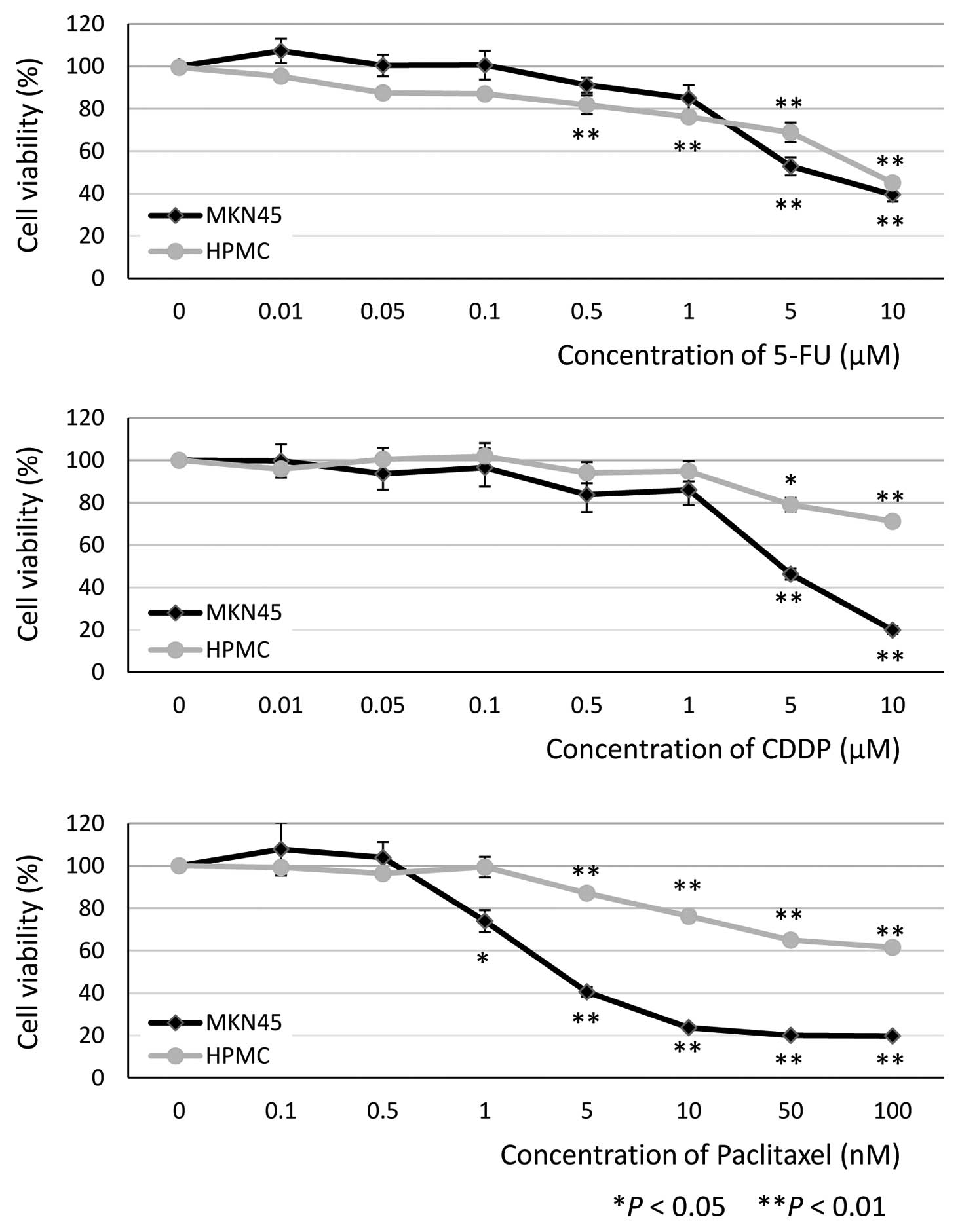
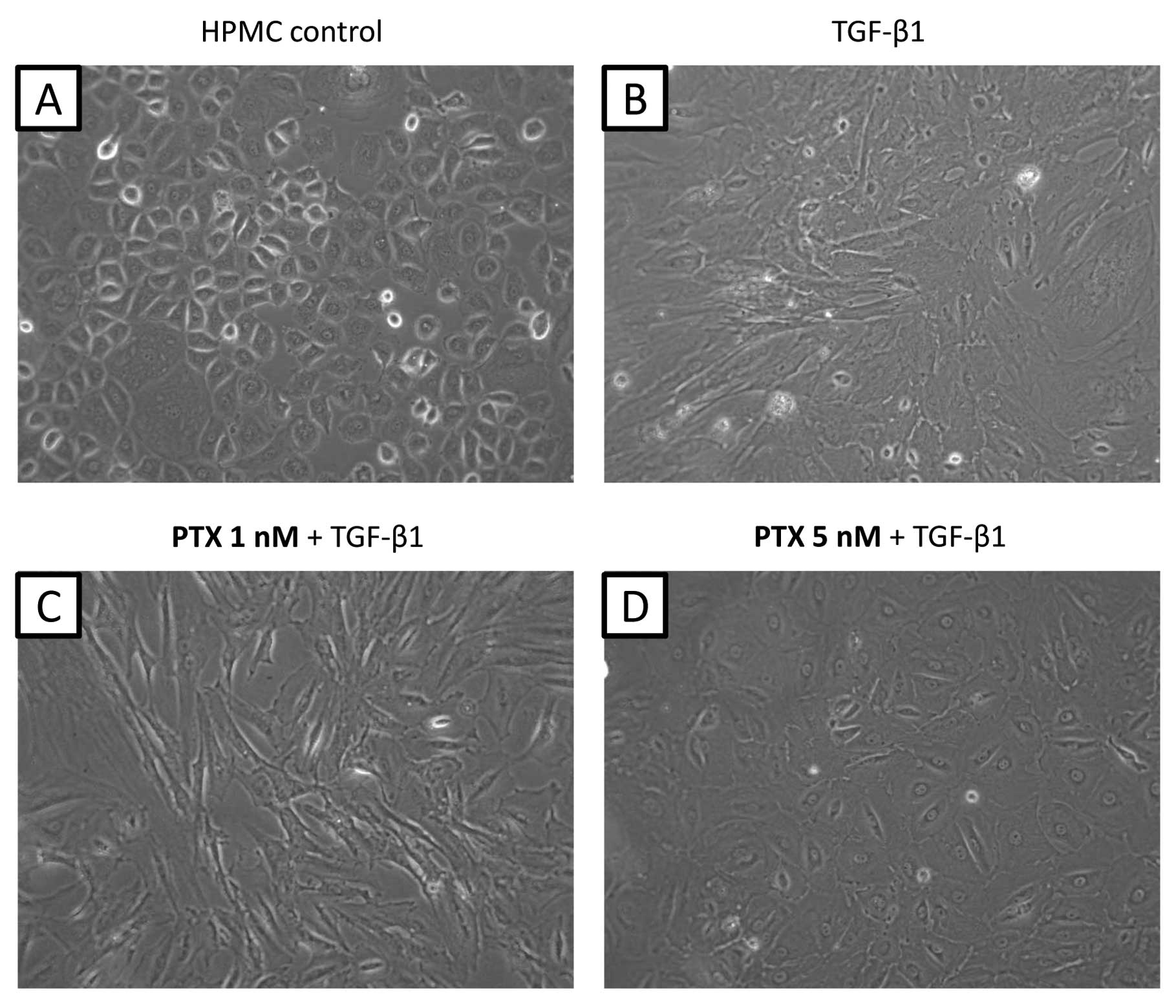
