Introduction
Preeclampsia (PE) is the main cause of maternal
mortality and morbidity worldwide. Marked decreases in eclampsia
rates and maternal mortality and morbidity rates have been recorded
in developed countries over the past five decades (1). The persistent high occurrence in
developing countries has been reported to be mainly due to
inadequate prenatal care (2). PE
complications lead to an annual maternal mortality rate of
>5,000. The maternal mortality rates in developing countries are
>15%, compared with rates of 0-1.8% in developed countries due
to lack of access to appropriate maternal care (3). However, studies have suggested that
eculizumab, a targeted inhibitor of complement protein C5, may
result in significant clinical improvement for patients with severe
PE/HELLP syndrome at the 26th week of pregnancy, resulting in
complete normalization of laboratory parameters (4). Additionally, the human protein
α1-microglobulin (A1M), an endogenous antioxidation protection
protein, has been shown to protect the placenta from
hemoglobin-induced damage and restore the blood-placental barrier,
suggesting that A1M may contribute to future pharmacological
treatment for PE (5). Genetic
mutations and regulatory mechanisms in PE require clarification to
identify an effective and safe strategy for its treatment.
G protein γ 7 (GNG7) is a subunit of a
heterotrimeric G protein with ubiquitous expression in various
tissues but low expression in cancer (6). This protein may be associated with
transmembrane signaling pathways and involved in cell
contact-induced growth arrest, thus repressing uncontrolled cell
proliferation in multicellular organisms (7). Data from previous studies suggest
that the components of G protein signaling pathways, including
regulator of G-protein signaling 2 and rs4606, may have effects on
the progression and risk of PE (8). In addition, the mammalian target of
rapamycin (mTOR) signaling pathway can be inhibited by GNG7 which
is involved in autophagy and cell death (6). mTOR, which is a serine/threonine
protein kinase that encompasses two multiprotein complexes (mTORC1
and mTORC2), has been demonstrated to be a downstream marker of the
phosphoinositide 3-kinase/AKT signaling pathway (9). Of critical importance, mTOR, which
is ubiquitously expressed in cells, can be regarded as a
therapeutic target for cancer in humans (10). In addition, activation of the mTOR
signaling pathway has been demonstrated to improve the pregnancy
outcomes of rats induced by N-carbamylglutamate (11). A previous study confirmed that
suppression of the mTOR signaling pathway induces the occurrence of
PE through decreasing the invasion ability of trophoblast cells
(12). Consequently, GNG7 and the
mTOR signaling pathway may be involved in the proliferation and
apoptosis of placental cytotrophoblasts in PE. Therefore, the
present study examined the effects of GNG7 on PE through the mTOR
signaling pathway to provide a theoretical basis for PE treatment
in the near future.
Materials and methods
Ethics statement
The present study was conducted in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The
protocol was approved by the Institutional Animal Care and Use
Committee of Second Xiangya Hospital, Central South University
(Changsha, China).
Model establishment
A total of 45 specific pathogen-free Sprague-Dawley
rats (30 females and 15 males) aged 2-3 months and weighing 200-220
g were purchased from the Medical Experimental Animal Center of
Guangdong Province (Guangdong, China). The mice were acclimatized
for 1 week and housed in well-ventilated cages at 25-26°C with a
relative humidity of ~70%, regular ultraviolet disinfection, light
exposure between 6:00 a.m. and 6:00 p.m., and free intake of food
and water. The rats were then mated (2:1 female:male), and vaginal
smears were collected the following morning and observed under a
microscope. The day on which the presence of sperm was observed on
the smears was defined as pregnancy day 0. A total of 25 pregnant
rats were randomly classified into the PE group (12 rats) and the
normal group (10 rats) on the 5th to 10th days of pregnancy, and
the remaining three rats were used for the follow-up experiments.
From the 10th day of pregnancy, NG-nitro-L-arginine methyl ester at
a dose of 100 mg/(kg·day) was continuously and subcutaneously
injected into the rats in the PE group, and the same volume of
normal saline was injected into the rats in the normal group. PE
manifestations in the rats a included higher systolic blood
pressure (BP) of 110.12±5.73 mmHg and urine protein (6.33±0.42
mg/24 h) on the 18th day of pregnancy compared with those in the
normal group, indicating successful establishment of the PE model
(13).
The mother rats were sacrificed following cesarean
delivery to collect the uterine placenta. Part of the uterine
placenta tissue was used for extraction and detection of total
protein and RNA, and the isolation and culture of primary
cytotrophoblasts; and the other part was preserved for the other
follow-up experiments.
BP measurement
The BP of the rats was measured with the tail-cuff
method using a Coda noninvasive BP system (Kent Scientific
Corporation, Torrington, CT, USA) on the 6th, 12th and 18th days of
pregnancy. The BP measurement was performed at 8:00 a.m. with the
following requirements: First, the rats were fasted for 2 h prior
to measurement and then placed on a preheated thermostat (37°C) for
10 min prior to measurement; subsequently, during measurement, the
room temperature was set at 25°C, and the BP of the rats was
measured using the preadjusted tail-cuff device with the clamp
placed at the root of the rat tails when their heart rates were
stable. The BP of each rat was measured three times and then
averaged.
Automatic biochemical analysis
Urine was collected from the rats in each group on
the 6th, 12th and 18th days of pregnancy to detect the urine
protein levels through full automatic biochemical analysis.
Following 10 min of centrifugation of the urine at 717 × g and 4°C,
the sediment was removed. Biuret reagent (HPBIO-R1253, Pengpai
Biotech Company, Shanghai, China) was then added to the urine
samples to detect urine proteins. On the 21st day of pregnancy, the
rat placental tissues were obtained from the uterus following
cesarean delivery; part of the tissue was used for the extraction
and detection of total protein and RNA and for primary
cytotrophoblast isolation, and the remainder was fixed for use in
the other follow-up experiments.
Hematoxylin and eosin (H&E)
staining
The placental tissues preserved in 4% formalin were
obtained, dehydrated in gradient alcohol (75, 80, 90, 95 and 100%)
twice for 1.5 h each series, and cleared with xylene twice for 8
min each time. Subsequently, the tissues were embedded in paraffin
and sliced into 5-7-µm-thick slices using a microtome
(RM2016; Leica, Shanghai, China). The slices were then incubated at
55°C, deparaffinized in xylene and hydrated with gradient alcohol
at concentrations of 100, 95, 85 and 75% (3 min each
concentration). The tissues were then stained with hematoxylin for
8 min, washed with water for 2 min, differentiated with
hydrochloric alcohol for 5 sec, and washed with water for 2 min.
The tissues were treated with 0.25% ammonia for 1 min and washed
with water for 2 min. Subsequently, the tissues were stained using
eosin for 30 sec and washed with water for 2 min prior to
dehydration twice with a gradient alcohol series (85, 95 and 100%;
2 min for each) and xylene clearing twice (5 min each). When the
staining was completed, the sample slides were mounted using
neutral balsam and sealed with clean cover slips. The
histopathological changes in the placenta were observed under a
microscope (XSP-2C; Bingyu Optical Instrument Co., Ltd., Shanghai,
China).
Immunohistochemistry
The placental tissues immersed in 4%
paraformaldehyde at 4°C were collected and dehydrated. The
paraffin-embedded tissues were sectioned into 5-µm-thick
slices, dewaxed, and stained with H&E. Following air-drying at
room temperature for 24 h, the slices were placed into a
phosphate-buffered saline (PBS) container (pH 7.4) and diluted with
0.05 mol/dm3 tris(hydroxymethyl)aminomethane
hydrochloride containing 1% bovine serum albumin (BSA; Yancheng
Saibao Biotechnology Co., Ltd., Yancheng, China) at pH 7.6. The
slices were labeled with a modified biotinylated anti-rat
immunoglobulin for 15 min. The slices were then incubated with
primary antibodies at 4°C overnight, including polyclonal rabbit
anti-4E-BP1, 4E-binding protein 1 (4E-BP1; 1:100, cat. no. ab2606),
phosphoprotein 70 ribosomal protein S6 kinase (p70S6K; 1:100, cat.
no. ab59208) and mTOR (1:100, cat, no. ab2732), and then washed
three times in PBS for 5 min per wash. The above antibodies were
all procured from Abcam (Cambridge, MA, USA). Subsequently, the
slices were incubated with the secondary antibody (goat anti-rabbit
immunoglobulin G, IgG, 1:1,000, cat. no. ab6721; Abcam) at 37°C for
30 min. The staining results were observed and images were captured
under an optical microscope (14). Five high-magnification fields with
100 cells per field were randomly selected for each slice. The
staining intensity was graded as follows: <10% positive cells,
negative; ≥10 and <50%, positive; and ≥50% positive cells,
strongly positive.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the placental tissue
samples or transfected cytotrophoblast cells using the TRIzol
reagent one-step method according to the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
extracted RNA was then reverse transcribed into complementary DNA
(cDNA) following the two-step instructions of the PrimeScript™ RT
Reagent kit (RR037Q; Takara Biotechnology, Co., Ltd., Dalian,
China) in a reaction system that contained 2 µl of 5X
PrimeScript buffer (for real time), 0.5 µl of PrimeScript RT
Enzyme mix I, 0.5 µl of the Oligo dT primer (50 µM),
0.5 µl of random 6-mers (100 µM), 2 µg of
total RNA and RNase-free dH2O to a total volume of 20
µl. The reaction was conducted under the following
conditions: Reverse-transcribed at 37°C for 15 min, inactivation of
reverse transcriptase at 85°C for 5 sec. The cDNA was temporarily
preserved at −80°C. Subsequent RT-qPCR analysis was conducted using
the TaqMan probe method, and the reaction system was performed
according to the instructions of the kit purchased from Marrone Bio
Innovations, Inc. (MBI, Davis, CA, USA). The primer sequences are
shown in Table I and the reaction
conditions were as follows: Predenaturation at 95°C for 30 sec and
40 cycles of denaturation at 95°C for 10 sec, annealing at 60°C for
20 sec and extension at 72°C for 10 sec. The reaction system
included 12.5 µl of Premix Ex Taq or SYBR-Green Mix, 1
µl of the forward primer, 1 µl of the reverse primer,
1-4 µl of cDNA and ddH2O to a total volume of 25
µl. The relative expression levels were determined using a
Real-Time PCR instrument (Bio-Rad iQ5; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) with glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) as an internal reference. The primer sequences shown in
Table I were synthesized by
Shanghai Generay Biotech Co., Ltd. (Shanghai, China), and the
reliability of the PCR results was evaluated using melting curve
analysis. The quantification cycle (Cq) was obtained and used in
the following formulas: ΔCq = Cq(target gene) -
Cq(GAPDH), and ΔΔCq = ΔCq(experiment group) -
ΔCq(control group) (15). The experiment was repeated three
times, and the average values were obtained.
