Introduction
Renal fibrosis is a common condition which can lead
to various types of progressive chronic kidney disease and even
end-stage renal failure. It is characterized by the activation of
renal fibroblasts and the accumulation of excessive amounts of
extracellular matrix (ECM) proteins (1,2).
Transforming growth factor-β1 (TGF-β1) is the most important growth
factor which regulates the transdifferentiation of tubular
epithelial cells into myofibroblasts in renal fibrosis (3). Previous studies have demonstrated
that TGF-β1 expression is markedly elevated in animal models of
renal fibrosis and in patients with glomerulonephritis (4,5).
Thus, the suppression of TGF-β1 signaling may be a potential target
with which to prevent progressive renal fibrosis.
WD40-repeat proteins are a large, highly conserved
family of adaptors implicated in various biological process, such
as signal transduction, gene transcriptional regulation, protein
modifications, cytoskeleton assembly, vesicular trafficking, DNA
damage and repair, cell death and cell cycle progression (6). The receptor for activated C-kinase 1
(RACK1) is a member of the WD40-repeat family of proteins and has
been reported to be implicated in the development of various
diseases (7-9). A previous study implied that RACK1
overexpression inhibited cardiomyocyte apoptosis following
myocardial ischemia/reperfusion in adult rats (10). The downregulation of RACK1 has
been shown to inhibit cell proliferation, along with invasion and
migration in vitro and in vivo in esophageal squamous
cell carcinoma (11). Moreover, a
previous study reported that RACK1 promoted the TGF-β1-mediated
activation of pro-fibrogenic pathways, as well as the
differentiation, proliferation and migration of hepatic stellate
cells (HSCs), and the depletion of RACK1 suppressed the progression
of thioacetamide-induced liver fibrosis in vivo (12). However, the role of RACK1 in renal
fibrosis remains unclear. Therefore, in this study, we investigated
the effects of RACK1 on TGF-β1-treated human proximal tubular
epithelial cells and aimed to elucidate the possible mechanisms
responsible for its anti-fibrotic effects.
Materials and methods
Specimen collection
Renal biopsy samples were collected by transparietal
puncture from 12 healthy individuals and 10 patients with renal
fibrosis, diagnosed on clinical, biological and histological
grounds. The samples were immediately stored in liquid nitrogen in
preparation for use. This study was approved by the Ethics
Committee of the First Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China), and written informed consent was
obtained from all participants prior to sample collection.
Cell culture and treatment
The human proximal tubular epithelial cell line,
HK-2, was purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). The HK-2 cells were maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 5%
fetal bovine serum (FBS) (both from Invitrogen life Technologies,
Carlsbad, CA, USA), 100 U/ml penicillin and 100 mg/ml streptomycin
(Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a humidified 5%
CO2 atmosphere. The HK-2 cells were seeded in the
complete medium containing 5% FBS at approximately 70% confluence
in 6-well culture plates. After 24 h, the complete medium was
replaced with serum-free medium for 24 h prior to treatment with
recombinant TGF-β1 (5 ng/ml; Sigma-Aldrich).
Small interfering RNA (siRNA)
transfection
siRNA targeting RACK1 or its corresponding negative
control was designed and synthesized from Sangon Biotech Co., ltd.
(Shanghai, China). For in vitro transfection, the HK-2 cells
were plated and grown to 70-90% confluency without antibiotics, and
then transfected with siRNA-RACK1 or siRNA-mock using
lipofectamine™ 2000 (Invitrogen life Technologies) according to the
manufacturer's instructions. The transfection efficiency was
evaluated by examining RACK1 mRNA and protein expression by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis, respectively.
RT-qPCR analysis
Total RNA was extracted from the HK-2 cells using
TRIzol reagent (Takara Biotechnology Co., ltd., Dalian, China).
Approximately 5 µg total RNA from each sample was reverse
transcribed into cDNA using SuperScript II Reverse Transcriptase
(Takara Biotechnology Co., ltd.). Quantitative (real-time) PCR was
performed on an ABI-Prism 7500 using Power SYBR-Green Master Mix 2X
(Applied Biosystems, Foster City, CA, USA). The following primers
were used: RACK1, 5′-TTC TCC TCT GAC AAC CGG CA-3′ (sense), 5′-GCC
ATC CTT GCC TCC AGA A-3′ (anti-sense); α-smooth muscle actin
(α-SMA), 5′-CTA TTC CTT CGT GAC TAC T-3′ (sense), 5′-ATG CTG TTA
TAG GTG GTG GTT-3′ (antisense); connective tissue growth factor
(CTGF), 5′-GGA AAA GAT TCC CAC CCA AT-3′ (sense), 5′-TGC TCC TAA
AGC CAC ACC TT (antisense); and β-actin, 5′-GGC AAA TTC AAC GGC ACA
GTC-3′ (sense), 5′-GCT GAC AAT CTT GAG TGA GTT-3′ (antisense). The
cycling conditions were as follows: pre-incubation at 95°C, 10 min;
PCR: 95°C, 15 sec and 59°C, 30 sec, 35 cycles; final elongation:
72°C, 10 min. β-actin was used as an internal control and the
expression levels of the relative genes were calculated using the
2−ΔΔCT method.
