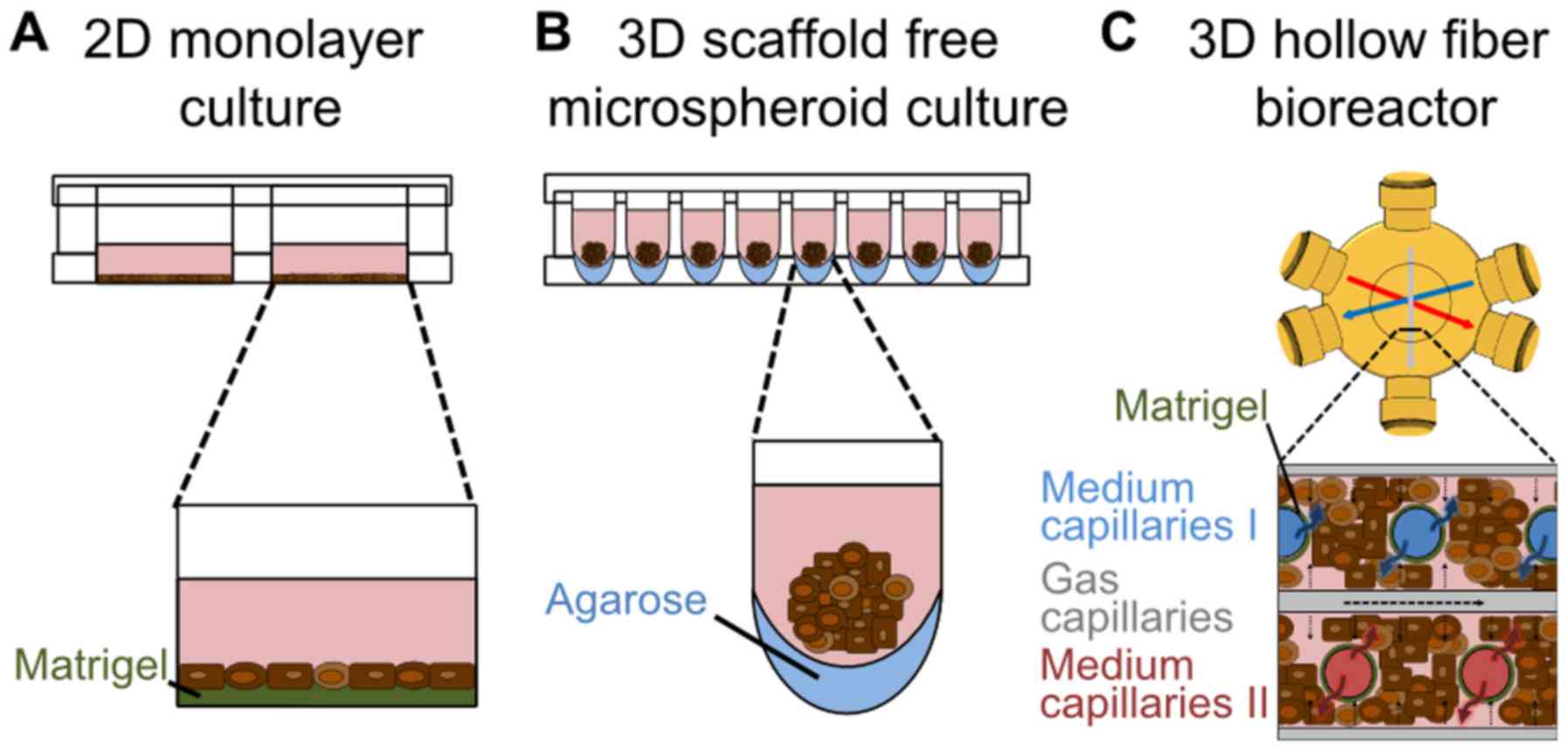
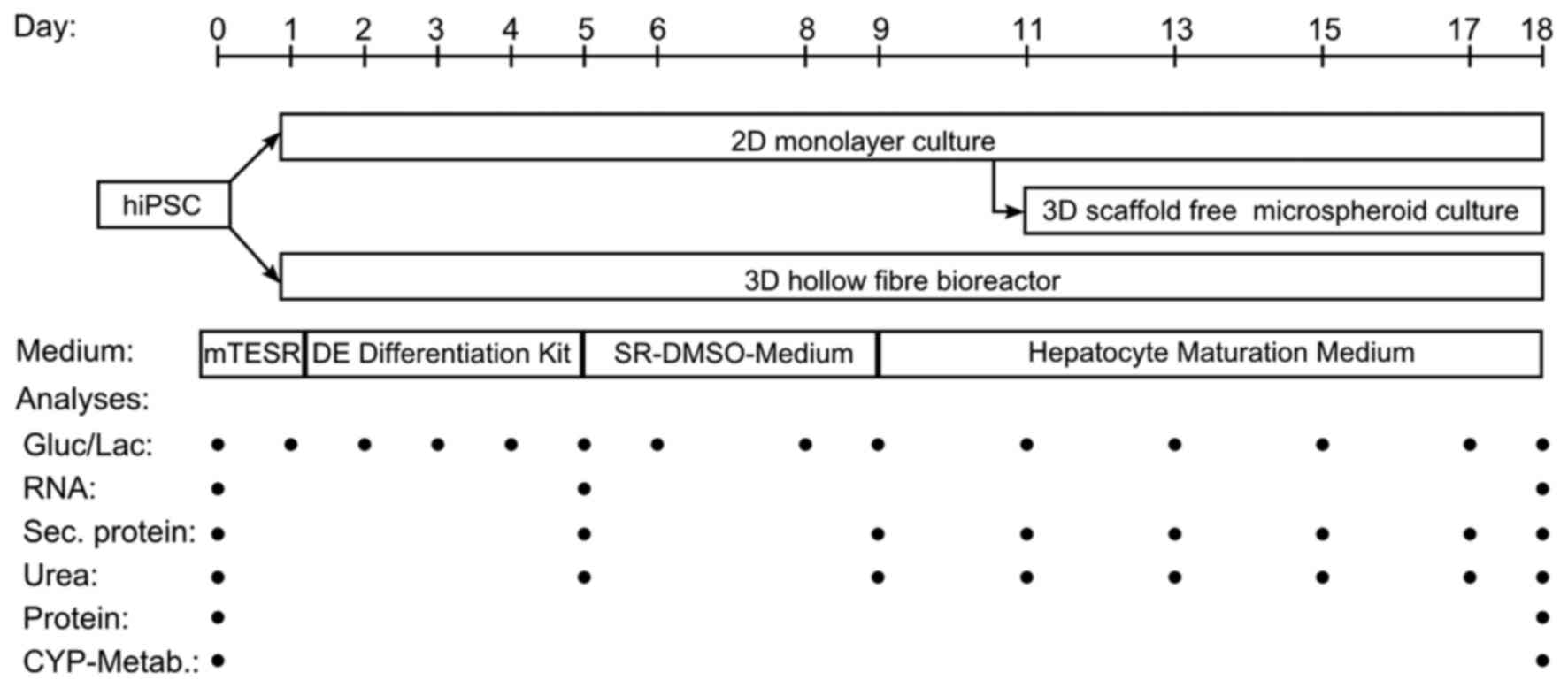
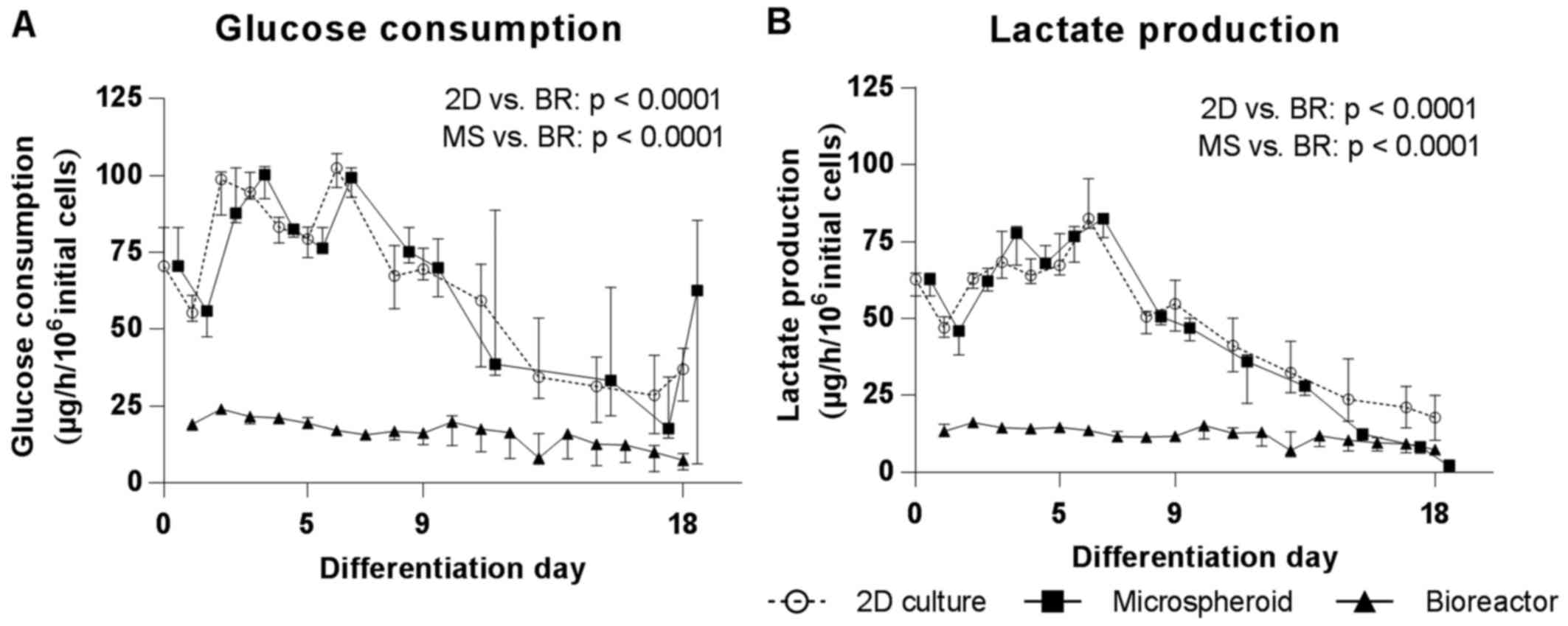
|
1
|
Lauschke VM and Ingelman-Sundberg M: The
importance of patient-specific factors for hepatic drug response
and toxicity. Int J Mol Sci. 17:17142016. View Article : Google Scholar :
|
|
2
|
Mueller SO, Guillouzo A, Hewitt PG and
Richert L: Drug biokinetic and toxicity assessments in rat and
human primary hepatocytes and HepaRG cells within the EU-funded
Predict-IV project. Toxicol In Vitro. 30:19–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rashid ST, Corbineau S, Hannan N,
Marciniak SJ, Miranda E, Alexander G, Huang-Doran I, Griffin J,
Ahrlund-Richter L, Skepper J, et al: Modeling inherited metabolic