 | Table IPrimer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer sequence
(5′-3′) |
|---|
| GNG7 | F:
TTAGGTGTGCCAGTGTGGTC |
| R:
CCATGGGCAGTTTGAGCAAC |
| 4E-BP1 | F:
AAAGGATCCGGGGGCAGCAGGTGCAGCCAGACCC |
| R:
AAACTCGAGTTACTGTGACTCTTCACCGACCCGCC |
| p70S6K | F:
CTACAGAGACCTGAAGCCGGAGA |
| R:
AATGTGTGCGTGACTGTTCCATC |
| mTOR | F:
ATGACGAGACCCAGGCTAA |
| R:
GCCAGTCCTCTACAATACGC |
| VEGF | F:
TGCTCCATTCTGTAGGCTCTG |
| R:
CTGGCCCATAAATCATCCC |
| TGF-β1 | F:
CGAGGTGACCTGGGCACCATCCATGAC |
| R:
CTGCTCCACCTTCTTGGGCTTGCGACCCAC |
| AP-2γ | F:
CGCATCCCTCACCTCTCATC |
| R:
TAATGATCGGCGGATTGCGA |
| AP-2α | F:
CGCGGATCCACCACCATGGGCATGCTTTGGAAACTGACGGATAAT |
| R:
CCCAAGCTTTCACTTTCTGTGTTTCTCTTCTTTG |
| Bax | F:
AGACACCTGAGCTGACCTTGGAG |
| R:
GTTGAAGTTGCCATCAGCAAAC |
| Bcl-2 | F:
TGAACCGGCATCTGCACAC |
| R:
CGTCTTCAGAGACAGCCAGGAG |
| GAPDH | F:
GGCACAGTCAAGGCTGAGAATG |
| R:
ATGGTGGTGAAGACGCCAGTA |
Western blot analysis
The total proteins were extracted from the placental
tissues isolated from each group of rats and the protein
concentration was measured using the bicinchoninic acid assay. The
tissues were treated with 5X sodium dodecyl sulfate lysis buffer
(P0013G; Beyotime Institute of Biotechnology, Beijing, China) and
boiled at 100°C for 5 min to denature the proteins. The proteins
(20 µl) were loaded onto a polyacrylamide gel composed of a
5% stacking gel and a 12% separating gel for electrophoresis.
Following transfer, the membrane was blocked for 1 h on a shaker at
room temperature using Tris-buffered saline + Tween (TBST) with 5%
BSA. The blocking solution was then discarded, and the membrane was
placed into a plastic slot and oscillated overnight at 4°C with the
following primary antibodies diluted with 5% BSA: Polyclonal rabbit
anti-rat GNG7 (1 µg/ml, cat. no. ab169493), polyclonal
rabbit anti-rat 4E-BP1 (1:1,000, cat. no. ab2606), polyclonal
rabbit anti-rat p70S6K (1:250, cat. no. ab9366), polyclonal rabbit
anti-rat mTOR (1:2,000, cat. no. ab2732), polyclonal rabbit
anti-rat phosphorylated (p-)4E-BP1 (1:1,000, cat. no. ab47365),
polyclonal rabbit anti-rat p-p70S6K (2 µg/ml, cat. no.
ab2571), monoclonal rabbit anti-rat mTOR (1:2,000, cat. no.
ab109268), polyclonal rabbit anti-rat vascular endothelial growth
factor (VEGF; 3 µg/ml, cat. no. ab11939), polyclonal rabbit
anti-rat transforming growth factor-β1 (TGF-β1; 3 µg/ml,
cat. no. ab92486), polyclonal rabbit anti-rat activator protein-2γ
(AP-2γ; 1:1,000, ab203691), polyclonal rabbit anti-rat AP-2α
(1:1,000, cat. no. ab108311), monoclonal rabbit anti-rat B-cell
lymphoma 2 (Bcl-2)-associated X protein (Bax; 1:1,000, cat. no.
ab53154), and monoclonal rabbit anti-rat Bcl-2; 1:500, cat. no.
ab59348) (all purchased from Abcam). GAPDH (1:1,000, cat. no.
ab8245, Abcam) was used as an internal reference. The following
day, the membrane was rinsed three times with TBST (10 min each),
incubated with a diluted secondary antibody (goat anti-rabbit IgG,
1:2,000, cat. no. ab6721; Abcam) at 4°C for 4-6 h and then washed
three times with TBST (15 min each). Chemiluminescence reagents A
and B (Yanhui Biological Co., Ltd., Shanghai, China) were mixed at
a 1:1 ratio and evenly dropped onto the nitrocellulose membrane for
image development. Using the gray value ratio of the target band to
the GAPDH band to represent relative protein expression, the
relative optical density (OD) value was analyzed for all bands. The
experiment was repeated three times, and the average values were
calculated.
Cell isolation, culture and
transfection
The placental tissues were cut into slices with a
thickness of ~3 mm and detached with 2.5 g/l of trypsin in a 37°C
water bath three times for 10 min each time. The obtained cell
suspension was then centrifuged at 225 × g for 5 min at 37°C,
washed twice with PBS and resuspended in Dulbecco's modified
Eagle's medium/F12 (HyClone; GE Healthcare Life Sciences, Logan,
UT, USA) containing 100 ml/l of fetal bovine serum (TBD Sciences,
Tianjin, China). The cells were then inoculated into the culture
plate and incubated in a 37°C and 5% CO2 incubator.
Epithelioid cells growing in a lamellar shape were observed under
an inverted microscope. The cells identified as cytotrophoblasts
without parenchymal cells were further cultured in a 37°C and 5%
CO2 incubator. Following cell passage, the
third generation cytotrophoblasts were used for transfection.
Placental cytotrophoblasts isolated from the normal
and PE rats were assigned into the normal group (cytotrophoblasts
isolated from normal rats), blank group (cytotrophoblasts isolated
from PE rats without any treatment), negative control (NC) group
(cytotrophoblasts isolated from PE rats transfected with the empty
plasmid), GNG7-siRNA group [cytotrophoblasts isolated from PE rats
transfected with the GNG7-small interfering (si)RNA plasmid], HIV-1
Tat group (cytotrophoblasts isolated from PE rats treated with the
mTOR activator HIV-1 Tat, 48-60; Seebio Biotech, Inc., Shanghai,
China), GNG7-siRNA + HIV-1 Tat group (cytotrophoblasts isolated
from PE rats transfected with the GNG7-siRNA plasmid and treated
with the mTOR activator HIV-1 Tat), GNG7-siRNA + dimethylsulfoxide
(DMSO) group (cytotrophoblasts isolated from PE rats treated with
GNG7-siRNA and DMSO) and GNG7-siRNA + rapamycin group
(cytotrophoblasts isolated from PE rats treated with GNG7-siRNA and
the mTOR inhibitor rapamycin). Placental cytotrophoblasts at the
logarithmic growth phase were inoculated into a 6-well plate and
transfected with Lipofectamine 2000 reagent in accordance with the
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.) when cell confluence reached 30-50%. Subsequently, 100 pmol
of GNG7-siRNA and the empty plasmid were separately diluted using
250 µl of Opti-MEM serum-free medium (Gibco; Thermo Fisher
Scientific, Inc.) to a final concentration of 50 nM and incubated
at room temperature for 5 min. Subsequently, 5 µl of
Lipofectamine 2000 was diluted with 250 µl of serum-free
Opti-MEM and incubated at room temperature for 5 min. The above two
dilutions were mixed together and incubated at room temperature for
20 min prior to inoculation into the cell culture plates. Following
6-8 h of incubation at 5% CO2 and 37°C, the cells were
cultured in complete medium for another 1 or 2 days and then used
for the follow-up experiments.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) assay
Cells in the logarithmic growth phase were
inoculated into a 96-well plate (1×105 cells/ml) and
assigned to six groups (8 wells per group; 100 µl per well)
and cultured in an incubator with 5% CO2 at 37°C. After
24, 48 and 72 h of culture, each well was supplemented with 10
µl of MTT solution (5 mg/ml, SBJ-0190; Nanjing SenBeiJia
Biological Technology Co., Ltd., Nanjing, China) and incubated for
an additional 4 h. The OD values of each well were measured at 570
nm using a microplate reader.
Flow cytometry
Following 48 h of transfection, the cell culture
medium was discarded, and the cells were washed with PBS once,
treated with 0.25% trypsin and then collected. Annexin V and
propidium iodide (PI) staining (R&D Systems, Inc., Minneapolis,
MN, USA) was conducted using an in situ apoptosis detection
kit. The cells were suspended in 80 µl of balanced salt
solution that included 10 mmol/l of HEPES, 140 mmol/l of sodium
chloride and 2.5 mmol/l of calcium chloride (pH 7.4). To each cell
suspension, 10 µl of Annexin V (10 µg/ml) and 10
µl of PI reagent (50 µg/ml) were added. The cells
were mixed and incubated at room temperature for 15 min, and then
400 µl of buffer salt solution was added. Apoptosis and the
cell cycle distribution were immediately analyzed by flow
cytometry. Control tubes of unstained cells, PI single-stained
cells and Annexin V-stained cells were included to set the flow
cytometric compensation. Subsequently, the cells were analyzed
using a flow cytometer (FACSCalibur; BD Biosciences, Franklin
Lakes, NJ, USA), and the cell cycle distribution and induction of
apoptosis were evaluated when red fluorescence was detected at an
excitation wavelength of 488 nm. The experiment was repeated three
times.
