Introduction
The International Association for the Study of Pain
(IASP) defines neuropathic pain as 'an unpleasant sensory and
emotional experience associated with actual or potential tissue
damage described in terms of such damage' (1) (https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698).
Neuropathic pain can be associated with a variety of diseases or
injuries affecting the sensory nervous system through various
mechanisms. Typically, neuropathic pain is characterized by a wide
range of different symptoms and clinical manifestations associated
with both sensitivity reduction and enhancement (2).
The mechanisms for the development of neuropathic
pain are complex and are not yet fully understood. Sensory
information from peripheral structures is processed and transmitted
to central nuclei located in several parts of the brain, including
the hippocampus (3). The
hippocampus is part of the brain limbic system and the main
structure that provides spatial and contextual memory, as well as
playing an important role in the mechanisms of emotions formation.
This area of the brain is associated with the perception of chronic
pain, in particular its affective-motivational component (4). Hippocampal neurogenesis in adult
animals is known to be involved in learning and memory (5). The dysregulation of adult
neurogenesis may result in various neurological disorders,
including depression (6), anxiety
(7), stress (8) and cognitive disturbance (9,10).
Changes in hippocampal plasticity caused by painful stimuli, may
result in the development of neuropathic pain, namely cognitive and
emotional effects. Pain is a condition characterized by subjective
multifaceted symptoms. It has been demonstrated that chronic pain
can cause both sensory impairments and various functional disorders
(anxiety, amnesia, insomnia and depression) (4).
The manifestation of pain largely depends on the
type of pain, sex (11), the age
of the individual (12) and
previous experience of pain perception (13). The influence of age on the pain
cognitive and emotional consequences can be explained by anatomical
and morphological alterations in neurons and glial environments.
Such age-related changes are usually associated with an altered
neuronal transmission, the sensitivity of receptors involved in the
pain perception and modulation. In addition, changes in neuronal
electrophysiological parameters, such as the duration of excitatory
post-synaptic potentials, synaptic delay and the amplitude of
inhibitory postsynaptic potentials, may be crucial (14,15). Alterations in these parameters are
primarily associated with the age-related change in the number of
excitatory and inhibitory synapses on neurons (12,16,17). The relevance of neuropathic pain
studied in aged animals is due to the fact that a large proportion
of the population suffering from painful neuropathic disorders
refers to an older age group (18).
In our previous study (19), we investigated the effects of
neuropathic pain on the microglial state and neurogenesis within
the hippocampus in young mice. In this study, we aimed to clarify
the mechanisms of behavioral changes characteristic of animals with
neuropathic pain. In addition, we examined the effects of alkyl
glycerol ethers (AGE) treatment on the parameters under study. This
study was devoted to the evaluation of neuropathic pain cognitive
effects underlying mechanisms, as well as testing the
pharmacologically active substance based on AGE, which can affect
the state of hippocampal microglia and neurogenesis.
Materials and methods
Animals and surgery
Experiments were performed as previously described
(19) using 28 18-month old male
C57BL/6 mice with an average weight of 45 g. The animals were
housed 2-4 per cage with a 12-h light/dark cycle and had ad
libitum access to food and water. In order to reduce the amount
of stress place on the animals, the mice were handled for only 5
min once a day over a period of 5 consecutive days prior to the
experiments. In addition, glial cultures were obtained from the
cerebral cortex of 7 1-day-old Wistar rat pups. The animals were
raised at the 'A.V. Zhirmunsky National Scientific Center of Marine
Biology', Far Eastern Branch of the Russian Academy of Sciences
(Vladivostok, Russia). All procedures performed using animals were
approved by the Animal Ethics Committee at the National Scientific
Center of Marine Biology Far Eastern Branch, Russian Academy of
Sciences (Vladivostok, Russia) according to the Laboratory Animal
Welfare guidelines. Neuropathic pain was induced using the model of
chronic constriction injury (CCI) of the sciatic nerve, as
previously described (20). In
brief, the animals were anesthetized with sodium pentobarbital [50
mg/kg, administered intraperitoneally (i.p.)]. Subsequently, the
right sciatic nerve was exposed and 3 ligatures (3 silk gut
sutures; Ethicon US, LLC., Virginia Beach, VA, USA) were placed
around the nerve proximal to the trifurcation at a distance of 1 mm
between each ligature. The ligatures were tightened loosely until a
slight twitching of the ipsilateral hindlimb. The animals in the
sham-operated group underwent surgery identical to that described
above, but without nerve ligation.
Preparation of AGE
The liver from the squid Berryteuthis
magister was obtained from Nakhodka Active Marine Fishery Base
(Nakhodka, Russia) and stored at −200°C. The extraction of total
lipids was performed as previously described (19) and as described in the study by
Bligh and Dyer (1959); the saponification of lipids was carried out
using a conventional technique (21). Following hydrolysis and
acidification, the lipid mixture was dissolved in acetone at a
ratio of 1:5 at room temperature and incubated for 24 h at −20°C,
as previously described (22).
The recrystallization of the obtained sediment was performed for
the complete separation of AGE from the other lipids. The AGE
content in the resulting product was >99%, where chimyl alcohol
was the main component (94%) (19).
Animal treatment
The AGE preparation was administered to the mice as
a water emulsion at a dose of 250 mg/kg by oral gavage, as
previously described (19). The
period of administration was 2 weeks from the day that the surgery
took place. The mice (n=28) were divided into 4 different groups as
follows: i) The sham-operated group (n=7) in which the mice were
treated with the vehicle (water); ii) the CCI group (n=7) in which
the animals subjected to sciatic nerve constriction injury were
treated with the vehicle (water); iii) the CCI + AGE group (n=7) in
which the mice subjected to sciatic nerve constriction injury were
treated with AGE; and iv) the AGE group (n=7) in which the
sham-operated mice were treated with AGE. The animals were
sacrificed 14 days after the surgery took place on the day of the
final administration of AGE.
Behavioral tests
All the mice were exposed to behavioral tests 2
weeks after surgery the took place, as previously described
(19). The determination of
thermal hyperalgesia was carried out on a weekly basis. All the
tests were performed during the light cycle between 7:00 a.m. and
7:00 p.m. In order to minimize olfactory cues from previous trials,
each apparatus was thoroughly cleaned with 10% ethanol after each
use on each animal. In order to allow the animals to adapt and to
minimize the stress placed on the animals associated with their new
environment, the animals were placed into the test apparatus for 10
min for 3 days prior to the day of the test. On the day of the
test, the mice were left in their home cages in the room used for
the experiment for 2 h prior to the onset of the behavioral test.
All behavioral tests were performed on days 13-14 of the
experiment. The testing of cold allodynia was carried out on the 6,
9, 11th and 14 day of the experiment.
Thermal allodynia
Thermal allodynia was measured using the hot plate
test (Cold/Hot Plate Analgesia Meter no. 05044; Columbus
Instruments, Columbus, OH, USA), as previously described (19,23). The test was carried out in a
chamber with 30-cm acrylic walls on a metal plate with dimensions
of 30x30 cm. The temperature of the cold plate was maintained at
+4°C, and the cut-off latency was 60 sec. The mice were placed on
the cold plate, and the total lifting time of the injured hind paw
was recorded. The contact time of the limb with the cold plate is
significantly reduced in the presence of damage accompanied by pain
response (24).
Spontaneous locomotor activity
Locomotor activity was evaluated as previously
described (19) by placing each
mouse into the center of a clear Plexiglas circular open-field
arena (diameter, 60 cm; height, 40 cm) and allowing the mouse a
period of 5 min to explore its surroundings. Bright overhead
lighting was approximately 500 lux inside the arena. The area of
the chamber was divided into 37 squares. The behavior of the mice
was continuously monitored and recorded using a video camera which
was placed over the apparatus. In the subsequent analysis, the
number of crossed squares was determined.