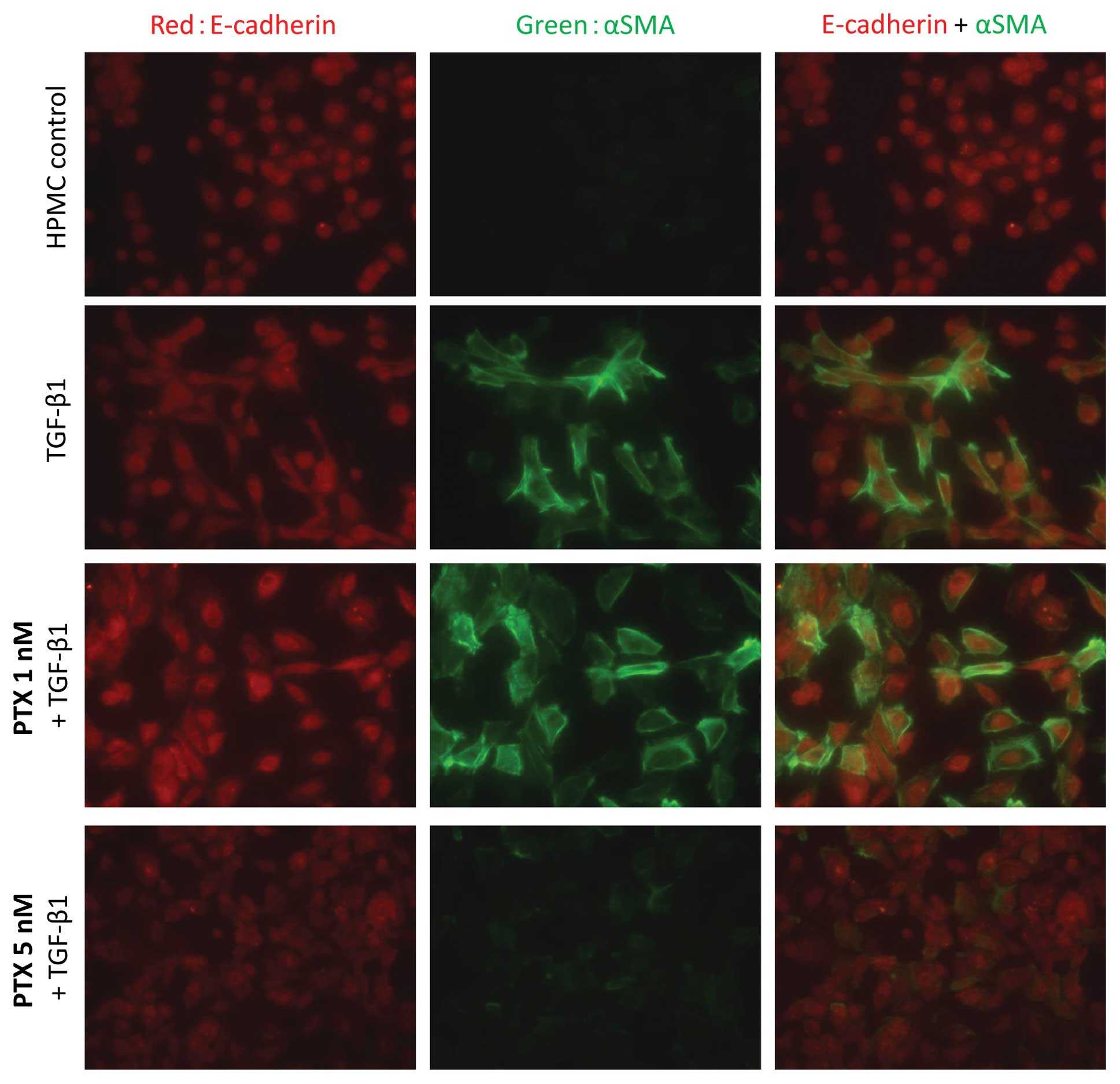
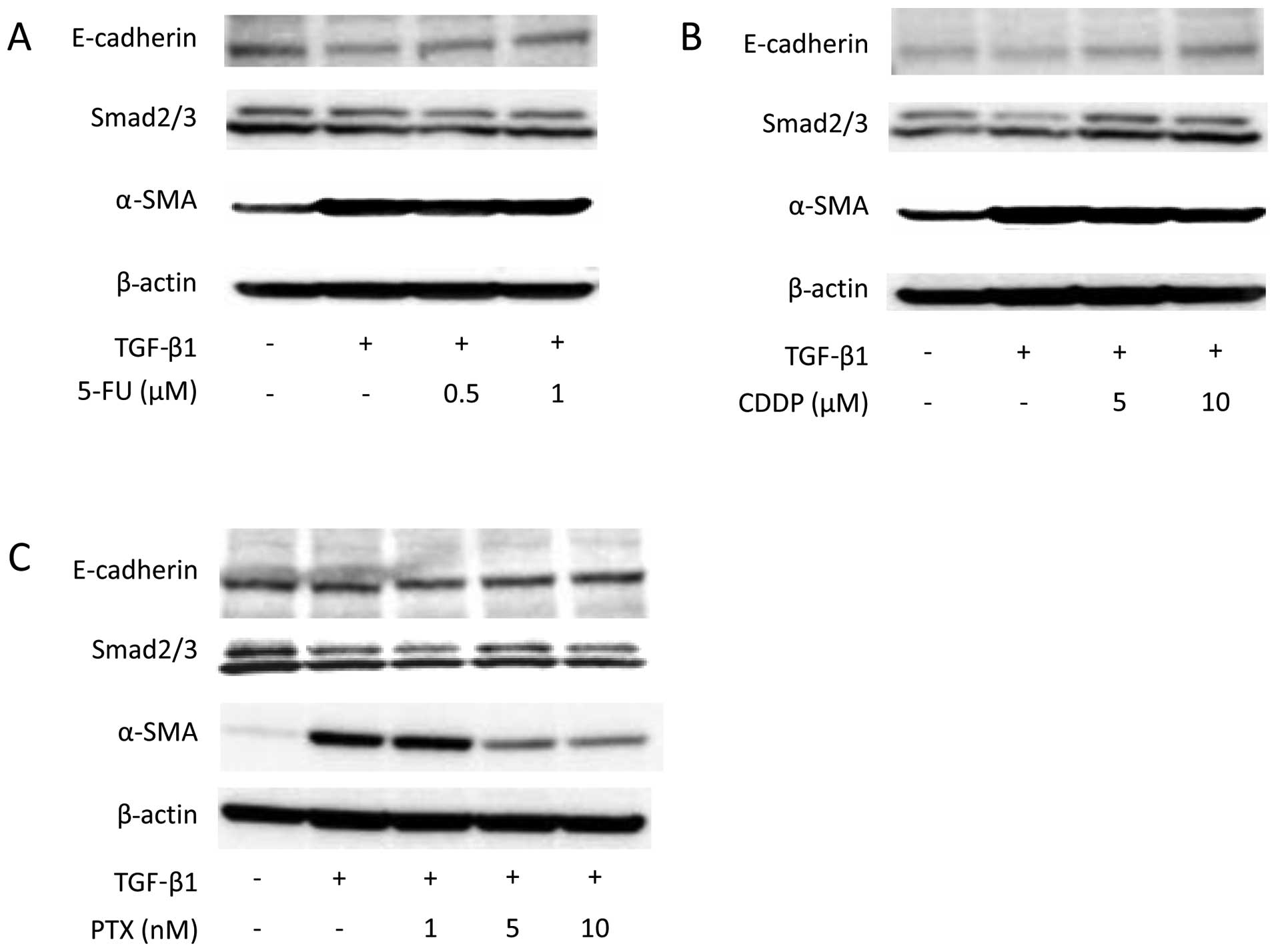
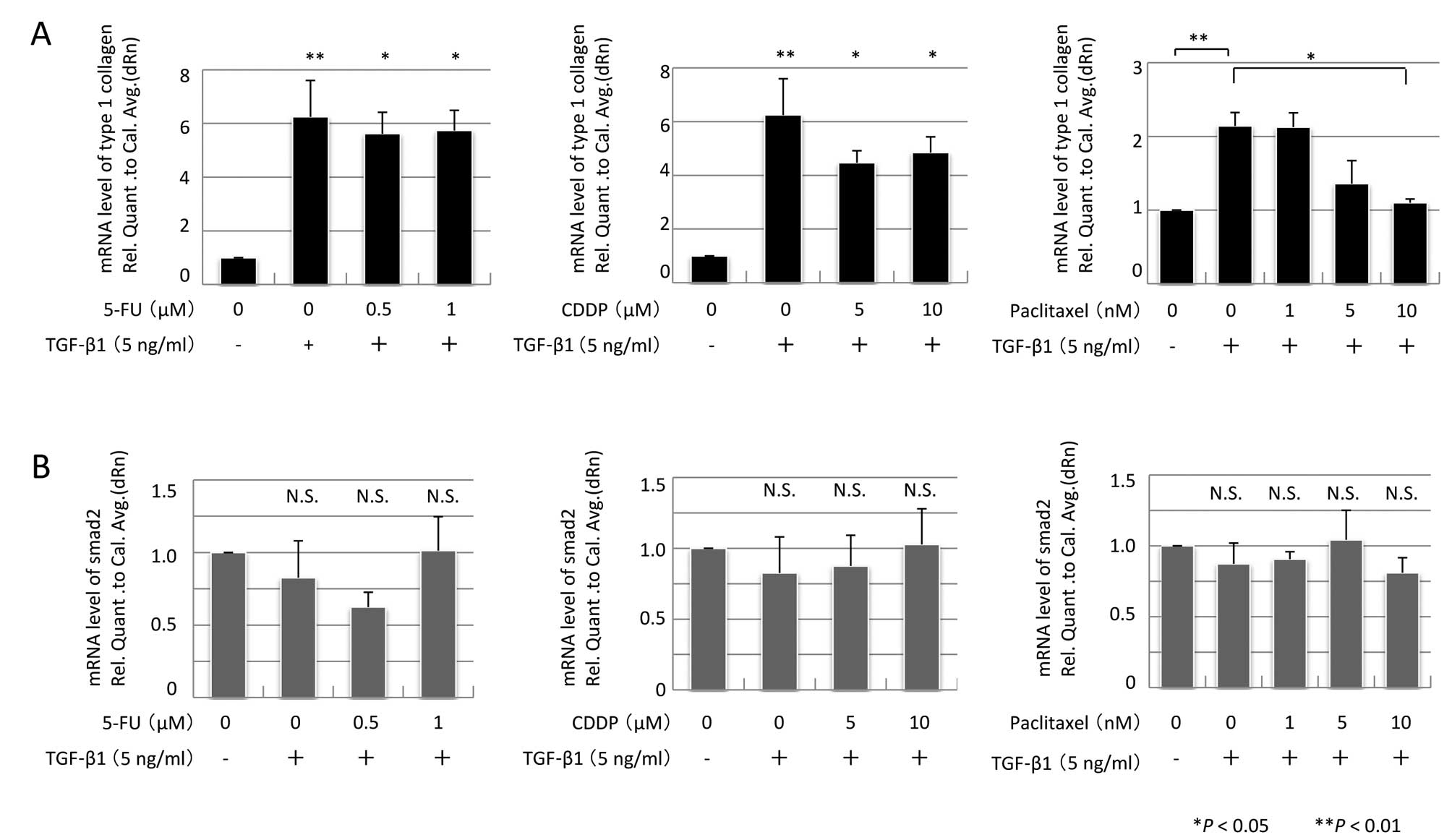
