Introduction
Long-term alcohol abuse may cause chronic injuries
to the myocardium and be further associated with an increased
incidence of alcoholic cardiomyopathy (ACM). ACM refers to a
specific cardiomyopathy that has frequently been observed in
patients who have a history of long-term alcohol abuse, but
excludes organic heart diseases, such as congenital heart disease,
valvular heart disease, coronary heart disease, hypertension and
myocarditis (1). Scholars
hypothesize that almost all ACM patients exhibit varying degrees of
myocardial remodeling, including the remodeling of cardiac
collagen, i.e., myocardial fibrosis (2). Myocardial fibrosis, a major
pathological process in myocardial remodeling, leads to impaired
systolic and diastolic function, which is closely associated with
the occurrence and development of chronic heart failure, arrhythmia
or even sudden cardiac death. However, there remains no specific
treatment that effectively alleviates myocardial fibrosis caused by
long-term alcohol abuse. Therefore, in-depth investigations into
the underlying mechanism of myocardial fibrosis in ACM are
conducive to establish novel preventive and therapeutic strategies
to alleviate myocardial fibrosis, and what is more, to reduce the
risk of heart failure in ACM patients.
Autophagy is an evolutionarily conserved process and
a key mechanism for the maintenance of cellular homeostasis, which
has been associated with the degradation and recycling of damaged
or unnecessary proteins and organelles to promote cell survival
under stressful conditions. Autophagy has been demonstrated to be
involved in a series of physiological and pathological processes
(3,4). Accumulating evidence indicates that
fundamental autophagy is an essential cellular protective and
cellular self-aid behavior in harsh environments, beyond this
range, it will lead to cell damage or cell death. Excessive
autophagy is stimulated via lysosome-driven degradations in
response to a variety of extracellular and intracellular stresses,
including deprivation of nutrients and growth factors. Numerous
studies have confirmed that myocardial cells, stimulated by
multiple pathological factors, such as ischemia reperfusion, high
blood glucose and overload, are generally associated with
excessively activated autophagy (5–7).
High levels of autophagy causes a large loss of myocardial cells
(8,9), which is considered as a crucial
event in the progression of myocardial remodeling and heart failure
(10). Long-term alcohol intake
may give rise to increasing expression levels of
microtubule-associated protein 1 light chain 3 type II (LC3-II) and
autophagy related 7 (Atg7), which are autophagy-associated proteins
in heart tissues. By contrast, myocardial injuries caused by
alcohol intake may be markedly improved when autophagy is inhibited
by 3-methyladenine (3-MA) (11).
The above-mentioned studies indicate a potential correlation
between autophagy and alcohol-induced myocardial injuries. MicroRNA
(miRNA), as a small non-coding endogenous RNA, causes degradation
of messenger RNA (mRNA) or blocks the translation process of mRNA
into protein, and participates in and regulates multiple biological
processes, such as cell proliferation, differentiation and
apoptosis. A previous study revealed that the variation in
expression profiles of miRNA may be involved in the regulation of
myocardial remodeling and cell autophagy (12), and extensive interactions have
been observed between phosphatidylinositol-4,5-bisphosphate
3-kinase (PI3K)/AKT and transforming growth factor (TGF)-β1, which
are involved in the regulation of myocardial fibrosis, autophagy,
miR-21 and miR-221. In a study on patients with ACM conducted by
Jing et al (13), it was
found that differential expression of miRNAs may also participate
in the occurrence and development of ACM (13).
Hydrogen sulfide (H2S) is recognized as
the third most common endogenously produced gaseous signaling
molecule in mammalian systems [the top two being nitric oxide (NO)
and carbon monoxide (CO)]. Numerous studies have demo nstrated its
protective effect on the brain, liver, kidney, heart, lung and
other organs. H2S is well-known as a potent vasodilator
and signaling molecule under normal and pathophysiological
conditions (14). However, the
protective effect of H2S on ACM, as well as its
intrinsic mechanism remain unknown. Studies have demonstrated that
H2S improves the left ventricular function of smoking
rats by downregulating autophagy (15) and provides a protective effect on
the brain in traumatic brain injuries (16). In a study of mouse models with
myocardial ischemia or inflammatory injuries, it was identified
that H2S reduces ischemic injury and inhibits
inflammatory responses in the myocardium of mice by upregulating
the expression level of miRNA-21 (17). Thus, H2S may alleviate
myocardial fibrosis in ACM by regulating the expression levels of
miRNA and cell autophagy; therefore, the present study established
a mouse model of ACM to observe the effect of H2S on
myocardial fibrosis and cell autophagy in ACM. Furthermore, the
mechanisms by which H2S affected proteins in the
PI3K/AKT signaling pathways involved in the regulation of autophagy
and the expression levels of relevant miRNA were investigated.
Materials and methods
Animals and reagents
The experimental protocol was approved by the Animal
Ethics Committee of the University of South China (Hengyang,
China). Male Kunming mice (n=44; weight, 16–21 g) were purchased
from the Animal Experimental Center of the University of South
China. Animals were housed separately, under a controlled
temperature (24°C) and a normal phase light-dark cycle (light from
8:00 a.m. to 8:00 p.m.), with free access to food and water or
ethanol solution. Sodium hydrosulfide (NaHS) and
D,L-propargylglycine (PAG) were purchased from Sigma-Aldrich; Merck
KGaA (Darmstadt, Germany). Cell lysis buffer for western blotting,
phenylmethanesulfonyl fluoride, a bicinchoninic acid (BCA) protein
assay kit (cat. no. P0012), SDS-PAGE gel preparation kit (cat. no.
P0012A) and chloral hydrate were all purchased from Beyotime
Institute of Biotechnology (Shanghai, China). Polyvinylidene
fluoride (PVDF) membranes and prestained color protein molecular
weight marker were purchased from Sigma-Aldrich; Merck KGaA. Rabbit
polyclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
cat. no. BM16243), rabbit polyclonal anti-matrix metalloproteinase
8 (MMP8; cat. no. BA2201), rabbit polyclonal anti-MMP13 (cat. no.
BA2204), rabbit polyclonal anti-MMP14 (cat. no. BA1278), rabbit
polyclonal anti-collagen I (cat. no. BA0325) and rabbit polyclonal
anti-TGF-β1 (cat. no. BA0290) were all purchased from Wuhan Boster
Biological Technology, Ltd. (Wuhan, China). Rabbit polyclonal
anti-MMP17 (cat. no. bs-1862R) and rabbit polyclonal anti-tissue
inhibitor of metalloproteinase 1 (TIMP1; cat. no. bs-0415R) were
both purchased from Beijing Biosynthesis Biotechnology Co., Ltd.
(Beijing, China). In addition, rabbit monoclonal anti-Beclin 1
(cat. no. 3495), rabbit monoclonal anti-autophagy-related protein 3
(Atg3; cat. no. 3415), rabbit monoclonal anti-Atg7 (cat. no. 8558)
and rabbit polyclonal anti-PI3K (cat. no. 4249) and rabbit
polyclonal anti-AKT1 (cat. no. 75692) were all purchased from Cell
Signaling Technology, Inc., (Danvers, MA, USA). Horseradish
peroxidase-labeled goat anti-rabbit IgG (cat. no. 074-1506) was
purchased from KPL, Inc. (Gaithersburg, MD, USA).
ACM model
Kunming male mice (n=44) were divided randomly into
the following four groups (n=11/group) subsequent to 7-day
environment adaptive feeding: Control group, ACM model group (model
group), ACM model with NaHS treatment group (NaHS group) and ACM
model with PAG treatment group (PAG group). The ACM model was
established via the drinking of 4% ethanol solution (used as the
sole source of drinking water) freely for 12 weeks, according to
the study by Ge and Ren (18) and
mice in the control group had free access to clean water. The 4%
ethanol solution and clean water were refreshed every morning. Mice
in the NaHS group received a daily intraperitoneal injection of 50
µmol/kg NaHS, mice in the PAG group received a daily
intraperitoneal injection of 40 mg/kg PAG (endogenous
H2S production enzyme inhibitor), and mice in the
control and model groups were intraperitoneally injected with
physiological saline of the same volume every day. Twelve weeks
later, animals were sacrificed following anesthesia induced by
chloral hydrate at a dose of 350 mg/kg. Cardiac tissue samples were
harvested for pathological sections, and morphological changes on
the myocardium were observed via Masson's and Van Gieson's (VG)
staining and used to judge whether the ACM model had been
successfully established.
Histopathological examination
Myocardial tissue samples were fixed with 4%
polyformaldehyde (Beyotime Institute of Biotechnology), washed with
water, dehydrated with alcohol, embedded in paraffin (Beyotime
Institute of Biotechnology) and sliced into sections (thickness, 4
µm). The sections were stained using a Masson's staining kit
(cat. no. SBJ-0288) and VG staining kit (cat. no. SBJ-0297) (both
from Nanjing Senbeijia Biological Technology Co., Ltd., Nanjing,
China) according to the manufacturer's instructions and observed
under a light microscope (Motic BA210; Motic Medical Diagnostic
Systems Co., Ltd., Xiamen, China).