Working memory
The working memory of the mice in this study was
examined as previously described (19) using the Y-maze spontaneous
alternation test. Y-maze testing was carried out using an apparatus
with 3 equal arms (length, 30 cm; width, 10 cm; and height, 40 cm),
made of opaque acrylic glass. Each mouse was placed at the center
of the maze and was given a period of 5 min with which to explore
its new environment. An arm entry was scored when the mouse entered
the arm with all 4 paws. The total number of entries (N) and the
number of 'correct' triplets (M, consecutive choices of each of the
3 arms without re-entries) was evaluated. The alternation rate was
computed according to the following formula: R (%) = Mx100/(N-2),
as previously described (25).
Novel object recognition
The novel object recognition test was carried out
according to the method described in the study by Bevins and
Besheer (26). During the
training session, each animal was placed for 10 min into a chamber
with 2 identical plastic objects located on the left and right
sides of the arena. The animal was then returned to the home cage
for a 60-min (for the testing of short-term memory) or 24-h (for
the testing of long-term memory) retention interval. For testing,
each animal was placed in a test arena, where one of the items was
replaced with a new one. The animal was placed equidistantly from
both objects. The animal was then left in the testing chamber where
it could freely move behind the 2 objects for 5 min. Mouse behavior
was continuously recorded using a video camera which was placed
over the apparatus. The criterion of the exploration of the object
was the location of the nose of the animals at a distance not >2
cm from the object. The discrimination ratio was calculated as the
time spent on a new object divided by the total time spent
exploring both objects. The objects and arenas were carefully
cleaned with 70% ethanol between the tests.
Immunohistochemistry
The collection of material for subsequent
histological analysis was performed as previously described
(19) on day 14 after the
surgery. The mice were anesthetized with sodium pentobarbital (50
mg/kg, administered i.p.) and then received a transcardial
perfusion with 100 ml of ice-cold saline (~4°C; (pH 7.2). The mouse
brain were immediately removed and divided into 2 hemispheres. The
right hemisphere was post-fixed for 12 h at 4°C in fresh-buffered
4% paraformaldehyde (PFA). The hippocampus from the left hemisphere
was extracted and frozen at −70°C for further use in biochemical
analysis. The use of this method allows the use of material
obtained from the same animal for both immunohistochemical and
biochemical analyses. Following fixation with PFA, the tissue
samples were embedded in paraffin blocks and sectioned at a
thickness of 10 µm, using a Leica rotary microtome (RM 2245; Leica
Biosystems, Buffalo Grove, IL, USA). Following deparaffinization,
sagittal paraffin-embedded sections were incubated in 3% hydrogen
peroxide to block endogenous peroxidase prior to
immunohistochemical staining. Following 3 washes in 0.1 M phosphate
buffer (pH 7.2), the sections were treated for 60 min in a 2%
bovine serum albumin solution (sc-2323; Santa Cruz Biotechnology,
Santa Cruz, CA, USA) and 0.25% Triton X-100 (Sigma, St. Louis, MO,
USA). The slices were then incubated with primary antibodies
(anti-Iba-1 rabbit polyclonal antibodies, 1:500, ab108539;
anti-CD86 rabbit monoclonal antibodies, 1:1,000, ab53004;
anti-doublecortin antibody, 1:500, ab18723; anti-PCNA mouse
monoclonal antibodies, 1:500, ab29; NeuN rabbit monoclonal
antibodies 1:1,000, ab177487) (all from Abcam, Cambridge, MA, USA)
on a glass slide in a humidified chamber at 4°C for 24 h. Following
3 washes with 0.1 M phosphate buffer (pH 7.2), the sections were
incubated in a secondary antibody (anti-rabbit conjugated to
horseradish peroxidase, 1:100, PI-1000; anti-mouse conjugated to
horseradish peroxidase, 1:100, PI-2000, Vector Laboratories,
Burlingame, CA, USA; fluorescent anti-mouse, 1:500, ab150108;
fluorescent anti-rabbit, 1:500, ab150080) (both from Abcam)
solution for 45 min. After washing with 0.1 M phosphate buffer (pH
7.2), sections were treated for 5-10 min with chromogen (DAB Plus;
Thermo Fisher Scientific, Waltham, MA, USA) to elicit the
immunoperoxidase reaction. The slices were subsequently washed with
0.1 M phosphate buffer (pH 7.2), dehydrated and embedded in Dako
Toluene-Free Mounting Medium (CS705; Dako, Carpinteria, CA,
USA).
Immunostaining
In order to evaluate the activity of
microglia/macrophages in the mouse hippocampi, immunostaining was
performed using anti-Iba-1 rabbit polyclonal antibodies (1:500,
ab108539) and anti-CD86 rabbit monoclonal antibodies (1:1,000,
ab53004; both from Abcam, Cambridge, MA, USA). For the analysis of
neurogenesis, the determination of the number of newly-formed
neurons in the dentate gyrus (DG) subgranular zone (SGZ) was
performed using anti-doublecortin (anti-DCX) antibody (1:500,
ab18723; Abcam). Appropriate secondary antibodies conjugated to
horseradish peroxidase (PI-1000, anti-rabbit; PI-2000, anti-mouse)
were used according to the manufacturer's instructions (1:100;
Vector Laboratories, Burlingame, CA, USA). Proliferating cell
nuclear antigen (PCNA)-immunoreactivity was determined using
anti-PCNA mouse monoclonal antibodies (1:500, ab29) and NeuN rabbit
monoclonal antibodies (1:1,000, ab177487; both from Abcam) in the
DG SGZ. Appropriate fluorescent secondary antibodies (anti-mouse,
ab150108; anti-rabbit, ab150080) were used according to the
manufacturer's instructions (1:500; Abcam). Following incubation
with the antibodies (4°C, 24 h for primary antibodies and room
temperature, 45 min for secondary antibodies), the sections were
embedded in Fluoroshield mounting medium with DAPI (ab104139;
Abcam).
Image analysis
For immunoperoxidase, staining images were obtained
on a Zeiss AxioScope A1 microscope equipped with an AxioCam 503
color and AxioVision software (Carl Zeiss, Oberkochen, Germany).
For immunofluorescence staining, the slides were examined under a
confocal laser scanning microscope (LSM 710 META; Carl Zeiss).
Microphotographs were captured and stored as TIFF files. Images
were processed and analyzed using ImageJ software (NIH, Bethesda,
MD, USA). For DCX and PCNA quantification, every 6th section
through the entire brain hemisphere was used. The cells within the
SGZ were counted on both blades of the DG. The calculation was
performed using only whole cells containing visible nuclei. The DG
area was multiplied by the section thickness and expressed in cubic
millimeters. The number of immunopositive cells per mm3
was calculated using the following formula: d =
(106xn)/(Sxl), where 'd' indicates the cell density, 'n'
the number of immunopositive cells, 'S' the SGZ area
(µm2), 'l' the thickness of the slice and
'106' the coefficient for converting µm2 into
mm2.
The quantification of Iba1- and CD86-immunopositive
cells was performed using every 6th section. To this end,
micro-graphs were processed using the following steps: Converting
the image to grayscale mode, background subtraction and
binarization. Subsequently, the area occupied by Iba-1- and
CD86-immunopositive microglia within the CA1 hippocampal region was
calculated. All measurements were performed by an operator who was
blinded to the identity of the sections.
Cell culture
Primary microglial cultures were from 1-day-old
Wistar rat pups according to a previously published protocol
(Fig. S1) (27). The cells were maintained in
DMEM/F12 with 10% fetal bovine serum, non-essential amino acids and
penicillin streptomycin (all from Gibco/Thermo Fisher Scientific)
in a T75 flask and grown with 5% CO2 in an incubator at
37°C. For cell passaging, 0.05% TrypsinEDTA (Gibco/Thermo Fisher
Scientific) was used for 5 min. Microglia were visu-alized by
immunocytochemistry for Iba-1 (rabbit polyclonal antibody, 1:500,
ab108539; Abcam; goat anti-rabbit IgG (H+L) secondary antibody,
Alexa Fluor 488, 1:200, A27034; Thermo Fisher Scientific). A
solution of AGE in 96% ethanol was added to the culture medium to
obtain a final concentration of 5 µM followed by culture for 24 h.