disorders of the liver using human induced pluripotent stem cells.
J Clin Invest. 120:3127–3136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tafaleng EN, Chakraborty S, Han B, Hale P,
Wu W, Soto-Gutierrez A, Feghali-Bostwick CA, Wilson AA, Kotton DN,
Nagaya M, et al: Induced pluripotent stem cells model personalized
variations in liver disease resulting from α1-antitrypsin
deficiency. Hepatology. 62:147–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Si-Tayeb K, Lemaigre FP and Duncan SA:
Organogenesis and development of the liver. Dev Cell. 18:175–189.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hay DC, Fletcher J, Payne C, Terrace JD,
Gallagher RC, Snoeys J, Black JR, Wojtacha D, Samuel K, Hannoun Z,
et al: Highly efficient differentiation of hESCs to functional
hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl
Acad Sci USA. 105:12301–12306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hannan NRF, Segeritz CP, Touboul T and
Vallier L: Production of hepatocyte-like cells from human
pluripotent stem cells. Nat Protoc. 8:430–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Czysz K, Minger S and Thomas N: DMSO
efficiently down regulates pluripotency genes in human embryonic
stem cells during definitive endoderm derivation and increases the
proficiency of hepatic differentiation. PLoS One. 10:e01176892015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szkolnicka D, Farnworth SL,
Lucendo-Villarin B and Hay DC: Deriving functional hepatocytes from
pluripotent stem cells. Curr Protoc Stem Cell Biol. 30:1G.5.1–12.