Transmission electronic microscopy
observation
Left ventri cular myocardial tissue samples were cut
into slices and fixed with 2.5% glutaraldehyde (Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China), post-fixed with 1% osmium
tetraoxide (Absin Bioscience Inc., Shanghai, China), rinsed with
phosphoric acid rinse solution (Beyotime Institute of
Biotechnology), dehydrated using a series of acetone (Beyotime
Institute of Biotechnology) at different concentrations, embedded
and solidified, and cut into slices (thickness, 50-100 nm). The
ultrathin slices were stained using 3% uranyl acetate (Shanghai
Fortune Biological Technology Co., Ltd., Shanghai, China) and lead
nitrate (Tanyun Industry Fine Chemical Co., Ltd., Yingkou, China)
at 37°C for 30 min. Samples were observed under a transmission
electron microscopy and the images were saved.
Expression of collagen I detected by
immunohistochemistry
Myocardial tissue samples were fixed with 4%
polyformaldehyde, embedded in paraffin, cut into slices (thickness,
10 µm), dewaxed and hydrated. The slices were incubated with
3% hydrogen peroxide (Beyotime Institute of Biotechnology), washed
with phosphate-buffered saline (PBS; Beyotime Institute of
Biotechnology), blocked with 10% normal goat serum (Beyotime
Institute of Biotechnology) at 37°C for 10 min, incubated at 37°C
with rabbit polyclonal anti-collagen I (dilution 1:100) for 90 min,
washed with PBS, incubated at 37°C with secondary antibodies
(dilution 1:2,000) for 15 min, washed with PBS, incubated at 37°C
with horseradish peroxidase-labeled streptavidin (Beyotime
Institute of Biotechnology) for 20 min, washed with PBS, colored
with color developing reagent (Beyotime Institute of
Biotechnology), stained with hematoxylin (Beyotime Institute of
Biotechnology) at 37°C for 20 min, dehydrated with alcohol and
sealed with neutral resins.
Western blot analysis
Total proteins were extracted in cell dialysis
buffer containing protease inhibitors and quantified using a BCA
Protein Assay kit. The proteins were denatured, separated (40
µg protein/lane) by electrophoresis equipped with 10%
SDS-PAGE and transferred to a PVDF membrane (200 mA, 110 min). The
membranes were blocked with Tris-buffered saline (Well-Biology Co.,
Ltd., Changsha, China) with Tween-20 (TBST; Beyotime Institute of
Biotechnology) containing 5% skimmed milk at 37°C for 1 h. The
membranes were incubated overnight at 4°C with primary antibody
diluted at the following appropriate concentrations: MMP8 (1:400),
MMP13 (1:400), MMP14 (1:400), MMP17 (1:400), TIMP1 (1:400), TGF1
(1:400), Beclin 1 (1:1,000), Atg3 (1:1,000), Atg7 (1:1,000), PI3K
(1:1,000) and AKT1 (1:1,000). Following washing with TBST buffer
three times, the membranes were incubated with secondary antibody
(1:8,000) for 1 h at 37°C. Stripes were visualized using an
enhanced chemiluminescence detection reagent (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) and analyzed using Alpha
Imager 2200 (ProteinSimple, San Jose, CA, USA). GAPDH served as the
internal reference.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from myocardial tissue of
mice in each group using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Concentration of extracted RNA
was measured using an ultraviolet spectrophotometer (Agilent
Technologies, Inc., Santa Clara, CA, USA) and the integrity of RNA
was analyzed with a gel imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The RT reaction was manipulated with mi-RNA
specific RT primer (GenScript Co., Ltd., Nanjing, China) using an
RT polymerase chain reaction kit (cat. no. K1622; MBI Fermentas;
Thermo Fisher Scientific, Inc.). RT-qPCR was performed and analyzed
on a Thermo Scientific PikoReal Real-Time PCR system (PikoReal 96;
Thermo Fisher Scientific, Inc.). Expression levels of miR-21,
miR-221, miR-133a and miR-199a were detected by qPCR using a Taqman
mi-RNA assay probe (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and U6 was used as loading control for quantitation of
miRNAs. RT was performed under the following conditions: 37°C for
15 min, 42°C for 50 min and 85°C for 5 min. The acquired cDNA was
subjected to qPCR as follows: 50°C for 2 min, 95°C for 10 min, 5
sec at 95°C and 30 sec at 60°C for 40 cycles. Relative
quantification of miR-21, miR-221, miR-133a and miR-199a expression
were calculated using the 2−ΔΔCt method.
Statistical analysis
Data are presented as the mean ± standard deviation
(mean ± SD). Differences among groups were evaluated by one-way
analysis of variance using SPSS software (version 18.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of H2S on body weight,
heart weight and heart/body weight ratio
Twelve weeks after the intervention, the following
numbers of mice had survived in each group: Control group, n=11;
model group, n=9; NaHS group, n=10; and PAG group, n=9. As
demonstrated in Table I, the
differences of body weight, heart weight and heart/body weight
ratio in each group were not statistically significant.
 | Table IBW, HW and HW/BW ratio in each group
(mean ± SD). |
Table I
BW, HW and HW/BW ratio in each group
(mean ± SD).
| Groups | Nos. | BW
(g) | HW
(mg) | HW/BW
(mg/g) |
|---|
| Control | 11 | 43.96±3.58 | 222.12±29.96 | 5.06±0.60 |
| Model | 9 | 42.37±4.18 | 227.30±29.34 | 5.36±0.43 |
| Sodium
hydrosulfide | 10 | 42.60±3.25 | 221.20±36.94 | 5.18±0.73 |
|
L-propargylglycine | 9 | 40.97±3.10 | 227.37±24.86 | 5.57±0.71 |
Effect of H2S on
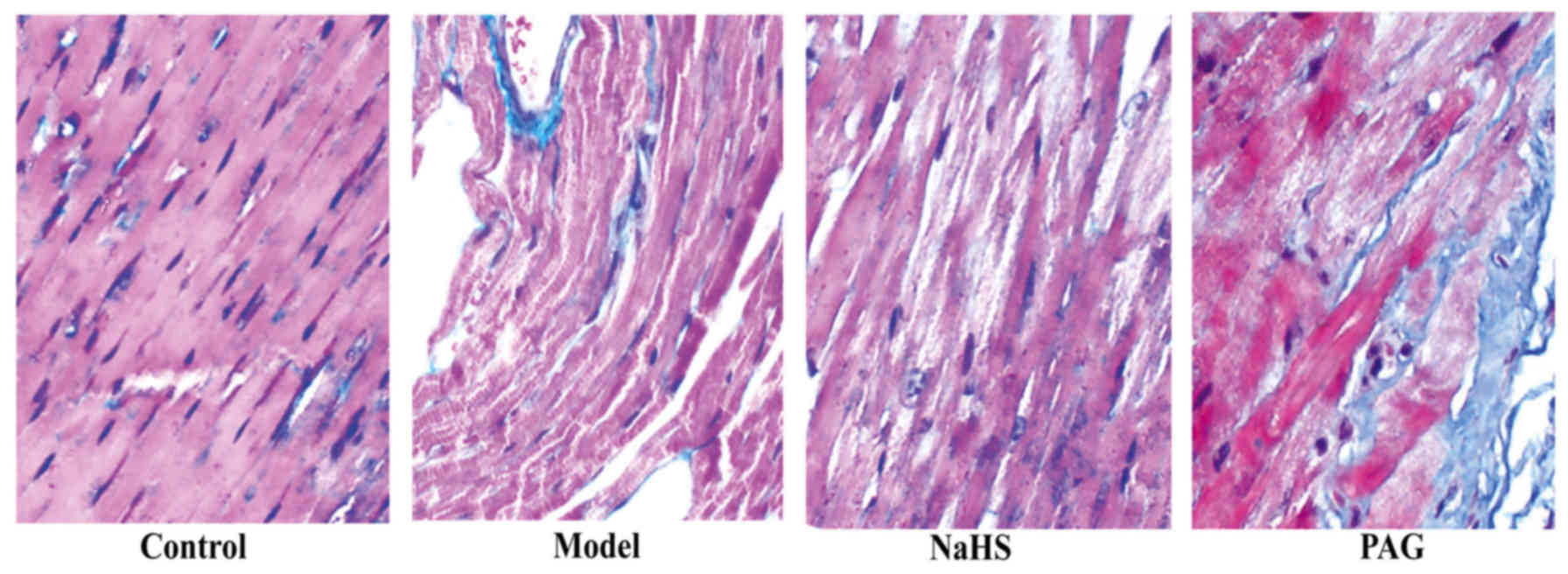
histopathological changes induced by ACM
In order to observe histopathological changes in
myocardial tissue samples, Masson's staining was used and the blue
staining indicated collagenous fibers in the myocardial tissue. As
reflected in Fig. 1, irregularly
arranged myocardial cells and increased fibrosis were observed in
the model group compared with the control group. The degree of
irregular arrangement of myocardial fibres and myocardial fibrosis
were significantly reduced following treatment with H2S
in the NaHS group. In addition, compared with the model group,
irregular arrangement of myocardial fibres and myocardial fibrosis
were observed in the PAG group.