Western blot analysis
Total protein was extracted from the HK-2 cells,
then washed with ice-cold PBS and lysed with RIPA cell lysis buffer
(Cell Signaling Technology, Inc., Danvers, MA, USA) containing a
phosphatase inhibitor and the protease inhibitor cocktail
(Sigma-Aldrich), by incubating on ice for 30 min. The protein
concentration was assayed using a micro BCA protein kit (Pierce,
Rockford, Il, USA). Forty micrograms of protein per lane were
separated by 10% SDS-PAGE and then transferred onto polyvinylidene
fluoride (PVDF) membranes (Millipore, Boston, MA, USA).
Non-specific binding sites were blocked with 5% (w/v) skim milk
(Bio-Rad laboratories, Inc., Hercules, CA, USA) in Tris-buffered
saline-Tween-20 (TBS-T) for 1 h at room temperature and the
membranes were then incubated with anti-RACK1 (1:1,000 dilution;
ab62735), anti-α-SMA (1:2,500 dilution; ab7817), anti-CTGF (1:1,000
dilution; ab94939), anti-p-Smad3 (1:2,500 dilution; ab51177), (all
from Abcam, Cambridge, UK) anti-Smad3 (1:1,000 dilution; sc-101154)
and anti-GAPDH (1:1,500 dilution; sc-47724) (all from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) antibodies at 4°C
overnight. The membranes were then washed and incubated with
horseradish peroxidase-conjugated secondary antibodies (sc-2005 and
sc-2004; Santa Cruz Biotechnology, Inc.). The absorbance values of
the target proteins were determined using Gel-Pro Analyzer version
4.0 software (Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis
All data are presented as the means ± standard
deviation (SD) based on at least 3 independent experiments.
Statistical analysis was performed using the Student's t-test and
ANOVA. A value of P<0.05 was considered to indicate a
statistically significant difference compared to the respective
control.
Results
RACK1 is highly expressed in renal
fibrotic tissues
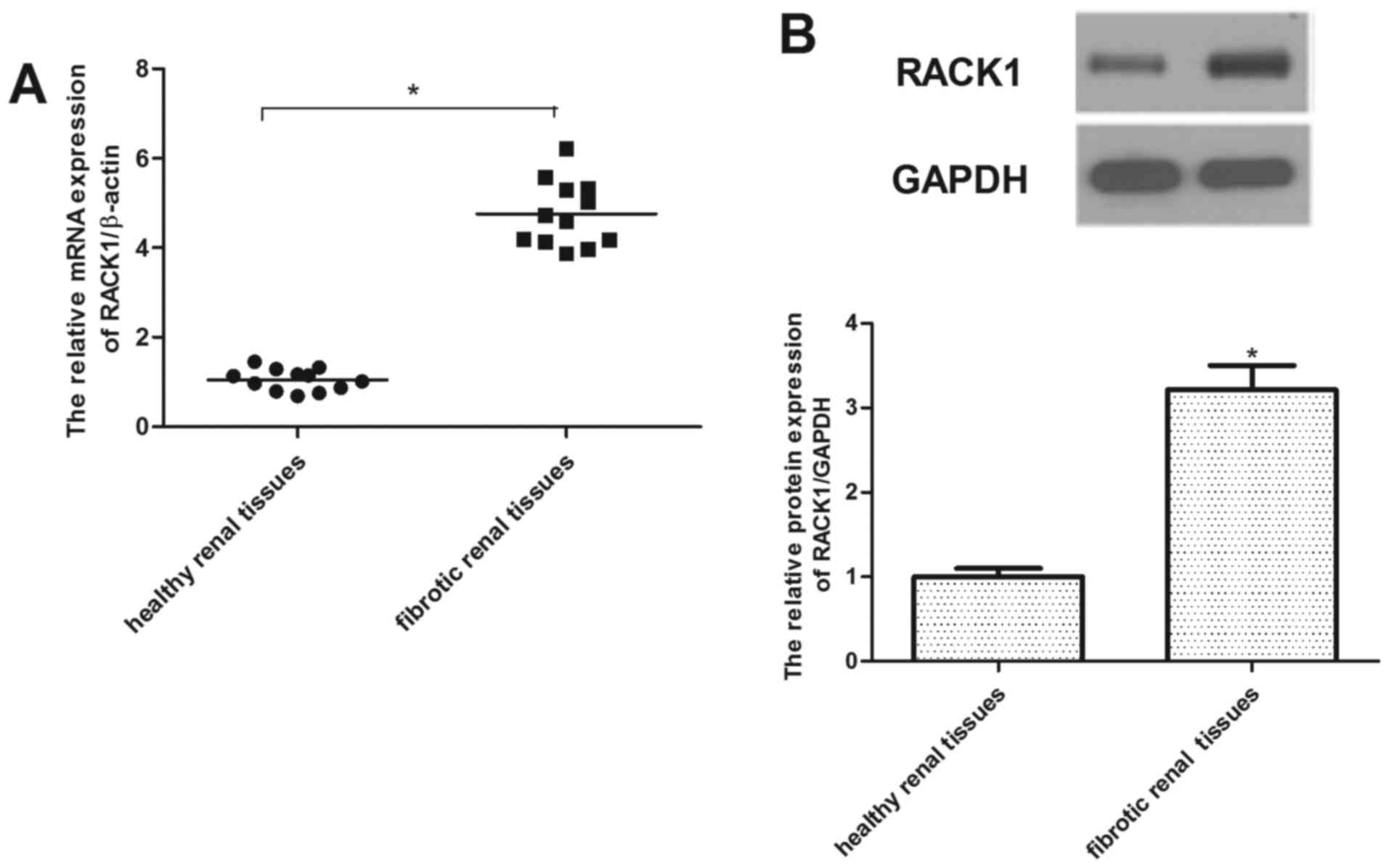
In order to examine the expression of RACK1 in renal
fibrosis, the mRNA transcription level of RACK1 was examined by
RT-qPCR. As shown in Fig. 1A,
RACK1 mRNA expression was markedly increased in the renal fibrotic
tissues, as compared with those of the control group. Similarly,
western blot analysis demonstrated that the protein expression of
RACK1 was also upregulated in the renal fibrotic tissues (Fig. 1B).
