Introduction
The reactive oxygen species (ROS) generated by
oxidative stress in the central nervous system (CNS) contribute to
the pathogenesis of neurodegenerative diseases, including
Parkinson's disease, Alzheimer's disease and amyotrophic lateral
sclerosis (1). Several effective
factors have been reported to protect neurons and attenuate
neuroinflammation against oxidative stress (1,2).
However, novel therapeutic strategies are required for the clinical
application of neuroprotective factors.
Several previous studies have demonstrated a role
for mesenchymal stem cells (MSCs) in promoting neuronal survival
and the recovery of pathological symptoms in stroke, spinal cord
injury and Parkinson's disease (3). In addition, experimental data have
demonstrated that trophic factors released from MSCs have a
therapeutic effect, termed a 'paracrine effect', promoting tissue
repair, including neuronal survival, differentiation, axonal
regeneration and endogenous angiogenesis (4).
In our previous study, the secretion of various
growth factors from human MSCs (hMSCs) were analyzed, with vascular
endothelial growth factor (VEGF), hepatocyte growth factor (HGF),
stem cell factor (SCF) and members of the insulin-like growth
factor binding protein (IGFBP) family observed. In addition, the
expression of IGFBP-4 and -6 was significantly enhanced in
hMSC-conditioned medium (hMSC-CM) (5). A total of six distinct IGFBPs,
designated IGFBP-1-6, function as carrier proteins for IGFs, and
modulate IGF activities, including cell survival, proliferation,
migration and differentiation in various cell types (6). IGFBPs are also important in
biological functions by IGF-independent mechanisms (7). Despite their sequence homology,
individual IGFBPs have distinct gene products and functional
properties (8). IGFBP-6 is
expressed in a variety of tissues, including the CNS, and its
expression is developmentally regulated. In several cell lines,
IGFBP-6 predominantly binds to IGF-2 and inhibits its function
(9). However, the effect of
IGFBP-6 on IGF-1 function remains to be fully elucidated,
particularly in non-cancer cells. IGF-1 is a widely studied
survival factor secreted from hMSCs and is involved in IGF-1
receptor (IGF-1R) signaling (10).
In the present study, a rat primary cortical neuron
culture was used to examine the effect of IGFBP-6 released from
hMSCs on neuronal cell death. The results suggested that IGFBP-6
was important in neuronal survival through activation of the Akt-
and IGF-1R-mediated signaling pathway.
Materials and methods
hMSC culture
Cryopreserved adult bone marrow-derived hMSCs were
purchased from Cambrex Bioscience (Walkersville, MD, USA). The
hMSCs (passages 4–10) were cultured in Dulbecco's modified Eagle's
medium (DMEM)-low glucose containing 10% fetal bovine serum (FBS)
(both from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
at 37°C with 5% CO2.
Primary cortical neuron-enriched cultures
and H2O2 treatment
All procedures were performed with the approval of
the Institutional Animal Care and Use Committee issued by Seoul
National University (Seoul, Korea). Primary cortical
neuron-enriched cultures were prepared from the cerebral cortices
of E17-day-old Sprague-Dawley rat embryos. In brief, the cortical
tissue was dissociated and cells were seeded in neurobasal-A medium
(NB; Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS.
After 24 h, the medium was replaced with NB containing 2% B27
(Gibco; Thermo Fisher Scientific, Inc.). These primary cortical
neuron-enriched cultures were maintained for 7–10 days, with the
medium replaced every 3 days. The primary cortical neurons were
prepared in 24-well tissue culture plates for
H2O2 treatment, based on a previously
reported method (8), in
serum-free NB containing 15 µM H2O2
for 15 min (Fig. 1A). The medium
was then replaced with serum-free NB or CM and cultured for 24 h at
37°C.
hMSC-CM
The hMSCs (5×104 cells/100 mm/dish) were
washed twice with phosphate-buffered saline (PBS; pH 7.4) and the
medium was replaced with serum-free NB. After 18 h, the medium was
collected and added to the H2O2-treated
primary cortical neuron-enriched cultures. For the IGFBP-6
inhibition experiment, 30 µg/ml of anti-IGFBP-6 antibody
(cat. no. MAB8762; R&D Systems, Inc., Minneapolis, MN, USA) was
added for pre-incubation with the hMSC-CM for 30 min at 37°C
(11).
PD98059, wortmannin and picropodophyllin
treatment
The H2O2-treated primary
cortical neuron-enriched cultures were pre-incubated with PD98059
(50 µM; Promega Corp., Madison, WI, USA), wortmannin (100
nM; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), or
picropodophyllin (PPP, 500 nM; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) diluted in the hMSC-CM for 15, 30 or 15 min at
37°C, respectively (12–14). Following pre-incubation with the
inhibitors, the cells were treated with 15 µM
H2O2 for 15 min.
Cell viability and apoptosis assays
Following treatment as described above, the
viability of the cells was then analyzed using the
3-(4,5-dimethylthiaziazol-3-yl)-2,5-diphenyltetrazolium bromide
(MTT) method. The cells were incubated with 1 mg/ml MTT (Sigma;
Merck Millipore) for 1 h at 37°C. The medium was carefully
aspirated, and dimethyl sulfoxide (DMSO) (150 µl) was added
to solubilize the colored formazan product. The optical density was
read at 554 nm. Terminal deoxynuceotidyl transferase dUTP nick-end
labeling (TUNEL) staining was performed using the In Situ
Cell Death Detection kit (Roche Diagnostics, Basel, Switzerland)
according to the manufacturer's protocol. In the ventral region of
the spinal cord slice cultures, the numbers of apoptotic cells were
counted (magnification, ×100). All images were captured using a
confocal laser-scanning microscope (FV300; Olympus, Tokyo,
Japan).
Immunoblotting
The primary cortical neuron-enriched cultures were
washed twice with cold PBS and lysed with RIPA buffer containing 50
mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.25% sodium
deoxycholate, 0.2 mg/ml leupeptin, 0.2 mg/ml aprotinin, 0.1 M
phenylmethylsulfonylfluoride, 1 mM Na3VO4 and
0.5 M NaF. The lysates were centrifuged at 13,500 × g for 15 min at
4°C, and 30 µg of the supernatants were loaded onto 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) gels. The membrane was blocked in Tris-buffered saline
containing 3% bovine serum albumin (BSA) for 1 h at room
temperature. It was incubated overnight at 4°C with the following
primary antibodies: Anti-Akt (1:1,000; cat. no. 9272S),
anti-phosphorylated (p)Akt (1:1,000; cat. no. 4058S) (both from
Cell Signaling Technology, Inc., Beverly, MA, USA), anti-B-cell
lymphoma 2-like protein 4 (Bax; 1:1,000; cat. no. 556467; BD
Pharmingen, San Diego, CA, USA), anti-cyclooxygenase (COX) IV
(1:1,000; cat. no. 4644S; Cell Signaling Technology, Inc.), and
anti-α-tubulin (1:5,000; cat. no. T5168; Sigma-Aldrich; Merck
Millipore). The primary antibodies were visualized using a
chemiluminescence detection system (Thermo Fisher Scientific, Inc.)
after incubation with goat anti-rabbit or anti-mouse horseradish
peroxidase-conjugated secondary antibodies (cat. nos. A0545 and
A2554; Sigma-Aldrich; Merck Millipore) for 1 h at room temperature.