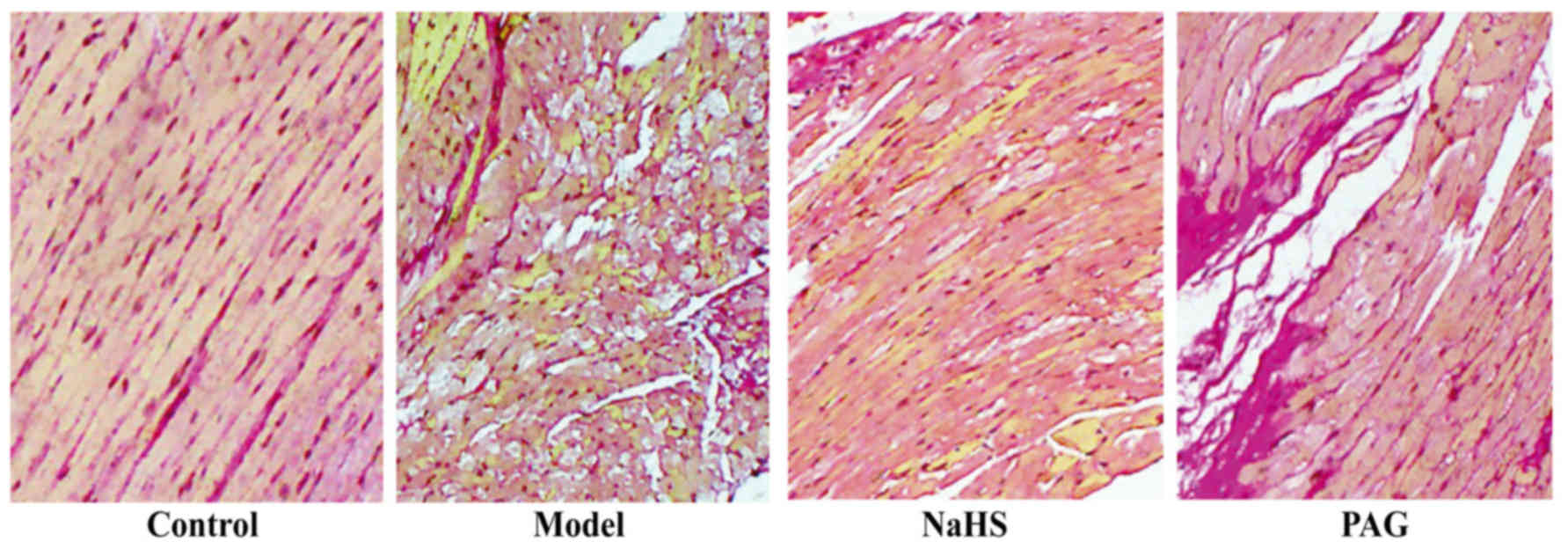
Effect of H2S on myocardial
collagen deposition
To evaluate the degree of myocardial collagen
deposition, VG staining was performed and red staining indicated
collagenous fibers in the myocardial tissue. As reflected in
Fig. 2, increased red-stained
collagenous fibers were observed in the model group compared with
the control group. In the NaHS group, myocardial collagen
deposition was decreased compared with in the model group. The
degree of myocardial collagen deposition was exacerbated in the PAG
group when compared with that in the model group.
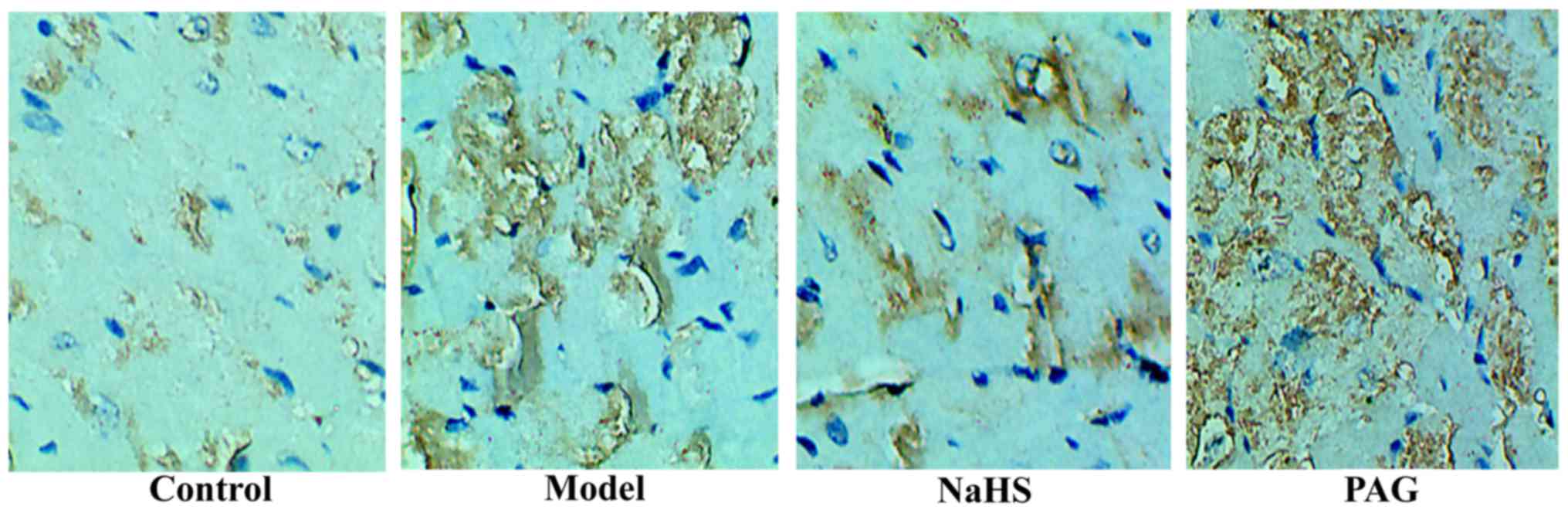
Effect of H2S on the
expression level of collagen I
The expression level of collagen I was detected by
immunohistochemistry to clarify myocardial fibrosis at the
molecular level. As reflected in Fig.
3, the expression level of collagen I was markedly increased in
the model group compared with that in the control group. Compared
with the model group, the expression level of collagen I was
markedly decreased in the NaHS group. Additionally, the expression
level of collagen I was significantly increased in the PAG group
compared with that in the model group.
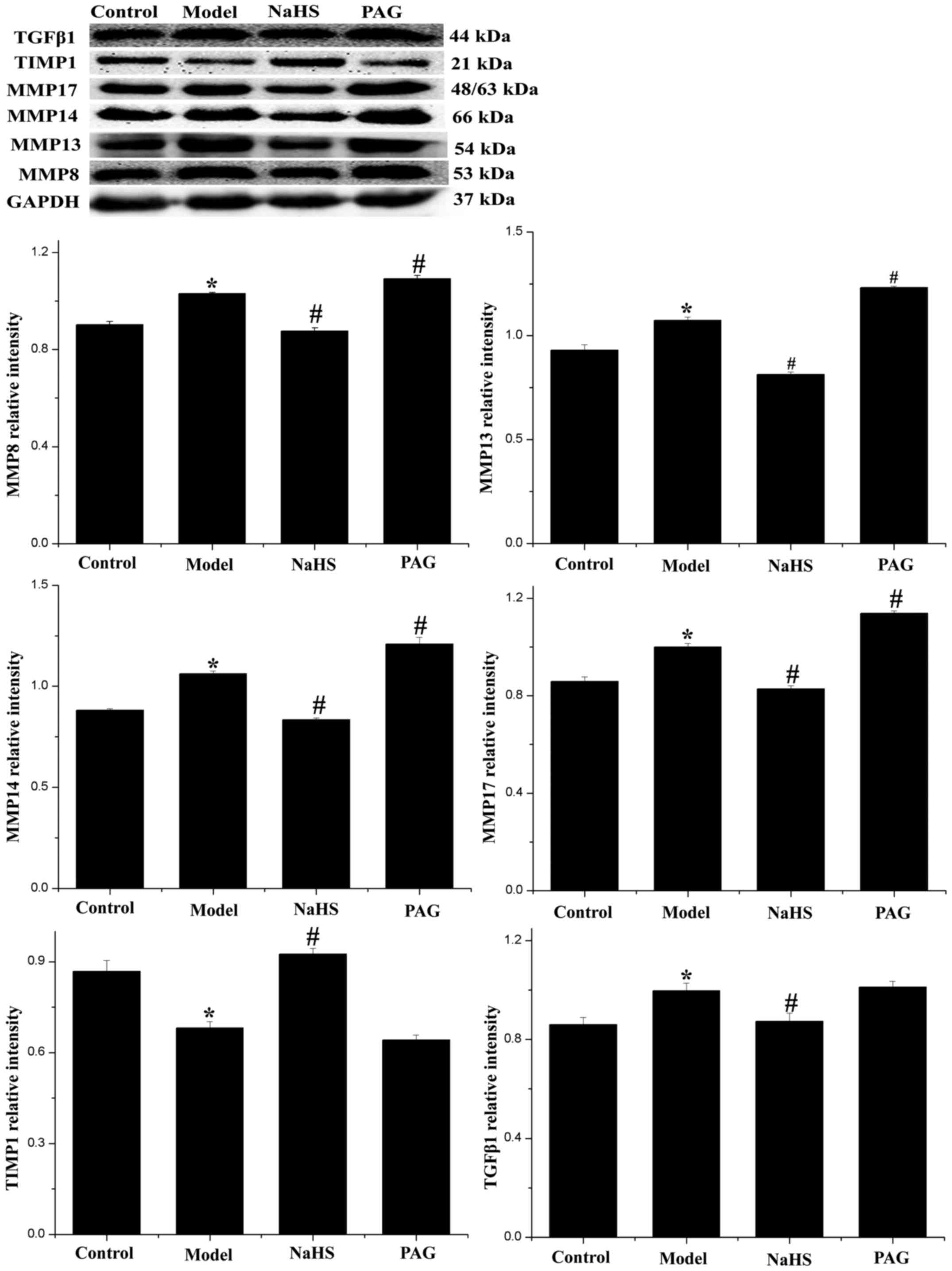
Effect of H2S on
fibrosis-associated proteins
It is generally hypothesized that the degree of
collagen synthesis and degradation is closely dependent on the
balance of MMPs and TIMPs (19,20). Furthermore, TGF-β1 is regarded as
a protein closely associated with myocardial fibrosis. For these
reasons, the expression levels of MMP8, MMP13, MMP14, MMP17, TIMP1
and TGF-β1 were detected by western blotting (Fig. 4). The results indicated that the
expression levels of MMP8, MMP13, MMP14, MMP17 and TGF-β1 were
significantly increased, while the expression level of TIMP1 was
significantly decreased in the model group, compared with the
control group (P<0.05). These changes were significantly
reversed in the NaHS group (P<0.05). Furthermore, compared with
the model group, the expression levels of MMP8, MMP13, MMP14 and
MMP17 were significantly increased (P<0.05), the expression
level of TGF-β1 was marginally increased, and the expression level
of TIMP1 was slightly decreased (P>0.05) in the PAG group.