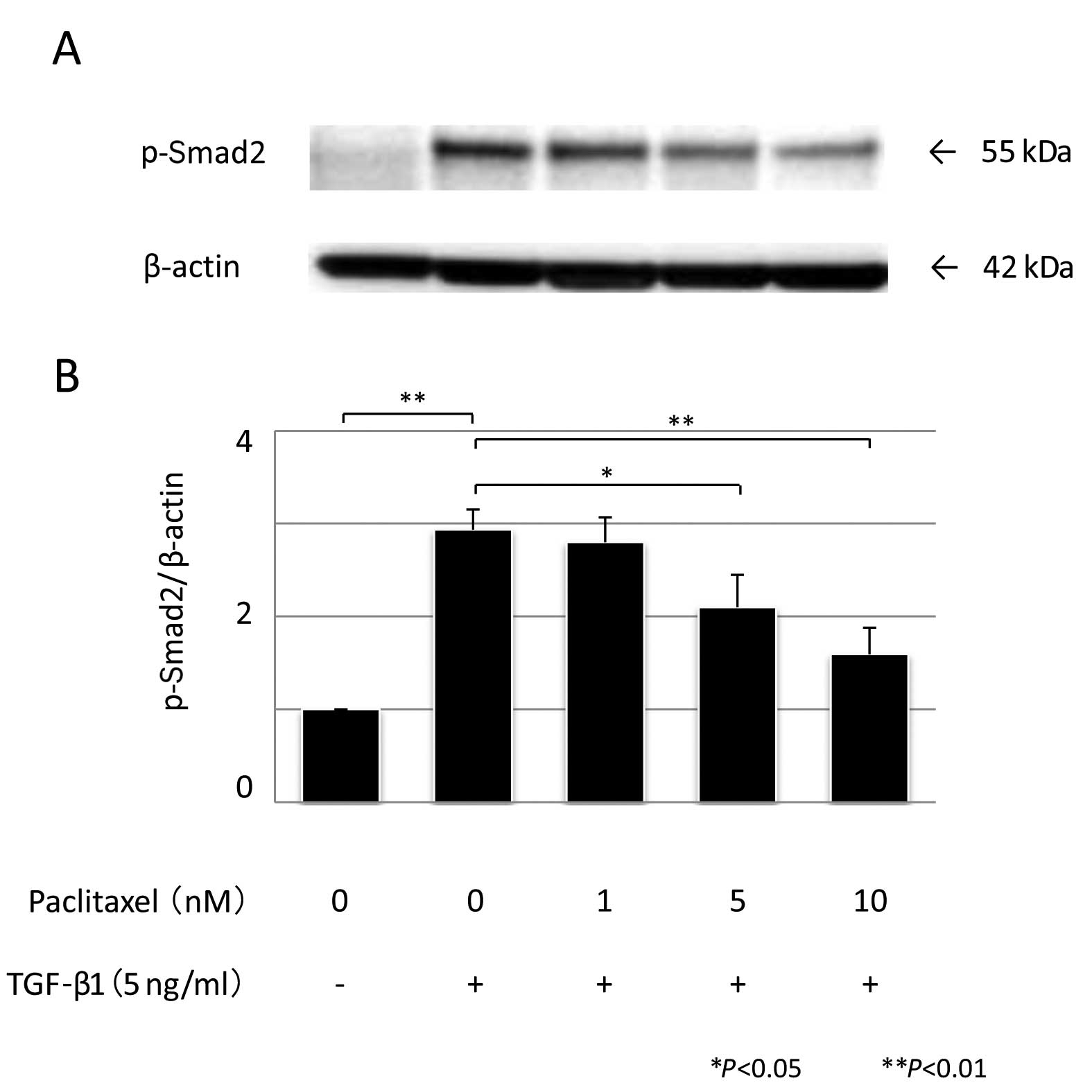
|
1
|
Fushida S, Kinoshita J, Yagi Y, Funaki H,
Kinami S, Ninomiya I, Fujimura T, Nishimura G, Kayahara M and Ohta
T: Dual anti-cancer effects of weekly intraperitoneal docetaxel in
treatment of advanced gastric cancer patients with peritoneal
carcinomatosis: a feasibility and pharmacokinetic study. Oncol Rep.