Mitochondrial and cytosolic fractions were isolated with the
Mitochondrial Fraction kit (Active Motif, Carlsbad, CA, USA) for
Bax analysis. ImageJ software 1.6.0 version (NIH, Bethesda, MD,
USA) was used for quantification of immunoblotting.
Enzyme-linked immunosorbent assay (ELISA)
for IGF-1 and IGF-2
The primary rat cortical neurons in 60-mm dishes
were cultured in serum-free base medium with the additional
treatment according to the experimental groups described above.
After 30 h, the supernatant was collected and centrifuged at 135 ×
g for 5 min at room temperature. The concentrations of IGF-1 or
IGF-2 in the supernatant were quantified using a rat IGF-1 ELISA
kit or a rat IGF-2 ELISA kit (cat. nos. ab213902 and ab213903;
Abcam), respectively, according to the manufacturer's protocols.
The optical density at 450 nm was measured using a microplate
reader (Epoch2; Bio-Tek Instruments, Inc., Winooski, VT, USA).
Organotypic spinal cord slice
culture
The organotypic slice cultures were generated as
previously described (15).
Briefly, the lumbar spinal cords of 16-day-old post-natal
Sprague-Dawley rats were removed after euthanizing with
CO2 gas. Nerve roots and excess connective tissue were
then collected in cold Hank's balanced salt solution (Gibco; Thermo
Fisher Scientific, Inc.) containing 6.4 mg/ml glucose. Using a
Mcllwain tissue chopper (Mickle Laboratory Engineering, Gomshall,
UK), the spinal cords were finely cut, and four slices were
carefully placed and co-cultured on a membrane insert
(Millicell-CM; EMD Millipore, Billerica, MA, USA) for 7 days at
37°C in a 6-well plate with 1 ml of culture media containing 50%
Eagle's minimum essential medium (Gibco; Thermo Fisher Scientific,
Inc.), 6.4 mg/ml glucose and 20 mM HEPES (Sigma; Merck Millipore).
The media was replaced twice a week.
Demyelination of organotypic spinal cord
slices and hMSC transplantation
At 7 days following the generation of the spinal
cord slice cultures, the slices were treated with 0.5 mg/ml of
lysolecithin (LPC; Sigma; Merck Millipore) for 17 h at 37°C to
induce demyelination, as previously described (15). The media was then replaced with
fresh media, with or without anti-IGFBP-6 antibody (30
µg/ml). The hMSCs (3×104 cells/2.5 µl)
were transplanted into the ventral region of the spinal cord slice
cultures using aspirator tube assemblies for microcapillary
pipettes (Sigma; Merck Millipore) and the cultures were incubated
for 1 week.
Statistical analysis
Statistical analysis was performed using the
language R (R Development Core Team 2010) and data were and
evaluated using one-way analysis of variance (ANOVA) analysis of
variance followed by the Newman-Keuls post hoc test. P<0.05 was
considered to indicate a statistically significant difference. All
data are presented as the mean ± standard error of the mean.
Results
Neuroprotective hMSC-CM signals by
IGFBP-6 through the activation of Akt in