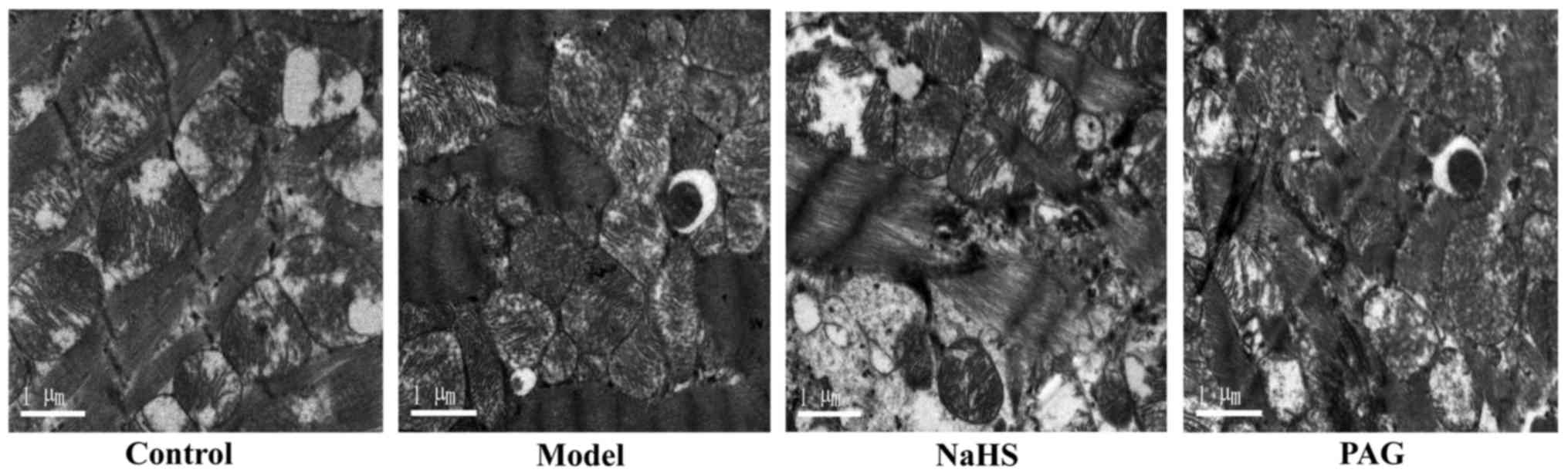
Effect of H2S on cardiomyocyte
autophagy
In order to evaluate autophagy level changes and the
effect of H2S on alcohol-induced autophagy in myocardial
tissue samples of mice with long-term alcohol intake, transmission
electron microscope was used to observe the autophagosomes in each
group. As demonstrated in Fig. 5,
autophagosomes were observed in the model and PAG groups, and no
observable autophagosomes were identified in the control and NaHS
groups.
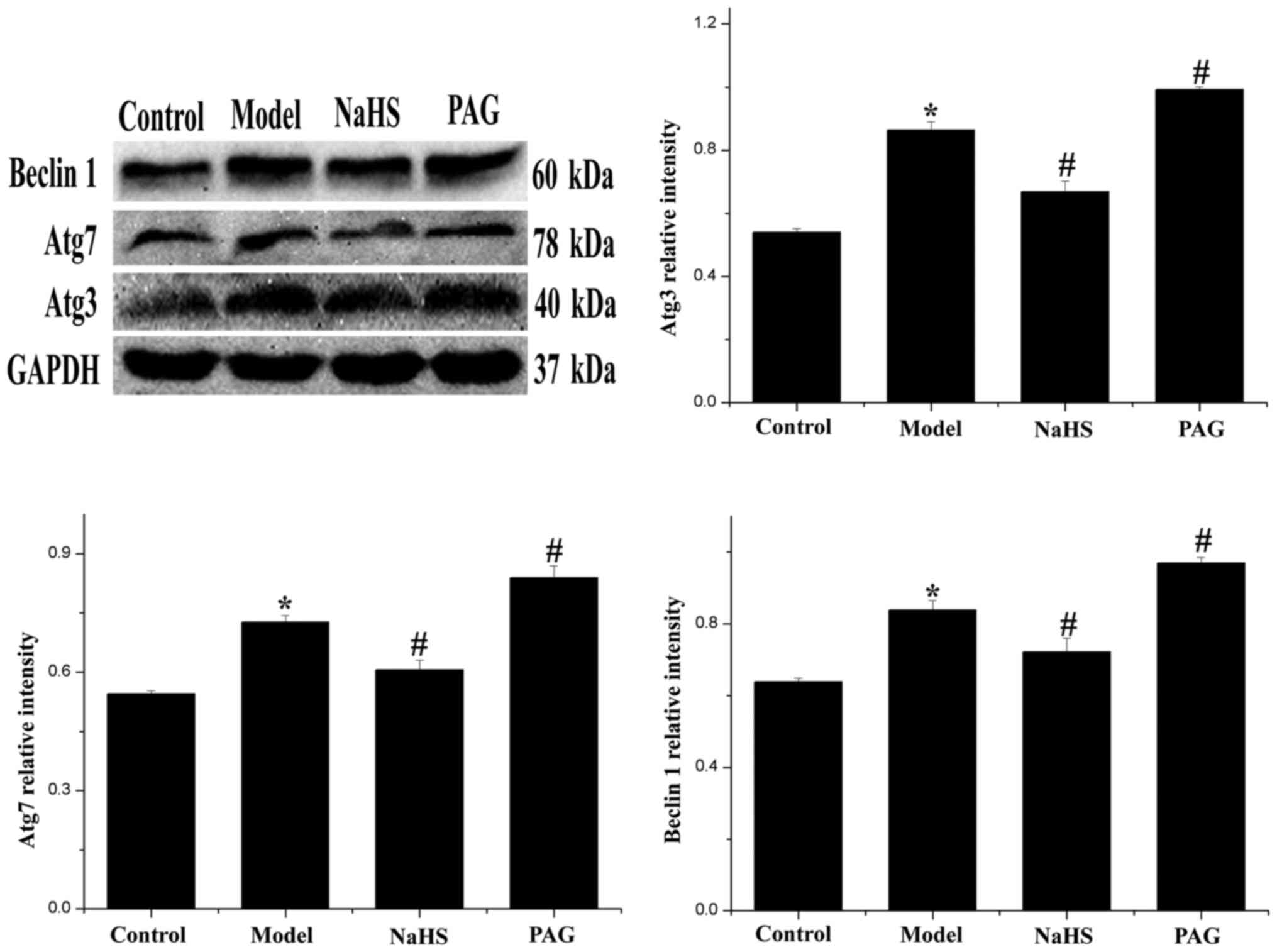
Effect of H2S on expression
levels of autophagy-associated proteins
In order to further clarify the level of autophagy
in myocardial tissue, autophagy-associated proteins, Beclin 1, Atg3
and Atg7 were detected by western blotting. As revealed in Fig. 6, expression levels of Beclin 1,
Atg3 and Atg7 were significantly increased in the model group
compared with the control group (P<0.05). After treatment with
H2S, expression levels of Beclin 1, Atg3 and Atg7 were
significantly decreased (P<0.05). Furthermore, compared with the
model group, expression levels of Beclin 1, Atg3 and Atg7 were
significantly increased in the PAG group (P<0.05).