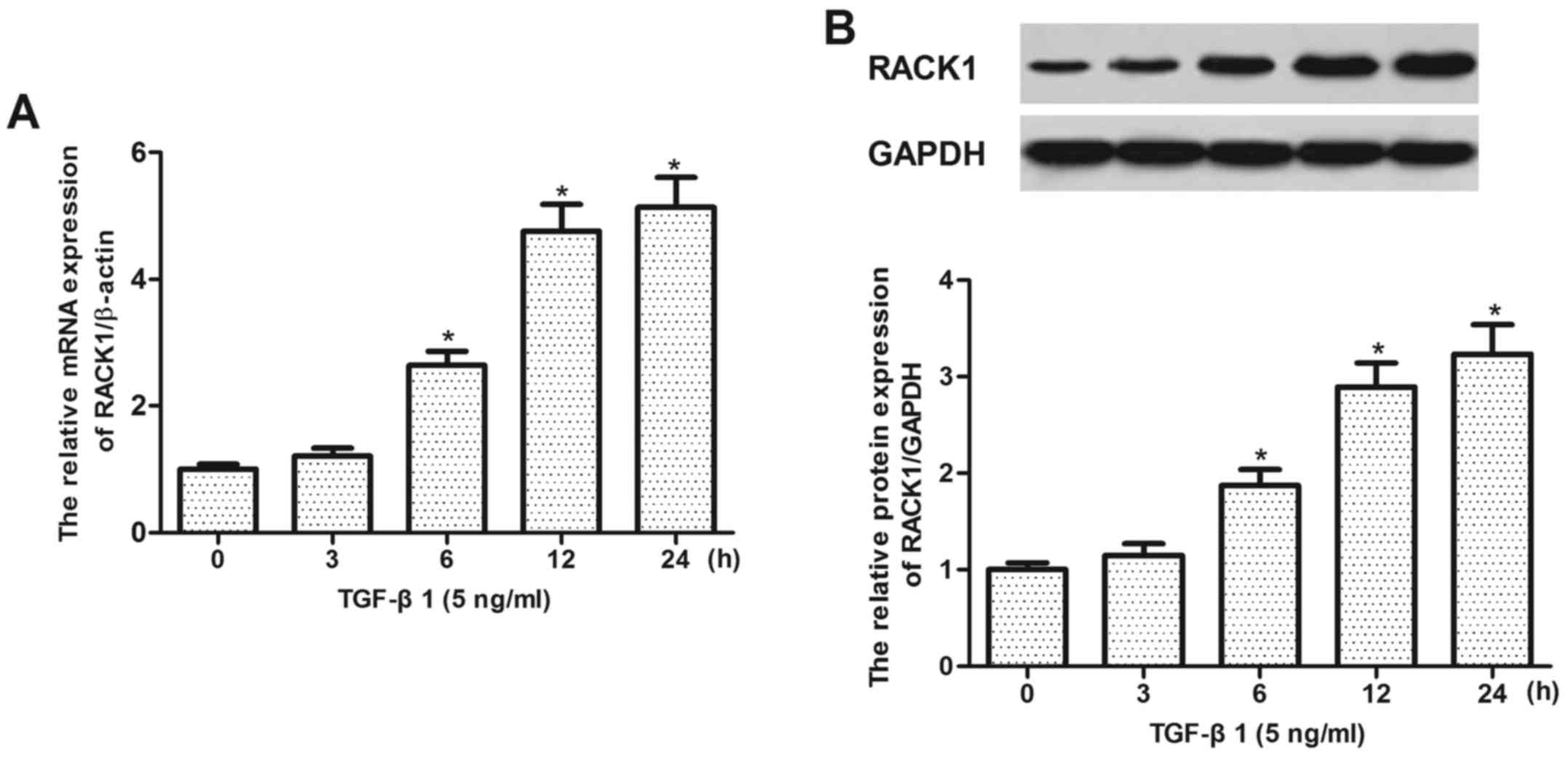
RACK1 expression is rapidly induced in
TGF-β1-treated HK-2 cells
We then examined the expression of RACK1 in
TGF-β1-treated HK-2 cells. As indicated in Fig. 2, the results revealed that TGF-β1
treatment significantly upregulated the mRNA and protein expression
of RACK1 in a time-dependent manner.