H2O2-treated primary neurons
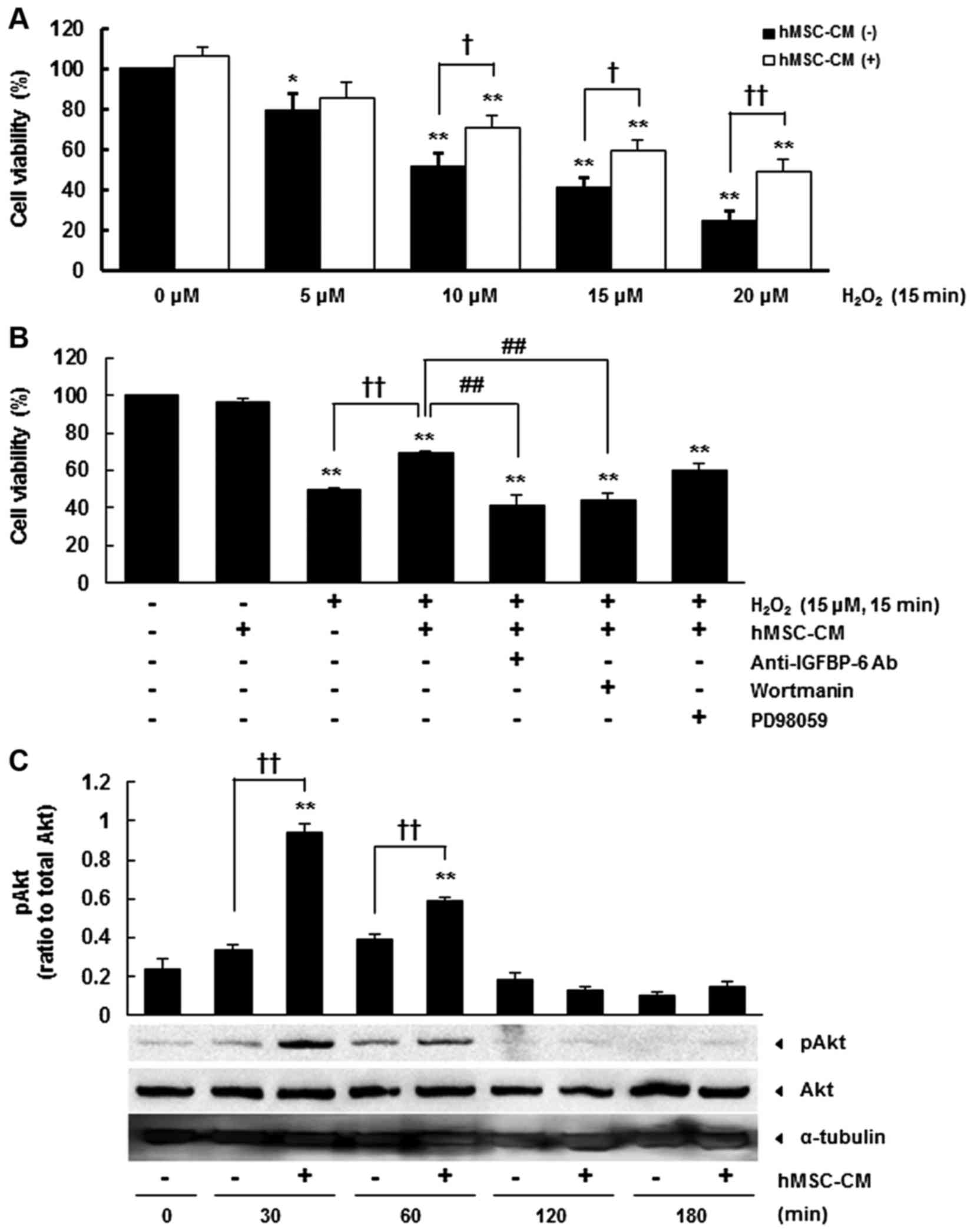
The present study examined the neuroprotective
effect of hMSC-CM using primary cortical neuron cultures damaged
using various concentrations of H2O2 (5–20
µM for 15 min), and found that hMSC-CM significantly
increased cell survival of the cortical neurons exposed to
H2O2 concentrations of 10, 15 and 20
µM (Fig. 1A). The
concentration of 15 µM H2O2 was
selected to induce cell death of cortical neurons in subsequent
experiments as this concentration resulted in m40.7±4.6% cell death
(Fig. 1A). In our previous study,
it was reported that transplanted hMSCs protected against cell
death in injured slice cultures and one of the growth factors
secreted by hMSCs was IGFBP-6 (5). Therefore, the present study aimed to
determine whether IGFBP-6 was responsible for the hMSC-CM-mediated
neuronal protection. Of note, the neutralizing antibody against
IGFBP-6 eliminated the hMSC-CM-mediated neuronal protection and
decreased cell viability to the level found in the
H2O2-treated culture (P<0.01) (Fig. 1B). These results indicated that
IGFBP-6 was crucial in the neuroprotection mediated by hMSC-CM.
To identify the signal transduction pathway
responsible for mediating neuroprotection by IGFBP-6 in hMSC-CM,
the present study examined the activation of various kinases known
to be involved in IGF signaling in primary cortical neurons.
Wortmannin, a pharmacological inhibitor of the phosphoinositide
3-kinase (PI3K)/Akt pathway, suppressed the hMSC-CM-mediated
neuroprotection of damaged primary cortical neurons (P<0.01)
(Fig. 1B), as did anti-IGFBP-6
antibody, which confirmed the importance of Akt. However, PD98059,
a pharmacological inhibitor of extracellular signal-regulated
kinase (ERK), did not affect the neuroprotection induced by hMSC-CM
(Fig. 1B). A significant increase
in pAkt was observed in the hMSC-CM-treated cells 30 min and 1 h
following hMSC-CM treatment, compared with that in the controls
under oxidative stress (P<0.01) (Fig. 1C). These results suggested that
the Akt signaling pathway was important in IGFBP-6-mediated
neuroprotection in primary cortical neurons.
Akt is activated by IGFBP-6 via
IGF-1R
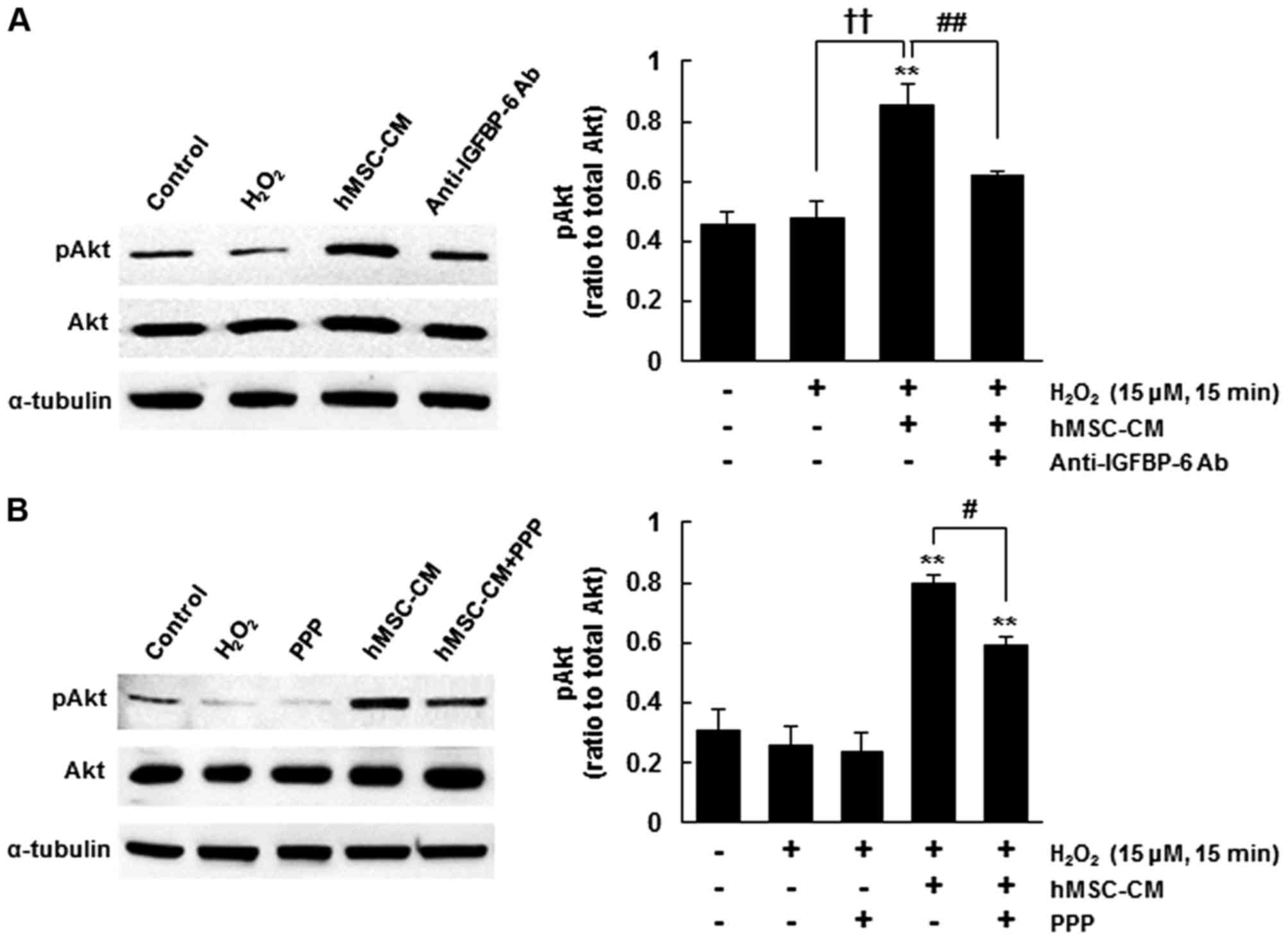
The neuroprotective IGFBP-6 signaling pathway may be
associated with the activation of Akt (Fig. 1B and C). To confirm this, the
phosphorylation level of Akt in hMSC-CM was determined following
H2O2 treatment, with or without anti-IGFBP-6
antibody incubation. The protein level of pAkt was significantly
increased in the hMSC-CM-treated primary cortical neurons following
the induction of oxidative stress (P<0.01) (Fig. 2A). However, pre-incubation with
the anti-IGFBP-6 antibody eliminated the hMSC-CM-mediated
activation of Akt and decreased the level of pAkt to the level
observed in the H2O2-treated condition
cultures (P<0.01) (Fig. 2A).
