Introduction
Endothelial cells (ECs) form a semi-permeable
monolayer that separates the bloodstream from underlying tissues
and regulate the infiltration of blood cells and proteins through
the vessel wall (1,2). A number of studies have previously
suggested that vascular endothelial dysfunction caused by increased
junctional permeability is a key event in the development and
progression of atherosclerosis and atherosclerotic cardiovascular
diseases (3,4). Chronic metabolic disorders, such as
hyperglycemia and dyslipidemia, promote endothelial dysfunction and
eventually leakage, resulting in increased monocyte diapedesis
(5-7). Endothelial dysfunction during
diabetes is closely associated with increased oxidized low-density
lipoprotein (oxLDL) levels mediated by high glucose (HG) conditions
(8). HG and oxLDL induce
oxidative stress by increasing the production of reactive oxygen
species (ROS) (9), which drive
inflammatory processes to mediate monocyte activation and
recruitment to the inflamed endothelium (10). Subsequently, endothelial
inflammation enhances the expression of intercellular adhesion
molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)
on ECs to potentiate monocyte adhesion prior to diapedesis into the
sub-endothelial space, which precedes atherosclerotic plaque
development (11).
Endothelial permeability is regulated by the close
cooperation between inter-endothelial cell adherens and tight
junctions, which form an endothelial barrier to control the
paracellular passage of blood constituents into the subendothelial
space (2,12-16). Endothelial integrity is
maintained by the highly coordinated opening and closing of
endothelial adherens and tight junctions (17). Vascular endothelial
(VE)-cadherin, a major constituent of adherens junctions, interacts
with the tight junction molecule zonula occludens-1 (ZO-1) to link
together adjacent ECs to regulate diapedesis (2,13). There is evidence that vascular
permeability and leukocyte invasion are promoted by the
phosphorylation of VE-cadherin (16,18) and the downregulation of ZO-1
expression (16).
Pharmacological compounds that can maintain the
homeostasis of endothelial junctions have attracted attention due
to their potential clinical applicability in alleviating vascular
diseases (19-21). Rosmarinic acid (RA; also known as
α-O-caffeoyl-3,4-dihydroxyphenyl lactic acid) is a polyphenolic
antioxidant that can be found in the Lamiaceae family of herbs and
mint, such as Rosmarinus officinalis (22). RA has been reported to exert
antioxidant and anti-inflammatory activities. In particular, the
antidiabetic and cardioprotective activities of RA have been
previously high-lighted (23,24). It was demonstrated that
oxLDL-mediated oxidative stress can upregulate thioredoxin
interacting protein (TXNIP) expression, which then binds to
nucleotide-binding domain and leucine-rich repeat containing
family, pyrin domain-containing 3 (NLRP3) to activate the NLRP3
inflammasome in ECs under HG conditions (25). RA was found to reverse this form
of oxLDL-mediated oxidative stress and NLRP3 inflammasome
activation (25). In addition,
the subsequent oxLDL-mediated IL-1β secretion in ECs was reversed
by RA treatment by downregulating ROS production, p38
phosphorylation and forkhead box O1 (FOXO1) protein expression in
ECs (25). However, the
potential effect of oxLDL-triggered NLRP3 activation on endothelial
integrity, permeability and monocyte transmigration remains poorly
understood. In addition, the potential effect of RA on these
oxLDL-mediated pathophysiological processes along with its
underlying mechanism remain unknown.
Therefore, in the present study, the effects of
oxLDL-triggered NLRP3 activation on the interaction between ECs and
monocytes, in addition to endothelial integrity and permeability,
under HG conditions, were evaluated. Specific research emphasis was
placed on the possible effects of RA on these oxLDL-mediated events
in the endothelium.
Materials and methods
Materials
Low-glucose DMEM (cat. no. SH30021.01), high-glucose
DMEM (cat. no. SH30243.01), RPMI-1640 (cat. no. SH30027.01), fetal
bovine serum (FBS; cat. no. SH30070.03), penicillin-streptomycin
100X solution (cat. no. SV30010) and 0.05% trypsin-EDTA (cat. no.
SH30236.01) were purchased from HyClone; Cytiva. RA (cat. no.
R4033) and human low-density lipoprotein (LDL; cat. no. 437644)
were provided by MilliporeSigma. Primary antibodies against ICAM-1
(cat. no. ab109361), VCAM-1 (cat. no. ab134047), phosphorylated
(p-)-VE cadherin (Y685; cat. no. ab119785), ZO-1 (cat. no. ab96587)
and TXNIP (cat. no. ab188865) were purchased from Abcam. Antibodies
against phosphorylated (p-)p-38 (cat. no. 9211s) and FOXO1 (cat.
no. 2880s) were purchased from Cell Signaling Technology, Inc.
Antibodies against p38 (cat. no. sc-535) and total VE-cadherin
(cat. no. sc-6458) were obtained from Santa Cruz Biotechnology,
Inc. TurboFect transfection reagent (cat. no. R0531) was purchased
from Thermo Fisher Scientific, Inc. Clarity Western Enhanced
chemiluminescence western blotting detection reagent (cat. no.
BR170-5061) was obtained from Bio-Rad Laboratories, Inc. BCECF
(cat. no. C3411) was obtained from Sigma-Aldrich; Merck KGaA.
SB203580 (cat. no. 1202) was obtained from Tocris Bioscience.
AS1842856 (cat. no. 16761) was procured from Cayman Chemical
Company. All other reagents, including MitoTEMPO (cat. no.
SML0737), Ac-YVAD-cmk (cat. no. SML0429), 2-mercaptoethanol,
protease inhibitor cocktail (cat. no. P8340),
2′,7′-dichlorodihy-drofluores-cein diacetate (cat. no. D6883),
N-acetyl-L-cysteine (NAC; cat. no. A9165), SB203580 (cat. no.
S8307) and the β-actin antibody (cat. no. a2066), were all obtained
from Sigma-Aldrich; Merck KGaA.
Preparation of oxLDL
oxLDL was prepared as described previously by Jin
et al (26). Briefly,
human LDL (2 mg/363 µl of 150 mM NaCl, pH 7.5, 0.01% EDTA)
was added with 1xPBS to make final concentration of 2 mg/1 ml.
Then, human LDL was dialyzed against PBS for 16 h at 4°C to remove
EDTA and then oxidized with 5 µM CuSO4 for 16 h
at 37°C. The extent of LDL oxidation was assessed by measuring the
formation of thiobarbituric acid-reactive substances (TBARS; cat.
no. 10009055; Cayman Chemical Company) at a wavelength of 540 nm
using a microplate reader (VersaMax Microplate Reader; Molecular
Devices, LLC). The level of TBARS for oxLDL and native LDL was
13.5±0.5 and 2.5±0.4, respectively.
Cell culture and treatment
The human umbilical vein endothelial cell line
EA.hy926 (cat. no. ATCC-CRL-2922) and human monocyte cell line
THP-1 were originally obtained from the American Type Culture
Collection. EA.hy926 cells and THP-1 cells were grown in DMEM and
RPMI-1640 medium, both supplemented with 10% FBS, 100 IU/ml
penicillin and 10 µg/ml streptomycin, respectively. All
cells were maintained in a humidified incubator at 37°C with 5%
CO2. ECs were cultured under HG conditions (25 mM) for
48 h at 37°C and pre-treated with Ac-YVAD-cmk (20 µM), NAC
(3 mM), mito-TEMPO (20 µM), AS1842856 (50 nM), SB203580 (0.5
µM) or RA (1, 10, 50 and 100 µM) prior to oxLDL
treatment for 1 h at 37°C. Subsequently, 50 µl oxLDL stock
solution (2 mg/ml) per 1 ml media was added for additional 24 h at
37°C.