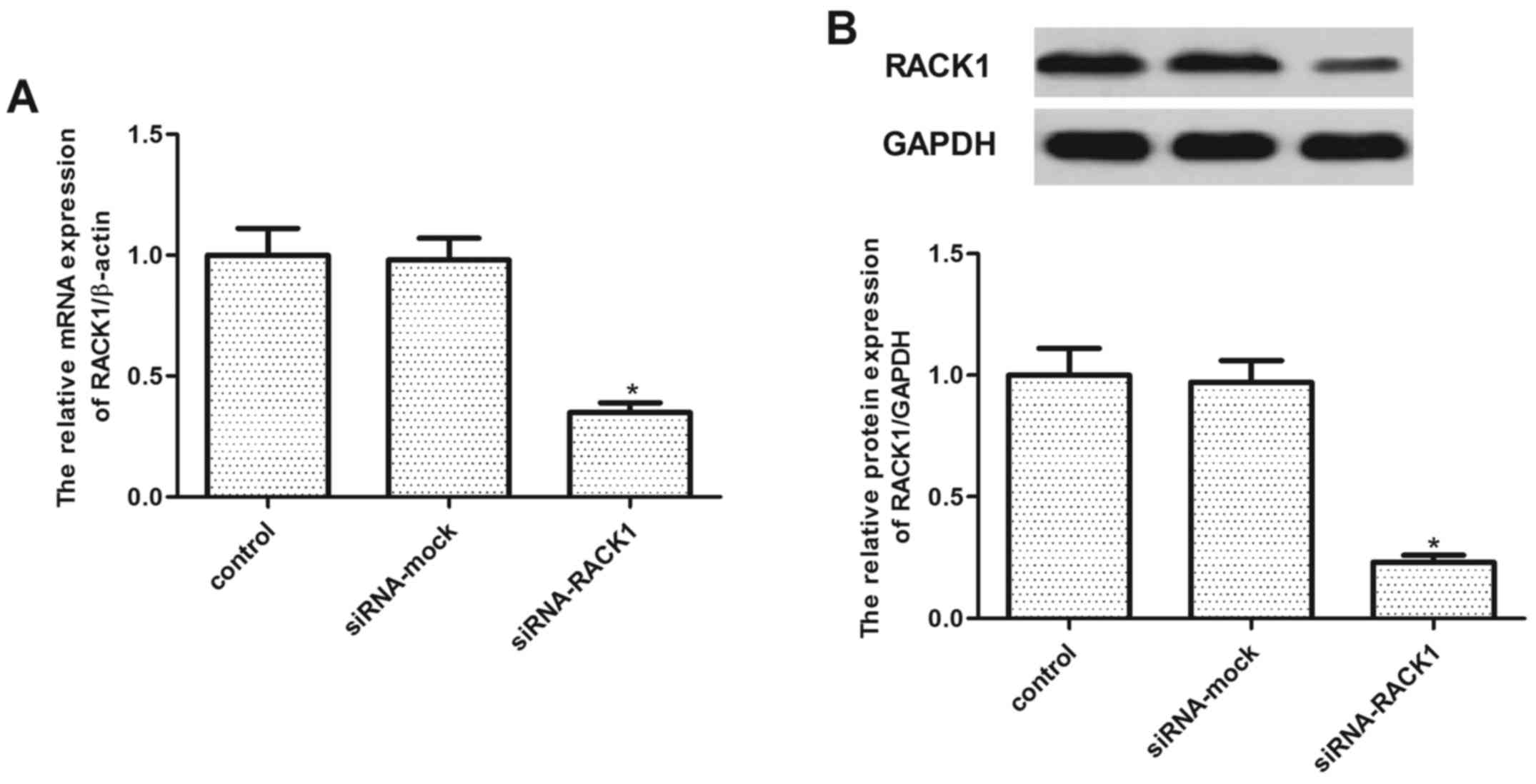
RACK1 silencing inhibits TGF-β1-induced
α-SMA and CTGF in HK-2 cells
To confirm the role of RACK1 in TGF-β1-induced renal
fibrogenesis, we used specific siRNA to knock down the expression
of RACK1. As shown in Fig. 3, the
RACK1 mRNA and protein expression levels in the
siRNA-RACK1-transfected group were significantly lower than those
in the siRNA-mock transfected group. We then evaluated the effects