Several studies have reported that the activation of Akt is
involved in the IGF-1R downstream pathway (16). Using PPP, an inhibitor of IGF-1R,
it was showed that the neuroprotective effect of hMSC-CM occurred
through the IGF-1R-mediated activation of Akt. PPP treatment
significantly reduced the hMSC-CM-mediated phosphorylation of Akt
(P<0.05) (Fig. 2B). These
results suggested that hMSC-CM protected the primary cortical
neurons against oxidative stress-induced cell death through the
activation of IGF-1R by IGFBP-6.
Modulation of Bax translocation by
IGFBP-6
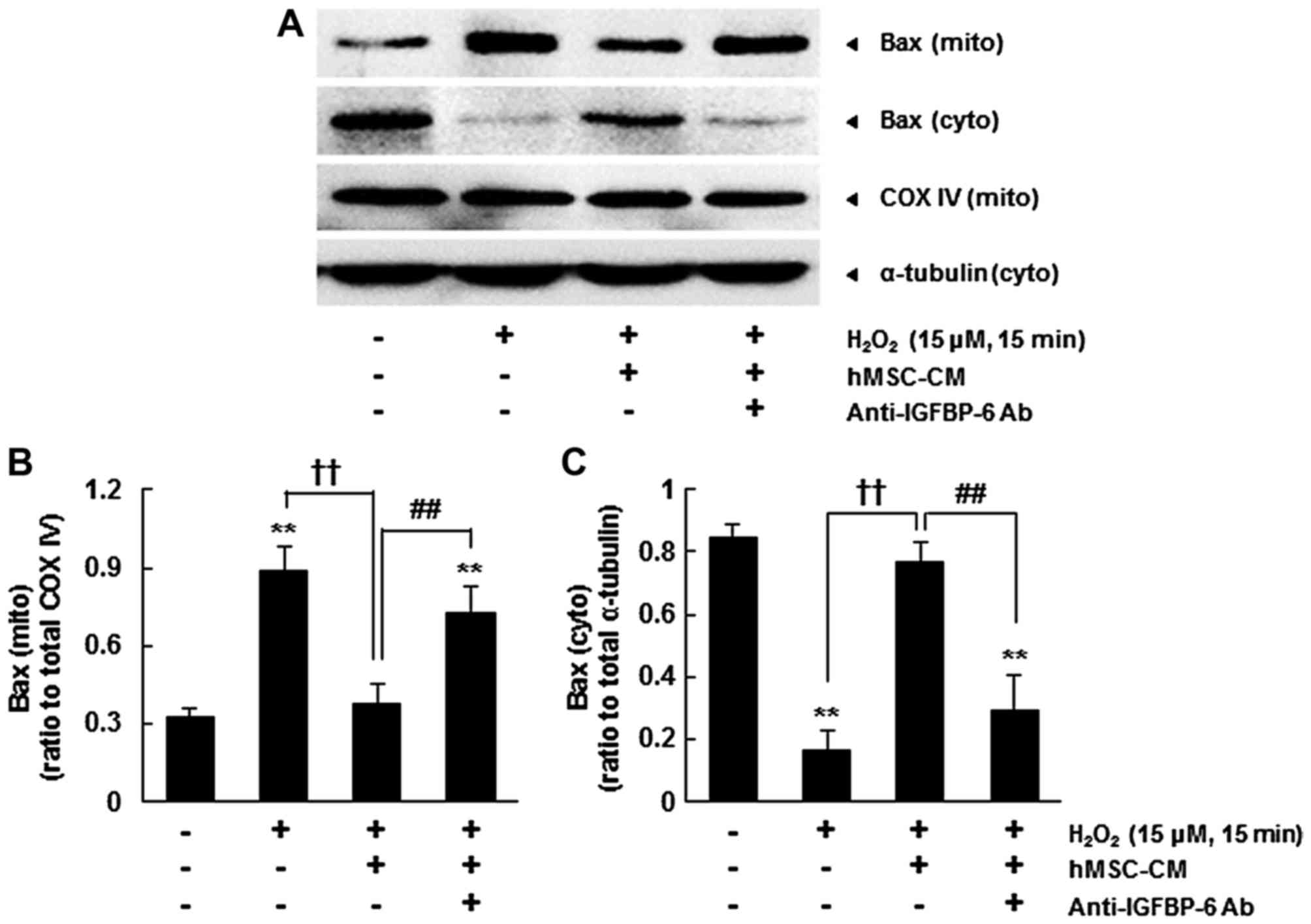
IGF-induced survival effects are regulated by Akt
signaling, which interrupts the mitochondria-mediated apoptotic
pathway and translocation of Bax, a regulator of apoptosis
(17). The majority of Bax
present in the cytosol translocates into the mitochondrial membrane
during apoptosis (18,19). Oxidative stress induced by
H2O2 treatment significantly increased the
level of Bax in the mitochondria (~2.8-fold vs. control) and
significantly reduced the cytosolic level of Bax (P<0.01)
(Fig. 3). By contrast, hMSC-CM
treatment inhibited the translocation of Bax and significantly
reduced the level of mitochondrial Bax induced by
H2O2 to the control level, restoring the
cytosolic level of Bax. However, pre-incubation with the
anti-IGFBP-6 antibody reversed the effect of hMSC-CM treatment and
increased the mitochondrial level of Bax following
H2O2 exposure (~1.9-fold vs. control),
whereas the cytosolic level of Bax was reduced (P<0.01)
(Fig. 3). These results suggested
that the IGFBP-6-induced activation of Akt inhibited the
translocation of endogenous Bax from the cytoplasm to mitochondria,
thereby promoting neuronal survival.