Gene silencing with siRNA
Cells were transfected with 100 nM TXNIP-targeting
small interfering RNA (siTXNIP; sense, 5′-GCC GUU AGG AUC CUG GCU
UTT-3′ and antisense, 5′-AAG CCA GGA UCC UCC UAA CGG CTT-3′;
Bioneer Corporation) or control small interfering RNA (siCTRL;
sense, 5′-CCU ACG CCA CCA AUU UCG U-3′ and antisense, 5′-ACG AAA
UUG GUG GCG UAG G-3′; Bioneer Corporation) by using the Turbofect
transfection reagent (cat. no. R0531; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. Briefly, ECs were
transfected with control siRNA (100 nM) or TNXIP siRNA (100 nM) in
the HG conditions (25 mM) for 48 h and changed with fresh medium
before being stimulated with oxLDL (100 µg/ml) for an
additional 12 h. After incubation for total 60 h at 37°C, gene
silencing efficiency was determined by western blot analysis.
Adhesion assay
EA.hy926 cells (4×105 cells/ml) were
seeded in six-well plates and cultured until ~90% confluence under
HG conditions (25 mM) for 48 h at 37°C before being treated with
oxLDL in the presence or absence of caspase-1, ROS, p38 MAPK and
TXNIP inhibitors or RA at the indicated concentrations (1, 10, 50
and 100 µM) for an additional 24 h at 37°C. THP-1 cells
(7×105 cells per well) were labeled with the BCECF
fluorescent dye for 30 min at 37°C, before being added onto the ECs
and incubated for 30 min at 37°C. Following incubation,
non-adherent cells were washed three times with PBS and the number
of THP-1 cells adhered onto the EC cells was counted using a
fluorescence microscope (Eclipse Ti-U, Nikon Corporation). Images
were acquired at ×200 magnification from five randomly selected
fields per well. Adhered cells were counted using ImageJ version
5.2.0 software (National Institutes of Health).
Transmigration assay
To study the transmigration of THP-1 cells through
an EC monolayer, a Transwell system (Falcon® Permeable
Support for 24-well Plate with 8.0-µm Transparent PET
Membrane; Corning, Inc.) was used. ECs (1×105 cells)
cultured in DMEM supplemented with 10% FBS, 100 IU/ml penicillin
and 10 µg/ml streptomycin were first seeded into the upper
chambers of the Transwell chamber and placed into a 24-well plate.
After 4 h at 37°C, THP-1 cells (5×105 cells) were then
added to the upper chambers of the Transwell chamber before RPMI
medium supplemented with 10% FBS was added into the lower chambers.
The migration chambers were then incubated for 24 h in a 37°C cell
culture incubator. The number of cells that migrated across the EC
monolayer into the lower chamber was immediately counted using 0.4%
tryptophan blue staining at room temperature and a Countess II
automated cell counter (Thermo Fisher Scientific, Inc.).
Proteins extraction and western
blotting
ECs were lysed in RIPA buffer (25 mM Tris-HCl, pH
7.4, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate and 0.1% SDS)
containing a protease inhibitor cocktail. The supernatant was
collected after centrifugation at 12,000 × g for 20 min at 4°C and
the protein concentration was quantified by using Bradford assay.
Aliquots of 20-60 µg total protein from the different
experimental groups were then analyzed. The cell homogenates were
denatured with 4X SDS sample buffer containing 5%
2-mercaptoethanol, boiled for 5 min at 95°C, loaded into a 8-10%
SDS-polyacrylamide gel and separated by electrophoresis for 200 min
at 100 V. The protein samples were then transferred onto PVDF
membranes for 90 min at 190 mA using a wet transfer system (Bio-Rad
Laboratories, Inc.). The membranes were blocked with 5% fat-free
dried milk in 1X Tris buffered saline-containing 0.05% Tween-20
(TBS-T) for 1 h at room temperature and then probed with ICAM-1
(1:1,000), VCAM-1 (1:1,000), p-VE-cadherin (1:1,000), VE-cadherin
(1:2,000), ZO-1 (1:1,000), p-p38 (1:1,000), p38 (1:2,000), FOXO-1
(1:500), TXNIP (1:1,000) or β-actin (1:5,000) primary antibodies
overnight at 4°C. Following primary antibody incubation, the
membranes were washed with 1X TBS-T and incubated with
HRP-conjugated anti-rabbit, anti-mouse or anti-goat secondary
antibodies (1:5,000) for 1 h at room temperature. The
immunoreactive bands were visualized by Clarity Western ECL reagent
according to the manufacturer's protocol. The relative level of
each protein was normalized to the level of β-actin as a loading
control. Protein densities were quantified using ChemiDOC™ XRS+
Systems with Image Lab™ Software Version 5.2 (Bio-Rad Laboratories,
Inc.).
Statistical analysis
The data were analyzed using GraphPad Prism 7
software (GraphPad Software, Inc.). One-way analyses of variance
followed by Tukey's multiple-range test or two-way analyses of
variance followed by Bonferroni's test were used to detect
significant differences among the mean values. Data are presented
as the means ± standard from ≥ three independent experimental
repeats. P<0.05 was considered to indicate a statistically
significant difference.
Results
oxLDL-mediated expression of ICAM-1 and
VCAM-1 in ECs under HG conditions is ROS-, p38-,
FOXO1/TXNIP-dependent but RA reduces oxLDL-mediated ICAM-1 and
VCAM-1 expression
In a previous study (25), RA reversed oxLDL-mediated NLRP3
inflammasome activation by downregulating ROS production, p38
phosphorylation and FOXO1 protein expression in ECs. Therefore, in
the present study, the effects of oxLDL-mediated NLRP3 activation
on the expression of adhesion molecules ICAM-1 and VCAM-1 under HG
conditions were first examined. Afterwards, the effects of RA on
oxLDL-mediated ICAM-1 and VCAM-1 expression were examined. After
the ECs were stimulated with oxLDL (100 µg/ml) under low
glucose or HG conditions, the expression of ICAM-1 and VCAM-1 were
found to be significantly increased, though the magnitude of
increase was markedly greater following stimulation under HG
conditions (Fig. S1). However,
HG conditions without oxLDL stimulation did not result in any
changes in the expression of ICAM-1 and VCAM-1 (Fig. S1). oxLDL-mediated ICAM-1 and
VCAM-1 expression under HG conditions were found to be
significantly reversed by the selective and irreversible inhibitor
of caspase-1 Ac-YVAD-cmk (Fig.
1A). Next, the effects of ROS production on oxLDL-mediated
ICAM-1 and VCAM-1 expression in ECs under HG conditions were
investigated. ROS scavengers mitoTEMPO and NAC were found to
significantly reverse oxLDL-induced ICAM-1 and VCAM-1 upregulation
in the ECs under HG conditions (Fig.
1B). In addition, the p38 and FOXO1 pathway was potently
activated in ECs treated with oxLDL under HG conditions, compared
to low glucose conditions (Fig.
S2). Therefore, the effects p38 and FOXO1 pathway activation on
the upregulation of ICAM-1 and VCAM-1 in ECs stimulated with oxLDL
under HG conditions were next tested. The results showed that the
p38 inhibitor SB203580 and the FOXO1 inhibitor AS1842856
significantly reversed the oxLDL-induced expression of ICAM-1 and
VCAM-1 in ECs under HG conditions (Fig. 1C). Subsequently, transfection
with siTXNIP, which significantly knocked down TXNIP expression
induced by oxLDL under HG conditions (Fig. S3), also significantly reversed
the oxLDL-induced expression of ICAM-1 and VCAM-1 in ECs under HG
conditions (Fig. 1D). Finally,
oxLDL-mediated ICAM-1 and VCAM-1 upregulation in ECs under HG
conditions was found to be significantly reduced by RA in a
dose-dependent manner (Fig. 1E).
Therefore, the RA-mediated decrease in ICAM-1 and VCAM-1 expression
in ECs was possibly dependent on the inhibition of ROS production,
p38 phosphorylation, FOXO1 and TXNIP induction.