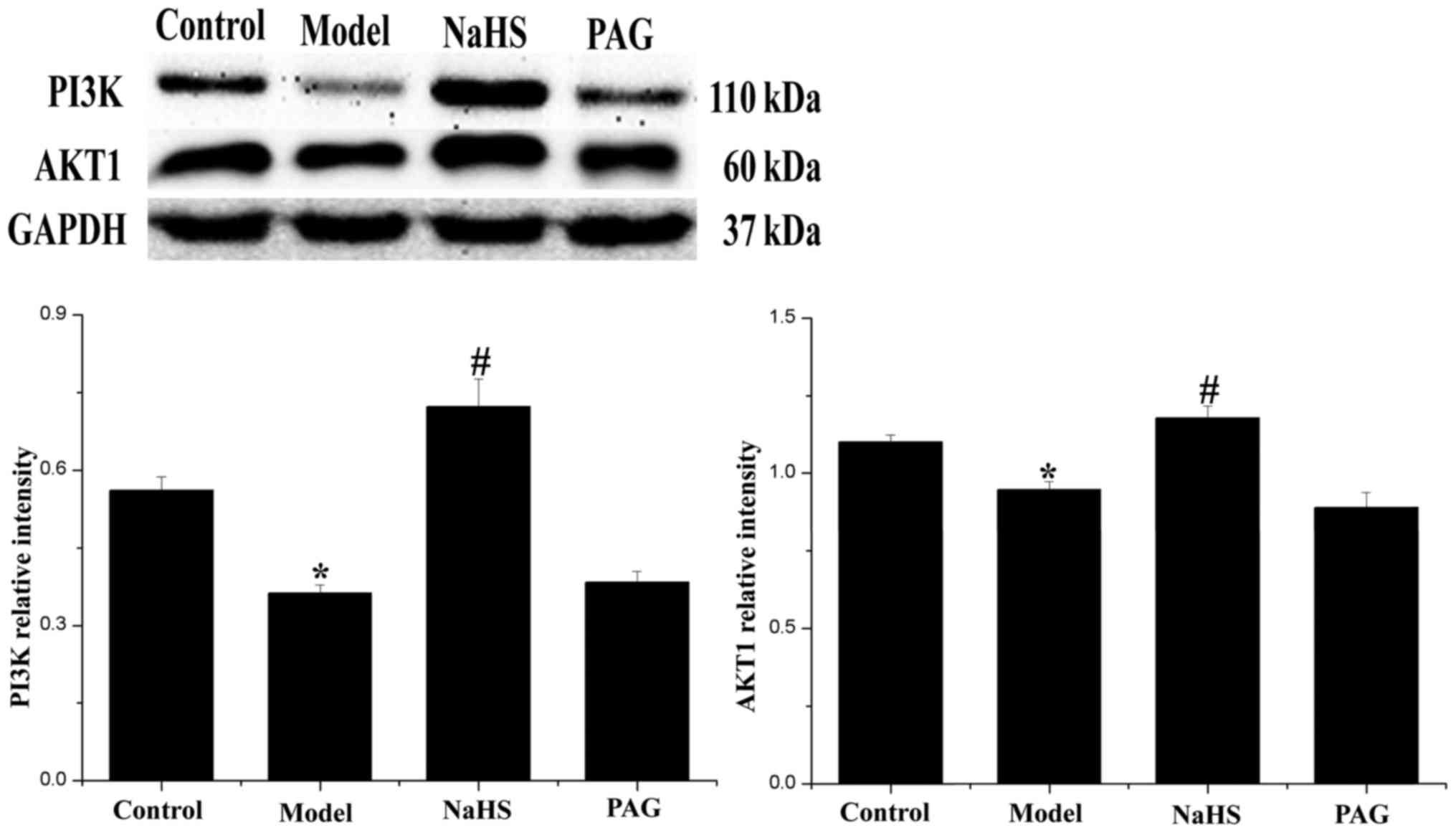
Effect of H2S on PI3K/AKT1
signaling expression
The expression levels of PI3K and AKT1 were detected
by western blotting and demonstrated PI3K/AKT1 as a classical
signaling pathway in the regulation of autophagy (Fig. 7). Compared with the control group,
the expression levels of PI3K and AKT1 were significantly decreased
in the model group (P<0.05). In addition, changes were
significantly reversed following treatment with H2S in
the NaHS group (P<0.05). Compared with the model group, the
expression levels of PI3K and AKT1 were not significantly decreased
in the PAG group (P>0.05).
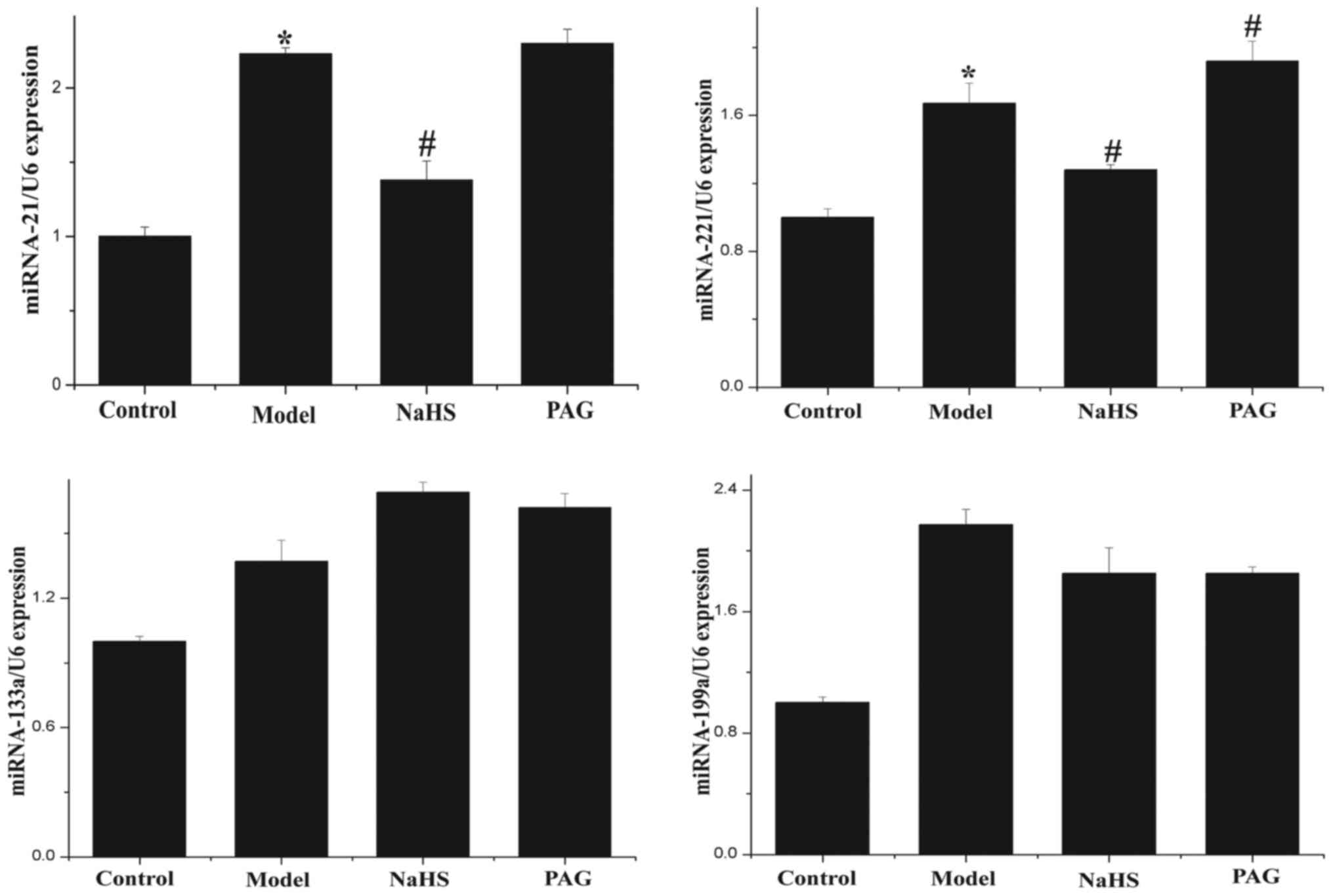
Effect of H2S on expression
levels of miR-21, miR-221, miR-133a and miR-199a
RT-qPCR was used to detect the expression levels of
miR-21, miR-221, miR-133a and miR-199a in myocardial tissue
samples. As shown in Fig. 8,
expression levels of miR-21 and miR-221 were significantly
increased in the model group compared with the control group
(P<0.05). Following treatment with H2S, the
expression levels of miR-21 and miR-221 were significantly
decreased (P<0.05). Furthermore, compared with the model group,
the level of miR-221 expression was significantly increased in the
PAG group (P<0.05). The level of miR-21 expression was
marginally greater in the PAG group than that in the model group,
although the difference was not statistically significant
(P>0.05). The differences between miR-133a and miR-199a
expression levels in each group were not identified to be
statistically significant.
Discussion
Currently, the incidence rate of ACM has been
increasing gradually as a result of wide-spread alcohol abuse,
which renders ACM a major cause for heart failure and sudden
cardiac death in clinical practices (21,22). During the occurrence and
development of ACM, various pathological changes may occur in the
myocardium, including myocardial fibrosis. Existing studies have
indicated that myocardial fibrosis is a critical link in the
pathogenesis of ACM. For example, Law et al observed the
evident myocardial fibrosis in the twelfth and 14th week after mice
were administered with 4% ethanol aqueous solution (23). In addition, previous studies
reported that pathological variations, such as cardiac hypertrophy
and myocardial fibrosis, are observed in the myocardium of certain
ACM patients with no symptoms (24). Myocardial fibrosis refers to the
accumulation of excessive collagen fibers in the myocardial
interstitium, the disproportional ratio of all types of collagens
and the disorganized arrangement of collagen fibers (25). Many types of cardiovascular
disease, such as cardiomyopathy, hypertension and coronary heart
disease, exhibit obvious myocardial fibrosis in the advanced stage
of the disease course, which is one of the major pathological
features of myocardial remodeling. Myocardial remodeling induced by
myocardial fibrosis is closely associated with the pathogenesis of
cardiac insufficiency and cardiac arrhythmia, and is, therefore, a
key link determining the disease outcome and an important
therapeutic target. In the present study, myocardial fibers were
observed to be disorganized in mice with chronic alcohol intake in
large quantities, and evident myocardial collagen accumulation was
also identified in the myocardial tissues of mice in the model
group according to the Masson's staining and VG staining. In
addition, immunohistochemistry revealed that the expression level
of collagen I in the model group was significantly increased
compared with that in the control group. MMPs are an important
factor regulating the collagen accumulation of extracellular matrix
collagen, and TIMPs, as the specific inhibitors of MMPs, may
regulate the activity of MMPs, and the two jointly contribute to
regulating the synthesis and degradation of collagen in myocardial
tissues (26). Existing studies
have revealed that upregulated expression levels of MMPs and
downregulated expression levels of TIMPs may result in the
synthesis of collagen in the myocardial interstitium and increase
accumulation, further leading to the occurrence of myocardial
fibrosis (27–29). In the present study, at the
molecular level, the results indicated that protein expression
levels of MMP8, MMP13, MMP14 and MMP17 were significantly elevated
in the myocardial tissue samples of mice in the model group, and
the protein expression level of TIMP1 was markedly decreased,
indicating that the disproportionate MMPs/TIMP1 contributed to the
occurrence of myocardial fibrosis in mice with chronic alcohol
intake in large quantities.