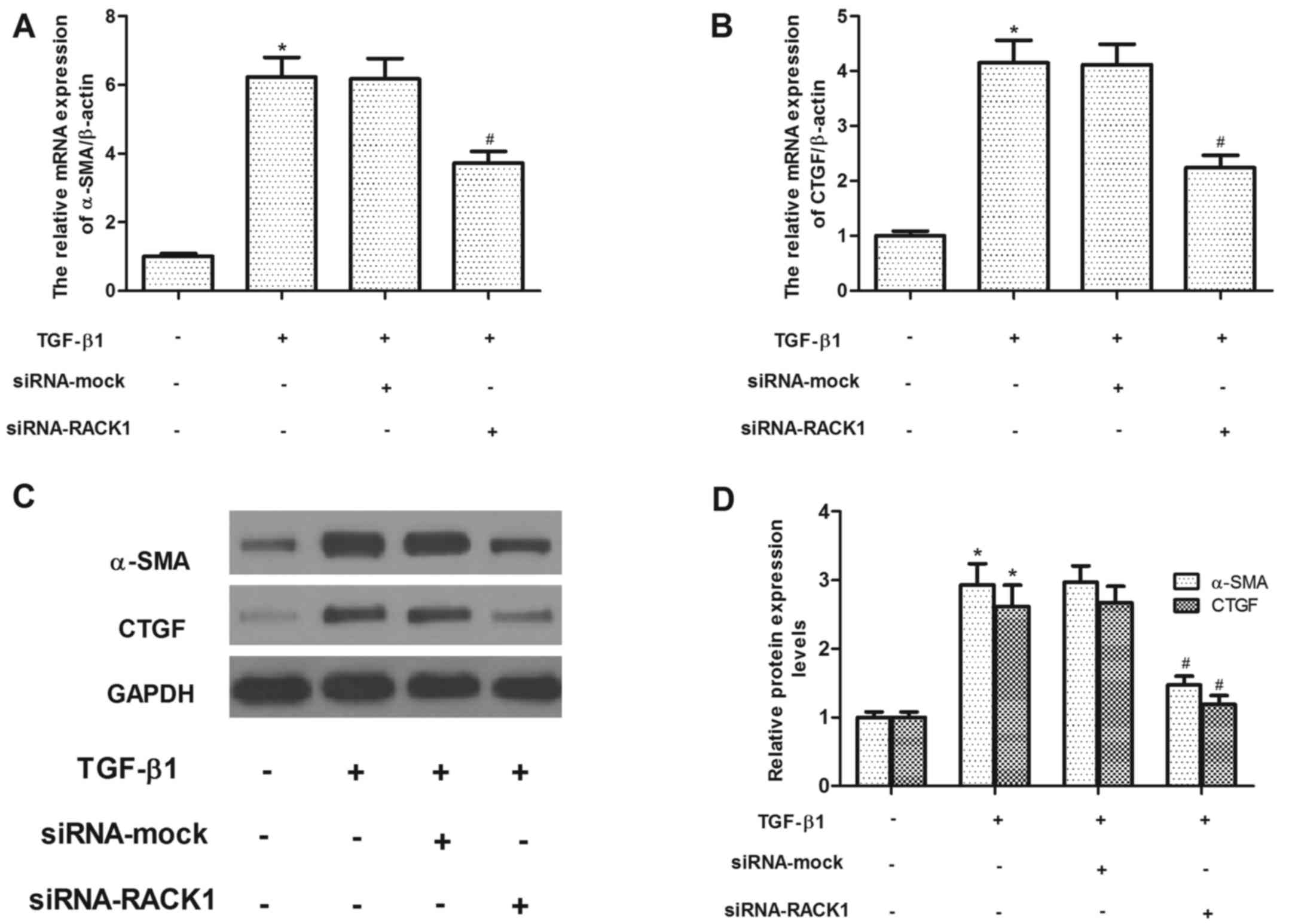
of siRNA-RACK1 on the α-SMA and CTGF expression levels in the
TGF-β1-treated HK-2 cells. As shown in Fig. 4A and C, TGF-β1 treatment greatly
increased the expression of α-SMA at both the mRNA and protein
level, while the knockdown of RACK1 inhibited the expression of
α-SMA in the TGF-β1-treated HK-2 cells. Similarly, RACK1 silencing
also suppressed the TGF-β1-induced increase in CTGF expression in
the HK-2 cells (Fig. 4A and
C).