The cells and culture medium were collected and frozen at −70°C for
use in enzyme-linked immunosorbent assay (ELISA).
ELISA
ELISA was carried out to quantify the hippocampal
levels of interleukin (IL)-1β, IL-10, CD86, CD163 and IL-1β, IL-10
and CD86 levels in the microglial cell culture. The hippocampi were
extracted from the left hemispheres, quickly frozen, and stored at
−70°C until use. Mouse IL-1β ELISA (ab100705), IL-10 ELISA
(ab100697; both from Abcam), CD86 ELISA (KA5061) and CD163 (KA4238;
both from Abnova, Taipei City, Taiwan) kits were used according to
the manufacturer's recommendations. The neural tissues or cells
with culture medium were homogenized on ice in the extraction
buffer recommended by the manufacturer (100 mM Tris, pH 7.4, 150 mM
NaCl, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100, 0.5% sodium
deoxycholate) with 1 mg/ml of protease inhibitor cocktail
(cOmplete) and 0.01 mg/ml of phosphatase inhibitor cocktail (P5726;
both from Sigma). The protein concentrations were determined using
a BCA protein assay kit (Pierce, Rockford, IL, USA). The absorbance
at 450 nm was measured with an iMark Microplate Absorbance Reader
(Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data are presented as the means ± SEM. 'n'
represents the number of animals for behavioral tests, and in the
immunohistochemistry assay and ELISA. The data obtained by the
behavioral tests, immunohistochemistry and ELISA were subjected to
statistical analysis using one-way ANOVA followed by a post hoc
Tukey's multiple comparison test. A value of P<0.05 was
considered to indicate a statistically significant difference. The
ELISA data for cell culture were subjected to statistical analysis
using a Student's t-test. All statistical tests were performed
using Microsoft Excel software (Microsoft, Tulsa, OK, USA) and
GraphPad prism 4 (GraphPad Software, San Diego, CA, USA).
Results
Behavioral responses to neuropathic pain
and AGE treatment
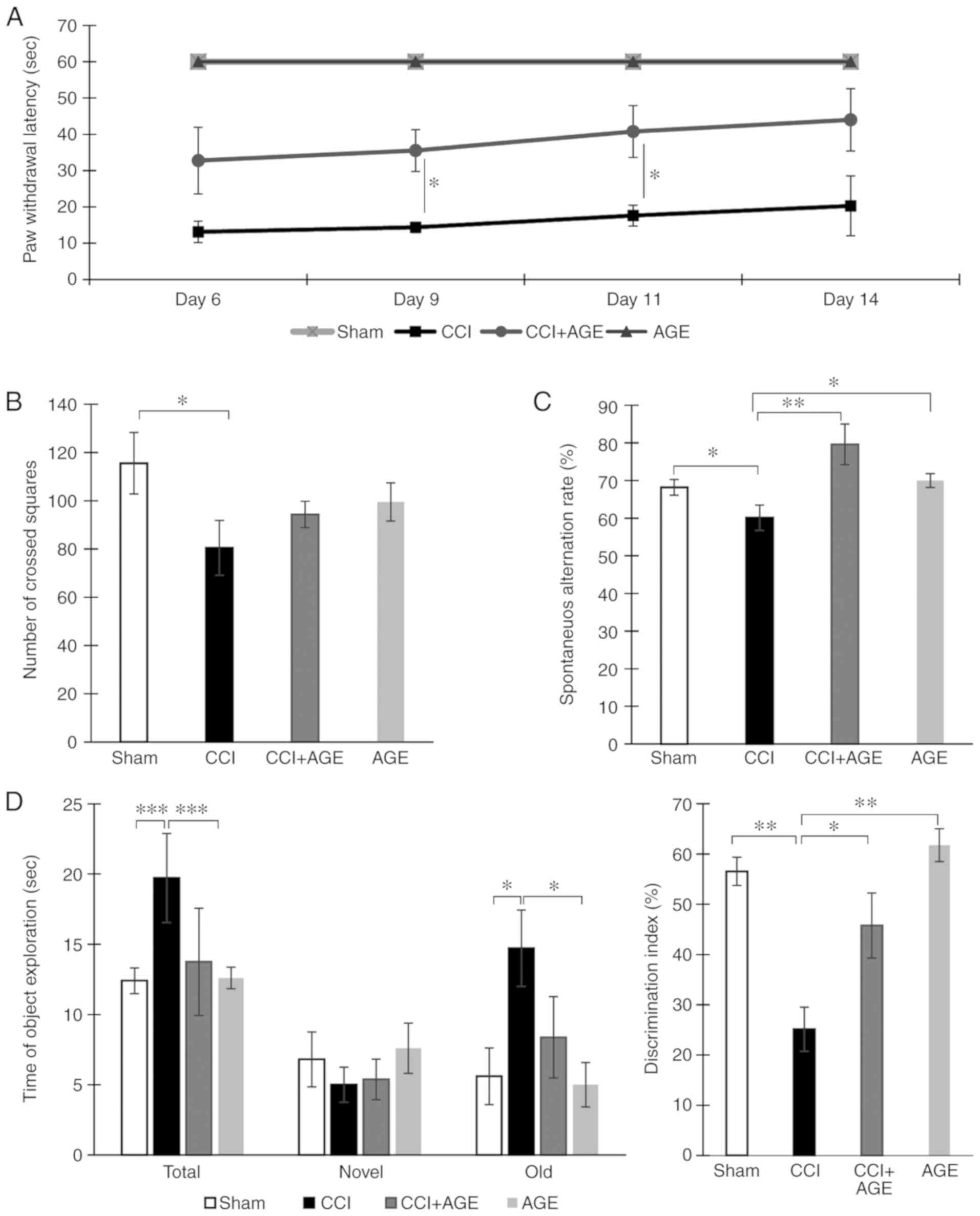
To confirm the presence of neuropathic pain, cold
allodynia was quantified using a 'hot/cold plate' device. The
animals were placed in the apparatus, and the moment of the injured
limb lifting was recorded. It was found that in the AGE-treated
animals, the moment of the injured limb lifting was significantly
delayed at 9 and 11 days following surgery (P<0.05). In the
sham-operated animals, no lifting of the injured limb was observed
(Fig. 1A).
The testing of locomotor activity in the 'open
field' apparatus revealed a significant decrease in the number of
crossed squares (115.55±12.73, sham-operated group vs. 80.50±11.35,
CCI group; P<0.05). However, in the CCI + AGE group, this
indicator did not differ significantly from the level of that in
the CCI group (Fig. 1B). Testing
in the Y-maze revealed that sciatic nerve ligation in older animals
leads to a decrease in the spontaneous alternation rate
(68.19±2.09, sham-operated group vs. 60.12±3.36, CCI group;
P<0.05). In the AGE-treated animals however, a similar decrease
was not observed. The behavioral indicators of the AGE-treated
sham-operated animals did not differ significantly from those of
the mice in the sham-operated group (Fig. 1C).
A study of long-term and short-term memory was
carried out using a test termed 'novel object recognition'. The
testing of short-term memory did not reveal significant differences
with the sham-operated group. However, the testing of long-term
memory revealed a decrease in the discrimination index in the CCI
group. At the same time, an increase in time spent with the 'old'
object was found. In the CCI + AGE group, no significant
differences were observed with the sham-operated group. The testing
of the sham-operated animals treated with AGE did not reveal any
significant differences compared to the vehicle-treated
sham-operated animals (Fig.
1D).
Hippocampal neurogenesis in CCI and AGE
treatment
In order to determine the level of hippocampal
neurogenesis in aged animals with neuropathic pain, PCNA (cell
proliferation and repair marker) and DCX (a marker of newly formed
neurons) were used for immunohistochemical staining. It should be
noted that the expression levels of these markers in the aged
animals were significantly lower than those in young animals in our
previous study (19). In this
study however, the induction of neuropathic pain significantly
decreased the level of PCNA expression in the DG SGZ of the
hippocampus (2,268.05±427.71, sham-operated group vs.