Enzyme-linked immunosorbent assay
(ELISA)
The super-natants were collected from cell cultures
in each group. The soluble fms-like tyrosine kinase 1 (sFlt-1) and
soluble endoglin (sEng) levels were determined according to the
instructions of the ELISA kits (Abcam). The absorbance value
A450 was measured at the 450 nm wavelength. Three
parallel wells were set for each sample, and the mean value was
obtained.
Statistical analysis
All data were processed with SPSS 21.0 statistical
software (IBM Corp., Armonk, NY, USA). Measurement data are
expressed as the mean ± standard deviation, and differences in
normally distributed and homogeneous data between two groups were
examined with an unpaired t-test. One-way analysis of variance
(ANOVA) was performed for comparison among multiple groups.
Comparisons of normally distributed data among multiple groups were
performed using Tukey's post hoc test. A repeated measures ANOVA
was conducted to assess cell viability at different time points.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PE model establishment with higher
systolic/diastolic BP and urine protein levels
To verify successful establishment of the PE model,
tail-cuff BP measurement and automatic biochemical analysis were
performed. Prior to pregnancy, no significant differences in
systolic/diastolic BP or urine protein were observed between the
normal and PE rats (P<0.05). On the 12th and 18th days of
pregnancy, the systolic/diastolic BP and urine protein were
increased in the PE rats compared with those in the normal rats. As
shown in Table II, the
systolic/diastolic BP and urine protein values were all increased
in the PE rats, suggesting that the PE model was successfully
established.
 | Table IIBP and urinary protein levels prior
to modeling and on days 12 and 18 after modeling. |
Table II
BP and urinary protein levels prior
to modeling and on days 12 and 18 after modeling.
| Indicator | Normal group
(n=10) | PE group (n=12)
|
|---|
| Pre-modeling | Day 12 after
modeling | Day 18 after
modeling |
|---|
| Systolic BP
(mmHg) | 108.74±9.03 | 114.90±11.06 |
132.78±12.35a |
148.23±13.64a |
| Diastolic BP
(mmHg) | 81.29±8.12 | 79.81±6.42 | 95.45±7.67a | 104.95±9.43a |
| 24-h urine protein
(mg) | 6.51±0.84 | 6.68±0.71 | 8.56±0.52a | 11.37±1.14a |
Proliferation and differentiation of
placental cytotropho- blasts are suppressed in PE rats
H&E staining was performed to observe
histopathological changes of the placenta in the normal and PE
rats. The results (Fig. 1A and B)
demonstrated that the cytotrophoblasts in the placental villi
invaded into the mesometrium in the normal rats, whereas the
placental villi in the PE rats showed limited cytotrophoblast
invasion, narrow spiral arterioles, hyperplastic endothelial cells,
capillary occlusion, placental tissue apoptosis, detachment of
endothelial cells from the basilar membrane, presence of
macrophages in the thrombus area and transformation of
cytotrophoblasts into syncytiotrophoblasts (16). Additionally, the placental villi
in the PE rats were characterized by nonuniform placental decidual
mesenchymal cells, decidual vascular endothelial cell fibrosis and
increased decidual fibrous necrosis. Therefore, the proliferation
and differentiation of placental cytotrophoblasts were inhibited in
the PE rats.
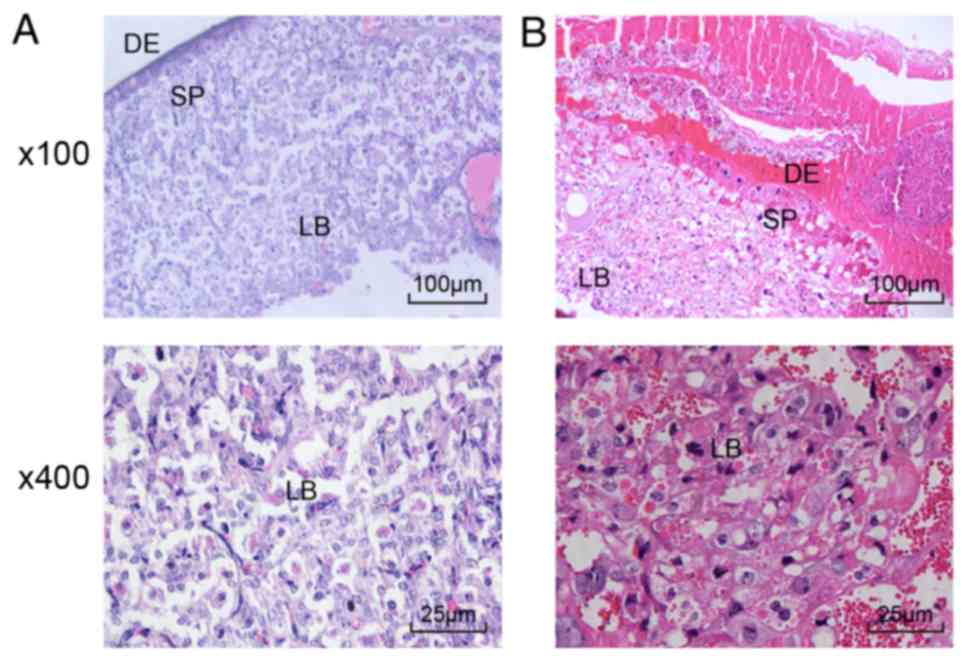 | Figure 1Proliferation and differentiation of
placental trophoblasts are suppressed in PE rats (magnification,
×100). (A) H&E staining of placental tissues of normal rats. In
normal rats, the placental villi were dominated by
syncytiotrophoblasts. (B) H&E staining of placental tissues of
PE rats. In the PE rats, the placental villi were characterized by
cytotrophoblast cell proliferation, immature villi, nonuniform
placental decidua mesenchymal cells, decidual vascular endothelial
cell fibrosis and notably increased decidual fibrous necrosis.
H&E, hematoxylin and eosin; PE, preeclampsia; LB, labyrinth;
DE, decidua; SP, spongiotrophoblast. |
4E-BP1, p70S6K and mTOR are downregulated
in PE placental tissues
Immunohistochemical staining was performed to
measure the positive expression rates of the 4E-BP1, p70S6K and
mTOR proteins in placental tissues from the normal and PE rats. The
immunohistochemical staining (Fig.
2A-D) showed that the 4E-BP1, p70S6K and mTOR proteins were
mainly expressed in the cytoplasm and that the positive cells were
light yellow, tan or tan brown in color. Compared with that in the
normal rats, positive expression of the 4E-BP1, p70S6K and mTOR
proteins was evidently reduced in the placental tissues from the PE
rats (P<0.05). These data revealed that the mTOR signaling
pathway was inactivated in the placental tissues of the PE
rats.
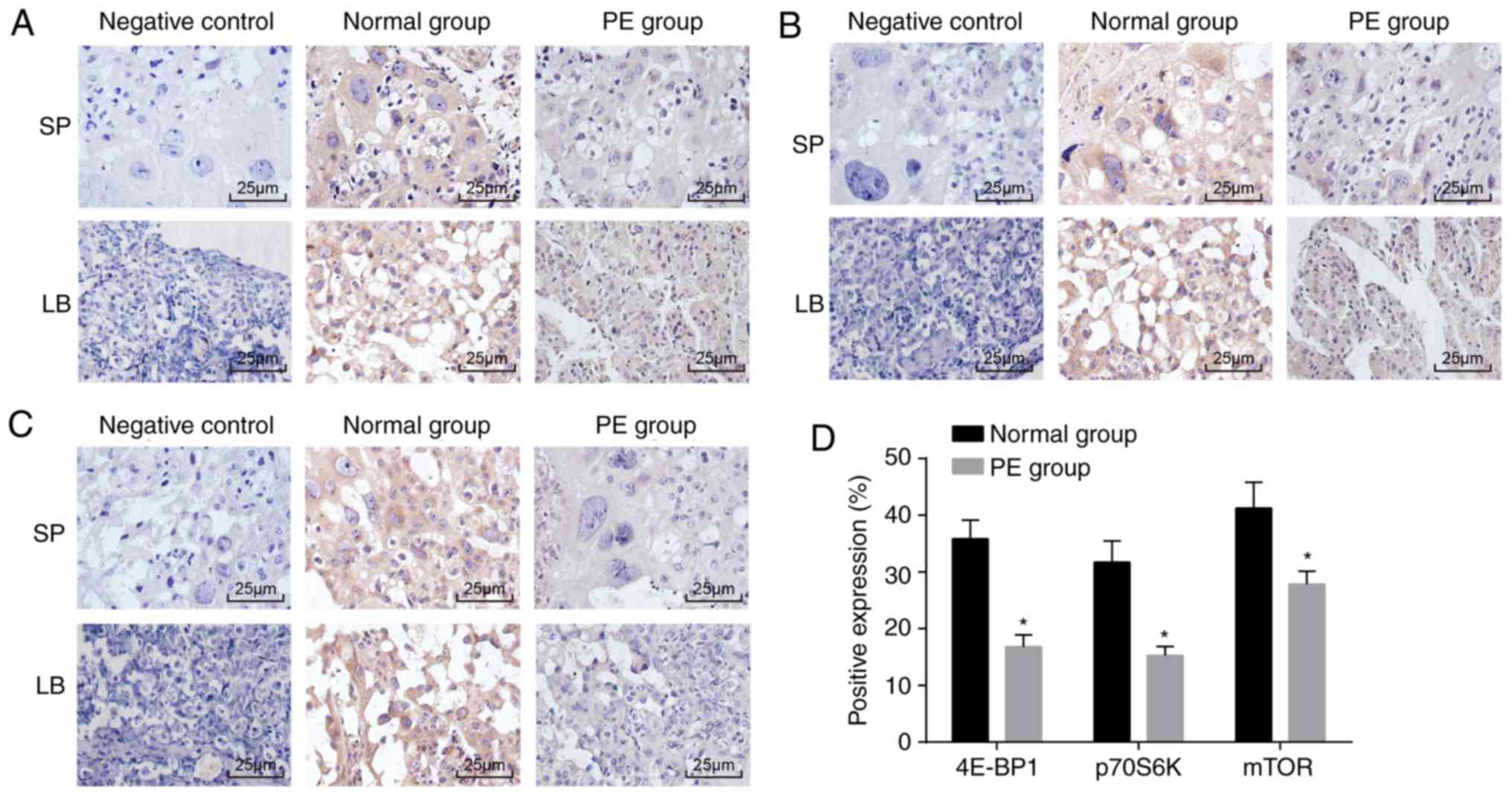 | Figure 24E-BP1, p70S6K and mTOR are reduced
in placental tissues from PE rats. (A) Immunohistochemical staining
showing positive expression of mTOR (magnification, ×400); (B)
positive expression of 4E-BP1 in placental tissues of PE rats
detected by immunohistochemical staining (magnification, ×400); (C)
positive expression of p70S6K in the PE placenta detected by
immunohistochemical staining (magnification, ×400); (D) protein
expression of 4E-BP1, p70S6K and mTOR in placental tissues from PE
rats; *P<0.05 compared with the normal rats. The
measurement data are expressed as the mean ± standard deviation and
were analyzed with an unpaired t-test. The sample size of the
normal group was n=10 and of the PE group was n=12; mTOR, mammalian
target of rapamycin; 4E-BP1, 4E-binding protein 1; p70S6K,
phosphoprotein 70 ribosomal protein S6 kinase; PE, preeclampsia;
LB, labyrinth; SP, spongiotrophoblast. |
GNG7 is upregulated and the mTOR
signaling pathway is inactivated in PE rats
RT-qPCR and western blot analyses were conducted to
detect the mRNA and protein expression levels of GNG7 and mTOR
signaling pathway-related genes in the normal and PE rats; the
results are shown in Fig. 3A-C.