19:1305–1310. 2008.
|
|
2
|
Shimada S, Tanaka E, Marutsuka T, Honmyo
U, Tokunaga H, Yagi Y, Aoki N and Ogawa M: Extensive intraoperative
peritoneal lavage and chemotherapy for gastric cancer patients with
peritoneal free cancer cells. Gastric Cancer. 5:168–172. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yonemura Y, Kawamura T, Nojima N, Bandou
E, Keizou T, Fujita H, Michiwa Y, Fujimura T, Fushida S, Ajisaka H
and Miwa K: Postoperative results of left upper abdominal
evisceration for advanced gastric cancer. Hepatogastroenterology.
47:571–574. 2000.PubMed/NCBI
|
|
4
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H and
Takeuchi M: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): a phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shirao K, Boku N, Yamada Y, Yamaguchi K,
Doi T, Takiuchi H, Nasu J, Nakamura K, Fukuda H and Ohtsu A:
Randomized phase III study of 5-fluorouracil continuous infusion
(5FUci) versus methotrexate and 5-FU sequential therapy (MF) in
gastric cancer with peritoneal metastasis (JCOG0106). Proc ASCO.
27(Suppl 15): abs. 4545. 2009.
|
|
6
|
Tsukada T, Fushida S, Harada S, Yagi Y,
Kinoshita J, Oyama K, Tajima H, Fujita H, Ninomiya I, Fujimura T
and Ohta T: The role of human peritoneal mesothelial cells in the
fibrosis and progression of gastric cancer. Int J Oncol.
41:476–482. 2012.PubMed/NCBI
|
|
7
|
Lv ZD, Na D, Ma XY, Zhao C, Zhao WJ and Xu
HM: Human peritoneal mesothelial cell transformation into
myofibroblasts in response to TGF-β1 in vitro. Int J Mol Med.
27:187–193. 2011.PubMed/NCBI
|
|
8
|
Hay ED: An overview of
epithelio-mesenchymal transformation. Acta Anat. 154:8–20. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nawshad A, Lagamba D, Polad A and Hay ED:
Transforming growth factor-beta signaling during
epithelial-mesenchymal transformation: implications for
embryogenesis and tumor metastasis. Cells Tissues Organs.
179:11–23. 2005. View Article : Google Scholar
|
|
12
|
Miyazono K, Suzuki H and Imamura T:
Regulation of TGF-beta signaling and its roles in progression of
tumors. Cancer Sci. 94:230–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong C, Li Z, Alvarez R Jr, Feng XH and
Goldschmidt-Clermont PJ: Microtubule binding to Smads may regulate
TGF beta activity. Mol Cell. 5:27–34. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Donaldson KL, Goolsby GL, Kiener PA and
Wahl AF: Activation of p34cdc2 coincident with taxol-induced
apoptosis. Cell Growth Differ. 5:1041–1050. 1994.PubMed/NCBI
|
|
16
|
Gelmon K: The taxoids: paclitaxel and
docetaxel. Lancet. 344:1267–1272. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishigami H, Kitayama J, Kaisaki S,
Hidemura A, Kato M, Otani K, Kamei T, Soma D, Miyato H, Yamashita H
and Nagawa H: Phase II study of weekly intravenous and
intraperitoneal paclitaxel combined with S-1 for advanced gastric
cancer with peritoneal metastasis. Ann Oncol. 21:67–70. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Markman M, Bundy BN, Alberts DS, Fowler
JM, Clark-Pearson DL, Carson LF, Wadler S and Sickel J: Phase III
trial of standard-dose intravenous cisplatin plus paclitaxel versus
moderately high-dose carboplatin followed by intravenous paclitaxel
and intraperitoneal cisplatin in small-volume stage III ovarian
carcinoma: an intergroup study of the Gynecologic Oncology Group,
Southwestern Oncology Group and Eastern Cooperative Oncology Group.