965.03±253.97, CCI group; cells/1 mm3; P<0.05). In
the AGE-treated animals, no significant reduction in the number of
PCNA-positive cells was observed. In addition, we found a marked
decrease in DG SGZ DCX expression in the mice in the CCI group
(4,957.92±764.05, sham-operated group vs. 2,744.27±676.06, CCI
group; cells/1 mm3; P<0.05). In the animals treated
with AGE, a similar decrease was not observed (2,157.27±373.49
cells/1 mm3) (Fig.
2).
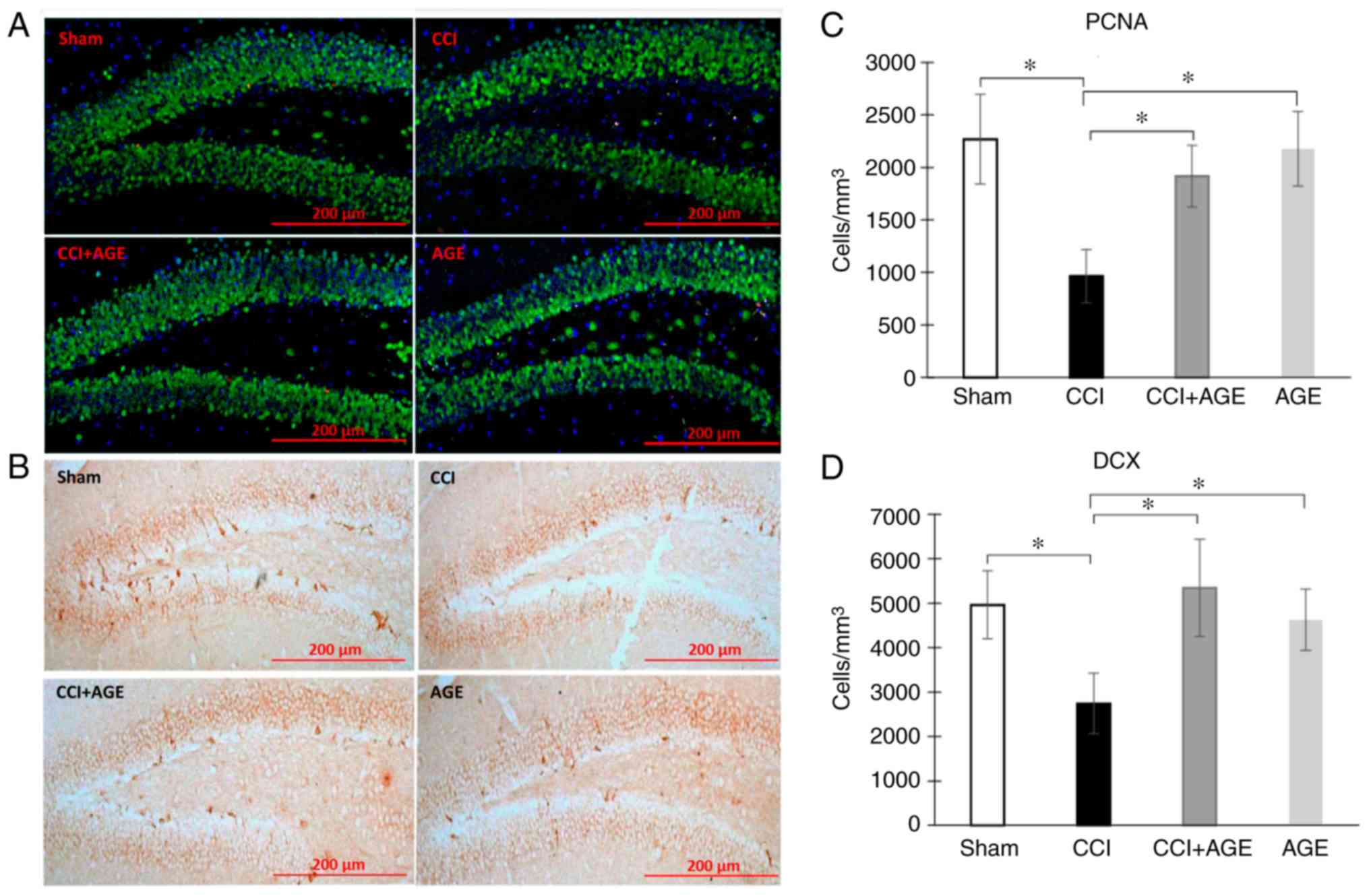 | Figure 2Proliferating cells and immature
neurons in the DG SGZ. Representative images of (A) PCNA and (B)
DCX expression in the SGZ of sham-operated mice, and CCI, CCI + AGE
and AGE group mice. (C) Histogram showing the changes in the number
of proliferating cells (by PCNA) in the DG between the
sham-operated mice (red, PCNA; green, NeuN; blue, DAPI), and the
CCI, CCI + AGE and AGE groups. (D) Histogram showing the changes in
the number of immature neurons (by DCX) between the sham-operated
mice, and the CCI, CCI + AGE and AGE group mice. Data are the means
± SEM, n=7 per group, *P<0.05. Scale bar, 200 µm.
Images were taken at x200 magnification. DG, dentate gyrus; SGZ,
subgranular zone; PCNA, proliferating cell nuclear antigen; DCX,
doublecortin; AGE, alkyl glycerol ethers; Sham, sham-operated; CCI,
chronic constriction injury. |
Alterations in microglia changes with CCI
and AGE treatment
In order to evaluate alterations in microglial
activity following peripheral neurotrauma and AGE administration,
we performed the immunohistochemical detection of the
Iba-1-positive resident microglia pool and CD86-positive in the
hippocampal areas CA1 and DG. Iba-1 is a protein that is
specifically expressed in all microglia (28). CD86 is a protein expressed in
pro-inflammatory microglia secreting pro-inflammatory cytokines,
such as IL-6, IL-1. (29). The
use of these markers allowed the quantification of any alterations
in the hippocampal microglial phenotype following sciatic nerve
injury and AGE treatment. We observed a significant increase in the
Iba-1-positive stained area in the CCI group in both the CA1 region
(5.34±0.38, sham-operated group vs. 6.73±0.51, CCI group;
P<0.05) and the DG of the hippocampus (4.92±0.33, sham-operated
group vs. 6.30±0.43 CCI group; P<0.05). In the animals treated
with AGE following sciatic nerve lesion, an increase in Iba-1
expression was also observed (7.83±0.71 in the CA1; P<0.05).
CD86 immunohistochemical detection revealed a significant increase
in immunopositive staining in the CCI group compared with the
sham-operated animals in the CA1 hippocampal region (13.44±0.64,
sham-operated group vs. 17.59±0.70 CCI group; P<0.01) and the DG
(19.48±1.44, sham-operated group vs. 23.44±1.36, CCI group;
P<0.05). In the AGE-treated animals, no significant increase in
the CD86-immunopositive area staining was observed (15.28±0.66 in
the CA1 and 19.41±1.29 in the DG) (Fig. 3).