Extracellular levels of IGF-1 and IGF-2
by IGFBP-6 in H2O2-treated primary cortical
neurons
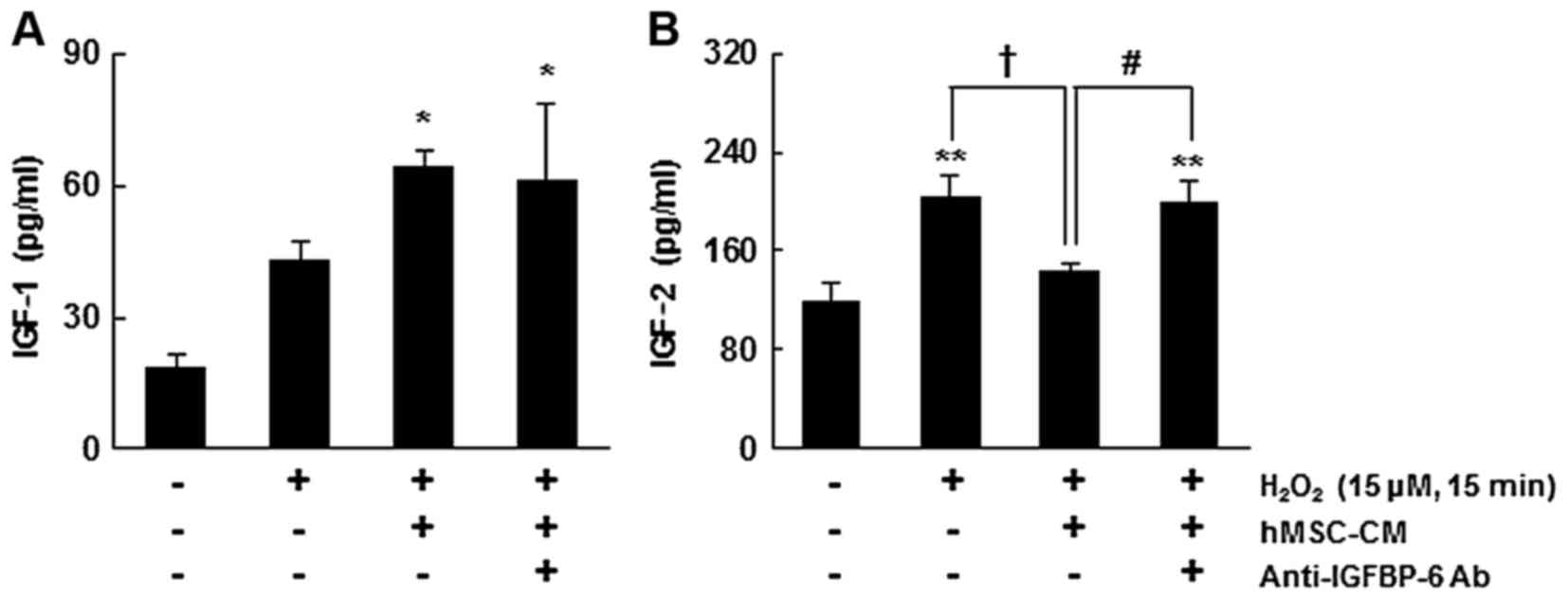
As shown in Fig.
2B, the neuroprotective effect of IGFBP-6 was regulated by
IGF-1R signaling. However, IGFBP-6 has a higher binding affinity
for IGF-2 than for IGF-1 and inhibits IGF-2 (9). Therefore, ELISA was used in the
present study to examine the extracellular levels of IGF-1 or IGF-2
in damaged primary cortical neurons, with or without IGFBP-6, in
hMSC-CM. Oxidative stress significantly increased the extracellular
level of IGF-2, but not that of IGF-1, in the primary cortical
neurons (P<0.01) (Fig. 4). By
contrast, hMSC-CM treatment significantly increased the
extracellular level of IGF-1 (P<0.05) (Fig. 4A), and reversed the higher level
of extracellular IGF-2 induced by H2O2 to the
control level (P<0.01) (Fig.
4B). Treatment with hMSC-CM but without IGFBP-6 also
significantly increased the extracellular level of IGF-1, compared
with that in the control (P<0.05) (Fig. 4A). However, the enhanced level of
extracellular IGF-2 in the cortical neurons damaged by
H2O2 was maintained, even when treated with
hMSC-CM without IGFBP-6 (P<0.05) (Fig. 4B). These results indicated that
IGFBP-6 significantly inhibited the oxidative stress-induced
increase in extracellular levels of IGF-2, but did not directly
regulate the extracellular levels of IGF-1.
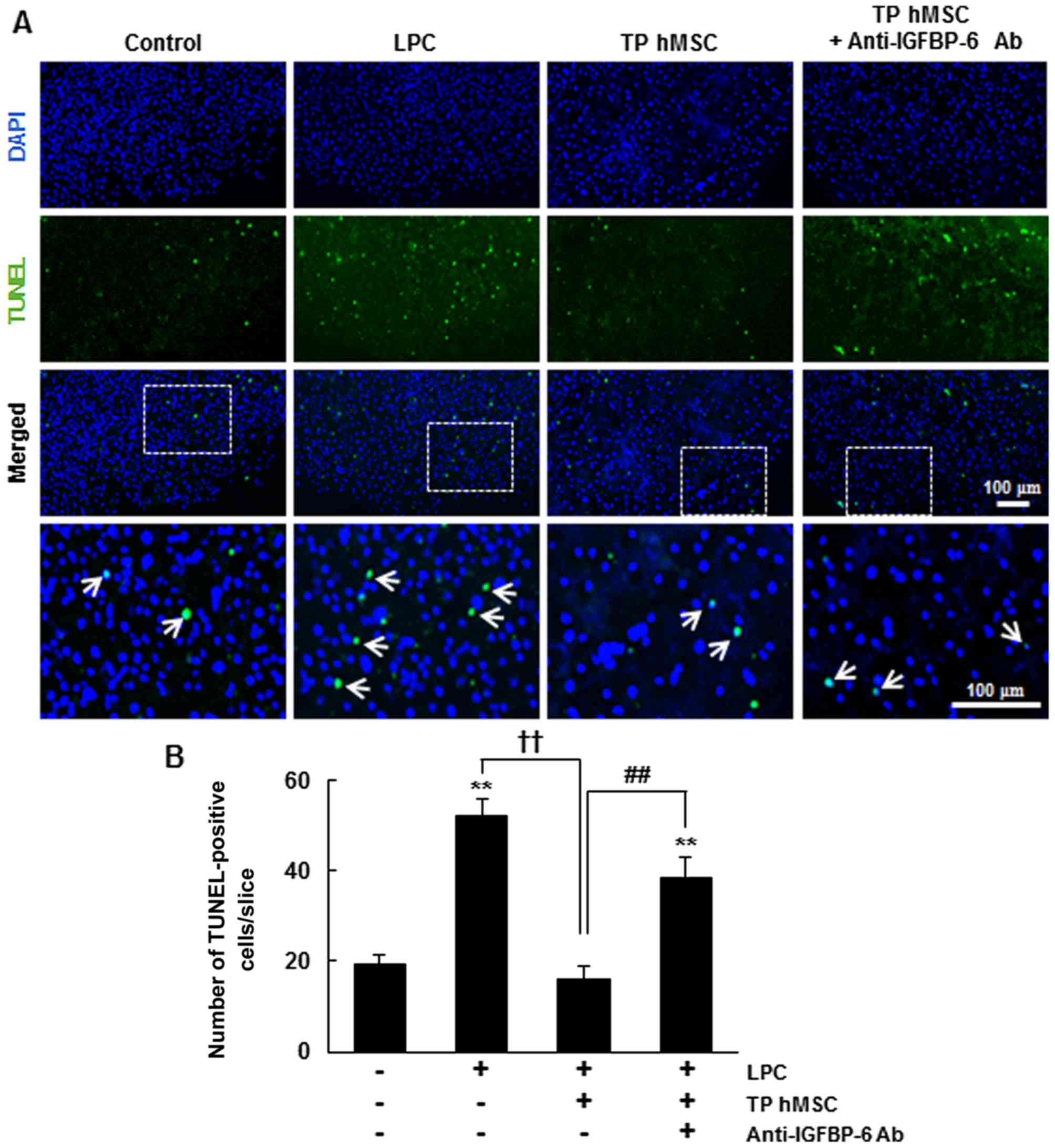
Effect of hMSC transplantation in an ex
vivo model of spinal cord injury