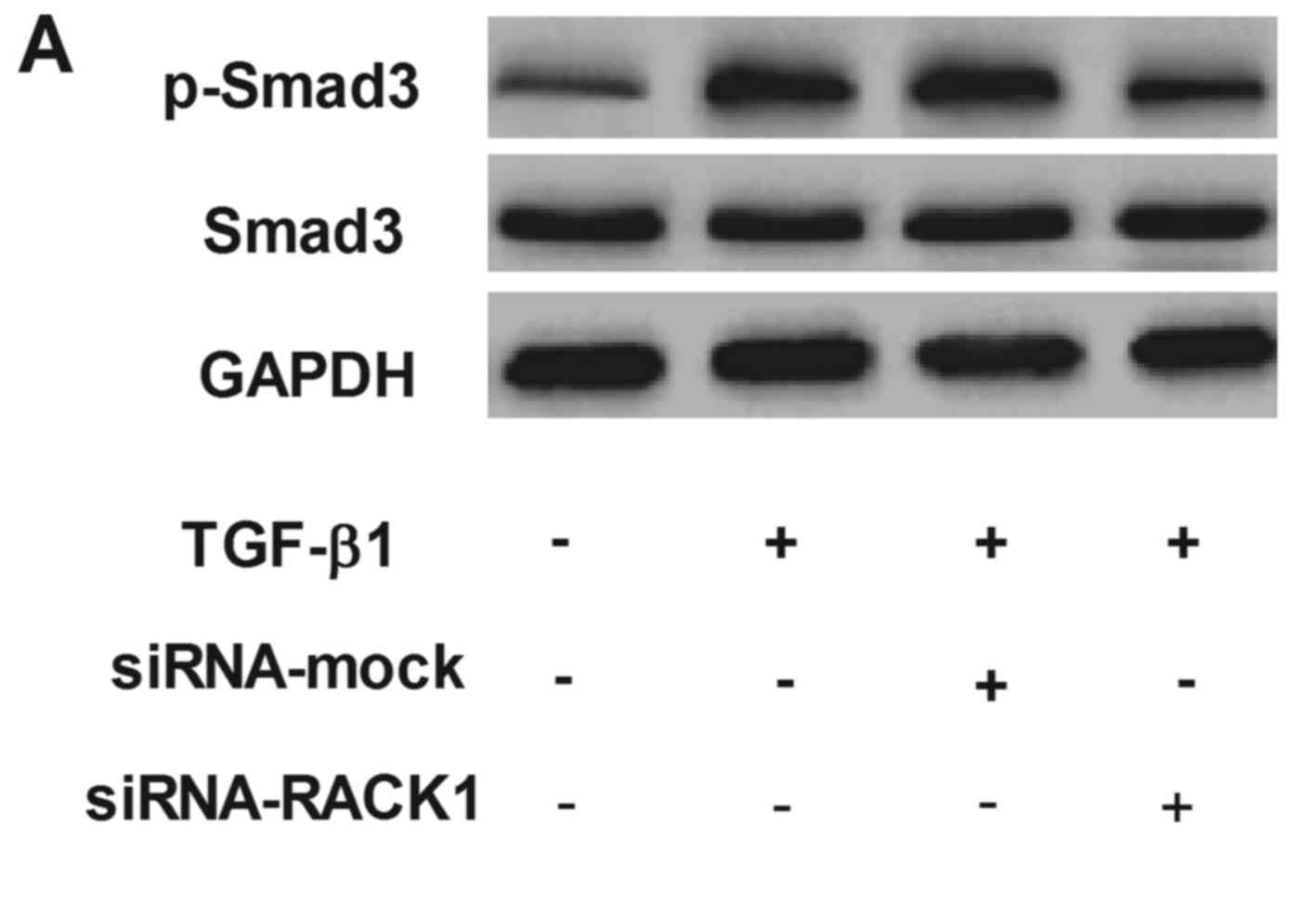
RACK1 silencing inhibits the expression
of phosphorylated Smad3 in TGF-β1-treated HK-2 cells
Smad3 is a critical transcription factor that
mediates cellular fibrotic response to TGF-β1 (13). Therefore, in this study, we
examined the effect of RACK1 on the expression of p-Smad3 and Smad3
in TGF-β1-treated HK-2 cells. As shown in Fig. 5, compared with the control, TGF-β1
treatment markedly increased the expression of phosphorylated
Smad3, while the knockdown of RACK1 inhibited the expression of
phosphorylated Smad3 in the TGF-β1-treated HK-2 cells. The
silencing of RACK1 had no effect on the expression of Smad3.
Discussion
The main findings of the present study were the
following: i) RACK1 was highly expressed in renal fibrotic tissues
and TGF-β1-treated HK-2 cells; ii) RACK1 silencing inhibited the
TGF-β1-induced increase in the expression of α-SMA and CTGF in HK-2
cells; iii) RACK1 silencing inhibited the expression of
phosphorylated Smad3 in the TGF-β1-treated HK-2 cells. To the best
of our knowledge, these data demonstrate for the first time the
role of RACK1 in renal fibrosis.
A previous study demonstrated that the expression of
RACK1 was increased in activated HSCs (12). However, RACK1 was also found to be
involved in dermal fibrosis, and the expression of RACK1 was found
to be significantly decreased in keloid fibroblasts (14). Therefore, RACK1 has a
pro-fibrogenic or anti-fibrogenic function depending on the cell
type or context. In the present study, we observed that RACK1 was
highly expressed in renal fibrotic tissues and TGF-β1-treated HK-2
cells. These findings suggest that RACK1 may have a pro-fibrogenic
function in the development of renal fibrosis.