2014. View Article : Google Scholar
|
|
12
|
Tasnim F, Phan D, Toh YC and Yu H:
Cost-effective differentiation of hepatocyte-like cells from human
pluripotent stem cells using small molecules. Biomaterials.
70:115–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwartz RE, Trehan K, Andrus L, Sheahan
TP, Ploss A, Duncan SA, Rice CM and Bhatia SN: Modeling hepatitis C
virus infection using human induced pluripotent stem cells. Proc
Natl Acad Sci USA. 109:2544–2548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bukong TN, Lo T, Szabo G and Dolganiuc A:
Novel developmental biology-based protocol of embryonic stem cell
differentiation to morphologically sound and functional yet
immature hepatocytes. Liver Int. 32:732–741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siller R, Greenhough S, Naumovska E and
Sullivan GJ: Small-molecule-driven hepatocyte differentiation of
human pluripotent stem cells. Stem Cell Reports. 4:939–952. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Asplund A, Pradip A, van Giezen M,
Aspegren A, Choukair H, Rehnström M, Jacobsson S, Ghosheh N, El
Hajjam D, Holmgren S, et al: One standardized differentiation
procedure robustly generates homogenous hepatocyte cultures
displaying metabolic diversity from a large panel of human
pluripotent stem cells. Stem Cell Rev. 12:90–104. 2016. View Article : Google Scholar
|
|
17
|
Kim JH, Jang YJ, An SY, Son J, Lee J, Lee
G, Park JY, Park HJ, Hwang DY, Kim JH, et al: Enhanced metabolizing
activity of human ES cell-derived hepatocytes using a 3D culture
system with repeated exposures to xenobiotics. Toxicol Sci.
147:190–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park H-J, Choi Y-J, Kim JW, Chun HS, Im I,
Yoon S, Han YM, Song CW and Kim H: Differences in the epigenetic
regulation of cytochrome P450 genes between human embryonic stem
cell-derived hepatocytes and primary hepatocytes. PLoS One.
10:e01329922015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baxter M, Withey S, Harrison S, Segeritz
CP, Zhang F, Atkinson-Dell R, Rowe C, Gerrard DT, Sison-Young R,
Jenkins R, et al: Phenotypic and functional analyses show stem
cell-derived hepatocyte-like cells better mimic fetal rather than
adult hepatocytes. J Hepatol. 62:581–589. 2015. View Article : Google Scholar :
|
|
20
|
Godoy P, Schmidt-Heck W, Natarajan K,
Lucendo-Villarin B, Szkolnicka D, Asplund A, Björquist P, Widera A,
Stöber R, Campos G, et al: Gene networks and transcription factor
motifs defining the differentiation of stem cells into
hepatocyte-like cells. J Hepatol. 63:934–942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kondo Y, Iwao T, Nakamura K, Sasaki T,
Takahashi S, Kamada N, Matsubara T, Gonzalez FJ, Akutsu H, Miyagawa
Y, et al: An efficient method for differentiation of human induced
pluripotent stem cells into hepatocyte-like cells retaining drug
metabolizing activity. Drug Metab Pharmacokinet. 29:237–243. 2014.
View Article : Google Scholar
|
|
22
|
Cameron K, Tan R, Schmidt-Heck W, Campos
G, Lyall MJ, Wang Y, Lucendo-Villarin B, Szkolnicka D, Bates N,
Kimber SJ, et al: Recombinant laminins drive the differentiation
and self-organization of hESC-derived hepatocytes. Stem Cell
Reports. 5:1250–1262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takayama K, Inamura M, Kawabata K,
Katayama K, Higuchi M, Tashiro K, Nonaka A, Sakurai F, Hayakawa T,
Furue MK, et al: Efficient generation of functional hepatocytes
from human embryonic stem cells and induced pluripotent stem cells
by HNF4alpha transduction. Mol Ther. 20:127–137. 2012. View Article : Google Scholar
|
|
24
|
Watanabe H, Takayama K, Inamura M,
Tachibana M, Mimura N, Katayama K, Tashiro K, Nagamoto Y, Sakurai
F, Kawabata K, et al: HHEX promotes hepatic-lineage specification
through the negative regulation of eomesodermin. PLoS One.
