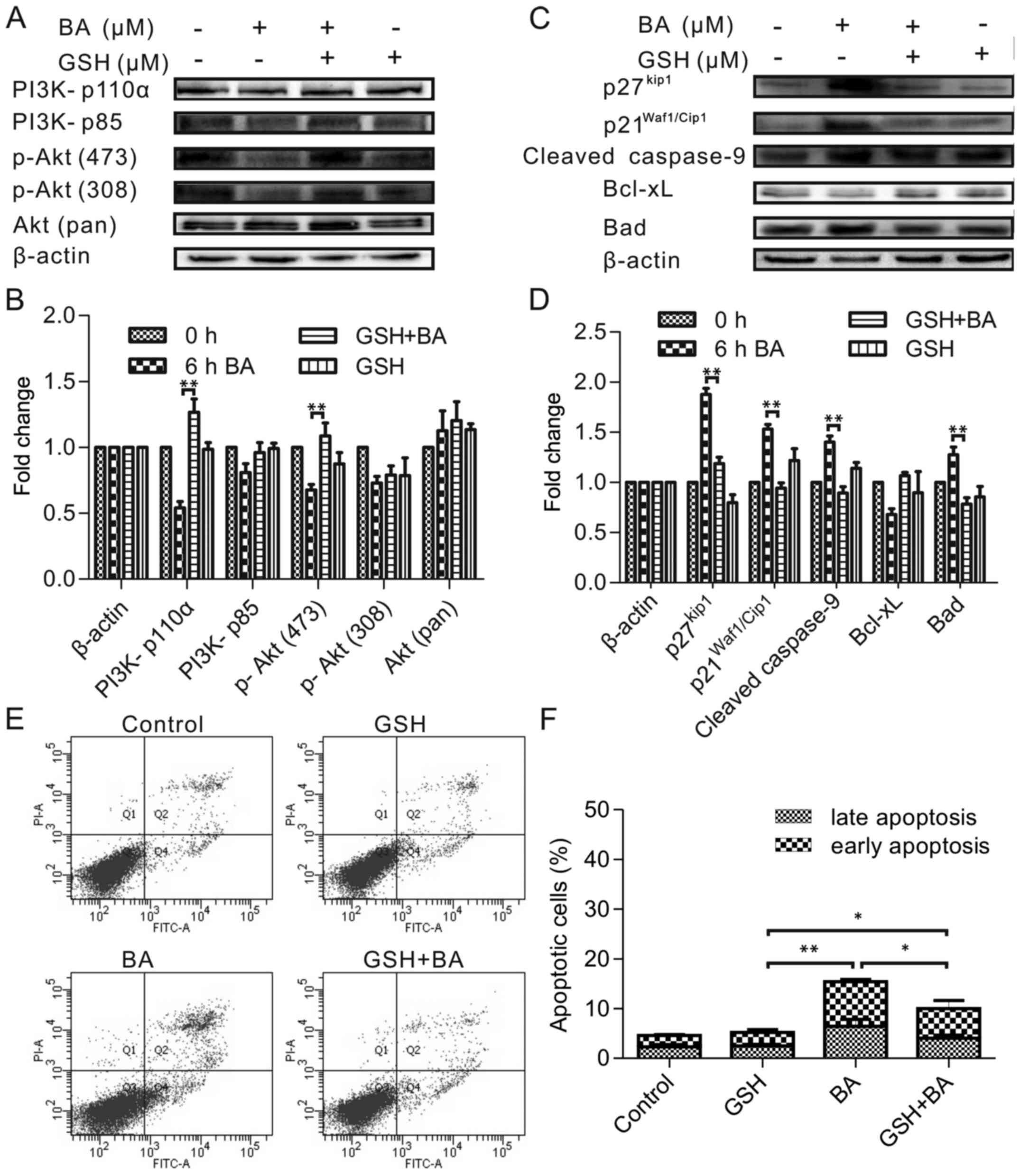
Antioxidants prevented PI3K and Akt
phosphorylation and apoptosis induction
The authors assessed the potential ability of
PI3K/Akt to protect HeLa cells from apoptosis, focusing on its
interventions upstream and downstream of ROS events. To unravel the
molecular mechanism involved in ROS accumulation and explore the
relationship between the ROS and the PI3K/Akt pathway, GSH (ROS
inhibitor) was used to pretreatment of HeLa cells before treatment
with 30 µmol/l BA for 6 h. According to the previous result,
6 h was the appropriate time because the expression of tested
proteins had changed by BA treatment at 6 h. As Fig. 5A and B shown the GSH prevented the
BA-induced inhibition of PI3K (p110a) and phospho-Akt (Ser473),
meanwhile this change in the PI3K and Akt phosphorylation pattern
correlated with the effects on other downstream substrates
(p27Kip, p21Waf1/Cip1) and mitochondrial
proteins (cleaved caspase-9, Bad) comparison with control cells
(Fig. 5C and D). Therefore, these
results suggested that ROS was upstream factor that could regulate
the PI3K/Akt signaling pathway and the mitochondrial pathway.