In our previous study, it was demonstrated that the
transplantation of hMSCs significantly increased cell survival in
LPC-treated demyelinated organotypic spinal cord slice cultures
(15). Demyelination is a
pathological feature of several neurological disorders and is
caused by oxidative stress in neuroinflammation (20). The present study used an ex
vivo model of spinal cord injury to examine whether hMSCs exert
their neuroprotective role through IGFBP-6. Demyelination by LPC
treatment notably increased the average number of TUNEL-stained
cells per slice, compared with that in the untreated control,
whereas transplantation of hMSCs significantly decreased the
average number of TUNEL-stained cells per slice by 31±5.5%,
compared with that in the LPC-treated slices (P<0.01) (Fig. 5). In addition, pre-incubation of
the LPC-treated slices with anti-IGFBP-6 antibody resulted in a
marked reversal of the anti-apoptotic effect of hMSC
transplantation. Anti-IGFBP-6 antibody treatment in the
hMSC-transplanted slices increased the average number of
TUNEL-stained cells per slice, compared with that of the
LPC-treated slices. These results indicated that IGFBP-6 was
critical for hMSC-mediated cell survival in the demyelinated
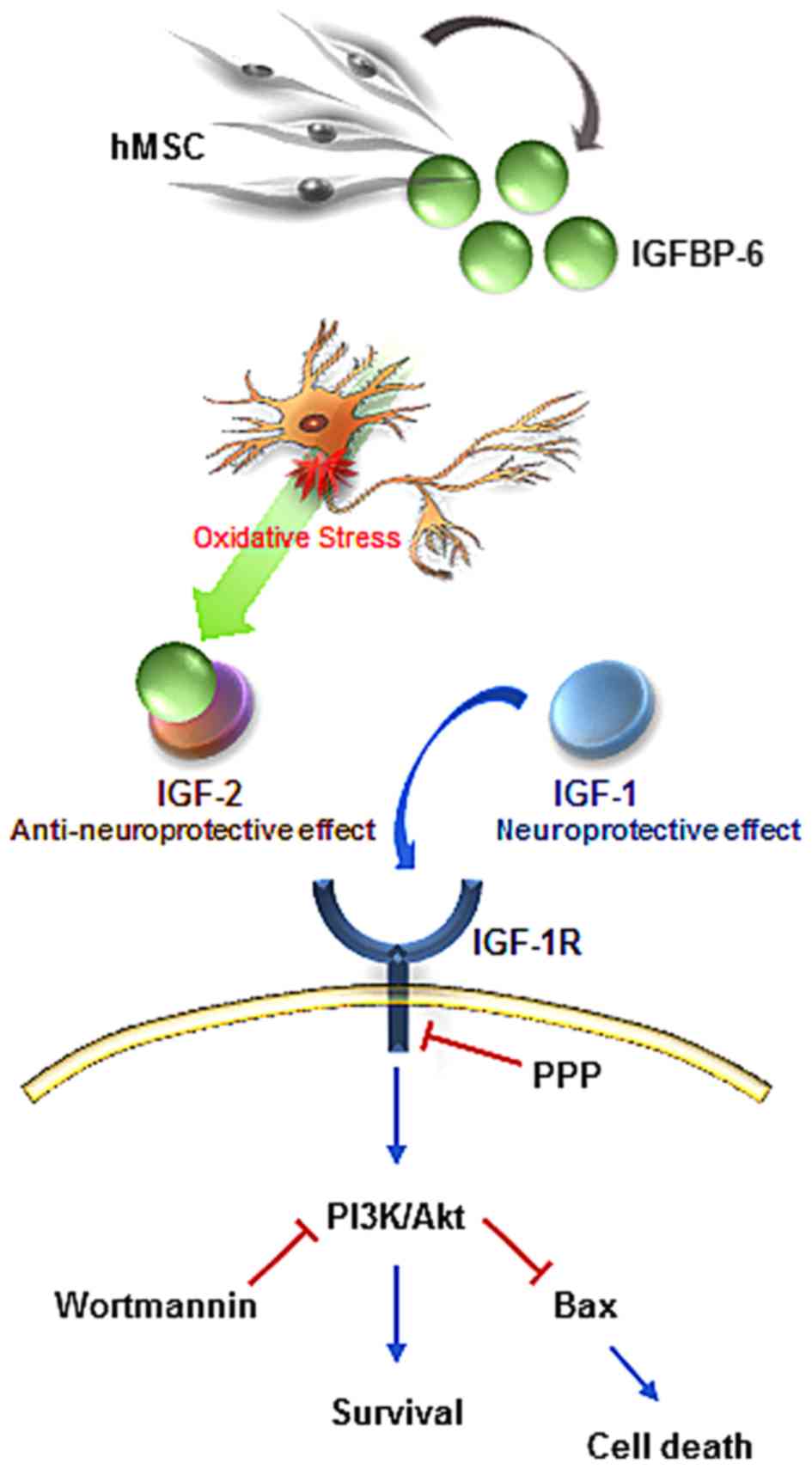
organotypic spinal cord slice cultures. Taken together, these
results suggested that IGFBP-6 was important in neuronal survival
through activation of the Akt- and IGF-1R-mediated signaling
pathway (Fig. 6).