In contrast to those of the normal rats, the mRNA and protein
levels of 4E-BP1, p70S6K, mTOR, VEGF, TGF-β1, AP-2γ and Bcl-2, and
the extent of 4E-BP1, p70S6K and mTOR phosphorylation were
significantly decreased in the placental tissues of the PE rats,
whereas the mRNA and protein expression levels of GNG7, AP-2α and
Bax were elevated (all P<0.05). These results showed that GNG7
was expressed at high levels in the placental tissues of the PE
rats and that mTOR signaling pathway activation was inhibited in
PE.
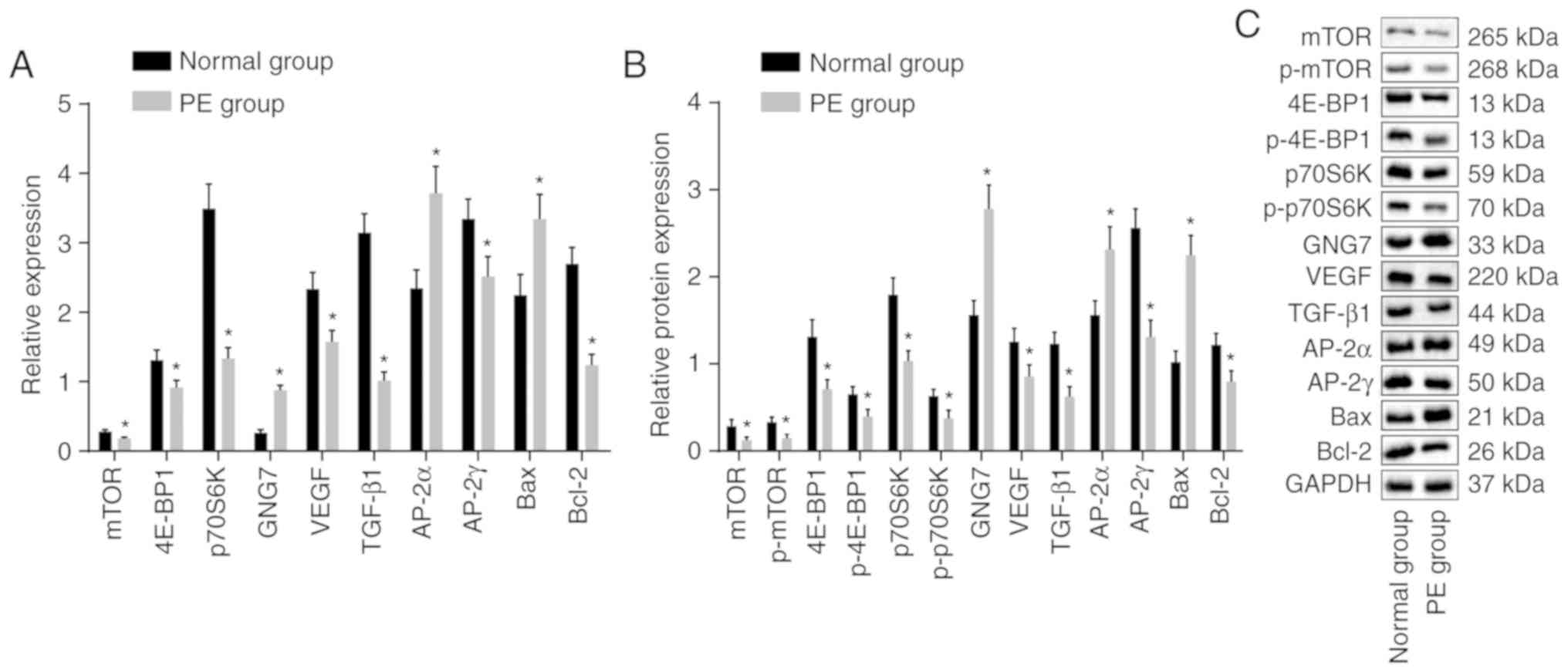 | Figure 3GNG7 is expressed at a high level in
placental tissues of PE rats and mTOR signaling pathway activation
is suppressed. (A) mRNA expression of 4E-BP1, p70S6K, mTOR, VEGF,
TGF-β1, AP-2γ, Bcl-2, GNG7, AP-2α and Bax in the PE rats,
determined by reverse transcription-quantitative polymerase chain
reaction analysis. (B) Protein levels of 4E-BP1, p70S6K, mTOR,
VEGF, TGF-β1, AP-2γ, Bcl-2, GNG7, AP-2α and Bax, and the extent of
4E-BP1, p70S6K and mTOR phosphorylation in the PE rats were
measured from (C) blots obtained by western blot analysis.
*P<0.05, compared with the normal group. The mRNA and
protein level measurement data are expressed as the mean ± standard
deviation and were analyzed with an unpaired t-test. The sample
sizes of the normal and PE groups were 10 and 12, respectively. PE,
preeclampsia; GNG7, G protein γ 7; 4E-BP1, 4E-binding protein 1;
p70S6K, phosphoprotein 70 ribosomal protein S6 kinase; mTOR,
mammalian target of rapamycin; VEGF, vascular endothelial growth
factor; TGF-β1, transforming growth factor-β1; AP-2γ, activator
protein-2γ; AP-2α, activator protein-2α; Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X protein; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase; p-, phosphorylated. |
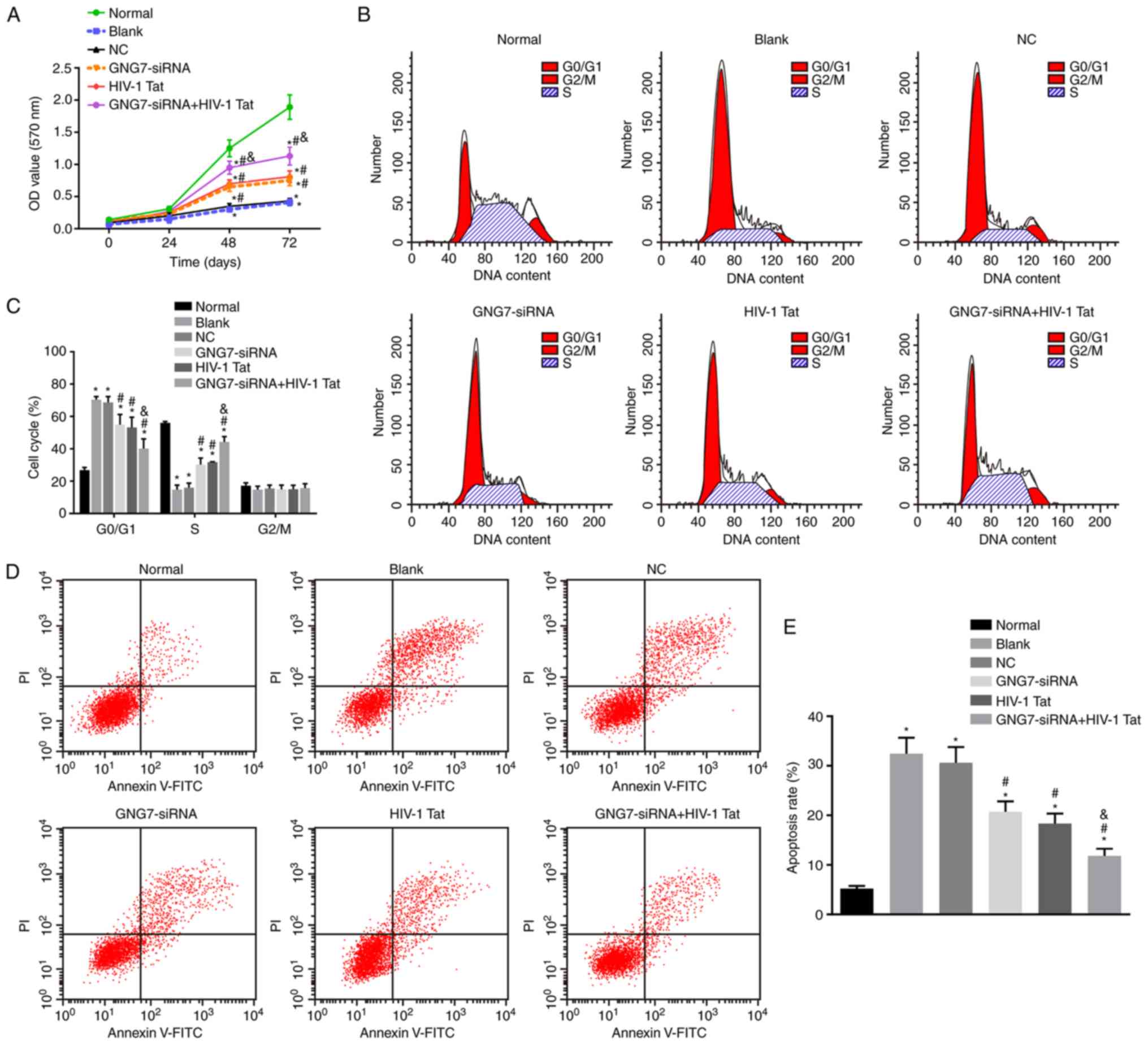
GNG7 silencing activates the mTOR
signaling pathway to enhance proliferation and differentiation and
suppress apoptosis of placental cytotrophoblasts in PE rats
To analyze the effects of GNG7 silencing on the
proliferation, differentiation and apoptosis of placental
cytotrophoblasts in the PE rats, RT-qPCR and western blot analyses
were performed to examine the mRNA and protein levels of GNG7, mTOR
signaling pathway-related factors (4E-BP1, p70S6K and mTOR), cell
proliferation-related factors (VEGF and TGF-β1), cell
differentiation-related factors (AP-2α and AP-2γ) and cell
apoptosis-related factors (Bax and Bcl-2); the results are shown in
Fig. 4A-E. Compared with those of
the normal group, the mRNA and protein levels of 4E-BP1, p70S6K,
mTOR, VEGF, TGF-β1, AP-2γ and Bcl-2 and the extent of 4E-BP1,
p70S6K and mTOR phosphorylation were markedly decreased in the
remaining five groups, whereas the mRNA and protein levels of GNG7,
AP-2α and Bax were notably increased (all P<0.05). No
significant difference was found in the expression of these genes
between the blank and NC groups (P>0.05). Compared with the
blank and NC groups, the GNG7-siRNA, HIV-1 Tat and GNG7-siRNA +
HIV-1 Tat groups exhibited elevated mRNA and protein levels 4E-BP1,
p70S6K, mTOR, VEGF, TGF-β1, AP-2γ and Bcl-2, and phosphorylation of
4E-BP1, p70S6K and mTOR, but reduced mRNA and protein levels of
AP-2α and Bax (all P<0.05). The mRNA and protein levels of GNG7
in the GNG7-siRNA and GNG7-siRNA + HIV-1 Tat groups were markedly
decreased compared with those in the blank and NC groups (all
P<0.05). Compared with the GNG7-siRNA and HIV-1 Tat groups, the
GNG7-siRNA + HIV-1 Tat group presented with increased mRNA and