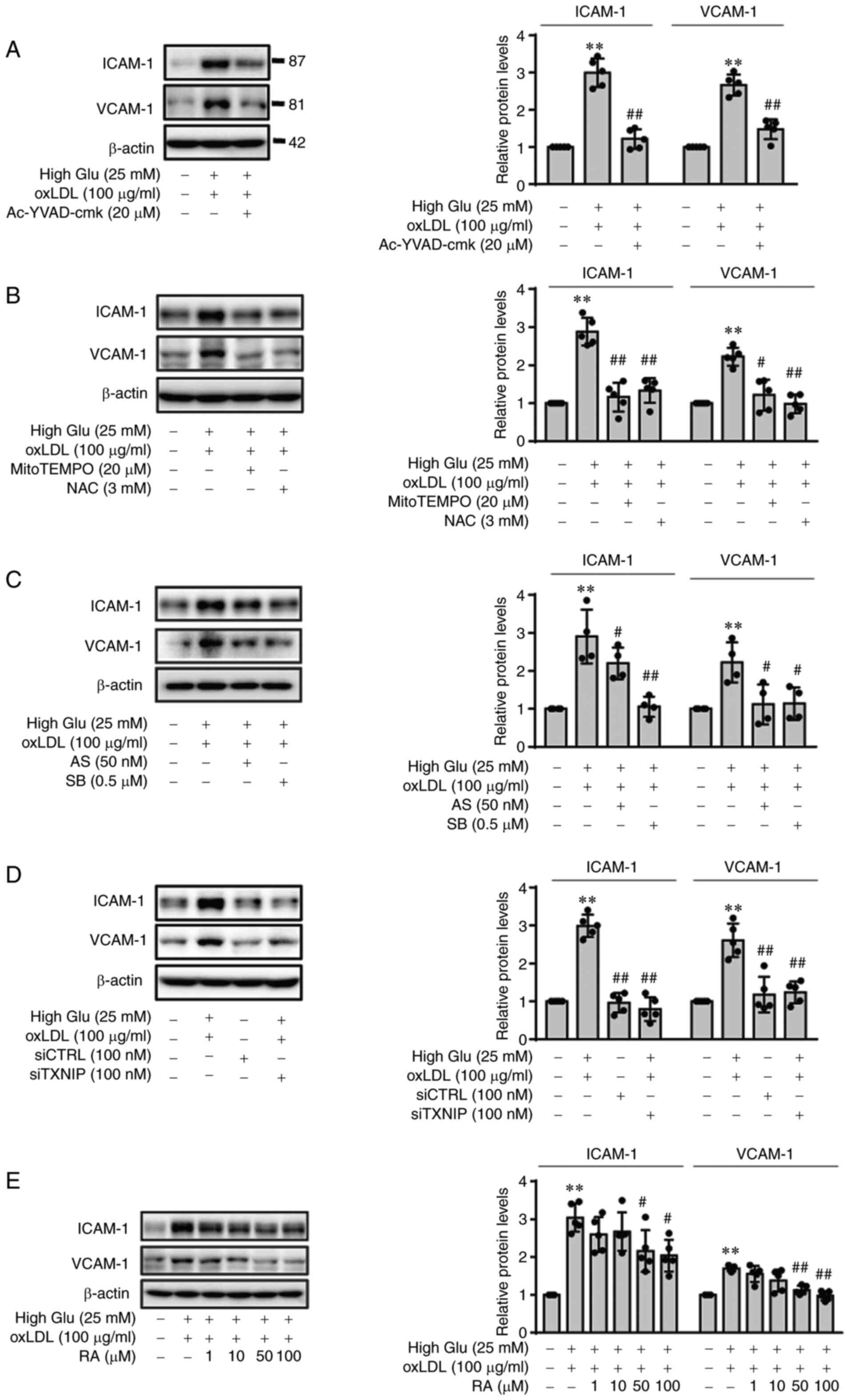 | Figure 1RA inhibits oxLDL-mediated ICAM-1 and
VCAM-1 expression in ECs n under HG conditions by downregulating
inflammasome activation in a ROS production, p38 MAPK and
FOXO1/TXNIP-dependent manner. ECs were cultured under HG conditions
(25 mM) for 48 h and pre-treated with (A) an irreversible inhibitor
of IL-1β Ac-YVAD-cmk (20 µM), (B) the ROS inhibitor NAC (3
mM) or the mitochondrial ROS inhibitor mitoTEMPO (20 µM) or
(C) the FOXO1 inhibitor AS1842856 (50 nM) or the p38 MAPK inhibitor
SB203580 (0.5 µM), prior to oxLDL treatment. The ECs were
then lysed 24 h later before ICAM-1 and VCAM-1 protein expression
were measured by western blotting. (D) CTRL siRNA- or TXNIP
siRNA-transfected ECs were cultured under HG conditions (25 mM) for
48 h before being stimulated with oxLDL for 24 h. ICAM-1 and VCAM-1
protein expression were measured in the lysates by western
blotting. (E) ECs were cultured under HG conditions (25 mM) for 48
h and stimulated with oxLDL for 24 h in the presence of RA (0, 1,
10, 50 and 100 mM), before ICAM-1 and VCAM-1 protein expression
were measured by western blotting. Band densities were quantified
and relative protein expression are presented as the mean ± SD from
five independent experiments. **P<0.01 vs. untreated
control. #P<0.05 and ##P<0.01 vs. HG +
oxLDL. RA, Rosmarinic acid; ICAM-1, intercellular adhesion
molecule-1; VCAM-1, vascular cell adhesion molecule-1; ECs,
endothelial cells; High Glu or HG, high glucose; ROS, reactive
oxygen species; FOXO1, forkhead box O1; TXNIP1, thioredoxin
interacting protein; NAC, N-acetyl-L-cysteine; oxLDL, oxidized low
density lipoprotein; AS, AS1842856; SB, SB203580; si,
small-interfering; CTRL, control. |
RA reverses oxLDL-induced endothelial
junction permeability under HG conditions
Next, to determine if oxLDL-induced inflammasome
activation can increase EC permeability under HG conditions,
VE-cadherin phosphorylation and ZO-1 expression were measured in
ECs following stimulation with oxLDL under HG conditions.
Consistent with the ICAM-1 and VCAM-1 expression results, oxLDL
stimulation under HG conditions significantly increased the
phosphorylation of VE-cadherin and decreased the expression of ZO-1
in ECs, which was in turn significantly reversed by pretreatment
with Ac-YVAD-cmk (Fig. 2A), ROS
scavengers mitoTEMPO and NAC (Fig.
2B) or p38 and FOXO1 inhibitors SB203580 and AS1842856,
respectively (Fig. 2C). TXNIP
knockdown also significantly decreased VE-cadherin phosphorylation
whilst restoring ZO-1 downregulation in the presence of oxLDL under
HG conditions (Fig. 2D).
Furthermore, RA was found to reverse EC dysfunction caused by oxLDL
treatment under HG conditions by restoring endothelial junction
integrity under HG conditions. As shown in Fig. 2E, RA significantly reversed the
oxLDL-induced increases in p-VE-cadherin levels under HG conditions
and oxLDL-induced decreases in ZO-1 expression under HG conditions
at 50 and 100 µM.