To further ascertain the relevance between apoptosis
and ROS, we pre incubated HeLa cells with 30 mM GSH before the 30
µmol/l BA treatment for 24 h. As presented in Fig. 5E and F, the apoptosis of cells
treated with GSH before BA was inhibited significantly (P<0.05)
compared to the positive control just incubated with GSH.
These results supported that ROS was an important
factor for regulating the PI3K/Akt signaling pathway and the
mitochondrial pathway involved in the BA-induced apoptosis
mechanism.
Discussion
The aim of the present study was to elucidate the
molecular mechanisms of apoptosis effects of BA and explore the
specific cellular targets or signaling pathways in HeLa cells. As
noted in previous studies, BA could induce HeLa apoptosis (14); however, the molecular mechanisms
of this process are not fully understood.
A concentration of 30 µmol/l BA was chosen
for subsequent treatments of HeLa cells to study the apoptosis
initiation by BA. Different screening methods demonstrated that BA
exhibited a cytotoxic activity in a time-dependent manner in the
present study. The growth of the HeLa cells was significantly
inhibited after 12 h treatment (Fig.
1B), and the typical morphology of apoptosis was also showed
after 12 h treatment (Fig. 1C).
Meanwhile, 12 h was the apoptosis initiation time of HeLa cells
exposed to 30 µmol/l BA because it caused a significant
increase of apoptosis cells at 12 h (Fig. 1D and E). Therefore, 12 h is a
critical treatment time to induce inhibition, and it was assumed
that the relevance factors involved in apoptosis process should be
activated by BA before 12 h.
BA appears to target the mitochondrial PT pore
directly in most previous results (6), thus, the authors firstly figured out
the expression level of cleavage caspase-9 to find an appropriate
monitor time for other proteins, because the caspase-9 is crucial
for mitochondrial pathway and its activation is relative later than
other proteins. As Fig. 2A
indicated, after 6 h treatment with 30 µmol/l BA, the
expression of cleaved caspase-9 was prominently increased, thus,
before and including 6 h treatment time was chosen to detect the
PI3K/Akt signaling factors.
The PI3K/Akt signaling pathway represents an
important anticancer target especially for mitochondrial apoptosis
because the regulation of mitochondrial respiratory activities was
affected by Akt through protein translation pathway (15,18,19). Growth factors and hormones trigger
a PI3K phosphorylation event, which, in turn, coordinates cell
growth, cell cycle entry, cell migration and cell survival
(20). In addition, the PI3K
pathway exerts its function through the downstream molecule Akt to
regulate various cell functions, including cell proliferation, cell
transformation, cell apoptosis, tumor growth and angiogenesis
(20). Given the significant role
of PI3K/Akt in cancer cells, the authors determined whether BA
treatment changed the PI3K expression and the phosphorylation
status of Akt in HeLa cells.
Western blotting was used to verify phosphorylation
of PI3Ks' catalytic subunit (p110) and an adapter subunit (p85),
which induces the phosphorylation of Akt at two key regulatory
sites: Threonine 308 (Thr308) and serine 473 (Ser473) (21). These proteins were inhibited in
does- and time-treatment (Fig.
2). Although some proteins showed a fluctuating trend when
treated with a small dose, Fig. 2C
and D still suggested that BA could inhibit the PI3K/Akt
signaling pathway in a relative does- dependent manner in early
time.
The Akt protein modulates cyclin-dependent kinase
inhibitors, p27Kip and p21Waf1/Cip1, that
inhibit cell cycle progression (22). In addition, Akt has been
implicated as an anti-apoptotic factor in many different cell death
processes (e.g., Akt regulates the apoptotic machinery and
inactivates proapoptotic proteins, such as Bad, which controls the
release of cytochrome c from mitochondria) (12,23). Therefore, the authors designed two
parts to measure the cell cycle and mitochondrial pathway.