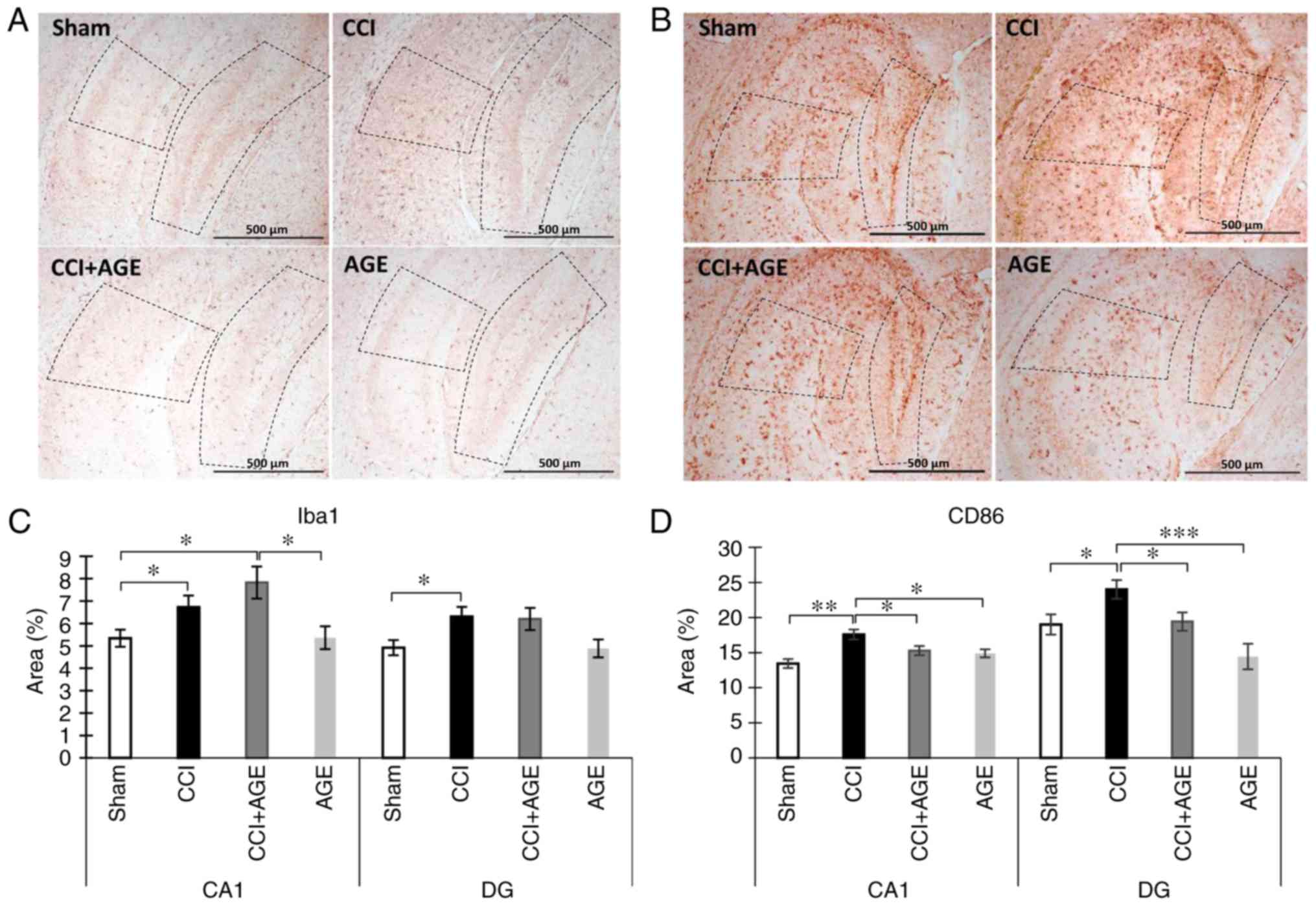 | Figure 3Effects of CCI and AGE treatment on
microglial activation. (A) Representative images of Iba-1
immunohistochemically-stained slides. (B) Representative images of
CD86 immunohistochemically-stained slides. (C) Histogram
demonstrating the percentage of area covered by Iba-1-positive
staining in the CA1 hippocampal region at 14 days after surgery.
Data are the means ± SEM, n=7 per group, *P<0.05. (D)
Histogram demonstrating the percentage of area covered by
CD86-positive staining in the CA1 hippocampal region at 14 days
after surgery, %. Data are the means ± SEM, n=7 per group,
*P<0.05, **P<0.01 and
***P<0.001. Scale bar, 500 µm. Images were taken at
x100 magnification. The dotted line indicates the boundaries of the
CA1 and DG regions. AGE, alkyl glycerol ethers; Sham,
sham-operated; CCI, chronic constriction injury; DG, dentate
gyrus. |
Hippocampal expression of inflammatory
mediators and microglial/macrophage markers
Classical (M1) microglial activation is associated
with the production of pro-inflammatory cytokines, such as IL-1β,
IL-6, tumor necrosis factor (TNF)-α, superoxide, nitric oxide,
reactive oxygen species. Alternatively (M2)-activated microglia
produce anti-inflammatory cytokines, including IL-4, IL-10,
transforming growth factor (TGF)-β and insulin-like growth factor
(IGF)-1 to provide tissue repair, reconstruction of the
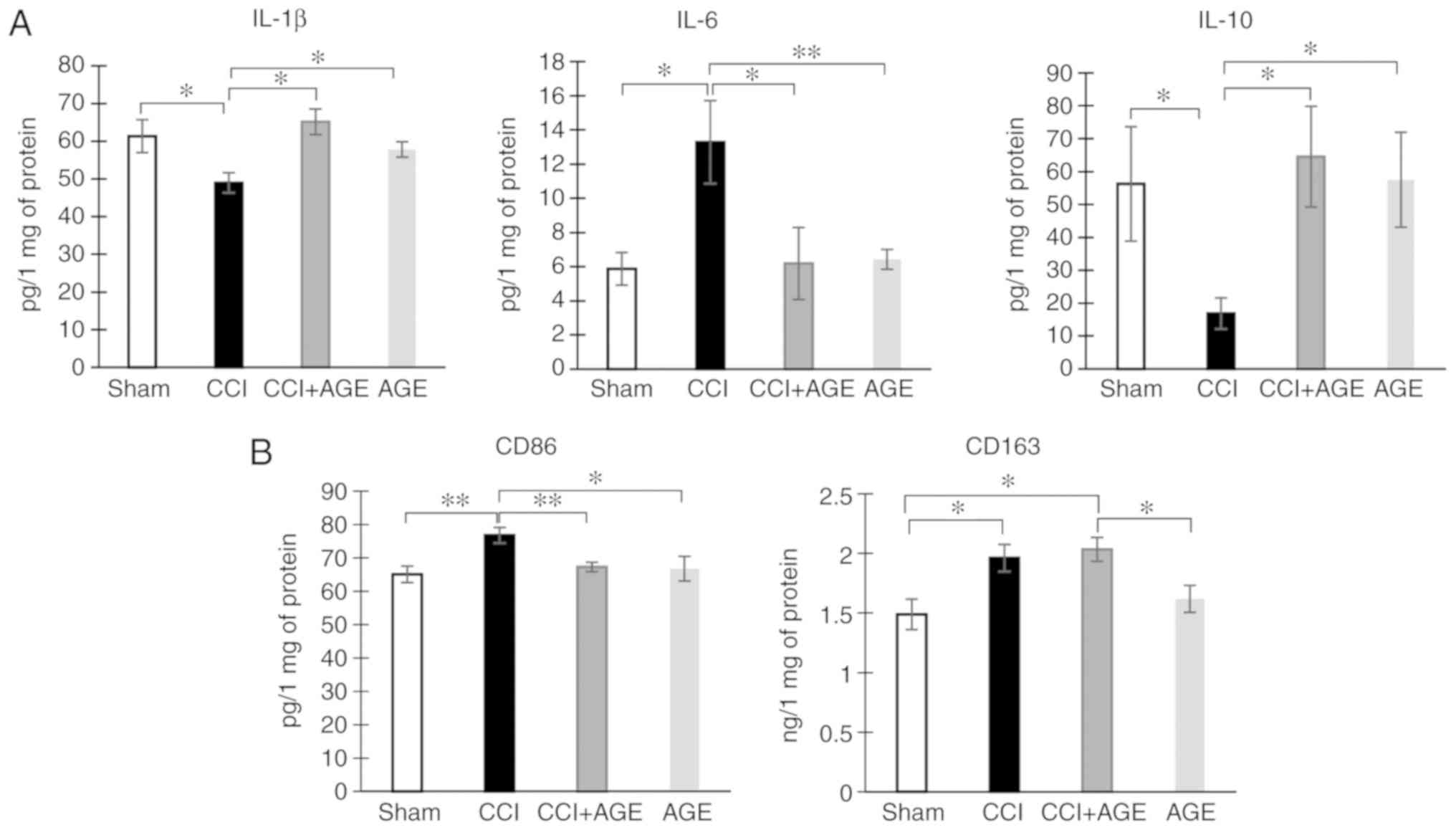
extracellular matrix and phagocytosis of cell debris (29). In this study, we used IL-1β and
IL-6 as markers of pro-inflammatory microglia, and IL-10 as a
marker of anti-inflammatory microglia. The study of hippocampal
cytokine expression was carried out by ELISA. We found that the
induction of neuropathic pain reduced the hippocampal levels of the
pro-inflammatory cytokine, IL-1β, at 2 weeks after surgery
(61.32±4.39, sham-operated group vs. 48.99±2.68, CCI group;
P<0.05). However, in AGE-treated animals with CCI, this
indicator was at the level of the sham-operated group (65.16±3.43
in CCI + AGE). Notably, a completely opposite effect was observed
for another pro-inflammatory cytokine, IL-6. The induction of
neuropathic pain caused an increase in the hippocampal IL-6 level
(5.86±0.95, sham-operated group vs. 13.27±2.43, CCI group;
P<0.05), while AGE administration prevented the development of
this effect (6.17±2.10, CCI + AGE group). The study of the
expression of the anti-inflammatory cytokine, IL-10, revealed a
decrease in mice with neuropathic pain (56.25±16.875, sham-operated
group vs. 17.37±4.73, CCI group), and in the AGE-treated animals,
the expression was at the level of that in the sham-operated group
(Fig. 4A).