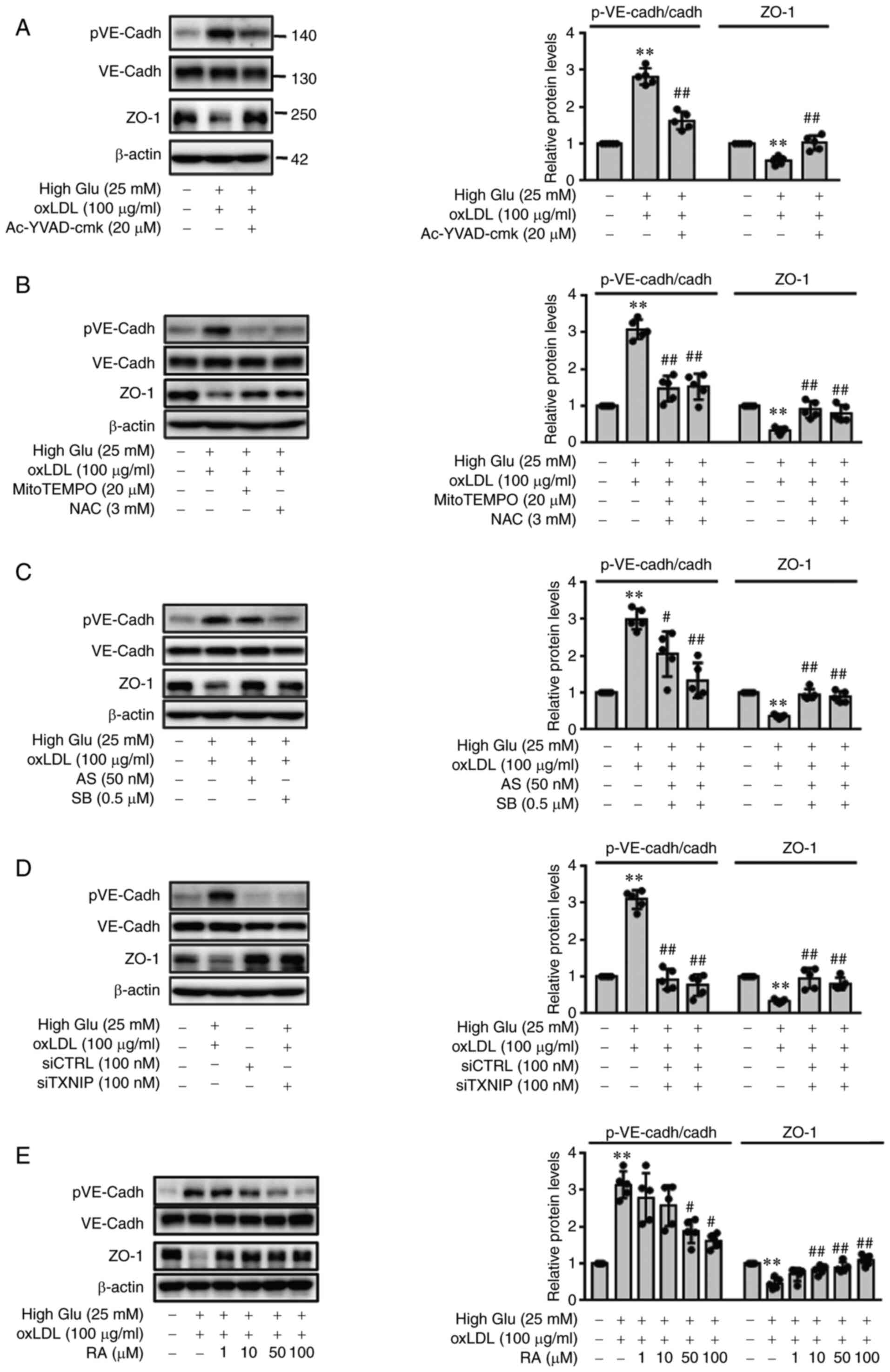 | Figure 2oxLDL promotes endothelial
permeability under HG conditions by phosphorylating VE-cadherin and
reducing the expression of ZO-1 in a RA-dependent manner. (A-E)
pVE-cadherin and ZO-1 protein levels in the cell lysates were
measured by western blot analysis. ECs cultured under HG conditions
were pre-treated with (A) Ac-YVAD-cmk (20 µM), (B) NAC (3
mM) or mitoTEMPO (20 µM) or (C) AS1842856 (50 nM) or
SB203580 (0.5 µM) for 1 h, before being stimulated with
oxLDL for 24 h. (D) CTRL siRNA- or TXNIP siRNA-transfected ECs were
cultured under HG conditions (25 mM) for 48 h and stimulated with
oxLDL for 24 h. (E) ECs were cultured under HG conditions (25 mM)
for 48 h and then stimulated with oxLDL for an additional 24 h in
the presence or absence of RA (0, 1, 10, 50 and 100 µM).
Band densities were quantified and relative protein levels are
presented as the mean ± SD from five independent experiments.
**P<0.01 vs. Control; #P<0.05 and
##P<0.01 vs. HG + oxLDL group. RA, Rosmarinic acid;
p, phosphorylated; VE-cadh, vascular endothelial cadherin; ZO-1,
zonula occludens; ECs, endothelial cells; High Glu or HG, high
glucose; TXNIP1, thioredoxin interacting protein; NAC,
N-acetyl-L-cysteine; oxLDL, oxidized low density lipoprotein; AS,
AS1842856; SB, SB203580; si, small-interfering; CTRL, control. |
Inhibitory effects of RA on HG- and
oxLDL-induced THP-1 monocyte adhesion to ECs is dependent on the
ROS-TXNIP/NLRP3 signaling pathway
Next, the effects of inhibiting the expression of
adhesion molecules in ECs on THP-1 adhesion to ECs were next
investigated. Consistent with the expression data of adhesion
molecules aforementioned, Ac-YVAD-cmk (Fig. 3A), the ROS scavengers MitoTEMPO
and NAC (Fig. 3B) and both p38
and FOXO1 inhibitors (Fig. 3C)
significantly reversed the oxLDL-induced adhesion of THP-1
monocytes to ECs under HG conditions. In addition, Fig. 3D revealed that TXNIP knockdown
significantly reversed oxLDL-triggered THP-1 cells adhesion to ECs
under HG conditions. RA was also found to significantly diminish
oxLDL-induced THP-1 monocyte adhesion to ECs under HG conditions
(Fig. 3E). These findings
suggest that RA effectively inhibits oxLDL-mediated THP-1 adhesion
to ECs under HG conditions through downregulation of adhesion
molecule (ICAM-1 and VCAM-1) expression.