A growing body of evidence suggests that the ECM
plays an important role in the pathogenesis of renal fibrosis. CTGF
acts as a downstream target of pro-fibrotic genes and is involved
in the formation of the ECM proteins, fibronectin and collagen
(15,16). Its expression is considered a
molecular hallmark of renal fibrosis. Yokoi et al reported
that CTGF antisense treatment significantly inhibited the induction
of CTGF and ECM genes and reduced renal fibrotic area in rat
obstructive nephropathy (17). In
epithelial-mesenchymal transition (EMT), the loss of tubular
epithelial cell adhesion molecules, such as E-cadherin, is replaced
by α-SMA, which is one of the interstitial myofibroblast activation
markers (18). In addition,
TGF-β1 can induce α-SMA expression and decreases adhesive protein
expression in human renal tubular epithelia cells (19). In line with these results, herein,
we observed that TGF-β1 induced the expression of α-SMA and CTGF in
HK-2 cells, while RACK1 silencing inhibited the TGF-β1-induced
increase in the expression of α-SMA and CTGF in HK-2 cells. These
results suggest that RACK1 silencing markedly attenuated renal
fibrosis via the reduction of ECM expression in TGF-β1-stimulated
HK-2 cells.
There is evidence to indicate that the TGF-β1/Smad
signaling pathway plays a critical role in the development of renal
fibrosis (20-23). During fibrogenesis, the activated
type I receptor kinase phosphorylates Smad2 and 3, which forms an
oligomeric complex with Smad4 that translocates into the nucleus to
regulate the transcription of target genes in collaboration with
various co-activators and co-repressors (24). In chronic kidney disease, TGF-β
overexpression induces renal fibrosis, while TGF-β inhibition
suppresses the expression of TGF-β1 and type I collagen in
tubulointerstitial cells in unilateral ureteral obstruction
(UUO)-induced renal fibrosis (25,26). Phosphorylated Smad3 is highly
expressed in the obstructed kidney (27), and Smad3 promotes renal fibrosis
by directly binding to the promoter region of collagens to trigger
their production (28), while the
deletion of Smad3 inhibits fibrogenesis in a number of rodent
models and the inhibition of Smad3 prevented
endothelial-myofibroblast transition and renal fibrosis in type-1
diabetic kidney disease (29). In
the present study, we found that RACK1 silencing inhibited the
expression of phosphorylated Smad3 in TGF-β1-treated HK-2 cells.
These results suggest that RACK1 silencing attenuates renal
fibrosis by suppressing the activation of the TGF-β1/Smad3
signaling pathway in HK-2 cells.
In conclusion, the present findings indicate that
RACK1 silencing attenuates renal fibrosis by suppressing the
activation of the TGF-β1/Smad3 signaling pathway in HK-2 cells.
Thus, RACK1 may serve as a novel regulator of renal fibrosis.
References
|
1
|
Boor P, Ostendorf T and Floege J: Renal
fibrosis: novel insights into mechanisms and therapeutic targets.
Nat Rev Nephrol. 6:643–656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eddy AA: Molecular basis of renal
fibrosis. Pediatr Nephrol. 15:290–301. 2000. View Article : Google Scholar
|
|
3
|
Stahl PJ and Felsen D: Transforming growth
factor-β, basement membrane, and epithelial-mesenchymal
transdifferentiation: implications for fibrosis in kidney disease.
Am J Pathol. 159:1187–1192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
López-Hernández FJ and López-Novoa JM:
Role of TGF-β in chronic kidney disease: an integration of tubular,
glomerular and vascular effects. Cell Tissue Res. 347:141–154.
2012. View Article : Google Scholar
|
|
5
|
Mozes MM, Böttinger EP, Jacot TA and Kopp
JB: Renal expression of fibrotic matrix proteins and of
transforming growth factor-β (TGF-β) isoforms in TGF-β transgenic
mice. J Am Soc Nephrol. 10:271–280. 1999.PubMed/NCBI
|
|
6
|
Zhang C and Zhang F: The multifunctions of
WD40 proteins in genome integrity and cell cycle progression. J
Genomics. 3:40–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kiely PA, Sant A and O'Connor R: RACK1 is
an insulin-like growth factor 1 (IGF-1) receptor-interacting
protein that can regulate IGF-1-mediated Akt activation and
protection from cell death. J Biol Chem. 277:22581–22589. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berns H, Humar R, Hengerer B, Kiefer FN
and Battegay EJ: RACK1 is up-regulated in angiogenesis and human
carcinomas. FASEB J. 14:2549–2558. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Battaini F, Pascale A, Paoletti R, Govoni
S and Battaini F: The role of anchoring protein RACK1 in PKC
activation in the ageing rat brain. Trends Neurosci. 20:410–415.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian L, Shi J, Zhang C, Lu J, Lu X, Wu K,
Yang C, Yan D, Zhang C, You Q, et al: Downregulation of RACK1 is
associated with cardiomyocyte apoptosis after myocardial
ischemia/reperfusion injury in adult rats. In Vitro Cell Dev Biol
Anim. 52:305–313. 2016. View Article : Google Scholar
|
|
11
|
Hu F, Tao Z, Wang M, Li G, Zhang Y, Zhong
H, Xiao H, Xie X and Ju M: RACK1 promoted the growth and migration
of the cancer cells in the progression of esophageal squamous cell
carcinoma. Tumor Biol. 34:3893–3899. 2013. View Article : Google Scholar
|
|
12
|
Jia D, Duan F, Peng P, Sun L, Liu X, Wang
L, Wu W, Ruan Y and Gu J: Up-regulation of RACK1 by TGF-β1 promotes
hepatic fibrosis in mice. PloS One. 8:e601152013. View Article : Google Scholar
|
|
13
|
Sato M, Muragaki Y, Saika S, Roberts AB
and Ooshima A: Targeted disruption of TGF-β1/Smad3 signaling
protects against renal tubulointerstitial fibrosis induced by
unilateral ureteral obstruction. J Clin Invest. 112:1486–1494.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou P, Shi L, Li Q and Lu D:
Overexpression of RACK1 inhibits collagen synthesis in keloid
fibroblasts via inhibition of transforming growth factor-β1/Smad
signaling pathway. Int J Clin Exp Med. 8:15262–15268. 2015.