For cell cycle part, as shown in Fig. 3A and B, although the protein
expression continuously increased with time, the BA treatment of
HeLa cells caused a drastic upregulation in the expression of
p21Waf1/Cip1 and p27Kip proteins after the
inhibition of the phosphorylation of pAkt (Fig. 3A); this finding is consistent with
the fact that p21Waf1/Cip1 and p27Kip are
substrates of the PI3K/Akt pathway (24). The flow cytometry result indicated
that BA arrested HeLa cells in the G0/G1 phase after the inhibition
of the PI3K/Akt pathway; this finding is in line with the protein
expression profile of p21Waf1/Cip1 and p27Kip
can arrest cell proliferation at the G1/S transition (25). Actually, numerous lines of
evidence have indicated that anticancer drugs induce tumor
regression through the induction of cell cycle arrest and/or
apoptosis (26,27), and Kang et al (28) also proved that the PI3K/Akt
pathway is involved in the apoptosis process by thioridazine. The
present observations suggested that the inhibition of the PI3K/Akt
pathway by BA in HeLa cells led to cell cycle arrest.
At the same time, in the apoptotic process, the
mitochondrial pathway is a central event that seals the cell's
fate, and it is particularly important for BA-induced apoptosis
(6,29). BA has been reported to induce
apoptosis via direct mitochondrial perturbations of Bcl-2 family
proteins, such as antiapoptotic Bcl-xL and proapoptotic Bad
(29,30). As noted in the authors' previous
studies, the 14-3-3 protein was inhibited in HeLa cells by BA
(14), and the interaction
between Bad and 14-3-3 causes Bad to be retained in the cytoplasm,
thus preventing Bad from dimerizing with Bcl-xL at the mitochondria
and mediating the release of Bax from Bcl-xL. Moreover, the
PI3K/Akt signaling pathway phosphorylates Bad at Ser155 in the BH3
domain that plays a critical role in blocking the dimerization of
Bad and Bcl-xL (17,31). Therefore, Bad and Bcl-xL were
measured to evaluate the relationship among these proteins by
western blotting for treatments of durations. Because of caspase-9
significantly increasing after 6 h (Fig. 4), the authors also examined
caspase-9 influenced by Akt because previous research demonstrated
the involvement of the PI3K/Akt pathway in the suppression of the
cytochrome c-induced processing of pro-caspase-9 and reduced
caspase activity (32).
To better understand the effectiveness of BA in
targeting the mitochondria of HeLa cells, alterations in the MMP
were directly determined. Loss of MMP is a near-universal hallmark
and a critical step for subsequent cell death (3,33).
Thus, the result (Fig. 4C and D)
showed that the mitochondria pathway was involved in the effects of
the BA treatment of HeLa cells. At the same time, ROS played an
important role in apoptosis induction because it is involved in MMP
and cell death induction (34,35). Hence, the generation of ROS was
monitored. Combined with activation time point, the decrease in MMP
started from 1 h of treatment after the generation of ROS 0.5 h,
confirming that the loss of MMP may be due to an increased ROS
level. These data illustrated the role of BA in enhancing ROS and
inducing apoptotic death in HeLa cells. The generation of ROS,
which induced disruption of mitochondrial function with a
concurrent loss of MMP, was important for the BA-treatment effects
on HeLa cells.
The results described above suggested that ROS
played a prominent role in BA-induced apoptosis and that PI3K/Akt
was also influenced by BA at an early time. Therefore, we then
investigated the relationship between ROS and the PI3K/Akt
signaling pathway. GSH is the key antioxidative regulator of
intracellular redox status and is commonly used to antagonize the
effects of ROS or to protect against programmed cell death
(36,37). Therefore, GSH was selected to
investigate the importance of ROS in the apoptosis process mediated
by BA. Western blot results supported that ROS was an important
upstairs factor for regulating the PI3K/Akt signaling pathway and
the mitochondrial pathway, as Fig.
5 indicated, PI3K (p110a), phospho-Akt (Ser473),
p27Kip, p21Waf1/Cip1, caspase-9 and Bad were
protected by GSH. At the same time, GSH could prevent HeLa cells
apoptosis that supposes that ROS was a critical regulator for
apoptosis in BA treatment (Fig. 5E
and F). These results supported the view that PI3K/Akt
signaling and the mitochondrial pathway were regulated by ROS and
acted in opposition in the balance of cell survival and death.