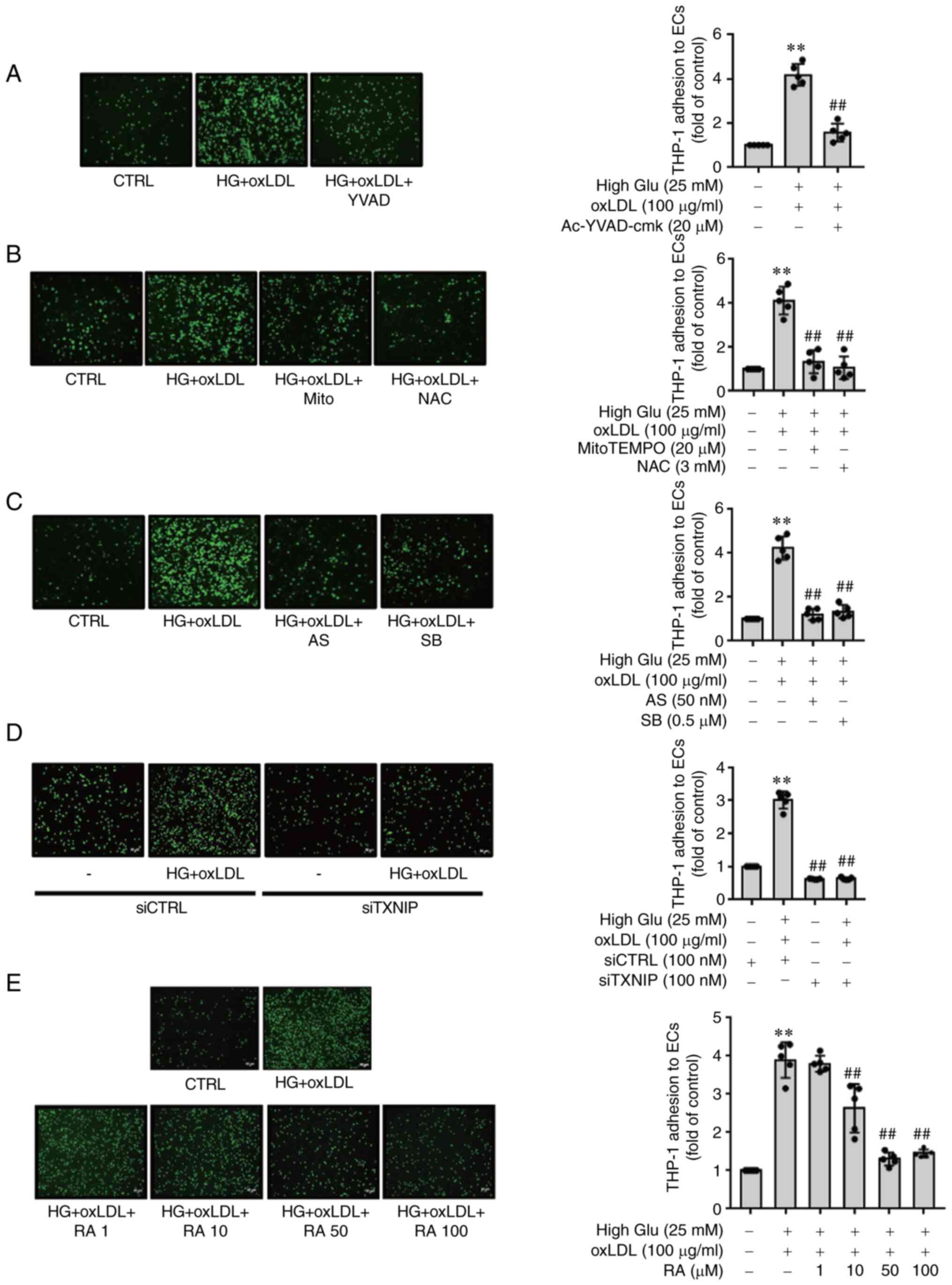 | Figure 3RA reduces the oxLDL-induced
adherence of THP-1 monocytes onto the endothelial monolayer under
HG conditions by downregulating reactive oxygen species production,
p38 MAPK activation and forkhead box O1/thioredoxin interacting
protein-induced inflammasome activation. (A-E) THP-1 cells
(7×105 cells/ml) were added to the ECs. After 30 min at
37°C, cell suspensions were withdrawn and the ECs were gently
washed three times with PBS. The adherent cells were then counted
under a fluorescence microscope. ECs cultured under HG conditions
were pretreated with (A) Ac-YVAD-cmk (20 µM), (B) NAC (3 mM)
or mitoTEMPO (20 µM) or (C) AS1842856 (50 nM) or SB203580
(0.5 µM) for 1 h before being stimulated with oxLDL for 24
h. (D) CTRL siRNA- or TXNIP siRNA-transfected ECs were cultured
under HG conditions (25 mM) for 48 h and stimulated with oxLDL for
24 h. (E) ECs were cultured under HG conditions (25 mM) for 48 h
and stimulated with oxLDL for an additional 24 h in the presence of
RA (0, 1, 10, 50 and 100 µM). Values are expressed as the
mean ± SD from five independent experiments. **P<0.01
vs. Control; ##P<0.01 vs. HG + oxLDL group. RA,
Rosmarinic acid; oxLDL, oxidized low density lipoprotein; ECs,
endothelial cells; High Glu or HG, high glucose; TXNIP1,
thioredoxin interacting protein; NAC, N-acetyl-L-cysteine; AS,
AS1842856; SB, SB203580; si, small-interfering; CTRL, control. |
oxLDL increases THP-1 monocyte diapedesis
under HG conditions in a manner that can be reversed by RA
Subsequently, the effects of oxLDL on THP-1
transmigration through the EC monolayer under HG conditions and the
effects of RA on THP-1 transmigration through ECs induced by oxLDL
and HG were all examined (Fig.
4A). oxLDL-treated ECs under HG conditions resulted in
significantly higher THP-1 transmigration levels through ECs
compared with those in untreated control cells, which were in turn
significantly reversed by Ac-YVAD-cmk (a caspase-1 inhibitor),
MitoTEMPO and NAC (ROS scavengers), SB203580 (a p38 MAPK inhibitor)
and AS1842856 (a FOXO1 inhibitor) treatment (Fig. 4B). THP-1 transmigration was
significantly reduced on TXNIP siRNA-transfected ECs treated with
oxLDL under HG conditions compared with that on CTRL
siRNA-transfected ECs (Fig. 4C).
RA also significantly reversed oxLDL-induced monocyte
transmigration through ECs under HG conditions at 50 and 100
µM (Fig. 4D). These
findings suggest that RA can alleviate oxLDL-induced endothelial
hyperpermeability and consequent THP-1 transmigration under HG
conditions.
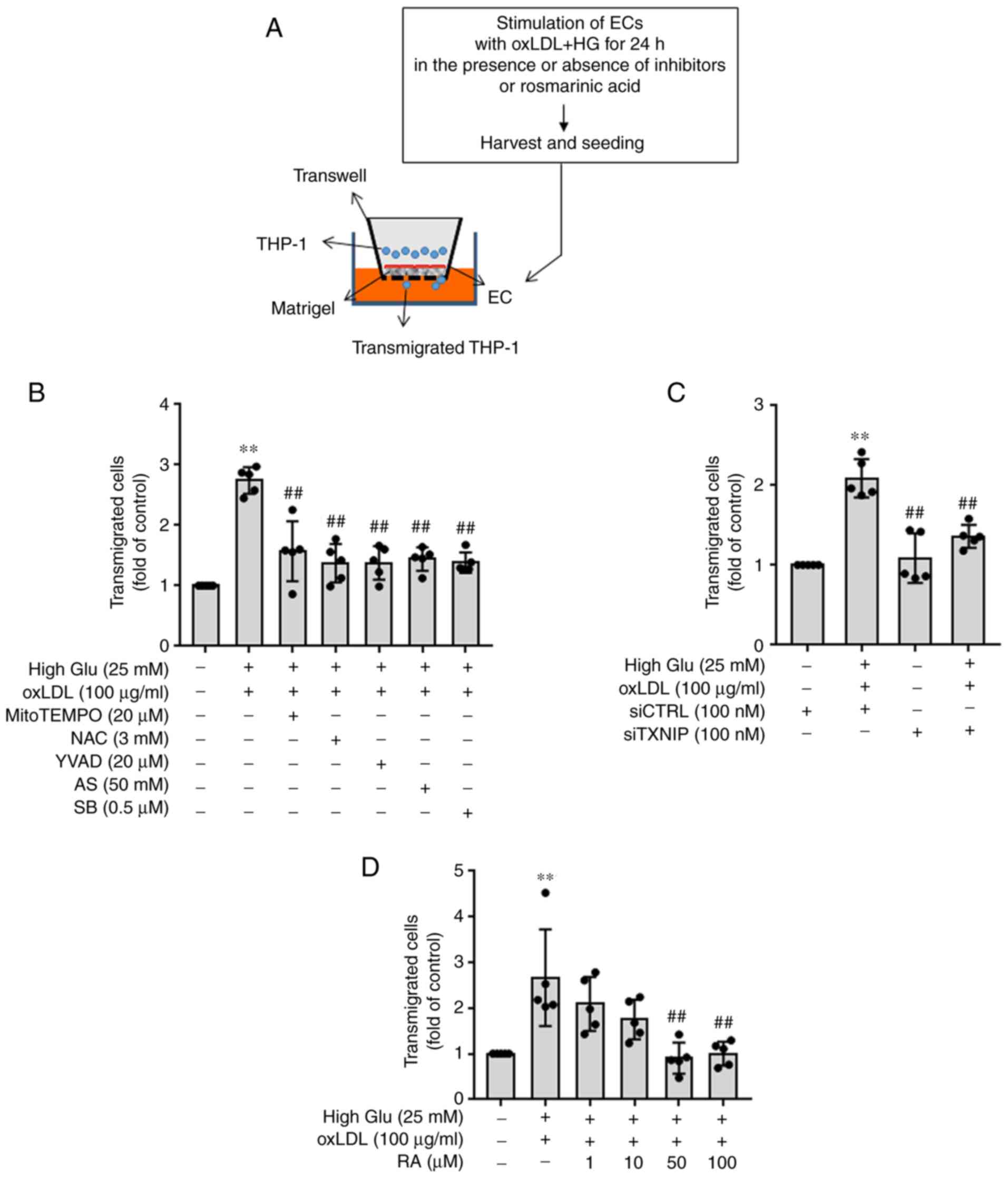 | Figure 4oxLDL and HG treatment promotes the
transmigration of monocytes through ECs by modulating the
inflammasome activation pathway in a manner that can be reversed by
RA. (A) ECs were added to Matrigel-coated insert wells. ECs
cultured under HG conditions (25 mM) for 48 h were stimulated with
oxLDL in the presence of inhibitors or RA for 24 h before being
harvested and added to Matrigel-coated insert wells. THP-1 cells
were then added to ECs 4 h later. The migration chambers were
incubated for 24 h. The number of cells that migrated across the EC
monolayer into the lower chamber was counted using tryptophan blue
staining and a Countess II automated cell counter. (B) ECs cultured
under HG conditions (25 mM) were pretreated with mitoTEMPO (20
µM), NAC (3 mM), Ac-YVAD-cmk (20 µM), AS1842856 (50
nM) or SB203580 (0.5 µM) for 1 h before being stimulated
with oxLDL for an additional 24 h. (C) CTRL siRNA- or TXNIP
siRNA-transfected ECs were cultured under HG conditions (25 mM) and
stimulated with oxLDL. (D) ECs cultured under HG conditions (25 mM)
were stimulated with oxLDL for an additional 24 h in the presence
or absence of RA (0, 1, 10, 50 and 100 µM). Values are
expressed as the mean ± SD from five independent experiments.