The study of hippocampal microglial/macrophage
markers using ELISA revealed an increased expression of CD86
(65.06±2.48, CCI group vs. 76.76±2.34, CCI + AGE group; P<0.01),
whereas in the AGE-treated mice, an increase in this protein was
not observed. The study of hippocampal CD163 expression, a marker
of anti-inflammatory microglial/macrophages, revealed a significant
increase in the animals with CCI (P<0.01). At the same time, in
the AGE-treated mice with CCI, CD163 was also significantly higher
than the levels in the sham-operated group (P<0.01) (Fig. 4B).
Effects of AGE treatment on microglial
cytokine production in vitro
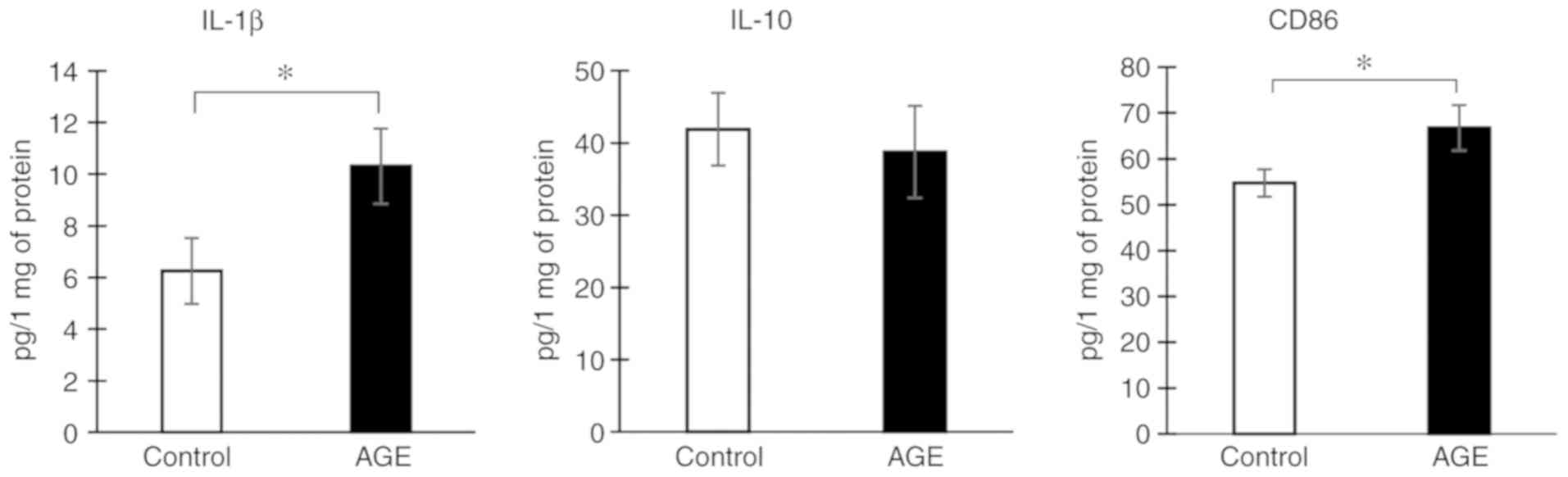
To determine the effects of AGE treatment on the
state of microglial cells in vitro, the drug was added to
the culture medium at a concentration of 5 µM and after 24 h, the
culture medium with the cells was examined by ELISA. It was found
that AGE treatment increased the production of the pro-inflammatory
cytokine, IL-1β, by 40%. However, the concentration of IL-10
remained unaltered. In addition, a slight, yet significant increase
in the expression of the pro-inflammatory microglial marker, CD86,
was found (P<0.05). Thus, AGE treatment induced microglial
M1-like polarization in our in vitro experiment (Fig. 5).
Discussion
In this study, we examined the effects of
neuropathic pain and AGE therapy on the state of microglia,
neurogenesis in the hippocampus and pain behavior in aged mice. The
use of 18-month-old animals is beneficial due to reduced
neurogenesis intensity (30).
This circumstance, as expected, allows us to obtain a clearer
picture of immunohistochemical and biochemical changes observed
following sciatic nerve ligation. Of note, in some cases, the age
of the animals contributes to the development of opposite effects
compared to our previous results obtained in young animals
(19). Namely, in 18-month-old
mice, the induction of neuropathic pain caused a decrease in
locomotor activity, while in three-month-old mice an increase in
locomotor activity was observed. Such age-related features are
usually associated with altered neurotransmission, as well as the
sensitivity of receptors involved in pain perception and
modulation. In addition, a similar effect may be associated with
age-related changes in the number of excitatory and inhibitory
synapses on neurons (12). These
factors affect the manifestation of pain-related behaviors
exhibited by experimental animals, depending on age. However, in
both cases, significant changes in the spatial working memory rate
are observed, which emphasizes the involvement of the hippocampal
formation in the pathogenesis of neuropathic pain. The violation of
spatial orientation due to the involvement of the hippocampus can
be explained by the presence of direct axonal projections from
layer III of the medial entorhinal cortex into CA1 hippocampal
region.
In addition to working spatial memory, in this
study, we investigated long-term memory in mice with CCI and AGE
treatment. We observed a decrease in the discrimination index in
animals with CCI. Moreover, the index was reduced by increasing the
time spent with the 'old' object in animals with CCI. We
hypothesized that this may occur due to a decrease in locomotor
activity in animals with neuropathic pain and as a result increase
in time spent with objects. Thus, the result certainly suggests
that neuropathic pain leads to long-term memory impairment, and AGE
prevents this effect. However, we do not exclude the effect of
reduced locomotor activity on this indicator.
The observed changes in glial marker expression may
be a consequence of those anatomical connections associated with
pain ascending systems (4). Thus,
an increase in the total microglia population (Iba-1+
cells) percentage with a simultaneous increase in the activation of
proinflammatory M1 microglia was found 2 weeks after surgery in CA1
and DG hippocampal regions. According to the testing on the hot
plate, this time was accompanied by a maximum pain response to the
nerve ligation among the entire observation period.
AGE treatment prevented the activation of
pro-inflammatory (M1) microglia with a simultaneous increase in the
number of Iba1-positive microglia. This may indirectly indicate the
phenotypic conversion of microglia to the anti-inflammatory
phenotype. The results of the ELISA confirm this assumption,
demonstrating that AGE decreased the levels of the pro-inflammatory
cytokine, IL-6, and increased those of the anti-inflammatory
cytokine, IL-10, within the hippocampus. The results of
immunohistochemistry were also confirmed by ELISA, which
demonstrated an increase in the CD86 concentration in the CCI group
and the prevention of such an increase in the AGE-treated mice.