|
|
15
|
Blom IE, Goldschmeding R and Leask A: Gene
regulation of connective tissue growth factor: new targets for
antifibrotic therapy? Matrix Biol. 21:473–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao X, Li J, Huang H and Li X: Connective
tissue growth factor stimulates renal cortical myofibroblast-like
cell proliferation and matrix protein production. Wound Repair
Regen. 16:408–415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yokoi H, Mukoyama M, Nagae T, Mori K,
Suganami T, Sawai K, Yoshioka T, Koshikawa M, Nishida T, Takigawa
M, et al: Reduction in connective tissue growth factor by antisense
treatment ameliorates renal tubulointerstitial fibrosis. J Am Soc
Nephrol. 15:1430–1440. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao Y, Liu J, Peng Y, Xiong X, Huang L,
Yang H, Zhang J and Tao L: GSTA3 attenuates renal interstitial
fibrosis by inhibiting TGF-beta-induced tubular
epithelial-mesenchymal transtion and fibronection expression. PloS
One. 11:e01608552016. View Article : Google Scholar
|
|
19
|
Wei MG, Sun W, He WM, Ni L and Yang YY:
Ferulic acid attenuates TGF-β1-induced renal cellular fibrosis in
NRK-52E cells by inhibiting Smad/IlK/Snail pathway. Evid Based
Complement Alternat Med. 2015:6197202015. View Article : Google Scholar
|
|
20
|
Samarakoon R, Overstreet JM, Higgins SP
and Higgins PJ: TGF-β1 → SMAD/p53/USF2 → PAI-1 transcriptional axis
in ureteral obstruction-induced renal fibrosis. Cell Tissue Res.
347:117–128. 2012. View Article : Google Scholar
|
|
21
|
Murphy M, Docherty NG, Griffin B, Howlin
J, McArdle E, McMahon R, Schmid H, Kretzler M, Droguett A, Mezzano
S, et al: IHG-1 amplifies TGF-β1 signaling and is increased in
renal fibrosis. J Am Soc Nephrol. 19:1672–1680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang C, Shen S, Ma Q, Chen J, Gill A,
Pollock CA and Chen XM: Blockade of KCa3.1 ameliorates renal
fibrosis through the TGF-β1/Smad pathway in diabetic mice.
Diabetes. 62:2923–2934. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han Y, Lu JS, Xu Y, Zhang L and Hong BF:
Rutin ameliorates renal fibrosis and proteinuria in
5/6-nephrectomized rats by anti-oxidation and inhibiting activation
of TGFβ1-smad signaling. Int J Clin Exp Pathol. 8:4725–4734.
2015.
|
|
24
|
Shi Y and Massagué J: Mechanisms of TGF-β
signaling from cell membrane to the nucleus. Cell. 113:685–700.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hwang M, Kim HJ, Noh HJ, Chang YC, Chae
YM, Kim KH, Jeon JP, Lee TS, Oh HK, Lee YS, et al: TGF-β1 siRNA
suppresses the tubulointerstitial fibrosis in the kidney of
ureteral obstruction. Exp Mol Pathol. 81:48–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schnaper HW, Jandeska S, Runyan CE,
Hubchak SC, Basu RK, Curley JF, Smith RD and Hayashida T: TGF-beta
signal transduction in chronic kidney disease. Front Biosci
(landmark Ed). 14:2448–2465. 2009. View
Article : Google Scholar
|
|
27
|
Liu N, Tolbert E, Pang M, Ponnusamy M, Yan
H and Zhuang S: Suramin inhibits renal fibrosis in chronic kidney
disease. J Am Soc Nephrol. 22:1064–1075. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vindevoghel L, Lechleider RJ, Kon A, de
Caestecker MP, Uitto J, Roberts AB and Mauviel A: SMAD3/4-dependent
transcriptional activation of the human type VII collagen gene
(COL7A1) promoter by transforming growth factor β. Proc Natl Acad
Sci USA. 95:14769–14774. 1998. View Article : Google Scholar
|
|
29
|
Lan HY: Diverse roles of TGF-β/Smads in
renal fibrosis and inflammation. Int J Biol Sci. 7:1056–1067. 2011.
View Article : Google Scholar :
|