**P<0.01 vs. Control; ##P<0.01 vs. HG +
oxLDL. RA, Rosmarinic acid; oxLDL, oxidized low density
lipoprotein; ECs, endothelial cells; High Glu or HG, high glucose;
TXNIP1, thioredoxin interacting protein; NAC, N-acetyl-L-cysteine;
AS, AS1842856; SB, SB203580; si, small-interfering; CTRL,
control. |
Discussion
Endothelial dysfunction is considered to be one of
the first stages of atherosclerosis under hyperglycemic and
dyslipidemic conditions (27).
Endothelial dysfunction increases the expression of adhesion
molecules ICAM-1 and VCAM-1 by ECs, which increases the adhesion of
monocytes to ECs to facilitate their diapedesis into the
subendothelial space, which activates atherosclerotic plaque
generation (11). Various
pathological conditions, including diabetes and obesity, perturb
the integrity of cell junctions, resulting in the increased
movement of monocytes and plasma lipoproteins into the intima
(20,28). Therefore, regulating the
interaction between monocytes and ECs, in addition to endothelial
permeability, would greatly limit the instigation of atherogenesis.
The overexpression of endothelial adhesion molecules has been
previously reported to be key for the pathogenesis of vascular
diseases, such as atherosclerosis (29,30). In addition, HG conditions and
oxLDL further contribute to the development of atherosclerosis by
increasing the expression of ICAM-1 and VCAM-1 (31,32). Previous studies have documented
that inflammatory cytokines, such as TNF-α, IL-33 and IL-1β, was
positively associated with the increased expression of VCAM-1 and
ICAM-1 in vascular and cardiac cells, leukocyte adhesion to ECs and
endothelial hyperpermeability (33-35).
RA has been previously reported to exert beneficial
effects on the cardiovascular system because of its antioxidant and
anti-inflammatory properties. It reduced adriamycin-induced
cardiotoxicity in H9C2 cells by inhibiting ROS production (36), in addition to inhibiting
atherosclerotic plaque formation in Apolipoprotein E−/−
mice fed on a high-cholesterol diet, by reducing the serum levels
of proinflammatory cytokines TNF-α and IL-1β (37). In a previous study, it was shown
that RA protected ECs by inhibiting oxLDL-mediated NLRP3
inflammasome activation and resultant IL-1β production under HG
conditions (25). Therefore, for
further study, the present study investigated the effects of RA on
oxLDL-mediated interactions between monocytes and ECs, endothelial
permeability and transmigration of monocytes through endothelial
monolayers. ECs stimulated with oxLDL under HG conditions were
found to exhibit significantly higher expression levels of adhesion
molecules ICAM-1 and VCAM-1, which resulted in increased THP-1
monocyte adhesion onto EC monolayer. oxLDL-induced endothelial
inflammation under HG conditions has been reported to be
orchestrated by ROS-mediated p38 phosphorylation, FOXO1/TXNIP
induction and IL-1β production (25,38,39). Correspondingly, ECs pretreated
with the irreversible inhibitor of caspase-1 (an IL-1β converting
enzyme), ROS scavengers and p38 and FOXO1 inhibitors all showed
significant reductions in oxLDL-induced ICAM-1 and VCAM-1 protein
expression under HG conditions. Notably, TXNIP knockdown also
reversed HG- and oxLDL-mediated overexpression of ICAM-1 and VCAM-1
in ECs. In particular, inhibitors of ROS, p38 MAPK, FOXO1 and
TXNIP, all of which are involved in the expression of the adhesion
molecules ICAM-1 and VCAM-1, significantly reduced oxLDL-induced
THP-1 monocyte adhesion to ECs under HG conditions. RA, which was
previously found to downregulate the activity of the
p38/FOXO1/TXNIP pathway and inhibit inflammasome activation in
oxLDL- and HG-stimulated ECs (22), diminished oxLDL-mediated
upregulation of ICAM-1 and VCAM-1 expression in ECs under HG
conditions whilst significantly reducing THP-1 monocyte adhesion to
ECs in a dose-dependent manner. oxLDL-induced expression of
adhesion molecules (ICAM-1 and VCAM-1) was found to be markedly
higher under HG conditions compared with that stimulated under low
glucose conditions. In addition, oxLDL treatment increased the
levels of NLRP3 activation pathway mediators p-p38, FOXO-1 and
TXNIP under low glucose conditions, which were enhanced under HG
conditions. However, HG without oxLDL stimulation did not exert any
changes in the expression of ICAM-1 and VCAM-1 whilst weakly
increasing p-p38, FOXO-1 and TXNIP levels. These results suggest
that oxLDL enhances the inflammatory response under HG conditions
but not under low glucose conditions or HG conditions without
oxLDL.
Endothelial inflammation alters junction integrity
and increases endothelial permeability (40,41). Vascular permeability and monocyte
diapedesis are increased by the phosphorylation of VE-cadherin, a
major component of adherens junctions (18,42), and by the downregulation of ZO-1,
a key molecule of tight junctions (16). HG and oxLDL were found to
increase VE-cadherin phosphorylation but decrease ZO-1 expression
in ECs in the present study, which in turn significantly induced
THP1 monocyte transmigration through ECs. Pretreatment of the ECs
with ROS scavengers, inhibitors of caspase-1, p38 MAPK and FOXO1
and transfection with TXNIP siRNA all reversed the oxLDL-induced
phosphorylation of VE-cadherin and ZO-1 downregulation under HG
conditions. In addition, inhibiting ROS/p38 MAPK/TXNIP inflammasome
pathway activation significantly reduced monocyte diapedesis
through the EC monolayers. RA also reversed the oxLDL-increased
phosphorylation of VE-cadherin and downregulation of ZO-1
expression in a dose-dependent manner, which resulted in the
blockage of monocyte diapedesis through EC monolayers.
Taken together, results of the present study
revealed that oxLDL can trigger the overexpression of adhesion
molecules in ECs under HG conditions, which leads to the adhesion
of monocytes to ECs. In addition, oxLDL stimulation increases
endothelial permeability, promoting monocyte diapedesis. Based on
previous findings and the results of this study, RA effectively
prevented the expression of adhesion molecules and THP-1 monocyte
adhesion to ECs whilst inhibiting endothelial monolayer leakage and
THP-1 monocyte diapedesis, by regulating oxLDL-mediated ROS/p38
MAPK/FOXO1/TXNIP/inflammasome activation under HG conditions
(Fig. 5). These findings suggest
that RA exerts protective effects against hyperlipidemia- and
hyperglycemia-induced cardiovascular disease by modulating the
interaction between monocytes and ECs, as well as monocyte
diapedesis.