H2S, the third most common internal gas
signaling molecule and, like Co and No, is characterized as a type
of inorganic gas molecule with liposolubility that freely
penetrates the cell membrane. Endogenous H2S is
generated by L-cysteine under the catalytic effect of pyridoxal
phosphate-5-phosphate-dependent enzyme, although endogenous
H2S is usually produced by L-cysteine catalyzed by
cystathionine γ-lyase (CSE). It has been confirmed that
H2S participates in the regulation of various
physiological and pathological processes of the cardiovascular
system, and certain studies revealed that H2S exerts an
important protective role in many cardiac injury models, including
viral myocarditis, myocardial ischemia reperfusion injury and
diabetic cardiomyopathy (30–33). In addition, a previous study
reported that H2S alleviates the remodeling of the left
ventricle in rats with a history of long-term alcohol intake
(34). In the present study,
taking NaHS as the donor of H2S, an intervention was
performed using H2S against myocardial fibrosis in mice
with chronic alcohol intake in large quantities. The results
demonstrated that, following intervention with exogenous
H2S, a significant alleviation in the accumulation of
collagen fibers was observed in the myocardium, and obvious
decreases were identified in the expression level of collagen I,
and the expression levels of MMP8, MMP13, MMP14 and MMP17 were
significantly downregulated. In addition, the expression level of
TIMP1 was upregulated, indicating a marked improvement in the
disproportionate MMPs/TIMP1. By contrast, PAG, the irreversible
inhibitor of CSE, was used to inhibit the generation of endogenous
H2S (35), and
following the intervention with PAG in mice with chronic alcohol
intake in large quantities in the PAG group, the accumulation of
collagen fibers in the myocardium was identified to be severely
exacerbated, the expression of type-I collagen was upregulated, and
the disproportionate tendency between MMPs and TIMP1 became more
evident. These results indicate that H2S, a gaseous
signaling molecule, may be involved in the regulation of ACM.
Autophagy has been demonstrated to be involved in
various physiological and pathological processes. Autophagy is a
key mechanism for the maintenance of cellular homeostasis, by which
damaged organelles and unused proteins are destroyed and recycled
to relieve cellular stress and provide nutrients to promote cell
survival (36). When excessive
autophagy destroys the cytosol and organelles beyond a certain
threshold, autophagic cell death is induced, together with
apoptosis and necrosis (37).
Matsui et al found that the process of autophagy is
activated in response to energy crisis and oxidative stress under
the condition of cardiac ischemia reperfusion injury. Although
autophagy is protective during ischemia, it is detrimental during
reperfusion (38). The roles of
autophagy in the occurrence and development of ACM, i.e.,
protecting the cells from injuries or exacerbating the injuries,
predominantly depend on the position of autophagy and the disease
course, which is subsequently dependent on the profile and the
strength of stimulations given to cells (39). Overwhelming autophagy may cause
the death of autophagic cells. The loss of myocardial cells and the
augmentation of components in the extracellular matrix could
further facilitate pathological myocardial remodeling. A previous
study demonstrated that the autophagy level was significantly
elevated in the myocardium of transgenic mice that were
overexpressing Beclin 1 and under pressure-overload, and may also
cause pathological myocardial remodeling, although inhibiting
autophagy may markedly alleviate myocardial remodeling (40). In addition, Miyata et al
(41) identified that granulocyte
colony-stimulating factor may decrease fibrosis in the myocardial
interstitium by suppressing the autophagy level in myocardial
cells. These studies indicated that the upregulation of autophagy
is closely correlated with myocardial remodeling. A recent study
reported the association between autophagy and ACM, and that the
expression levels of LC3-II and Atg7, the autophagy-associated
proteins, were significantly elevated in the heart tissues of
murine with a history of long-term alcohol intake (11). Yet, H2S was also
reported by previous studies to participate in the regulation of
cell autophagy (42). In
addition, H2S has been identified to remit myocardial
ischemia reperfusion injury (43). While in the study performed by
Zhou et al, H2S improved the cardiac function of
smoking rats by downregulating cell autophagy in the myocardium
(15). Results in the present
study indicated that the levels of autophagy-associated protein
expression, such as Beclin 1, Atg3 and Atg7, of mice in the model
group with long-term alcohol intake were significantly higher than
those in the control group, and the autophagosomes were observed
under the transmission electron microscope. Expression levels of
Beclin 1, Atg3 and Atg7 were significantly decreased following
treatment with NaHS, indicating that H2S may
downregulate the autophagy that was excessively activated in the
myocardial tissues of mouse with a history of long-term alcohol
intake. These results indicated that endogenous H2S may
participate in the regulation mechanisms of ACM and myocardial
fibrosis resulting from long-term alcohol intake by regulating cell
autophagy.
It has been found that the PI3K/AKT1 signaling
pathway and TGF-β signaling pathway are involved in the regulation
of myocardial fibrosis and closely associated with the regulation
mechanism of autophagy (44). Lin
et al revealed that basic fibroblast growth factor (bFGF)
improved heart function recovery and increased the survival of
cardiomyocytes in a myocardial ischemia/reperfusion (I/R) model.
The role of bFGF in myocardial I/R recovery is associated with the
inhibition of excessive autophagy via activation of the
PI3K/Akt/mTOR signaling (45).
Wang et al found that HDL ameliorated mechanical
stress-induced cardiac hypertrophy and autophagy via Akt-dependent
mechanism (46). These results
indicate that the regulatory mechanism of autophagy is closely
associated with PI3K/AKT signaling pathway. A vital checkpoint that
negatively regulates autophagy is the mechanistic target of
rapamycin (mTOR), which is the downstream target of the PI3K/AKT
signaling pathway. Downregulation of the PI3K/AKT1 signaling
pathway has been demonstrated to activate autophagy (47). In the present study, the PI3K/AKT1
signaling pathway was markedly inhibited in the myocardium of the
model group, whereas H2S treatment was observed to
activate PI3K/AKT1 in mice following chronic alcohol exposure,
which indicated that H2S may protect against cardiac
autophagy induced by chronic alcohol exposure via regulation of the
PI3K/AKT1 signaling pathway. In the present study, the results
indicated that a significant decrease was identified in the
expression levels of PI3K/AKT in the myocardium of mice in the PAG
group, which was similar to the model group, indicating that
regulation of H2S in autophagy, via the PI3K/AKT
signaling pathway, may be the major mechanism by which the
myocardium could be protected from the fibrosis induced by
long-term alcohol intake. Similar with PI3K/AKT, TGF-β1 is also
critical in the pathogenesis of myocardial fibrosis and the two are
common signaling pathways involved in the regulatory mechanism of
autophagy. TGF-β1 is central in the pathogenesis of myocardial
fibrosis. TGF-β1 promotes the occurrence of myocardial fibrosis by
activating fibroblast hyperplasia and promoting the accumulation of
collagen in the extracellular matrix (48). A previous study identified that
the expression level of TGF-β1 was significantly increased in the
myocardium of a chronic iron overloaded mouse model with myocardial
fibrosis (49), and a
significantly increased expression level of TGF-β1 was identified
in the myocardial fibrosis induced by homocysteine (50). Upregulation of TGF-β1 may result
in widespread myocardial fibrosis with the induction of autophagy
(51). The results in the present
study indicated that the mechanism of myocardial interstitial
fibrosis in mice following chronic alcohol exposure may be
associated with the activation of TGF-β1 signaling pathways as the
autophagy level increases.
miRNAs are a class of naturally occurring,
endogenous small non-coding RNA molecules that provide a mechanism
for negative regulation of mRNA translation into proteins. miRNAs
are distinct from, but associated with siRNAs and regulate their
targets by either inhibiting mRNA translation or promoting mRNA
degradation (52). Previous
studies have indicated that miRNAs are essential in a number of
biological processes, including proliferation, differentiation,
apoptosis and development (53–55). Furthermore, the dysregulation of
miRNAs has been linked to various pathological settings and their
roles in cardiovascular diseases have been an area of intense
investigation (56). A variety of
studies have confirmed that miRNAs perform important regulatory
roles in the occurrence and development of many cardiovascular
diseases. Previous studies have identified that miRNAs are key
regulators of genes involved in the pathophysiology of fibrosis in
the heart (57). Increasing
evidence has demonstrated that several miRNAs, particularly,
miR-21, miR-221, miR-133a and miR-199a, have been implicated in the
control of myocardial fibrosis and reported to be closely
associated with the mechanism of myocardial remodeling (58). Thum et al found that the
expression level of miR-21 was significantly elevated in a heart
failure model; however, silenced miR-21 may alleviate myocardial
fibrosis and improve heart function (59). In addition, further studies
revealed that miR-21 initiates the extracellular signal-regulated
kinases-mitogen-activated protein kinase signaling pathway by
inhibiting the target gene, sprouty RTK signaling antagonist 1.