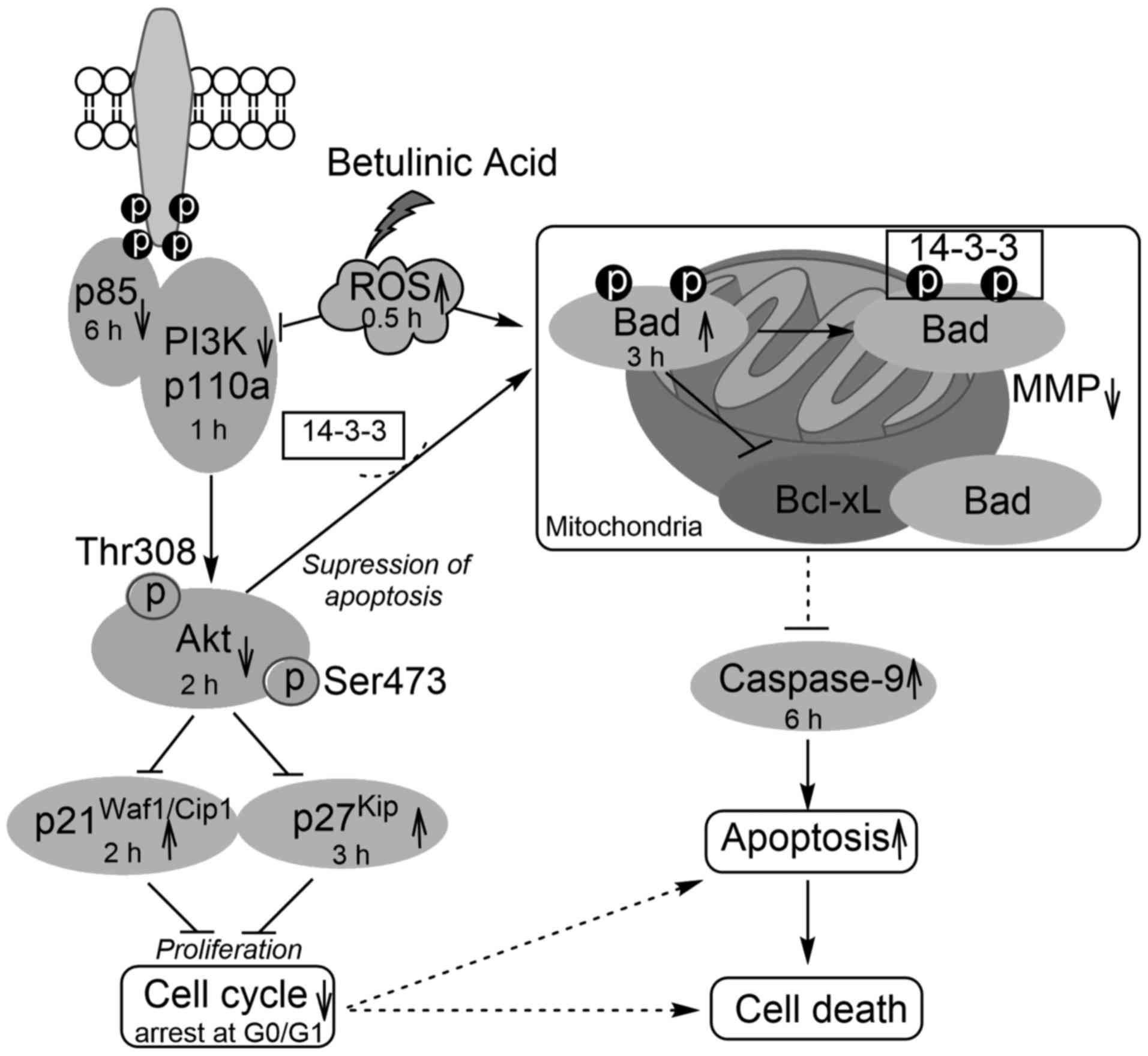
In conclusion, BA showed a strong inhibitory effect
on HeLa cell growth at pharmacological concentration (30
µmol/l). The present study confirmed that BA-induced
apoptosis involved the inhibition of PI3K (p110a and p85),
presented the deregulation of the Akt signaling pathway in response
to the generation of ROS, indicated that there is a decrease in the
mitochondrial potential, the activation of mitochondrial-regulated
Bad, caspase-9 and the cell cycle regulators p27Kip and
p21Waf1/Cip1 and the induction of G0/G1 phase arrest
(Fig. 6). These insights may be
helpful to understand the molecular mechanism of the BA antitumor
effect in HeLa cells and to enhance the development of BA for
clinical use.