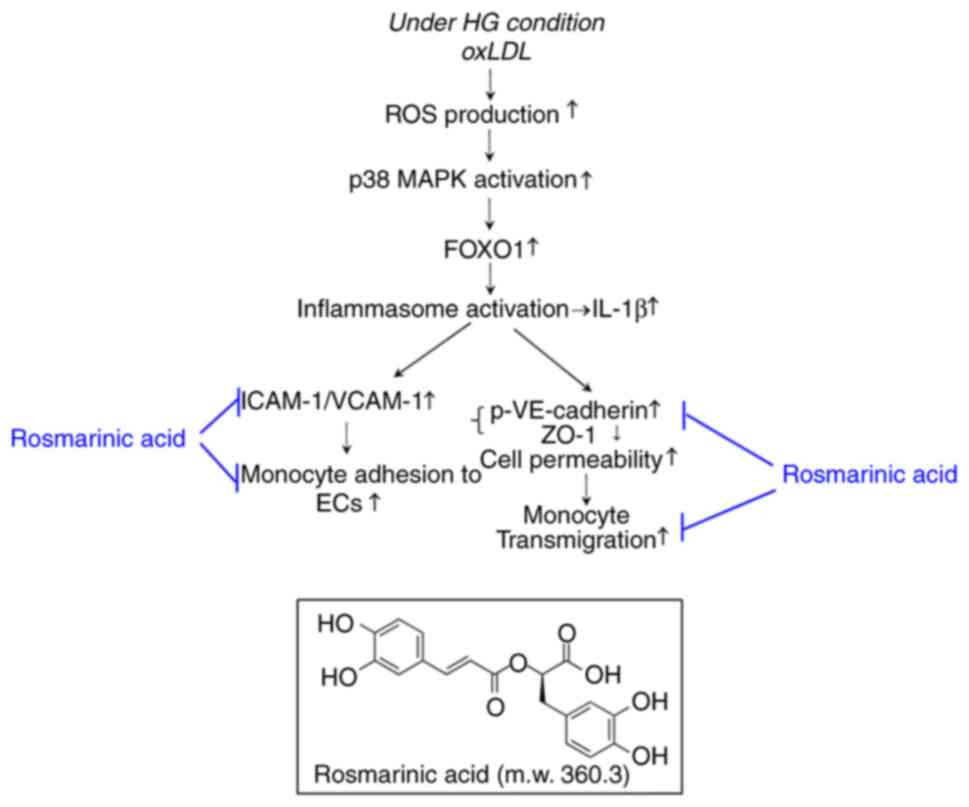 | Figure 5Schematic model of the underlying
mechanism of the rosmarinic acid-mediated reversal of
oxLDL-mediated endothelial dysfunction under HG conditions by
modulating the interaction between monocytes and ECs. oxLDL,
oxidized low density lipoprotein; HG, high glucose; ROS, reactive
oxygen species; FOXO1, forkhead box O1; ICAM1, intercellular
adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1;
ECs, endothelial cells; p-, phosphorylated; VE, vascular
endothelial; ZO-1, zonula occludens-1; m.w., molecular weight. |
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JBN performed the experiments and analyzed the data.
YSK and HJ performed the experiments and analyzed the data. SWP and
SPY contributed to the analysis and the interpretation of the data.
KRK provided important critical feedback on data interpretation and
revision. HJK designed the study, interpreted the data and revised
the manuscript. JBN, YSK and HJK confirmed the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by Basic Science Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Science, ICT and Future Planning (grant
no. NRF-2015R1A5A2008833) and by the Ministry of Education, Science
and Technology (grant no. 2021R1A2B5B01001446).
Abbreviations:
|
CTRL
|
control
|
|
ECs
|
endothelial cells
|
|
FOXO1
|
forkhead box O1
|
|
HG
|
high glucose
|
|
ICAM-1
|
intercellular adhesion molecule 1
|
|
NAC
|
N-acetyl-L-cysteine
|
|
NLRP3
|
nucleotide-binding domain and
leucine-rich repeat containing family, pyrin domain-containing
3
|
|
oxLDL
|
oxidized low density lipoprotein
|
|
RA
|
rosmarinic acid
|
|
ROS
|
reactive oxygen species
|
|
TXNIP
|
thioredoxin interacting protein
|
|
VCAM-1
|
vascular cells adhesion molecule 1,
VE-cadherin, vascular endothelial cadherin
|
|
ZO-1
|
zonula occludens-1
|
References
|
1
|
Sluiter TJ, van Buul JD, Huveneers S, Quax
PHA and de Vries MR: Endothelial barrier function and leukocyte
transmigration in atherosclerosis. Biomedicines. 9:3282021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taddei A, Giampietro C, Conti A, Orsenigo
F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S and
Dejana E: Endothelial adherens junctions control tight junctions by
VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol.
10:923–934. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Endothelial barrier and its abnormalities in cardiovascular
disease. Front Physiol. 6:3652015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sena CM, Pereira AM and Seiça R:
Endothelial dysfunction-a major mediator of diabetic vascular
disease. Biochim Biophys Acta. 1832:2216–2231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bai B, Yang Y, Wang Q, Li M, Tian C, Liu
Y, Aung LH, Li PF, Yu T and Chu XM: NLRP3 inflammasome in
endothelial dysfunction. Cell Death Dis. 11:7762020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katakami N: Mechanism of development of
atherosclerosis and cardiovascular disease in diabetes mellitus. J
Atheroscler Thromb. 25:27–39. 2018. View Article : Google Scholar :
|
|
7
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar :
|
|
8
|
Hamed S, Brenner B, Abassi Z, Aharon A,
Daoud D and Roguin A: Hyperglycemia and oxidized-LDL exert a
deleterious effect on endothelial progenitor cell migration in type
2 diabetes mellitus. Thromb Res. 126:166–174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ko YS, Jin H, Park SW and Kim HJ:
Salvianolic acid B protects against oxLDL-induced endothelial
dysfunction under high-glucose conditions by downregulating
ROCK1-mediated mitophagy and apoptosis. Biochem Pharmacol.
174:1138152020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akhmedov A, Rozenberg I, Paneni F, Camici
GG, Shi Y, Doerries C, Sledzinska A, Mocharla P, Breitenstein A,
Lohmann C, et al: Endothelial overexpression of LOX-1 increases
plaque formation and promotes atherosclerosis in vivo. Eur Heart J.
35:2839–2848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matheus AS, Tannus LR, Cobas RA, Palma CC,
Negrato CA and Gomes MB: Impact of diabetes on cardiovascular
disease: An update. Int J Hypertens. 2013:6537892013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Claesson-Welsh L, Dejana E and McDonald
DM: Permeability of the endothelial barrier: Identifying and
reconciling controversies. Trends Mol Med. 27:314–331. 2021.
View Article : Google Scholar :
|
|
13
|
Dejana E, Tournier-Lasserve E and
Weinstein BM: The control of vascular integrity by endothelial cell
junctions: Molecular basis and pathological implications. Dev Cell.
16:209–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giannotta M, Trani M and Dejana E:
VE-cadherin and endothelial adherens junctions: Active guardians of
vascular integrity. Dev Cell. 26:441–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chattopadhyay R, Dyukova E, Singh NK, Ohba
M, Mobley JA and Rao GN: Vascular endothelial tight junctions and
barrier function are disrupted by 15(S)-hydroxyeicosatetraenoic
acid partly via protein kinase C ε-mediated zona occludens-1
phosphorylation at threonine 770/772. J Biol Chem. 289:3148–3163.