This promotes the proliferation of fibroblasts and relevant
excretion of growth factors, thus accelerating the disease course
of myocardial fibrosis and myocardial remodeling. Furthermore, the
profibrogenic mechanism of miR-21 may be involved in regulating the
expression of TGF-βRIII, which is a negative regulation factor in
the TGF-β signaling pathway that inhibits the occurrence of
myocardial fibrosis. By experimenting on myocardial infarction
models, Liang et al confirmed that TGF-βRIII is the target
gene of miRNA-21 and the overexpression of miRNA-21 may inhibit the
expression of TGF-βRIII to initiate the TGF-β signaling pathway to
increase the content of collagen fibers (60). In the study by Wang et al,
it was found that overexpression of miR-221 induces the hypertrophy
of myocardial cells (61).
However, certain studies have indicated that miRNA-221 may
specifically inhibit the expression of P27 and phosphatase and
tensin homolog genes (62),
participate in the regulation of the PI3K/AKT signaling pathway and
cell autophagy (63), and
intimated that crosstalk exists between miRNA-21/miRNA-221 and
TGF-β, as well as PI3K/AKT signaling pathways, which jointly
participate in the regulation mechanism of cell autophagy and
myocardial fibrosis (64,65). The results of the present study
demonstrated that the expression levels of miR-21 and miR-221 in
the model group were significantly higher than those in the control
group; while compared with the model group, the expression levels
in the NaHS group were significantly downregulated. By contrast,
the expression level of miR-221 in the PAG group was significantly
higher than that in the model group, indicating that the
pathogenesis of ACM may be correlated with the upregulated
expression levels of miR-21 and miR-221. The abnormal expression in
miR-21 and miR-221 that regulates the miRNA may be the other
mechanism by which H2S improves myocardial fibrosis
following long-term alcohol consumption.
In conclusion, the results of the present study
indicated that H2S inhibits cell autophagy and
alleviates myocardial fibrosis by regulating the expression levels
of miR-21 and miR-221 via the TGF-β and PI3K/AKT signaling
pathways, which contributes to the research regarding the
pathogenesis of myocardial fibrosis in ACM and provides a novel
idea for the clinical treatment of ACM. However, the specific
mechanism of crosstalk between miRNA and TGF-β and PI3K/AKT
signaling pathways remains unknown in the occurrence and
development of ACM, and further studies are required to investigate
the clinical value and significance of the regulatory effect of
H2S on cell autophagy for the improvement of ACM and
myocardial fibrosis.
Acknowledgments
The present study was supported by National Natural
Science Foundation of China (grant no. 81270181).
References
|
1
|
Piano MR and Phillips SA: Alcoholic
cardiomyopathy: Pathophysiologic insights. Cardiovasc Toxicol.
14:291–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piano MR: Alcoholic cardiomyopathy:
Incidence, clinical characteristics, and pathophysiology. Chest.
121:1638–1650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Basu S, Rajakaruna S, Reyes B, Van
Bockstaele E and Menko AS: Suppression of MAPK/JNK-MTORC1 signaling
leads to premature loss of organelles and nuclei by autophagy
during terminal differentiation of lens fiber cells. Autophagy.
10:1193–1211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gottlieb RA and Mentzer RM Jr: Autophagy:
An affair of the heart. Heart Fail Rev. 18:575–584. 2013.
View Article : Google Scholar :
|
|
5
|
Jian J, Xuan F, Qin F and Huang R:
Bauhinia championii flavone inhibits apoptosis and autophagy via
the PI3K/Akt pathway in myocardial ischemia/reperfusion injury in
rats. Drug Des Devel Ther. 9:5933–5945. 2015.PubMed/NCBI
|
|
6
|
Younce CW, Wang K and Kolattukudy PE:
Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1
production and induction of a novel zinc-finger protein MCPIP.
Cardiovasc Res. 87:665–674. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu H, Rothermel BA and Hill JA: Autophagy
in load-induced heart disease. Methods Enzymol. 453:343–363. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma S, Wang Y, Chen Y and Cao F: The role
of the autophagy in myocardial ischemia/reperfusion injury. Biochim
Biophys Acta. 1852:271–276. 2015. View Article : Google Scholar
|
|
9
|
Jie X, Qin X, Cai X, Yang L, Xing Y, Li J,
Zhang L, Tang Y, Liu J, Zhang X, et al: Mitochondrial JNK
activation triggers autophagy and apoptosis and aggravates
myocardial injury following ischemia/reperfusion. Biochim Biophys
Acta. 1852:262–270. 2015. View Article : Google Scholar
|
|
10
|
Zhang L, Ding WY, Wang ZH, Tang MX, Wang
F, Li Y, Zhong M, Zhang Y and Zhang W: Early administration of
trimetazidine attenuates diabetic cardiomyopathy in rats by
alleviating fibrosis, reducing apoptosis and enhancing autophagy. J
Transl Med. 14:109–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo R, Hu N, Kandadi MR and Ren J:
Facilitated ethanol metabolism promotes cardiomyocyte contractile
dysfunction through autophagy in murine hearts. Autophagy.
8:593–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Rooij E, Sutherland LB, Liu N,
Williams AH, McAnally J, Gerard RD, Richardson JA and Olson EN: A
signature pattern of stress-responsive microRNAs that can evoke
cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A.
103:18255–18260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jing L, Jin C, Lu Y, Huo P, Zhou L, Wang Y
and Tian Y: Investigation of microRNA expression profiles
associated with human alcoholic cardiomyopathy. Cardiology.
130:223–233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calvert JW, Coetzee WA and Lefer DJ: Novel
insights into hydrogen sulfide - mediated cytoprotection. Antioxid
Redox Signal. 12:1203–1217. 2010. View Article : Google Scholar :
|
|
15
|
Zhou X, An G and Chen J: Hydrogen sulfide
improves left ventricular function in smoking rats via regulation
of apoptosis and autophagy. Apoptosis. 19:998–1005. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang M, Shan H, Chang P, Wang T, Dong W,
Chen X and Tao L: Hydrogen sulfide offers neuroprotection on
traumatic brain injury in parallel with reduced apoptosis and
autophagy in mice. PLoS One. 9:e872412014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toldo S, Das A, Mezzaroma E, Chau VQ,
Marchetti C, Durrant D, Samidurai A, Van Tassell BW, Yin C, Ockaili
RA, et al: Induction of microRNA-21 with exogenous hydrogen sulfide
attenuates myocardial ischemic and inflammatory injury in mice.
Circ Cardiovasc Genet. 7:311–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ge W and Ren J: mTOR-STAT3-notch
signalling contributes to ALDH2-induced protection against cardiac
contractile dysfunction and autophagy under alcoholism. J Cell Mol
Med. 16:616–626. 2012. View Article : Google Scholar
|
|
19
|
Guo H, Sa Y, Huang J, Wang Z, Wang L, Xie
M and Lv X: Urethral reconstruction with small intestinal submucosa
seeded with oral keratinocytes and TIMP-1 siRNA transfected
fibroblasts in a rabbit model. Urol Int. 96:223–230. 2016.
View Article : Google Scholar
|
|
20
|
Johnson KM and Crocker SJ: TIMP-1 couples
RhoK activation to IL-1β-induced astrocyte responses. Neurosci
Lett. 609:165–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schoppet M and Maisch B: Alcohol and the
heart. Herz. 26:345–352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laurent D and Edwards JG: Alcoholic
cardiomyopathy: Multigenic changes underlie cardiovascular
dysfunction. J Cardiol Clin Res. 2:10222014.PubMed/NCBI
|
|
23
|
Law BA, Levick SP and Carver WE:
Alterations in cardiac structure and function in a murine model of
chronic alcohol consumption. Microsc Microanal. 18:453–461. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dancy M and Maxwell JD: Alcohol and
dilated cardiomyopathy. Alcohol Alcohol. 21:185–198.
1986.PubMed/NCBI
|
|
25
|
Wang Y, Wu Y, Chen J, Zhao S and Li H:
Pirfenidone attenuates cardiac fibrosis in a mouse model of
TAC-induced left ventricular remodeling by suppressing NLRP3
inflammasome formation. Cardiology. 126:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
An Z, Yang G, He YQ, Dong N, Ge LL, Li SM
and Zhang WQ: Atorvastatin reduces myocardial fibrosis in a rat
model with post-myocardial infarction heart failure by increasing
the matrix metalloproteinase-2/tissue matrix metalloproteinase
inhibitor-2 ratio. Chin Med J (Engl). 126:2149–2156. 2013.
|
|
27
|
Dixon IM, Ju H, Reid NL, Scammell-La Fleur
T, Werner JP and Jasmin G: Cardiac collagen remodeling in the
cardiomyopathic Syrian hamster and the effect of losartan. J Mol
Cell Cardiol. 29:1837–1850. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cowan KN, Jones PL and Rabinovitch M:
Regression of hypertrophied rat pulmonary arteries in organ culture
is associated with suppression of proteolytic activity, inhibition
of tenascin-C, and smooth muscle cell apoptosis. Circ Res.
84:1223–1233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Polyakova V, Loeffler I, Hein S, Miyagawa
S, Piotrowska I, Dammer S, Risteli J, Schaper J and Kostin S:
Fibrosis in endstage human heart failure: Severe changes in
collagen metabolism and MMP/TIMP profiles. Int J Cardiol.