Acknowledgments
The present study was financially supported by the
Fundamental Research Funds for the Central Universities (grant no.
DL13EA02).
References
|
1
|
Pisha E, Chai H, Lee IS, Chagwedera TE,
Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown
DM, et al: Discovery of betulinic acid as a selective inhibitor of
human melanoma that functions by induction of apoptosis. Nat Med.
1:1046–1051. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuco V, Supino R, Righetti SC, Cleris L,
Marchesi E, Gambacorti-Passerini C and Formelli F: Selective
cytotoxicity of betulinic acid on tumor cell lines, but not on
normal cells. Cancer Lett. 175:17–25. 2002. View Article : Google Scholar
|
|
3
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rabi T, Shukla S and Gupta S: Betulinic
acid suppresses constitutive and TNFalpha-induced NF-kappaB
activation and induces apoptosis in human prostate carcinoma PC-3
cells. Mol Carcinog. 47:964–973. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eichenmüller M, von Schweinitz D and
Kappler R: Betulinic acid treatment promotes apoptosis in
hepatoblastoma cells. Int J Oncol. 35:873–879. 2009.PubMed/NCBI
|
|
6
|
Tan DF, Li Q, Rammath N, Beck A, Wiseman
S, Anderson T, al-Salameh A, Brooks J and Bepler G: Prognostic
significance of expression of p53 oncoprotein in primary (stage
I–IIIa) non-small cell lung cancer. Anticancer Res. 23:1665–1672.
2003.PubMed/NCBI
|
|
7
|
Mullauer FB, Kessler JH and Medema JP:
Betulinic acid induces cytochrome c release and apoptosis in a
Bax/Bak-independent, permeability transition pore dependent
fashion. Apoptosis. 14:191–202. 2009. View Article : Google Scholar
|
|
8
|
Eichenmüller M, Hemmerlein B, von
Schweinitz D and Kappler R: Betulinic acid induces apoptosis and
inhibits hedgehog signalling in rhabdomyosarcoma. Br J Cancer.
103:43–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng L, Xia TS, Wang YF, Zhou W, Liang
XQ, Xue JQ, Shi L, Wang Y and Ding Q: The apoptotic effect of D
Rhamnose β-hederin, a novel oleanane-type triterpenoid saponin on
breast cancer cells. PLoS One. 9:e908482014. View Article : Google Scholar
|
|
10
|
Kim JA, Åberg C, de Cárcer G, Malumbres M,
Salvati A and Dawson KA: Low dose of amino-modified nanoparticles
induces cell cycle arrest. ACS Nano. 7:7483–7494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang QH, Liu Y, Wu SS, Cui RR, Yuan LQ
and Liao EY: Ghrelin inhibits the apoptosis of MC3T3-E1 cells
through ERK and AKT signaling pathway. Toxicol Appl Pharmacol.
272:591–597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roy B, Pattanaik AK, Das J, Bhutia SK,
Behera B, Singh P and Maiti TK: Role of PI3K/Akt/mTOR and MEK/ERK
pathway in Concanavalin A induced autophagy in HeLa cells. Chem
Biol Interact. 210:96–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu T, Pang Q, Zhou D, Zhang A, Luo S, Wang
Y and Yan X: Proteomic investigation into betulinic acid-induced
apoptosis of human cervical cancer HeLa cells. PLoS One.