2014. View Article : Google Scholar
|
|
16
|
Tornavaca O, Chia M, Dufton N, Almagro LO,
Conway DE, Randi AM, Schwartz MA, Matter K and Balda MS: ZO-1
controls endothelial adherens junctions, cell-cell tension,
angiogenesis, and barrier formation. J Cell Biol. 208:821–838.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orsenigo F, Giampietro C, Ferrari A,
Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY,
Franco D, et al: Phosphorylation of VE-cadherin is modulated by
haemodynamic forces and contributes to the regulation of vascular
permeability in vivo. Nat Commun. 3:12082012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wessel F, Winderlich M, Holm M, Frye M,
Rivera-Galdos R, Vockel M, Linnepe R, Ipe U, Stadtmann A, Zarbock
A, et al: Leukocyte extravasation and vascular permeability are
each controlled in vivo by different tyrosine residues of
VE-cadherin. Nat Immunol. 15:223–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsieh SL, Wang JJ, Su KH, Kuo YL, Hsieh S
and Wu CC: Suppressive effects of the gynura bicolor ether extract
on endothelial permeability and leukocyte transmigration in human
endothelial cells induced by TNF-α. Evid Based Complement Alternat
Med. 2020:94137242020. View Article : Google Scholar
|
|
20
|
Lian D, Yuan H, Yin X, Wu Y, He R, Huang Y
and Chen Y: Puerarin inhibits hyperglycemia-induced
inter-endothelial junction through suppressing endothelial Nlrp3
inflammasome activation via ROS-dependent oxidative pathway.
Phytomedicine. 55:310–319. 2019. View Article : Google Scholar
|
|
21
|
Zhou X, Wu Y, Ye L, Wang Y, Zhang K, Wang
L, Huang Y, Wang L, Xian S, Zhang Y and Chen Y: Aspirin alleviates
endothelial gap junction dysfunction through inhibition of NLRP3
inflammasome activation in LPS-induced vascular injury. Acta Pharm
Sin B. 9:711–723. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim GD, Park YS, Jin YH and Park CS:
Production and applications of rosmarinic acid and structurally
related compounds. Appl Microbiol Biotechnol. 99:2083–2092. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karthik D, Viswanathan P and Anuradha CV:
Administration of rosmarinic acid reduces cardiopathology and blood
pressure through inhibition of p22phox NADPH oxidase in
fructose-fed hypertensive rats. J Cardiovasc Pharmacol. 58:514–521.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sotnikova R, Okruhlicova L, Vlkovicova J,
Navarova J, Gajdacova B, Pivackova L, Fialova S and Krenek P:
Rosmarinic acid administration attenuates diabetes-induced vascular
dysfunction of the rat aorta. J Pharm Pharmacol. 65:713–723. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nyandwi JB, Ko YS, Jin H, Yun SP, Park SW
and Kim HJ: Rosmarinic acid inhibits oxLDL-induced inflammasome
activation under high-glucose conditions through downregulating the
p38-FOXO1-TXNIP pathway. Biochem Pharmacol. 182:1142462020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin H, Ko YS, Park SW and Kim HJ:
P2Y2R activation by ATP induces oxLDL-mediated
inflammasome activation through modulation of mitochondrial damage
in human endothelial cells. Free Radic Biol Med. 136:109–117. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wan Z, Fan Y, Liu X, Zue J, Han Z, Zhu C
and Wang Z: NLRP3 inflammasome promotes diabetes-induced
endothelial inflammation and atherosclerosis. Diabetes Metab Syndr
Obes. 12:1931–1942. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hashimoto K, Kataoka N, Nakamura E,
Tsujioka K and Kajiya F: Oxidized LDL specifically promotes the
initiation of monocyte invasion during transendothelial migration
with upregulated PECAM-1 and downregulated VE-cadherin on
endothelial junctions. Atherosclerosis. 194:e9–e17. 2007.
View Article : Google Scholar
|
|
29
|
Santos JC, Cruz MS, Bortolin RH, Oliveira
KM, Araújo JN, Duarte VH, Silva AM, Santos IC, Dantas JM, Paiva MS,
et al: Relationship between circulating VCAM-1, ICAM-1, E-selectin
and MMP9 and the extent of coronary lesions. Clinics (Sao Paulo).
73:e2032018. View Article : Google Scholar
|
|
30
|
Rubio-Guerra AF, Vargas-Robles H, Serrano
AM, Lozano-Nuevo JJ and Escalante-Acosta BA: Correlation between
the levels of circulating adhesion molecules and atherosclerosis in
type-2 diabetic normotensive patients: Circulating adhesion
molecules and atherosclerosis. Cell Adh Migr. 3:369–372. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang Z, Cai X, Li S, Zhou H, Chu M, Shan
P and Huang W: Berberine-attenuated monocyte adhesion to
endothelial cells induced by oxidized low-density lipoprotein via
inhibition of adhesion molecule expression. Mol Med Rep. 7:461–465.
2013. View Article : Google Scholar
|
|
32
|
Chen JS, Chen YH, Huang PH, Tsai HY, Chen
YL, Lin SJ and Chen JW: Ginkgo biloba extract reduces
high-glucose-induced endothelial adhesion by inhibiting the
redox-dependent interleukin-6 pathways. Cardiovasc Diabetol.
11:492012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cejkova S, Kubatova H, Thieme F, Janousek
L, Fronek J, Poledne R and Lesna I: The effect of cytokines
produced by human adipose tissue on monocyte adhesion to the
endothelium. Cell Adh Migr. 13:293–302. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van Wetering S, van den Berk N, van Buul
JD, Mul FP, Lommerse I, Mous R, ten Klooster JP, Zwaginga JJ and
Hordijk PL: VCAM-1-mediated Rac signaling controls endothelial
cell-cell contacts and leukocyte transmigration. Am J Physiol Cell
Physiol. 285:C343–C352. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Demyanets S, Konya V, Kastl SP, Kaun C,
Rauscher S, Niessner A, Pentz R, Pfaffenberger S, Rychli K,
Lemberger CE, et al: Interleukin-33 induces expression of adhesion
molecules and inflammatory activation in human endothelial cells
and in human atherosclerotic plaques. Arterioscler Thromb Vasc
Biol. 31:2080–2089. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim DS, Kim HR, Woo ER, Hong ST, Chae HJ
and Chae SW: Inhibitory effects of rosmarinic acid on
adriamycin-induced apoptosis in H9c2 cardiac muscle cells by
inhibiting reactive oxygen species and the activations of c-Jun
N-terminal kinase and extracellular signal-regulated kinase.
Biochem Pharmacol. 70:1066–1078. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L, Tian J and Liang X: Regression of
atherosclerosis by rosmarinic acid via regulating lipid metabolism
and anti-inflammatory actions. J Mol Cell Cardiol. 44:P7192008.
View Article : Google Scholar
|
|
38
|
Jian D, Wang Y, Jian L, Tang H, Rao L,
Chen K, Jia Z, Zhang W, Liu Y, Chen X, et al: METTL14 aggravates
endothelial inflammation and atherosclerosis by increasing FOXO1
N6-methyladeosine modifications. Theranostics. 10:8939–8956. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liang B, Wang X, Zhang N, Yang H, Bai R,
Liu M, Bian Y, Xiao C and Yang Z: Angiotensin-(1-7) attenuates
angiotensin II-induced ICAM-1, VCAM-1, and MCP-1 expression via the
MAS receptor through suppression of P38 and NF-κB pathways in
HUVECs. Cell Physiol Biochem. 35:2472–2482. 2015. View Article : Google Scholar
|
|
40
|
Reglero-Real N, Colom B, Bodkin JV and
Nourshargh S: Endothelial cell junctional adhesion molecules: Role
and regulation of expression in inflammation. Arterioscler Thromb
Vasc Biol. 36:2048–2057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sukriti S, Tauseef M, Yazbeck P and Mehta
D: Mechanisms regulating endothelial permeability. Pulm Circ.
4:535–551. 2014. View
Article : Google Scholar
|
|
42
|
Gavard J: Endothelial permeability and
VE-cadherin: A wacky comradeship. Cell Adh Migr. 7:455–461. 2013.
View Article : Google Scholar
|



