9:e907912014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: Transcription factors and
medium suitable for initiating the differentiation of human induced
pluripotent stem cells to the hepatocyte lineage. J Cell Biochem.
117:2001–2009. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vinken M, Papeleu P, Snykers S, De Rop E,
Henkens T, Chipman JK, Rogiers V and Vanhaecke T: Involvement of
cell junctions in hepatocyte culture functionality. Crit Rev
Toxicol. 36:299–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramasamy TS, Yu JS, Selden C, Hodgson H
and Cui W: Application of three-dimensional culture conditions to
human embryonic stem cell-derived definitive endoderm cells
enhances hepatocyte differentiation and functionality. Tissue Eng
Part A. 19:360–367. 2013. View Article : Google Scholar
|
|
28
|
Sivertsson L, Synnergren J, Jensen J,
Björquist P and Ingelman-Sundberg M: Hepatic differentiation and
maturation of human embryonic stem cells cultured in a perfused
three-dimensional bioreactor. Stem Cells Dev. 22:581–594. 2013.
View Article : Google Scholar :
|
|
29
|
Gieseck RL III, Hannan NR, Bort R, Hanley
NA, Drake RA, Cameron GW, Wynn TA and Vallier L: Maturation of
induced pluripotent stem cell derived hepatocytes by 3D-culture.
PLoS One. 9:e863722014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takebe T, Sekine K, Enomura M, Koike H,
Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, et al:
Vascularized and functional human liver from an iPSC-derived organ
bud transplant. Nature. 499:481–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeilinger K, Schreiter T, Darnell M,
Söderdahl T, Lübberstedt M, Dillner B, Knobeloch D, Nüssler AK,
Gerlach JC and Andersson TB: Scaling down of a clinical
three-dimensional perfusion multicompartment hollow fiber liver
bioreactor developed for extracorporeal liver support to an
analytical scale device useful for hepatic pharmacological in vitro
studies. Tissue Eng Part C Methods. 17:549–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van de Bunt M, Lako M, Barrett A, Gloyn
AL, Hansson M, McCarthy MI, Beer NL and Honoré C: Insights into
islet development and biology through characterization of a human
iPSC-derived endocrine pancreas model. Islets. 8:83–95. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Friedrich J, Seidel C, Ebner R and
Kunz-Schughart LA: Spheroid- based drug screen: Considerations and
practical approach. Nat Protoc. 4:309–324. 2009. View Article : Google Scholar
|
|
34
|
Pfeiffer E, Kegel V, Zeilinger K,
Hengstler JG, Nüssler AK, Seehofer D and Damm G: Featured article:
Isolation, characterization, and cultivation of human hepatocytes
and non-parenchymal liver cells. Exp Biol Med (Maywood).
240:645–656. 2015. View Article : Google Scholar
|
|
35
|
Brzeszczyńska J, Johns N, Schilb A, Degen
S, Degen M, Langen R, Schols A, Glass DJ, Roubenoff R, Greig CA, et
al: Loss of oxidative defense and potential blockade of satellite
cell maturation in the skeletal muscle of patients with cancer but
not in the healthy elderly. Aging (Albany NY). 8:1690–1702. 2016.
View Article : Google Scholar
|
|
36
|
Vandesompele J, De Preter K, Pattyn F,
Poppe B, Van Roy N, De Paepe A and Speleman F: Accurate
normalization of real-time quantitative RT-PCR data by geometric
averaging of multiple internal control genes. Genome Biol.
3:RESEARCH00342002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
38
|
Liu J, Brzeszczynska J, Samuel K, Black J,
Palakkan A, Anderson RA, Gallagher R and Ross JA: Efficient
episomal reprogramming of blood mononuclear cells and
differentiation to hepatocytes with functional drug metabolism. Exp
Cell Res. 338:203–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Freyer N, Knöspel F, Strahl N, Amini L,
Schrade P, Bachmann S, Damm G, Seehofer D, Jacobs F, Monshouwer M,
et al: Hepatic differentiation of human induced pluripotent stem
cells in a perfused three-dimensional multicompartment bioreactor.