protein levels 4E-BP1, p70S6K, mTOR, VEGF, TGF-β1, AP-2γ and Bcl-2,
and phosphorylation of 4E-BP1, p70S6K and mTOR, but decreased mRNA
and protein levels AP-2α and Bax (all P<0.05). These results
suggested that silencing the expression of GNG7 led to enhanced
proliferation and differentiation of placental cytotrophoblasts in
the PE rats, but reduced apoptosis, via activation of the mTOR
signaling pathway.
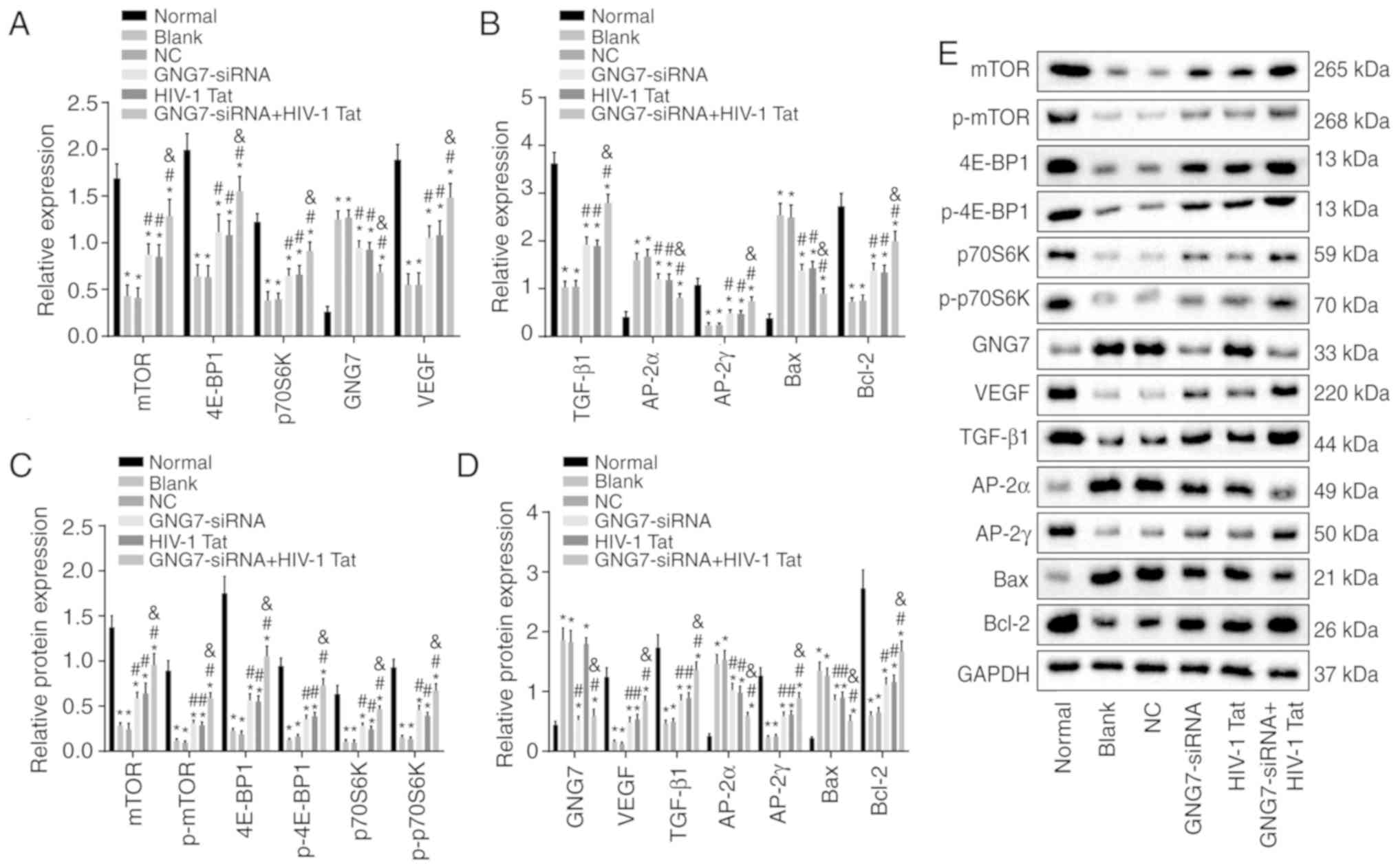 | Figure 4GNG7 silencing activates the mTOR
signaling pathway, promotes the proliferation and differentiation
of placental cytotrophoblasts in PE rats and inhibits apoptosis.
mRNA expression levels of (A) mTOR, 4E-BP1, p70S6K, GNG7, VEGF and
(B) TGF-β1, AP-2γ, AP-2α, Bcl-2 and Bax were determined in the PE
rats in response to GNG7-siRNA or HIV-1 Tat treatment or GNG7-siRNA
and HIV-1 Tat cotreatment by reverse transcription-quantitative
polymerase chain reaction analysis. (C) Protein levels of mTOR,
4E-BP1 and p70S6K, extent of 4E-BP1, p70S6K and mTOR
phosphorylation and (D) protein levels of GNG7, VEGF, TGF-β1,
AP-2γ, AP-2α, Bcl-2 and Bax were determined in the PE rats in
response to GNG7-siRNA or HIV-1 Tat treatment or GNG7-siRNA and
HIV-1 Tat cotreatment by western blotting. (E) Protein levels of
4E-BP1, p70S6K, mTOR, VEGF, TGF-β1, AP-2γ, Bcl-2, GNG7, AP-2α and
Bax, p-4E-BP1, p-p70S6K and p-mTOR. *P<0.05 compared
with the normal group; #P<0.05 compared with the
blank and NC groups; &P<0.05 compared with the
GNG7-siRNA and HIV-1 Tat groups. The measurement data are expressed
as the mean ± standard deviation and were analyzed by one-way
analysis of variance. The experiment was repeated three times; PE,
preeclampsia; GNG7, G protein γ 7; 4E-BP1, 4E-binding protein 1;
p70S6K, phosphoprotein 70 ribosomal protein S6 kinase; mTOR,
mammalian target of rapamycin; VEGF, vascular endothelial growth
factor; TGF-β1, transforming growth factor-β1; AP-2γ, activator
protein-2γ; AP-2α, activator protein-2α; Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X protein; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase; p-, phosphorylated; siRNA, small interfering RNA;
NC, negative control. |
GNG7 silencing promotes placental
cytotrophoblast prolif- eration and differentiation and inhibits
apoptosis in PE rats
Placental cytotrophoblasts from the six groups were
inoculated into a 96-well plate, and their viability was observed
using an MTT assay to investigate the ability of GNG7 to affect the
biological function of placental cytotrophoblasts. The results
(Fig. 5A) demonstrated that, 24 h
following the respective treatments, no significant difference was
present in the viability of the placental cytotrophoblasts in each
group (all P>0.05). However, cell viability was decreased over
time in the cytotrophoblasts isolated from the PE rats without
treatment, those treated with the empty plasmid, GNG7-siRNA or
HIV-1 Tat and those cotreated with GNG7-siRNA and HIV-1 Tat
compared with that of the cytotrophoblasts isolated from the normal
rats (P<0.05). Compared with the cytotrophoblasts without
treatment or those treated with the empty plasmid, the viability of
placental trophoblasts was increased significantly in the
cytotrophoblasts treated with GNG7-siRNA or HIV-1 Tat and those
cotreated with GNG7-siRNA and HIV-1 Tat (P<0.05). GNG7-siRNA and
HIV-1 Tat cotreatment resulted in higher cytotrophoblast viability
compared with treatment with either alone (P<0.05). Taken
together, these results show that the suppression of GNG7 enhanced
placental cytotrophoblast proliferation.