9:e1057682014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meinig JM and Peterson BR:
Anticancer/antiviral agent Akt inhibitor-IV massively accumulates
in mitochondria and potently disrupts cellular bioenergetics. ACS
Chem Biol. 10:570–576. 2015. View Article : Google Scholar :
|
|
16
|
Ma K, Liu Y, Zhu Q, Liu CH, Duan JL, Tan
BK and Zhu YZ: H2S donor, S-propargyl-cysteine, increases CSE in
SGC-7901 and cancer-induced mice: Evidence for a novel anti-cancer
effect of endogenous H2S? PLoS One. 6:e205252011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willis SN, Chen L, Dewson G, Wei A, Naik
E, Fletcher JI, Adams JM and Huang DC: Proapoptotic Bak is
sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by
BH3-only proteins. Genes Dev. 19:1294–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian F, Ding D and Li D: Fangchinoline
targets PI3K and suppresses PI3K/AKT signaling pathway in SGC7901
cells. Int J Oncol. 46:2355–2363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goo CK, Lim HY, Ho QS, Too HP, Clement MV
and Wong KP: PTEN/Akt signaling controls mitochondrial respiratory
capacity through 4E-BP1. PLoS One. 7:e458062012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thorpe LM, Yuzugullu H and Zhao JJ: PI3K
in cancer: Divergent roles of isoforms, modes of activation and
therapeutic targeting. Nat Rev Cancer. 15:7–24. 2015. View Article : Google Scholar :
|
|
22
|
Brazil DP and Hemmings BA: Ten years of
protein kinase B signalling: A hard Akt to follow. Trends Biochem
Sci. 26:657–664. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
del Peso L, González-García M, Page C,
Herrera R and Nuñez G: Interleukin-3-induced phosphorylation of BAD
through the protein kinase Akt. Science. 278:687–689. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reed SI: Keeping p27(Kip1) in the
cytoplasm: A second front in cancer's war on p27. Cell Cycle.
1:389–390. 2002. View Article : Google Scholar
|
|
25
|
Nakayama KI and Nakayama K: Ubiquitin
ligases: Cell-cycle control and cancer. Nat Rev Cancer. 6:369–381.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ortega S, Malumbres M and Barbacid M:
Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim
Biophys Acta. 1602:73–87. 2002.PubMed/NCBI
|
|
27
|
Lee SK, Zhang W and Sanderson BJ:
Selective growth inhibition of human leukemia and human
lymphoblastoid cells by resveratrol via cell cycle arrest and
apoptosis induction. J Agric Food Chem. 56:7572–7577. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang S, Dong SM, Kim BR, Park MS, Trink B,
Byun HJ and Rho SB: Thioridazine induces apoptosis by targeting the
PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells.
Apoptosis. 17:989–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rzeski W, Stepulak A, Szymański M,
Sifringer M, Kaczor J, Wejksza K, Zdzisińska B and
Kandefer-Szerszeń M: Betulinic acid decreases expression of bcl-2
and cyclin D1, inhibits proliferation, migration and induces
apoptosis in cancer cells. Naunyn Schmiedebergs Arch Pharmacol.
374:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Lu H, Wang Y, Liu C, Zhu W, Zheng
S and Wan F: Taurine induces the apoptosis of breast cancer cells
by regulating apoptosis-related proteins of mitochondria. Int J Mol
Med. 35:218–226. 2015. View Article : Google Scholar
|
|
31
|
Zou Y, Li Q, Jiang L, Guo C, Li Y, Yu Y,
Li Y, Duan J and Sun Z: DNA hypermethylation of CREB3L1 and Bcl-2
associated with the mitochondrial-mediated apoptosis via PI3K/Akt
pathway in human BEAS-2B cells exposure to silica nanoparticles.
PLoS One. 11:e01584752016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khan I, Guru SK, Rath SK, Chinthakindi PK,
Singh B, Koul S, Bhushan S and Sangwan PL: A novel triazole
derivative of betulinic acid induces extrinsic and intrinsic
apoptosis in human leukemia HL-60 cells. Eur J Med Chem.
108:104–116. 2016. View Article : Google Scholar
|
|
33
|
Hamanaka RB and Chandel NS: Mitochondrial
reactive oxygen species regulate cellular signaling and dictate
biological outcomes. Trends Biochem Sci. 35:505–513. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Behrend L, Henderson G and Zwacka RM:
Reactive oxygen species in oncogenic transformation. Biochem Soc
Trans. 31:1441–1444. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deeb D, Gao X, Jiang H, Janic B, Arbab AS,
Rojanasakul Y, Dulchavsky SA and Gautam SC: Oleanane triterpenoid
CDDO-Me inhibits growth and induces apoptosis in prostate cancer
cells through a ROS-dependent mechanism. Biochem Pharmacol.
79:350–360. 2010. View Article : Google Scholar
|
|
36
|
Chandel NS: Mitochondrial complex III: An
essential component of universal oxygen sensing machinery? Respir
Physiol Neurobiol. 174:175–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chandel NS: Mitochondrial regulation of
oxygen sensing. Adv Exp Med Biol. 661:339–354. 2010. View Article : Google Scholar : PubMed/NCBI
|