Biores Open Access. 5:235–248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hoffmann SA, Müller-Vieira U, Biemel K,
Knobeloch D, Heydel S, Lübberstedt M, Nüssler AK, Andersson TB,
Gerlach JC and Zeilinger K: Analysis of drug metabolism activities
in a miniaturized liver cell bioreactor for use in pharmacological
studies. Biotechnol Bioeng. 109:3172–3181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schyschka L, Sánchez JJ, Wang Z, Burkhardt
B, Müller-Vieira U, Zeilinger K, Bachmann A, Nadalin S, Damm G and
Nussler AK: Hepatic 3D cultures but not 2D cultures preserve
specific transporter activity for acetaminophen-induced
hepatotoxicity. Arch Toxicol. 87:1581–1593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rennert K, Steinborn S, Gröger M,
Ungerböck B, Jank AM, Ehgartner J, Nietzsche S, Dinger J, Kiehntopf
M, Funke H, et al: A microfluidically perfused three dimensional
human liver model. Biomaterials. 71:119–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Varum S, Rodrigues AS, Moura MB,
Momcilovic O, Easley CA IV, Ramalho-Santos J, Van Houten B and
Schatten G: Energy metabolism in human pluripotent stem cells and
their differentiated counterparts. PLoS One. 6:e209142011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tirona RG, Lee W, Leake BF, Lan LB, Cline
CB, Lamba V, Parviz F, Duncan SA, Inoue Y, Gonzalez FJ, et al: The
orphan nuclear receptor HNF4α determines PXR- and CAR-mediated
xenobiotic induction of CYP3A4. Nat Med. 9:220–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347:12604192015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miki T, Ring A and Gerlach J: Hepatic
differentiation of human embryonic stem cells is promoted by
three-dimensional dynamic perfusion culture conditions. Tissue Eng
Part C Methods. 17:557–568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
De Assuncao TM, Sun Y, Jalan-Sakrikar N,
Drinane MC, Huang BQ, Li Y, Davila JI, Wang R, O'Hara SP, Lomberk
GA, et al: Development and characterization of human-induced
pluripotent stem cell-derived cholangiocytes. Lab Invest.
95:684–696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Knöspel F, Jacobs F, Freyer N, Damm G, De
Bondt A, van den Wyngaert I, Snoeys J, Monshouwer M, Richter M,
Strahl N, et al: In vitro model for hepatotoxicity studies based on
primary human hepatocyte cultivation in a perfused 3D bioreactor
system. Int J Mol Sci. 17:5842016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Singh M, Berkland C and Detamore MS:
Strategies and applications for incorporating physical and chemical
signal gradients in tissue engineering. Tissue Eng Part B Rev.
14:341–366. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Uzel SG, Amadi OC, Pearl TM, Lee RT, So PT
and Kamm RD: Simultaneous or sequential orthogonal gradient
formation in a 3D cell culture microfluidic platform. Small.
12:612–622. 2016. View Article : Google Scholar :
|
|
51
|
Takayama K, Kawabata K, Nagamoto Y,
Kishimoto K, Tashiro K, Sakurai F, Tachibana M, Kanda K, Hayakawa
T, Furue MK, et al: 3D spheroid culture of hESC/hiPSC-derived
hepatocyte-like cells for drug toxicity testing. Biomaterials.
34:1781–1789. 2013. View Article : Google Scholar
|
|
52
|
Ginsberg G, Hattis D, Sonawane B, Russ A,
Banati P, Kozlak M, Smolenski S and Goble R: Evaluation of
child/adult pharmacokinetic differences from a database derived
from the therapeutic drug literature. Toxicol Sci. 66:185–200.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Goldring C, Antoine DJ, Bonner F, Crozier
J, Denning C, Fontana RJ, Hanley NA, Hay DC, Ingelman-Sundberg M,
Juhila S, et al: Stem cell-derived models to improve mechanistic
understanding and prediction of human drug-induced liver injury.
Hepatology. 65:710–721. 2017. View Article : Google Scholar
|