Discussion
The therapeutic effects of hMSCs have been
attributed to their multipotency to replace damaged or lost cells
and the secretion of paracrine factors (4). hMSCs promote neuronal survival and
neuritogenesis by secreting neurotrophic factors (21). hMSC-CM can increase neuronal
survival and neurite outgrowth, which is associated with higher
levels of secreted IGF-1, HGF, VEGF and TGF-β (22). However, the detailed function of
each paracrine factor in hMSC-CM remains to be fully elucidated.
IGFBP-6, one of the abundant growth factors released from hMSCs,
increases lifespan and decreases apoptosis (23). It also has direct mitogenic and
anti-apoptotic effects in Saos-2/B-10 cells, a human osteoblastic
osteosarcoma cell line (24).
The present study demonstrated for the first time,
to the best of our knowledge, that IGFBP-6 released from hMSCs
possessed neuroprotective effects in a primary cortical neuron
culture. IGFBP-6 in hMSC-CM protected primary cortical neurons
against the oxidative stress induced by H2O2.
The phosphorylation of Akt was markedly enhanced in damaged primary
cortical neurons treated with hMSC-CM, compared with that in cells
without hMSC-CM treatment, and the expression of pAkt inhibited
neuronal cell death. Additionally, the activation of Akt was
reduced following the addition of anti-IGFBP-6 inhibitory antibody
in the hMSC-CM, suggesting that IGFBP-6 regulated the level of
pAkt. It has been reported that the activation of Akt in neurons
prevents the translocation of pro-apoptotic Bax to the mitochondria
by inhibiting p53-mediated transactivation (25,26). The results of the present study
also demonstrated that hMSC-CM treatment prevented the
translocation of Bax to the mitochondria induced by
H2O2 treatment. However, incubation with the
anti-IGFBP-6 antibody reversed the effect of hMSC-CM treatment and
increased the level of mitochondrial Bax following
H2O2 treatment.
Consistent with the results of the present study,
several studies have shown a specific role of IGFBP-6 in cell
survival. IGFBP-6 inhibits the senescence and death of human
fibroblasts (23), and IGFBP-6
was found to be markedly upregulated in the brain following
hypoxic-ischemic (HI) injury, whereas IGF-2 was not (27). Another study reported that
treatment with IGF-2 increased neuronal loss in the hippocampus and
dentate gyrus during brain damage caused by HI injury, and
inhibited IGF-1-induced neuroprotection (28). As IGFBP-6 inhibits IGF-2 due to
its high binding affinity (Fig.
4) (9), the neuroprotective
effects of IGFBP-6 can be potentiated by free IGF-1 (27). IGF-1 regulates DNA synthesis, cell
growth and anti-apoptotic pathways, and induces neuronal
differentiation (29). In
general, IGF-1 and IGF-2 bind to IGF-1R, thereby inducing the
activation of the Akt and/or ERK pathways (16,30). The results of the present study
suggested that IGFBP-6 primarily activated Akt, rather than ERK
(Fig. 1B). In addition, the
significantly increased expression of pAkt following hMSC-CM
treatment was reversed by PPP treatment (Fig. 2B), indicating that IGFBP-6 may
enhance the phosphorylation of Akt through IGF-1R-mediated
signaling. These findings suggested that IGFBP-6 inhibited the
apoptotic effect of IGF-2 and regulated IGF-1R-mediated signaling
via free IGF-1, which activated the anti-apoptotic PI3K/Akt
signaling pathway.
The IGF-independent effects of IGFBP-6 (6) may also contribute to neuroprotection
in primary cortical neurons. Gli1, a full length transcriptional
activator of hedgehog signaling, maintains cell survival by
facilitating the transcription of Bcl-2 genes through binding to
promoter regions in IGFBP-6 (31,32) and mediates the survival of diffuse
large B-cell lymphoma cells by promoting the transcription of Akt
(33). This is consistent with
the finding of the present study that hMSC-CM containing IGFBP-6
increased the level of pAkt.
However, the function of IGFBP-6 remains
controversial. Several studies have reported on the
anti-proliferative activity of IGFBP-6. For example, IGFBP-6
predominantly binds to IGF-2 and inhibits IGF/insulin signaling to
suppress cell proliferation and survival in various cancer cell
lines (9,34). The present study provided insight
into IGFBP-6-mediated cellular survival mechanisms in the nervous
system. However, further investigations are required, particularly
regarding the detailed mechanisms of IGF-1 and IGF-1R signaling
through IGFBP-6. Taken together, the results of the present study
indicated that IGFBP-6 is an important neuronal survival factor
secreted from hMSCs, suggesting that IGFBP-6 is a desirable factor
for the treatment of neurodegenerative diseases.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(grant. no. M-SC, NRF-2015R1D1A1A01056950) funded by the Ministry
of Education, and by a grant from the Korean Health Technology
R&D Project (grant no. M-SC, A120476) of the Ministry of Health
and Welfare, Republic of Korea.
References
|
1
|
Reynolds A, Laurie C, Mosley RL and
Gendelman HE: Oxidative stress and the pathogenesis of
neurodegenerative disorders. Int Rev Neurobiol. 82:297–325. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uttara B, Singh AV, Zamboni P and Mahajan
RT: Oxidative stress and neurodegenerative diseases: A review of
upstream and downstream antioxidant therapeutic options. Curr
Neuropharmacol. 7:65–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008. View
Article : Google Scholar
|
|
4
|
Mezey E: The therapeutic potential of bone
marrow-derived stromal cells. J Cell Biochem. 112:2683–2687. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park HW, Lim MJ, Jung H, Lee SP, Paik KS
and Chang MS: Human mesenchymal stem cell-derived Schwann cell-like
cells exhibit neurotrophic effects, via distinct growth factor
production, in a model of spinal cord injury. Glia. 58:1118–1132.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Firth SM and Baxter RC: Cellular actions
of the insulin-like growth factor binding proteins. Endocr Rev.
23:824–854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hwa V, Oh Y and Rosenfeld RG: The
insulin-like growth factor-binding protein (IGFBP) superfamily.
Endocr Rev. 20:761–787. 1999.PubMed/NCBI
|
|
8
|
Hoyt KR, Gallagher AJ, Hastings TG and
Reynolds IJ: Characterization of hydrogen peroxide toxicity in
cultured rat forebrain neurons. Neurochem Res. 22:333–340. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bach LA: Recent insights into the actions
of IGFBP-6. J Cell Commun Signal. 9:189–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oskowitz A, McFerrin H, Gutschow M, Carter
ML and Pochampally R: Serum-deprived human multipotent mesenchymal
stromal cells (MSCs) are highly angiogenic. Stem Cell Res.