Subsequently, PI staining was performed to assess
the cell cycle distribution. The PI staining results (Fig. 5B and C) showed that an increased
cell proportion in G0/G1 phase and a
decreased cell proportion in S phase were the main cell cycle
changes of the cytotrophoblasts isolated from the PE rats without
treatment, those treated with the empty plasmid, GNG7-siRNA or
HIV-1 Tat, and those cotreated with GNG7-siRNA and HIV-1 Tat
compared with those of the cytotrophoblasts isolated from the
normal rats (all P<0.05). However, compared with the untreated
cytotrophoblasts or those treated with the empty plasmid, the
cytotrophoblasts treated with the GNG7-siRNA or HIV-1 Tat and those
cotreated with GNG7-siRNA and HIV-1 Tat exhibited decreased cell
arrest in the G0/G1 phase and increased cells
in S phase (all P<0.05). GNG7-siRNA and HIV-1 Tat cotreatment
led to a reduced proportion of cells in the
G0/G1 phase and an elevated proportion in the
S phase compared with the cells exposed to either treatment alone
(all P<0.05). Therefore, GNG7 gene silencing induced cell cycle
progression of the placental cytotrophoblasts in the PE rats.
Flow cytometric analysis, performed to evaluate
apoptosis of placental cytotrophoblast cells (Fig. 5D and E) showed increased apoptotic
rates in the untreated cytotrophoblasts, those treated with the
empty plasmid, GNG7-siRNA or HIV-1 Tat, and those cotreated with
GNG7-siRNA and HIV-1 Tat compared with the rate in cytotrophoblasts
isolated from the normal rats (all P<0.05). The GNG7-siRNA and
HIV-1 Tat treatments and GNG7-siRNA and HIV-1 Tat cotreatment
resulted in decreased cell apoptosis (all P<0.05). Compared with
GNG7-siRNA or HIV-1 Tat treatment, cell apoptosis was decreased by
GNG7-siRNA and HIV-1 Tat cotreatment (all P<0.05). Therefore,
GNG7 gene silencing inhibited cell apoptosis in the placental
cytotrophoblasts of the PE rats.
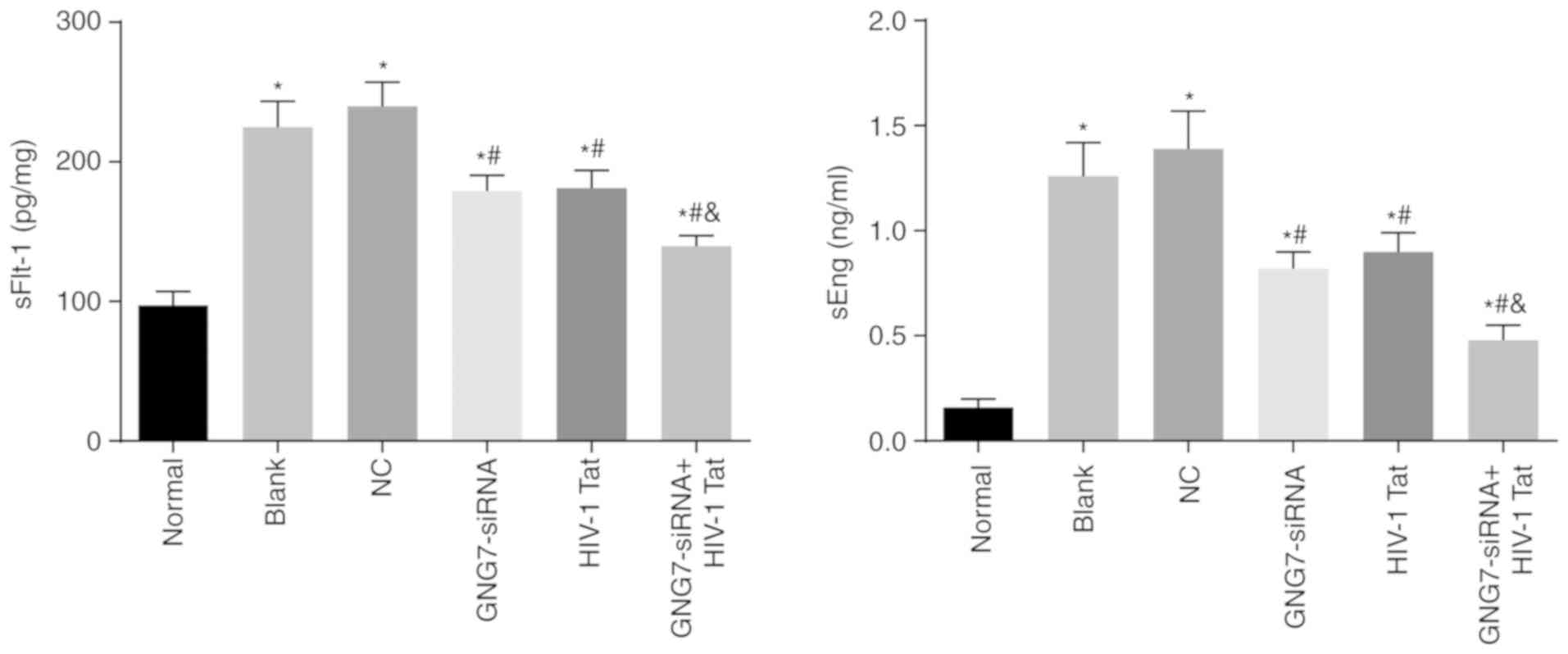
GNG7 depletion inhibits sFlt-1 and sEng
levels in PE rats
ELISA was conducted to measure the sFlt-1 and sEng
levels in the supernatants of placental cytotrophoblasts subjected
to different treatments. The results (Fig. 6) showed elevated sFlt-1 and sEng
levels in cytotrophoblasts isolated from the PE rats without
treatment, those treated with the empty plasmid, GNG7-siRNA or
HIV-1 Tat, and those cotreated with GNG7-siRNA and HIV-1 Tat
compared with those in cytotrophoblasts isolated from the normal
rats (all P<0.05). The cytotrophoblasts treated with GNG7-siRNA
or HIV-1 Tat and those cotreated with GNG7-siRNA and HIV-1 Tat
exhibited decreased levels of sFlt-1 and sEng (all P<0.05).
GNG7-siRNA and HIV-1 Tat cotreatment contributed to further
reduction of the levels of sFlt-1 and sEng compared with those of
either treatment alone (all P<0.05). Therefore, GNG7 gene
silencing suppressed the levels of sFlt-1 and sEng in the placental
cytotrophoblasts of the PE rats.
GNG7 knockdown enhances cell
proliferation and differentia- tion and represses apoptosis in
placental cytotrophoblasts from PE rats through activation of the
mTOR signaling pathway
To further examine whether GNG7 was associated with
enhanced cell proliferation and differentiation and inhibition of
apoptosis in placental cytotrophoblasts from PE rats through
regulation of the mTOR signaling pathway, the cytotrophoblasts were
cotransfected with GNG7-siRNA and rapamycin (mTOR inhibitor); cells
treated with GNG7-siRNA and DMSO served as a control. Compared with
those of the cells cotreated with GNG7-siRNA and DMSO, the mTOR
signaling pathway-related protein levels were decreased by
GNG7-siRNA and rapamycin cotreatment (Fig. 7A). Cell proliferation (Fig. 7B) and cell cycle arrest (Fig. 7C) were reduced, whereas cell
apoptosis (Fig. 7D) was
accelerated by GNG7-siRNA and rapamycin cotreatment. These results
demonstrated that GNG7 silencing activated the mTOR signaling
pathway, which promoted cell proliferation and differentiation and
inhibited apoptosis of the placental cytotrophoblasts from the PE
rats.