However, an interesting result was obtained as regards the
pro-inflammatory cytokine, IL-1β. The induction of neuropathic pain
caused a significant decrease in its production, while in the
AGE-treated animals, no significant decrease was observed. A
similar result has also been obtained previously (31), when a trend for decreased IL-1β
was observed within the thalamus at 10 days after surgery. However,
the mechanisms of this phenomenon remain unclear. Nevertheless, it
is known that the presence of IL-1β is necessary to maintain a
normal long-term potentiation, which emphasizes the involvement of
this cytokine in the processes of memory and learning (32). Thus, in young animals, the
increase in IL-1β levels in response to painful stimuli is
adaptive, while in aged animals, the production of this cytokine is
impaired due to impaired adaptation processes.
We also obtained an interesting result regarding the
anti-inflammatory cytokine, IL-10. In older mice, a decrease in the
concentration of IL-10 was found in the present study, while in
young animals with neuropathic pain an increase was previously
observed (19). This may also
indicate a disruption of the adaptive mechanisms in older animals.
In addition, an age-related decrease in IL-10 production in the
mouse brain has been shown to trigger a cascade of intracellular
events, leading to an increase in IL-6 gene expression (33). However, in this study, in animals
treated with AGE, the concentration of IL-10 in the hippocampus
remained at the level of the sham-operated group. The ELISA data
demonstrating the concentration of the anti-inflammatory
microglia/macrophage marker CD163 for pain and AGE administration
also give rise to speculation. Despite a decrease in IL-10 levels
in animals with neuropathic pain, we observed an increase in the
CD163 levels. At the same time, AGE administration had no effect on
the concentration of this marker. Perhaps this result is a
consequence of the fact that CD163-positive cells are peri-vascular
macrophages (34) and reflect the
peripheral immune response to the AGE administration. In addition,
it is possible that an increase in the production of IL-1β occurs
precisely due to the migration of peripheral macrophages. This can
be facilitated by AGE administration, which is known to be able to
increase the blood-brain barrier permeability [reviewed in
(35)].
To confirm the hypothesis about the induction of M2
microglial activation under the influence of AGE treatment, we
performed in vitro experiments using microglial cell
culture. Surprisingly, the addition of AGE to the culture medium at
a concentration of 5 µM resulted in an increase in IL-1β production
by microglial cells. In this case, the concentration of IL-10 in
cells and culture medium remained unchanged upon addition of AGE.
At the same time, we found an increase in CD86 concentration, which
was shown by ELISA. These data are consistent with those found in
the literature on the effect of AGE on cytokine production by
macrophages in vitro. For example, AGE contained in shark
liver oil has been shown to lead to an increase in interferon
(IFN)-γ production, without affecting the IL-4 level in splenic
mononuclear cells (36). However,
our in vivo immunohistochemical study revealed no
significant changes in the expression of glial markers, as well as
pro-inflammatory cytokines in AGE-treated sham-operated group (AGE)
compared to the sham-operated group. This indicates that in
vivo, the influence of AGE occurs indirectly, which makes it
possible to regulate already impaired functions and not to affect
the normally functioning organism.
The observed changes in the microglial state were
accompanied by a decrease in the intensity of hippocampal
neurogenesis during pain. At the same time, the basic level of
neurogenesis in aged mice was significantly lower than that in
young animals in our previous experiments (19). However, the administration of AGE
to animals prevented a decrease in both the number of proliferating
cells and the number of newly formed neurons. Probably, this
phenomenon is associated with a change in the state of microglia,
its pro- or anti-inflammatory transformation and the spectrum of
produced cytokines. As it is known, pro-inflammatory cytokines
inhibit the processes of neurogenesis. For example, in transgenic
mice with an increased production of IL-6 by astrocytes, a decrease
in the prolifera-tive activity in the SGZ neurons has been observed
(37). At the same time, an
increase in the production of anti-inflammatory cytokines is
usually associated with the stimulation of neuro-genesis (38,39). The observed effects of neuropathic
pain and treatment with AGE on working and long-term memory may be
the result of changes in neurogenesis (40).
The data on the pharmacological effects of AGE, in
particular the effect on the polarization of microglia, the
spectrum of produced cytokines and hippocampal neurogenesis
indicate the neuroprotective properties of this drug. However, the
opposite data obtained in in vivo and in vitro
experiments suggest the idea that there is no direct effect of AGE
on the brain microglial cells within a living organism. We still
aim to study the indirect mechanisms of AGE influence on the
microglial state and the processes of neurogenesis, although we
assume several possible mechanisms. For example, it was previously
shown that the administration of AGE to animals leads to an
increase in the plasmalogen concentration in various tissues
(41). It is the pronounced
anti-inflammatory activity of plasmalogen (42) that can underlie the observed
decrease in the pro-inflammatory activation of microglia and the
maintaining of the normal neurogenesis level. In general, we assume
that the effect of AGE on the central nervous system is complex and
is implemented through several mechanisms, including regulation of
plasmalogen synthesis, neurotrophic factors production, the
blood-brain barrier permeability, and several other mechanisms that
require detailed study.
In conclusion, neuropathic pain resulting from
disturbances in the peripheral nervous system often causes the
development of pathological processes of the central nervous
system. This study emphasizes that pain-induced behavioral deficit
is based on a neuroinflammatory response that covers primarily the
limbic system including the hippocampus, which is involved in
neuroplasticity, behavior, and cognition. Thus, an effective
treatment for the neuropathic pain cognitive and emotional
consequences may be precisely the effect on the functional activity
of brain resident macrophages. The drug based on AGE that we study
can affect the functions of microglial cells both in vitro
and in vivo. Taken together, these data suggest that AGE
prevent neuropathic pain-derived effects, including M1 microglial
activation, neurogenesis disruption and memory disturbances. At the
same time, the results of the in vitro experiments emphasize
the complexity of the mechanisms underlying AGE pharmacological
effects. This fact indicates AGE therapeutic potential and the
promise of its further study in terms of the neuropathic pain
pharmacological management.
Supplementary Materials
Abbreviations:
|
AGE
|
alkyl glycerol ethers
|
|
CCI
|
chronic constriction injury
|
|
DG SGZ
|
dentate gyrus subgranular zone
|
|
DCX
|
doublecortin
|
|
PFA
|
paraformaldehyde
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
ANOVA
|
one-way analysis of variance
|
Funding
The present study was supported by the Russian
Science Foundation (project no. 17-74-20006).
Availability of data and materials
All the data generated and analyzed in the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
AT and IM designed the study. AT and IM performed
the surgery. EE prepared the alkyl glycerol ethers. IM performed
the immunohistochemical analysis. AT performed the behavioral
tests, ELISA and image analysis. YK performed the in vitro
experiments. AT wrote the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Animal Ethics
Committee at National Scientific Center of Marine Biology Far
Eastern Branch, Russian Academy of Sciences, according to the
Laboratory Animal Welfare guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests to disclose.
Acknowledgments
Not applicable.
References
|
1
|
Merskey H and Bogduk N: Pain terms: A
current list with definitions and notes on usage: Classification of
chronic pain. 2nd ed. Seattle: IASP Task Force on Taxonomy, USA;
pp. 154–196. 1994, ISBN.
|
|
2
|
Baron R, Binder A and Wasner G:
Neuropathic pain: Diagnosis, pathophysiological mechanisms, and
treatment. Lancet Neurol. 9:807–819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McEwen BS: Plasticity of the hippocampus:
Adaptation to chronic stress and allostatic load. Ann N Y Acad Sci.