151:18–33. 2011. View Article : Google Scholar
|
|
30
|
Elrod JW, Calvert JW, Morrison J, Doeller
JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, et al:
Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury
by preservation of mitochondrial function. Proc Natl Acad Sci U S
A. 104:15560–15565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
King AL and Lefer DJ: Cytoprotective
actions of hydrogen sulfide in ischaemia-reperfusion injury. Exp
Physiol. 96:840–846. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qian H and Liu L: Protective effect of
hydrogen sulfide on mice with experimental viral myocarditis and
its mechanism. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 30:708–712.
2014.In Chinese. PubMed/NCBI
|
|
33
|
Zhou X, An G and Lu X: Hydrogen sulfide
attenuates the development of diabetic cardiomyopathy. Clin Sci
(Lond). 128:325–335. 2015. View Article : Google Scholar
|
|
34
|
Zhou X, Lu X, Xu W and Chen J: Protective
effects of hydrogen sulfide against chronic alcohol intake-induced
left ventricular remodeling in rats. Cardiovasc Drugs Ther.
27:221–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Awata S, Nakayama K, Suzuki I and Kodama
H: Effect of cysteine on the inactivation of cystathionine
gamma-lyase by D, L-propargylglycine. Acta Med Okayama. 43:329–335.
1989.PubMed/NCBI
|
|
36
|
Ashford TP and Porter KR: Cytoplasmic
components in hepatic cell lysosomes. J Cell Biol. 12:198–202.
1962. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zeglinski MR, Davies JJ, Ghavami S, Rattan
SG, Halayko AJ and Dixon IM: Chronic expression of Ski induces
apoptosis and represses autophagy in cardiac myofibroblasts.
Biochim Biophys Acta. 1863(6 Pt A): 1261–1268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matsui Y, Takagi H, Qu X, Abdellatif M,
Sakoda H, Asano T, Levine B and Sadoshima J: Distinct roles of
autophagy in the heart during ischemia and reperfusion: Roles of
AMP-activated protein kinase and Beclin 1 in mediating autophagy.
Circ Res. 100:914–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baehrecke EH: Autophagy: Dual roles in
life and death? Nat Rev Mol Cell Biol. 6:505–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu H, Tannous P, Johnstone JL, Kong Y,
Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA and Hill
JA: Cardiac autophagy is a maladaptive response to hemodynamic
stress. J Clin Invest. 117:1782–1793. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Miyata S, Takemura G, Kawase Y, Li Y,
Okada H, Maruyama R, Ushikoshi H, Esaki M, Kanamori H, Li L, et al:
Autophagic cardiomyocyte death in cardiomyopathic hamsters and its
prevention by granulocyte colony-stimulating factor. Am J Pathol.
168:386–397. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xiao T, Luo J, Wu Z, Li F, Zeng O and Yang
J: Effects of hydrogen sulfide on myocardial fibrosis and
PI3K/AKT1-regulated autophagy in diabetic rats. Mol Med Rep.
13:1765–1773. 2016. View Article : Google Scholar
|
|
43
|
Shui M, Liu X, Zhu Y and Wang Y: Exogenous
hydrogen sulfide attenuates cerebral ischemia-reperfusion injury by
inhibiting autophagy in mice. Can J Physiol Pharmacol. 22:1–6.
2016.
|
|
44
|
Sun L, Jin H, Sun L, Chen S, Huang Y, Liu
J, Li Z, Zhao M, Sun Y, Tang C, et al: Hydrogen sulfide alleviates
myocardial collagen remodeling in association with inhibition of
TGF-β/Smad signaling pathway in spontaneously hypertensive rats.
Mol Med. 20:503–515. 2015.
|
|
45
|
Lin L, Liu X, Xu J, Weng L, Ren J, Ge J
and Zou Y: High-density lipoprotein inhibits mechanical
stress-induced cardiomyocyte autophagy and cardiac hypertrophy
through angiotensin II type 1 receptor-mediated PI3K/Akt pathway. J
Cell Mol Med. 19:1929–1938. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang ZG, Wang Y, Huang Y, Lu Q, Zheng L,
Hu D, Feng WK, Liu YL, Ji KT, Zhang HY, et al: bFGF regulates
autophagy and ubiquitinated protein accumulation induced by
myocardial ischemia/reperfusion via the activation of the
PI3K/Akt/mTOR pathway. Sci Rep. 5:92872015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dany M, Rimmani HH, Matar SA and Hajj
Hussein I: mTORC2-Akt signaling axis is implicated in myocardial
compensation and fibrosis. J Biol Regul Homeost Agents. 29:745–753.
2015.
|
|
48
|
Usunier B, Benderitter M, Tamarat R and
Chapel A: Management of fibrosis: The mesenchymal stromal cells
breakthrough. Stem Cells Int. 2014:3402572014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Wang H, Cui L, Zhang Y, Liu Y,
Chu X, Liu Z, Zhang J and Chu L: Continuing treatment with Salvia
miltiorrhiza injection attenuates myocardial fibrosis in chronic
iron-overloaded mice. PLoS One. 10:e01240612015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Raaf L, Noll C, Cherifi Mel H, Samuel JL,
Delcayre C, Delabar JM, Benazzoug Y and Janel N: Myocardial
fibrosis and TGFB expression in hyperhomocysteinemic rats. Mol Cell
Biochem. 347:63–70. 2011. View Article : Google Scholar
|
|
51
|
Araki S, Izumiya Y, Rokutanda T, Ianni A,
Hanatani S, Kimura Y, Onoue Y, Senokuchi T, Yoshizawa T, Yasuda O,
et al: Sirt7 contributes to myocardial tissue repair by maintaining
transforming growth factor-β signaling pathway. Circulation.
132:1081–1093. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Condorelli G, Latronico MV and Dorn GW II:
MicroRNAs in heart disease: Putative novel therapeutic targets? Eur
Heart J. 31:649–658. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang M, Wang Y, Zang W, Wang H, Chu H, Li
P, Li M, Zhang G and Zhao G: Downregulation of microRNA-182
inhibits cell growth and invasion by targeting programmed cell
death 4 in human lung adenocarcinoma cells. Tumour Biol. 35:39–46.
2014. View Article : Google Scholar
|
|
54
|
Chen C, Jia KY, Zhang HL and Fu J: miR-195
enhances cardio-myocyte apoptosis induced by hypoxia/reoxygenation
injury via downregulating c-myb. Eur Rev Med Pharmacol Sci.
20:3410–3416. 2016.PubMed/NCBI
|
|
55
|
Yu J, Wu SW and Wu WP: A tumor-suppressive
microRNA, miRNA-485-5p inhibits glioma cell proliferation and
invasion by down-regulating TPD52L2. Am J Transl Res. 9:3336–3344.
2017.
|
|
56
|
Nouraee N and Mowla SJ: miRNA therapeutics
in cardiovascular diseases: Promises and problems. Front Genet.
6:2322015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Iacopo F, Lorenzo C, Calogero E, Matteo P,
Riccardo PN, Veronica S, Valentina B, Riccardo L, Cristian S, Maria
MC, et al: Review in translational cardiology: MicroRNAs and
myocardial fibrosis in aortic valve stenosis, a deep insight on
left ventricular remodeling. J Cardiovasc Echogr. 26:109–114. 2016.
View Article : Google Scholar
|
|
58
|
Chen S, Puthanveetil P, Feng B, Matkovich
SJ, Dorn GW II and Chakrabarti S: Cardiac miR-133a overexpression
prevents early cardiac fibrosis in diabetes. J Cell Mol Med.
18:415–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Thum T, Gross C, Fiedler J, Fischer T,
Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, et
al: MicroRNA-21 contributes to myocardial disease by stimulating
MAP kinase signalling in fibroblasts. Nature. 456:980–984. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liang H, Zhang C, Ban T, Liu Y, Mei L,
Piao X, Zhao D, Lu Y, Chu W and Yang B: A novel reciprocal loop
between micro- RNA-21 and TGFβRIII is involved in cardiac fibrosis.
Int J Biochem Cell Biol. 44:2152–2160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang C, Wang S, Zhao P, Wang X, Wang J,
Wang Y, Song L, Zou Y and Hui R: miR-221 promotes cardiac
hypertrophy in vitro through the modulation of p27 expression. J
Cell Biochem. 113:2040–2046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Garofalo M, Di Leva G, Romano G, Nuovo G,
Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P,
et al: miR-221&222 regulate TRAIL resistance and enhance
tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell.
16:498–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cheng M, Wu G, Song Y, Wang L, Tu L, Zhang
L and Zhang C: Celastrol-induced suppression of the miR-21/ERK
signalling pathway attenuates cardiac fibrosis and dysfunction.
Cell Physiol Biochem. 38:1928–1938. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yu X, Li R, Shi W, Jiang T, Wang Y, Li C
and Qu X: Silencing of microRNA-21 confers the sensitivity to
tamoxifen and fulvestrant by enhancing autophagic cell death
through inhibition of the PI3K-AKT-mTOR pathway in breast cancer
cells. Biomed Pharmacother. 77:37–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen Q, Zhou Y, Richards AM and Wang P:
Up-regulation of miRNA-221 inhibits hypoxia/reoxygenation-induced
autophagy through the DDIT4/mTORC1 and Tp53inp1/p62 pathways.
Biochem Biophys Res Commun. 474:168–174. 2016. View Article : Google Scholar : PubMed/NCBI
|