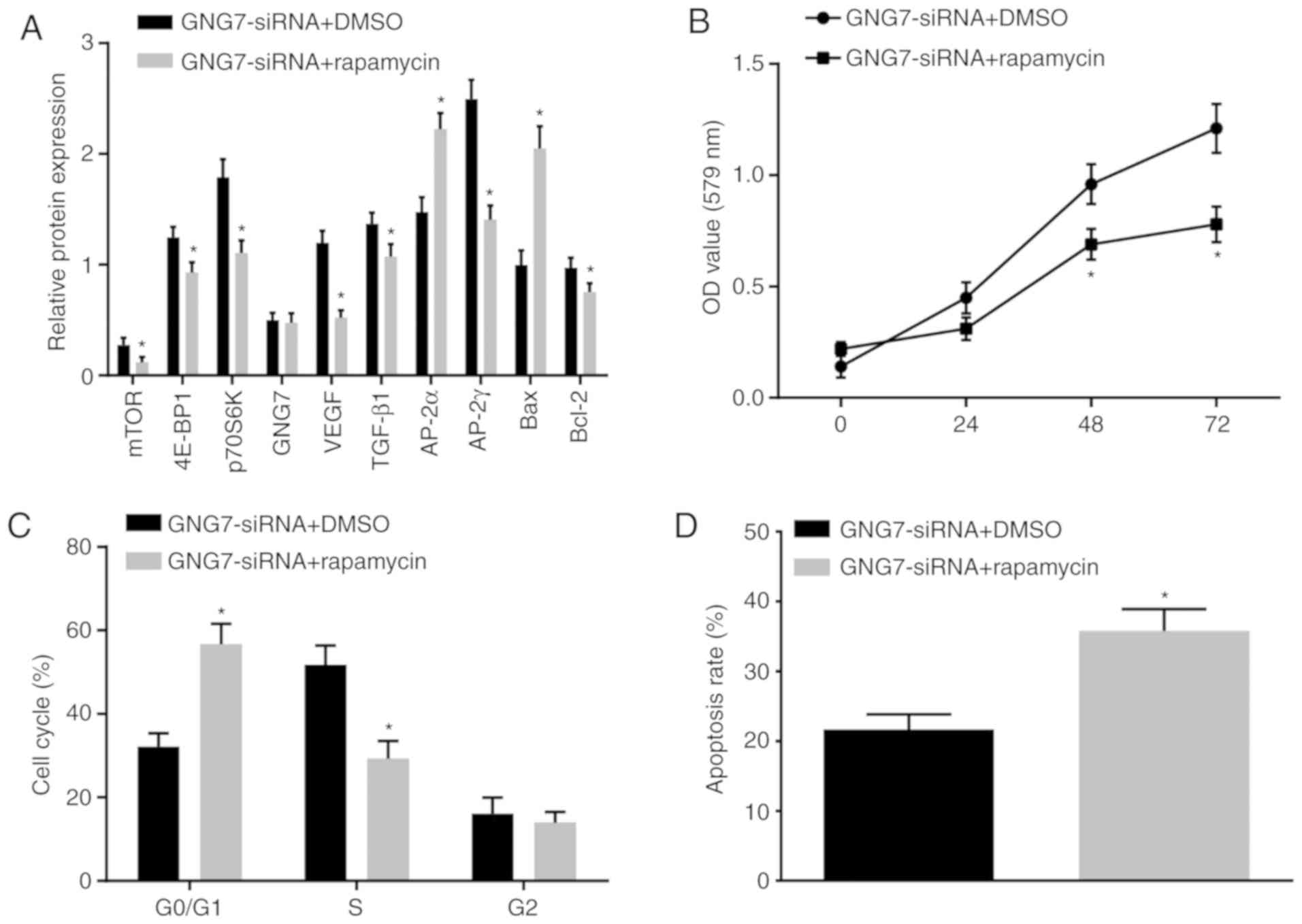 | Figure 7GNG7 depletion enhances cell
proliferation and restrains apoptosis in placental cytotrophoblasts
of PE rats through activating the mTOR signaling pathway. (A)
Protein levels of, 4E-BP1, p70S6K, mTOR, VEGF, TGF-β1, AP-2γ,
Bcl-2, GNG7, AP-2α and Bax in PE rats in response to cotreatment
with GNG7-siRNA and DMSO or rapamycin, as measured by western
blotting. (B) Cell proliferation in response to cotreatment with
GNG7-siRNA and DMSO or rapamycin, as detected by the
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
assay. (C) Cell cycle distribution in response to cotreatment with
GNG7-siRNA and DMSO or rapamycin, as detected by flow cytometry.
(D) Cell apoptosis in response to cotreatment with GNG7-siRNA and
DMSO or rapamycin, as assessed by flow cytometry. The test results
are measurement data expressed as the mean ± standard deviation and
analyzed by an independent sample t-test. The experiment was
repeated three times. *P<0.05 compared with the
GNG7-siRNA + DMSO group. GNG7, G protein γ 7; 4E-BP1, 4E-binding
protein 1; p70S6K, phosphoprotein 70 ribosomal protein S6 kinase;
mTOR, mammalian target of rapamycin; VEGF, vascular endothelial
growth factor; TGF-β1, transforming growth factor β1; AP-2γ,
activator protein-2γ; AP-2α, activator protein-2α; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein; NC, negative control;
siRNA, small interfering RNA; DMSO, dimethylsulfoxide; OD, optical
density. |
Discussion
PE is a serious pregnancy-specific disease that is
associated with increased morbidity and mortality rates of the
mother and the neonate (17).
Human extravillous trophoblast invasion is implicated in inadequate
arterial remodeling, resulting in a severe pregnancy disorder even
for early-onset PE (18). The
present study mainly examined the mechanism underlying how GNG7
affects the proliferation, differentiation and apoptosis of
placental cytotrophoblasts in PE model rats through activation of
the mTOR signaling pathway. The findings provide evidence that GNG7
gene silencing enhances the proliferation and differentiation of
placental cytotrophoblasts in PE rats via activation of the mTOR
signaling pathway.
In the present study, weak expression of mTOR and a
higher expression of GNG7 were found in the placental tissues and
cytotrophoblasts from the PE rats. Although loss of GNG7 has been
implicated in human cancer, including head and neck cancer
(7) and extrahepatic
cholangiocarcinoma (19), the
expression of GNG7 in PE remains unclear. In the present study,
GNG7 was elevated in the PE rats. Functionally, mTOR acts as a
connector of growth factor signals to energy status and nutrient
levels, thereby modulating protein metabolism and cell growth
(20). Unlike amino acid
transport proteins, the increase in the expression of mTOR is
unique to intrauterine growth restriction-associated pregnancy and
is not observed in PE (21). The
data obtained in the present study showed decreased expression of
4E-BP1 and p70S6K in PE rat placental tissues, which was indicative
of inactivation of the mTOR signaling pathway in PE.
To further analyze the potential effects of GNG7,
placental cytotrophoblasts isolated from normal and PE rats were
treated with an siRNA against GNG7 (GNG7-siRNA) and/or an mTOR
signaling pathway activator (HIV-1 Tat). The results showed that
the suppression of GNG7 activated the mTOR signaling pathway to
promote the proliferation and inhibit the apop-tosis of
cytotrophoblasts in PE rats. Activation of the mTOR signaling
pathway has been shown to promote the proliferation of invasive
pituitary adenoma cells (22). In
addition, the mTOR signaling pathway has been shown to be involved
in the proliferation and apoptosis of glioma cells (23). GNG7 gene silencing and mTOR
signaling pathway activation resulted in elevated mRNA and protein
levels of VEGF, TGF-β1, AP-2γ and Bcl-2, but reduced mRNA and
protein levels of AP-2α and Bax, which further suggested its
promoting effect on cell proliferation and differentiation and its
inhibitory effect on apoptosis. The AP-2 protein family includes
five transcription factors (α, β, γ, δ and ε), which are involved
in embryonic development (24).
As a sequence-specific DNA-binding transcription factor, AP-2α is
implicated in cell transformation and differentiation and
positively affects the EGF-dependent invasion of human trophoblasts
(25). The regulation of Bcl-2
and Bax mediated by AP-2α has been demonstrated to influence cell
apoptosis, which in turn results in PE pathogenesis (26). The Bcl-2 and Bax proteins belong
to the Bcl-2 family, have opposing functions and are involved in
the pathological process of PE under regulation by Ap-2α; elevation
of the Bcl-2 protein usually indicates an increased anti-apoptotic
function of cells, whereas elevation of the Bax protein indicates a
promotion of cell apoptosis (27). There is evidence that the elevated
expression of AP-2γ exerts a protective effect against PE, thereby
avoiding the increase in BP (28). p70S6K, which is a downstream
target of mTOR, acts as a protein synthesis regulator in cell
growth and proliferation (29).
VEGF has been shown to enhance neovascularization in animals and
human subjects, and its deficiency in PE is attributed to the lower
angiogenic capacity of the fetal endothelium (30,31). GNG7 gene silencing resulted in
elevated expression and phosphorylation of 4E-BP1, p70S6K and mTOR,
thus inducing activation of the mTOR signaling pathway.
Typically, PE is characterized by hypertensive
disorder in pregnancy, proteinuria and dysfunctions in other
maternal organs and tissues (32). Pregnancy-induced hypertension is a
particular complication during the gestation period, which refers
to pre-existing hypertension, gestational hypertension and PE
(33). Additionally, the
overexpression of sFlt-1 has been reported to induce hypertension
to limit fetal growth and provide support for the pathogenesis of
PE (34). Maynard et al
first reported the association between sFlt-1 and PE in 2003 and
showed that the level of sFlt-1 was markedly increased in patients
with PE (35). Another study
revealed that the level of sFlt-1 showed an increased tendency with
the deterioration of PE, which induced alterations of
cytotrophoblast cell morphology and function (36). Consistently, increased expression
levels of sFlt-1 and sEng were detected in the cytotrophoblasts of
PE rats in the present study. sEng is a homodimeric membrane
glycoprotein that is expressed in vascular endothelial cells and
serves as a cell surface coreceptor for TGF-β1, which affects
vascular homeostasis. Venkatesha et al found that
placenta-secreted sEng was a type of angiogenesis inhibitor, which
induced vascular damage through regulating TGF-β1 (37). The present study found that GNG7
gene silencing contributed to reduced levels of sFlt-1 and sEng in
cytotrophoblasts, thus alleviating disorders in the PE rats.
Consequently, the present study found that GNG7 gene
silencing inhibited cell apoptosis and promoted the proliferation
and differentiation of placental cytotrophoblasts in PE rats by
activating the mTOR signaling pathway. GNG7 was expressed at a high
level and the mTOR signaling pathway was inhibited during PE,
resulting in vascular endothelial dysfunction and placental
hypoxia. Inadequate trophoblastic invasion, inhibition of
proliferation and enhanced apoptosis of cytotrophoblasts were found
in the PE rats, which aggravated PE. By contrast, GNG7 gene
silencing reduced the restriction on the mTOR signaling pathway and
promoted the proliferation and differentiation of cytotrophoblasts
in the PE rats. Therefore, GNG7 can be suggested as a novel target
for PE treatment. Further investigation of the molecular mechanisms
of GNG7-targeted PE therapeutic methods is warranted. Additionally,
further efforts are expected to examine the clinical efficacy of
potential targeted therapy for patients with PE. Although
pregnancy-induced hypertension has the same clinical outcomes as
PE, the pathogenesis differs. Therefore, determining whether a
similar influence exists requires further investigation.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WSL and YLD designed the study. WSL and YLD collated
the data, designed and developed the database, performed data
analyses and produced the initial draft of the manuscript. WSL and
YLD contributed to drafting the manuscript. Both authors
contributed to the revised manuscript and have read and approved
the final submitted manuscript.