933:265–277. 2001. View Article : Google Scholar
|
|
4
|
Liu MG and Chen J: Roles of the
hippocampal formation in pain information processing. Neurosci
Bull. 25:237–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deng W, Aimone JB and Gage FH: New neurons
and new memories: How does adult hippocampal neurogenesis affect
learning and memory? Nat Rev Neurosci. 11:339–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sahay A and Hen R: Adult hippocampal
neurogenesis in depression. Nat Neurosci. 10:1110–1115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Revest JM, Dupret D, Koehl M, Funk-Reiter
C, Grosjean N, Piazza PV and Abrous DN: Adult hippocampal
neurogenesis is involved in anxiety-related behaviors. Mol
Psychiatry. 14:959–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dranovsky A and Hen R: Hippocampal
neurogenesis: Regulation by stress and antidepressants. Biol
Psychiatry. 59:1136–1143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saxe MD, Battaglia F, Wang JW, Malleret G,
David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER,
Santarelli L, et al: Ablation of hippocampal neurogenesis impairs
contextual fear conditioning and synaptic plasticity in the dentate
gyrus. Proc Natl Acad Sci USA. 103:17501–17506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Argoff CE: The coexistence of neuropathic
pain, sleep, and psychiatric disorders: A novel treatment approach.
Clin J Pain. 23:15–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fillingim RB, King CD, Ribeiro-Dasilva MC,
Rahim-Williams B and Riley JL III: Sex, gender, and pain: A review
of recent clinical and experimental findings. J Pain. 10:447–485.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leite-Almeida H, Almeida-Torres L,
Mesquita AR, Pertovaara A, Sousa N, Cerqueira JJ and Almeida A: The
impact of age on emotional and cognitive behaviours triggered by
experimental neuropathy in rats. Pain. 144:57–65. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reicherts P, Gerdes AB, Pauli P and Wieser
MJ: Psychological placebo and nocebo effects on pain rely on
expectation and previous experience. J Pain. 17:203–214. 2016.
View Article : Google Scholar
|
|
14
|
Li Q, Navakkode S, Rothkegel M, Soong TW,
Sajikumar S and Korte M: Metaplasticity mechanisms restore
plasticity and associativity in an animal model of Alzheimer's
disease. Proc Natl Acad Sci USA. 114:5527–5532. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lister JP and Barnes CA: Neurobiological
changes in the hippocampus during normative aging. Arch Neurol.
66:829–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmidt M, Dubin AE, Petrus MJ, Earley TJ
and Patapoutian A: Nociceptive signals induce trafficking of TRPA1
to the plasma membrane. Neuron. 64:498–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McQuail JA, Frazier CJ and Bizon JL:
Molecular aspects of age-related cognitive decline: The role of
GABA signaling. Trends Mol Med. 21:450–460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jones MR, Ehrhardt KP, Ripoll JG, Sharma
B, Padnos IW, Kaye RJ and Kaye AD: Pain in the elderly. Curr Pain
Headache Rep. 20:232016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tyrtyshnaia AA, Manzhulo IV, Sultanov RM
and Ermolenko EV: Adult hippocampal neurogenesis in neuropathic
pain and alkyl glycerol ethers treatment. Acta Histochem.
119:812–821. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bligh EG and Dyer WJ: A rapid method of
total lipid extraction and purification. Can J Biochem Physiol.
37:911–917. 1959. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ermolenko EV, Latyshev NA, Sultanov RM and
Kasyanov SP: Technological approach of 1-O-alkyl-sn-glycerols
separation from Berryteuthis magister squid liver oil. J Food Sci
Technol. 53:1722–1726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allen JW and Yaksh TL: Assessment of acute
thermal nociception in laboratory animals. Methods Mol Med.
99:11–23. 2004.PubMed/NCBI
|
|
24
|
Manzhulo IV, Ogurtsova OS, Kipryushina YO,
Latyshev NA, Kasyanov SP, Dyuizen IV and Tyrtyshnaia AA:
Neuron-astrocyte interactions in spinal cord dorsal horn in
neuropathic pain development and docosahexaenoic acid therapy. J
Neuroimmunol. 298:90–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lysenko LV, Kim J, Henry C, Tyrtyshnaia A,
Kohnz RA, Madamba F, Simon GM, Kleschevnikova NE, Nomura DK,
Ezekowitz RA and Kleschevnikov AM: Monoacylglycerol lipase
inhibitor JZL184 improves behavior and neural properties in Ts65Dn
mice, a model of down syndrome. PLoS One. 9:e1145212014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bevins RA and Besheer J: Object
recognition in rats and mice: A one-trial non-matching-to-sample
learning task to study 'recognition memory'. Nat Protoc.
1:1306–1311. 2006. View Article : Google Scholar
|
|
27
|
Saura J, Tusell JM and Serratosa J:
High-yield isolation of murine microglia by mild trypsinization.
Glia. 44:183–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahmed Z, Shaw G, Sharma VP, Yang C,
McGowan E and Dickson DW: Actin-binding proteins coronin-1a and
IBA-1 are effective microglial markers for immunohistochemistry. J
Histochem Cytochem. 55:687–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang YU and Weidong LE: Differential roles
of M1 and M2 microglia in neurodegenerative diseases. Mol
Neurobiol. 53:1181–1194. 2016. View Article : Google Scholar
|
|
30
|
Van Praag H, Shubert T, Zhao C and Gage
FH: Exercise enhances learning and hippocampal neurogenesis in aged
mice. J Neurosc. 25:8680–8685. 2005. View Article : Google Scholar
|
|
31
|
Apkarian AV, Baliki MN and Geha PY:
Towards a theory of chronic pain. Prog Neurobiol. 87:81–97. 2009.
View Article : Google Scholar :
|
|
32
|
Schneider H, Pitossi F, Balschun D, Wagner
A, Del Rey A and Besedovsky HO: A neuromodulatory role of
interleukin-1β in the hippocampus. Proc Natl Acad Sci USA.
95:7778–7783. 1998. View Article : Google Scholar
|
|
33
|
Ye SM and Johnson RW: An age-related
decline in interleukin-10 may contribute to the increased
expression of interleukin-6 in brain of aged mice.
Neuroimmunomodulation. 9:183–192. 2001. View Article : Google Scholar
|
|
34
|
Galea J, Cruickshank G, Teeling JL, Boche
D, Garland P, Perry VH and Galea I: The intrathecal
CD163-haptoglobin-hemoglobin scavenging system in subarachnoid
hemorrhage. J Neurochem. 121:785–792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iannitti T and Palmieri B: An update on
the therapeutic role of alkylglycerols. Mar Drugs. 8:2267–2300.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hajimoradi M, Hassan ZM, Pourfathollah AA,
Daneshmandi S and Pakravan N: The effect of shark liver oil on the
tumor infiltrating lymphocytes and cytokine pattern in mice. J
Ethnopharmacol. 126:565–570. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vallieres L, Campbell IL, Gage FH and
Sawchenko PE: Reduced hippocampal neurogenesis in adult transgenic
mice with chronic astrocytic production of interleukin-6. J
Neurosci. 22:486–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Perez-Asensio FJ, Perpiñá U, Planas AM and
Pozas E: Interleukin-10 regulates progenitor differentiation and
modulates neurogenesis on adult brain. J Cell Sci. 126:4208–4219.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pereira L, Font-Nieves M, Van den Haute C,
Baekelandt V, Planas AM and Pozas E: IL-10 regulates adult
neurogenesis by modulating ERK and STAT3 activity. Front Cell
Neurosci. 25:9–57. 2015.
|
|
40
|
McKim DB, Niraula A, Tarr AJ, Wohleb ES,
Sheridan JF and Godbout JP: Neuroinflammatory dynamics underlie
memory impairments after repeated social defeat. J Neurosci.
36:2590–2604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brites P, Ferreira AS, Da Silva TF, Sousa
VF, Malheiro AR, Duran M, Waterham HR, Baes M and Wanders RJ:
Alkyl-glycerol rescues plasmalogen levels and pathology of
ether-phospholipid deficient mice. PLoS One. 6:e285392011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ifuku M, Katafuchi T, Mawatari S, Noda M,
Miake K, Sugiyama M and Fujino T:
Anti-inflammatory/anti-amyloidogenic effects of plasmalogens in
lipopolysaccharide-induced neuroinflammation in adult mice. J
Neuroinflammation. 9:1972012. View Article : Google Scholar : PubMed/NCBI
|