6:215–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koike H, Ito K, Takezawa Y, Oyama T,
Yamanaka H and Suzuki K: Insulin-like growth factor binding
protein-6 inhibits prostate cancer cell proliferation: Implication
for anticancer effect of diethylstilbestrol in hormone refractory
prostate cancer. Br J Cancer. 92:1538–1544. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen DM: Urea-inducible Egr-1
transcription in renal inner medullary collecting duct (mIMCD3)
cells is mediated by extracellular signal-regulated kinase
activation. Proc Natl Acad Sci USA. 93:11242–11247. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu EH and Wong YH: Involvement of Gi/o
proteins in nerve growth factor-stimulated phosphorylation and
degradation of tuberin in PC-12 cells and cortical neurons. Mol
Pharmacol. 67:1195–1205. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wahane SD, Hellbach N, Prentzell MT, Weise
SC, Vezzali R, Kreutz C, Timmer J, Krieglstein K, Thedieck K and
Vogel T: PI3K-p110-alpha-subtype signalling mediates survival,
proliferation and neurogenesis of cortical progenitor cells via
activation of mTORC2. J Neurochem. 130:255–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho JS, Park H-W, Park S-K, Roh S, Kang
SK, Paik KS and Chang MS: Transplantation of mesenchymal stem cells
enhances axonal outgrowth and cell survival in an organotypic
spinal cord slice culture. Neurosci Lett. 454:43–48. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saltiel AR and Kahn CR: Insulin signalling
and the regulation of glucose and lipid metabolism. Nature.
414:799–806. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pang Y, Zheng B, Fan LW, Rhodes PG and Cai
Z: IGF-1 protects oligodendrocyte progenitors against
TNFalpha-induced damage by activation of PI3K/Akt and interruption
of the mitochondrial apoptotic pathway. Glia. 55:1099–1107. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wolter KG, Hsu Y-T, Smith CL, Nechushtan
A, Xi XG and Youle RJ: Movement of Bax from the cytosol to
mitochondria during apoptosis. J Cell Biol. 139:1281–1292. 1997.
View Article : Google Scholar
|
|
19
|
Hsu YT, Wolter KG and Youle RJ:
Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during
apoptosis. Proc Natl Acad Sci USA. 94:3668–3672. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ljubisavljevic S: Oxidative stress and
neurobiology of demyelination. Mol Neurobiol. 53:744–758. 2016.
View Article : Google Scholar
|
|
21
|
Crigler L, Robey RC, Asawachaicharn A,
Gaupp D and Phinney DG: Human mesenchymal stem cell subpopulations
express a variety of neuro-regulatory molecules and promote
neuronal cell survival and neuritogenesis. Exp Neurol. 198:54–64.
2006. View Article : Google Scholar
|
|
22
|
Nakano N, Nakai Y, Seo T-B, Yamada Y, Ohno
T, Yamanaka A, Nagai Y, Fukushima M, Suzuki Y, Nakatani T, et al:
Characterization of conditioned medium of cultured bone marrow
stromal cells. Neurosci Lett. 483:57–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Micutkova L, Diener T, Li C,
Rogowska-Wrzesinska A, Mueck C, Huetter E, Weinberger B,
Grubeck-Loebenstein B, Roepstorff P, Zeng R, et al: Insulin-like
growth factor binding protein-6 delays replicative senescence of
human fibroblasts. Mech Ageing Dev. 132:468–479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmid C, Keller C, Gosteli-Peter M and
Zapf J: Mitogenic and antiapoptotic effects of insulin-like growth
factor binding protein-6 in the human osteoblastic osteosarcoma
cell line Saos-2/B-10. Biochem Biophys Res Commun. 263:786–789.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brunet A, Datta SR and Greenberg ME:
Transcription-dependent and -independent control of neuronal
survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol.
11:297–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamaguchi A, Tamatani M, Matsuzaki H,
Namikawa K, Kiyama H, Vitek MP, Mitsuda N and Tohyama M: Akt
activation protects hippocampal neurons from apoptosis by
inhibiting transcriptional activity of p53. J Biol Chem.
276:5256–5264. 2001. View Article : Google Scholar
|
|
27
|
Beilharz EJ, Russo VC, Butler G, Baker NL,
Connor B, Sirimanne ES, Dragunow M, Werther GA, Gluckman PD,
Williams CE, et al: Co-ordinated and cellular specific induction of
the components of the IGF/IGFBP axis in the rat brain following
hypoxic-ischemic injury. Brain Res Mol Brain Res. 59:119–134. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guan J, Williams CE, Skinner SJ, Mallard
EC and Gluckman PD: The effects of insulin-like growth factor
(IGF)-1, IGF-2, and des-IGF-1 on neuronal loss after
hypoxic-ischemic brain injury in adult rats: Evidence for a role
for IGF binding proteins. Endocrinology. 137:893–898. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chesik D, De Keyser J and Wilczak N:
Insulin-like growth factor binding protein-2 as a regulator of IGF
actions in CNS: Implications in multiple sclerosis. Cytokine Growth
Factor Rev. 18:267–278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
LeRoith D, Werner H, Beitner-Johnson D and
Roberts CT Jr: Molecular and cellular aspects of the insulin-like
growth factor I receptor. Endocr Rev. 16:143–163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoon JW, Kita Y, Frank DJ, Majewski RR,
Konicek BA, Nobrega MA, Jacob H, Walterhouse D and Iannaccone P:
Gene expression profiling leads to identification of GLI1 binding
elements in target genes and a role for multiple downstream
pathways in GLI1 induced cell transformation. J Biol Chem.
277:5548–5555. 2002. View Article : Google Scholar
|
|
32
|
Xu XF, Guo C-Y, Liu J, Yang WJ, Xia YJ, Xu
L, Yu YC and Wang XP: Gli1 maintains cell survival by up-regulating
IGFBP6 and Bcl-2 through promoter regions in parallel manner in
pancreatic cancer cells. J Carcinog. 8:132009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Agarwal NK, Qu C, Kunkalla K, Liu Y and
Vega F: Transcriptional regulation of serine/threonine protein
kinase (AKT) genes by glioma-associated oncogene homolog 1. J Biol
Chem. 288:15390–15401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Duan C and Xu Q: Roles of insulin-like
growth factor (IGF) binding proteins in regulating IGF actions. Gen
Comp Endocrinol. 142:44–52. 2005. View Article : Google Scholar : PubMed/NCBI
|