Ethics approval and consent to
participate
The present study was conducted in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The
protocol was approved by the Institutional Animal Care and Use
Committee of Second Xiangya Hospital, Central South University
(Changsha, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ghulmiyyah L and Sibai B: Maternal
mortality from preeclampsia/eclampsia. Semin Perinatol. 36:56–59.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Phipps E, Prasanna D, Brima W and Jim B:
Preeclampsia: Updates in pathogenesis, definitions, and guidelines.
Clin J Am Soc Nephrol. 11:1102–1113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Staff AC, Benton SJ, von Dadelszen P,
Roberts JM, Taylor RN, Powers RW, Charnock-Jones DC and Redman CW:
Redefining preeclampsia using placenta-derived biomarkers.
Hypertension. 61:932–942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burwick RM and Feinberg BB: Eculizumab for
the treatment of preeclampsia/HELLP syndrome. Placenta. 34:201–203.
2013. View Article : Google Scholar
|
|
5
|
Hansson SR, Gram M and Akerstrom B: Fetal
hemoglobin in preeclampsia: A new causative factor, a tool for
prediction/diagnosis and a potential target for therapy. Curr Opin
Obstet Gynecol. 25:448–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J, Ji X, Li Z, Yang X, Wang W and
Zhang X: G protein gamma subunit 7 induces autophagy and inhibits
cell division. Oncotarget. 7:24832–24847. 2016.PubMed/NCBI
|
|
7
|
Hartmann S, Szaumkessel M, Salaverria I,
Simon R, Sauter G, Kiwerska K, Gawecki W, Bodnar M, Marszalek A,
Richter J, et al: Loss of protein expression and recurrent DNA
hypermethylation of the GNG7 gene in squamous cell carcinoma of the
head and neck. J Appl Genet. 53:167–174. 2012. View Article : Google Scholar :
|
|
8
|
Kvehaugen AS, Melien O, Holmen OL,
Laivuori H, Oian P, Andersgaard AB, Dechend R and Staff AC: Single
nucleotide polymorphisms in G protein signaling pathway genes in
preeclampsia. Hypertension. 61:655–661. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Populo H, Lopes JM and Soares P: The mTOR
signalling pathway in human cancer. Int J Mol Sci. 13:1886–1918.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gomez-Pinillos A and Ferrari AC: mTOR
signaling pathway and mTOR inhibitors in cancer therapy. Hematol
Oncol Clin North Am. 26:483–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeng X, Huang Z, Mao X, Wang J, Wu G and
Qiao S: N-carbamylglutamate enhances pregnancy outcome in rats
through activation of the PI3K/PKB/mTOR signaling pathway. PLoS
One. 7:e411922012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu D, Hong H, Huang X, Huang L, He Z, Fang
Q and Luo Y: CXCR2 is decreased in preeclamptic placentas and
promotes human trophoblast invasion through the Akt signaling
pathway. Placenta. 43:17–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amaral LM, Cornelius DC, Harmon A, Moseley
J, Martin JN Jr and LaMarca B: 17-hydroxyprogesterone caproate
significantly improves clinical characteristics of preeclampsia in
the reduced uterine perfusion pressure rat model. Hypertension.
65:225–231. 2015. View Article : Google Scholar
|
|
14
|
Serman L, Vlahovic M, Sijan M, Bulic-Jakus
F, Serman A, Sincic N, Matijevic R, Juric-Lekic G and Katusic A:
The impact of 5-azacytidine on placental weight, glycoprotein
pattern and proliferating cell nuclear antigen expression in rat
placenta. Placenta. 28:803–811. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Bergmann A, Ahmad S, Cudmore M, Gruber AD,
Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves ACh
Grone HJ, et al: Reduction of circulating soluble Flt-1 alleviates
preeclampsia-like symptoms in a mouse model. J Cell Mol Med.
14:1857–1867. 2010. View Article : Google Scholar
|
|
17
|
Grotegut CA: Prevention of preeclampsia. J
Clin Invest. 126:4396–4398. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Velicky P, Windsperger K, Petroczi K, Pils
S, Reiter B, Weiss T, Vondra S, Ristl R, Dekan S, Fiala C, et al:
Pregnancy-associated diamine oxidase originates from extravillous
trophoblasts and is decreased in early-onset preeclampsia. Sci Rep.
8:63422018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang M, Gong B, Li Y and Wang Y: Human
G-protein gamma 7 in extrahepatic cholangiocarcinoma and its
clinicopathological significance. Hematol Oncol Stem Cell Ther.
3:66–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giguere V: Canonical signaling and nuclear
activity of mTOR-a teamwork effort to regulate metabolism and cell
growth. FEBS J. 285:1572–1588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aiko Y, Askew DJ, Aramaki S, Myoga M,
Tomonaga C, Hachisuga T, Suga R, Kawamoto T, Tsuji M and Shibata E:
Differential levels of amino acid transporters System L and ASCT2,
and the mTOR protein in placenta of preeclampsia and IUGR. BMC
Pregnancy Childbirth. 14:1812014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou K, Fan YD, Wu PF, Duysenbi S, Feng
ZH, Du GJ and Zhang TR: MicroRNA-145 inhibits the activation of the
mTOR signaling pathway to suppress the proliferation and invasion
of invasive pituitary adenoma cells by targeting AKT3 in vivo and
in vitro. Onco Targets Ther. 10:1625–1635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang G, Liu M, Wang H, Yu S, Jiang Z, Sun
J, Han K, Shen J, Zhu M, Lin Z, et al: Centrosomal protein of 55
regulates glucose metabolism, proliferation and apoptosis of glioma
cells via the Akt/mTOR signaling pathway. J Cancer. 7:1431–1440.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orso F, Penna E, Cimino D, Astanina E,
Maione F, Valdembri D, Giraudo E, Serini G, Sismondi P, De Bortoli
M and Taverna D: AP-2alpha and AP-2gamma regulate tumor progression
via specific genetic programs. FASEB J. 22:2702–2714. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biadasiewicz K, Sonderegger S, Haslinger
P, Haider S, Saleh L, Fiala C, Pollheimer J and Knofler M:
Transcription factor AP-2α promotes EGF-dependent invasion of human
trophoblast. Endocrinology. 152:1458–1469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Jia L, Cui S, Shi Y, Chang A,
Wang P and Zhang Z: AP-2alpha-dependent regulation of Bcl-2/Bax
expression affects apoptosis in the trophoblast. J Mol Histol.
43:681–689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang A, Liu Q, Zhang J and Zheng R:
Berberine alleviates preeclampsia possibly by regulating the
expression of interleukin-2/interleukin-10 and Bcl-2/Bax. Int J
Clin Exp Med. 8:16301–16307. 2015.PubMed/NCBI
|
|
28
|
Schneider HA, Gembruch U, Fimmers R,
Schmitz J and Muller AM: Expression of AP-2γ in placentas of
patients with preeclampsia and of smokers. Arch Gynecol Obstet.
291:1015–1021. 2015. View Article : Google Scholar
|
|
29
|
Hu LY, Sun ZG, Wen YM, Cheng GZ, Wang SL,
Zhao HB and Zhang XR: ATP-mediated protein kinase B Akt/mammalian
target of rapamycin mTOR/p70 ribosomal S6 protein p70S6 kinase
signaling pathway activation promotes improvement of locomotor
function after spinal cord injury in rats. Neuroscience.
169:1046–1062. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wahl EA, Schenck TL, Machens HG and
Balmayor ER: VEGF released by deferoxamine preconditioned
mesenchymal stem cells seeded on collagen-GAG substrates enhances
neovascularization. Sci Rep. 6:368792016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Escudero C, Bertoglia P, Hernadez M, Celis
C, Gonzalez M, Aguayo C and Acurio J: Impaired A2A adenosine
receptor/nitric oxide/VEGF signaling pathway in fetal endothelium
during late- and early-onset preeclampsia. Purinergic Signal.
9:215–226. 2013. View Article : Google Scholar :
|
|
32
|
Khanabdali R, Shakouri-Motlagh A,
Wilkinson S, Murthi P, Georgiou HM, Brennecke SP and Kalionis B:
Low-dose aspirin treatment enhances the adhesion of preeclamptic
decidual mesenchymal stem/stromal cells and reduces their
production of pro-inflammatory cytokines. J Mol Med (Berl).
96:1215–1225. 2018. View Article : Google Scholar
|
|
33
|
Kintiraki E, Papakatsika S, Kotronis G,
Goulis DG and Kotsis V: Pregnancy-induced hypertension. Hormones
(Athens). 14:211–223. 2015. View Article : Google Scholar
|
|
34
|
Lu F, Longo M, Tamayo E, Maner W, Al-Hendy
A, Anderson GD, Hankins GD and Saade GR: The effect of
over-expression of sFlt-1 on blood pressure and the occurrence of
other manifestations of preeclampsia in unrestrained conscious
pregnant mice. Am J Obstet Gynecol. 196. pp. 396 e391–397;
discussion 396 e397. 2007, View Article : Google Scholar
|
|
35
|
Maynard SE, Min JY, Merchan J, Lim KH, Li
J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et
al: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may
contribute to endothelial dysfunction, hypertension, and
proteinuria in preeclampsia. J Clin Invest. 111:649–658. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ohkuchi A, Hirashima C, Suzuki H,
Takahashi K, Yoshida M, Matsubara S and Suzuki M: Evaluation of a
new and automated electrochemiluminescence immunoassay for plasma
sFlt-1 and PlGF levels in women with preeclampsia. Hypertens Res.
33:422–427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Venkatesha S, Toporsian M, Lam C, Hanai J,
Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, et al:
Soluble endoglin contributes to the pathogenesis of preeclampsia.
Nat Med. 12:642–649. 2006. View
Article : Google Scholar : PubMed/NCBI
|