J Clin Oncol. 19:1001–1007. 2001.
|
|
19
|
Sakurai Y, Yoshida I, Tonomura S, Sakai W,
Nakamura Y, Imazu H, Matsubara T and Ochiai M: Weekly
administration of paclitaxel attenuated rectal stenosis caused by
multiple peritoneal recurrence 8 years after the resection of
gastric carcinoma. Gastric Cancer. 6:243–249. 2003.
|
|
20
|
Jordan MA, Toso RJ, Thrower D and Wilson
L: Mechanism of mitotic block and inhibition of cell proliferation
by taxol at low concentrations. Proc Natl Acad Sci USA.
90:9552–9556. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Axel DI, Kunert W, Göggelmann C, Oberhoff
M, Herdeg C, Küttner A, Wild DH, Brehm BR, Riessen R, Köveker G and
Karsch KR: Paclitaxel inhibits arterial smooth muscle cell
proliferation and migration in vitro and in vivo using local drug
delivery. Circulation. 96:636–645. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colombo A, Drzewiecki J, Banning A, Grube
E, Hauptmann K, Silber S, Dudek D, Fort S, Schiele F, Zmudka K and
Guagliumi G: Randomized study to assess the effectiveness of slow-
and moderate-release polymer-based paclitaxel-eluting stents for
coronary artery lesions. Circulation. 108:788–794. 2003. View Article : Google Scholar
|
|
23
|
Stone GW, Ellis SG, Cox DA, Hermiller J,
O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J,
Popma JJ and Russell ME; TAXUS-IV Investigators: A polymer-based,
paclitaxel-eluting stent in patients with coronary artery disease.
N Engl J Med. 350:221–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Zhu S, Wang T, Hummers L, Wigley
FM, Goldschmidt-Clermont PJ and Dong C: Paclitaxel modulates
TGFbeta signaling in scleroderma skin grafts in immunodeficient
mice. PLoS Med. 2:1334–1442. 2005.PubMed/NCBI
|
|
25
|
Choi HS, Savard CE, Choi JW, Kuver R and
Lee SP: Paclitaxel interrupts TGF-beta1 signaling between
gallbladder epithelial cells and myofibroblasts. J Surg Res.
141:183–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yung S, Li FK and Chan TM: Peritoneal
mesothelial cell culture and biology. Perit Dial Int. 26:162–173.
2006.PubMed/NCBI
|
|
27
|
Wakayama T, Sai Y, Ito A, Kato Y, Kurobo
M, Murakami Y, Nakashima E, Tsuji A, Kitamura Y and Iseki S:
Heterophilic binding of the adhesion molecules poliovirus receptor
and immunoglobulin superfamily 4A in the interaction between mouse
spermatogenic and Sertoli cells. Biol Reprod. 76:1081–1090. 2007.
View Article : Google Scholar
|
|
28
|
Yagi Y, Fushida S, Harada S, Tsukada T,
Kinoshita J, Oyama K, Fujita H, Ninomiya I, Fujimura T, Kayahara M,
Kinuya S, Yashiro M, Hirakawa K and Ohta T: Biodistribution of
humanized anti-VEGF monoclonal antibody/bevacizumab on peritoneal
metastatic models with subcutaneous xenograft of gastric cancer in
mice. Cancer Chemother Pharmacol. 66:745–753. 2010. View Article : Google Scholar
|
|
29
|
Yashiro M, Chung YS, Nishimura S, Inoue T
and Sowa M: Fibrosis in the peritoneum induced by scirrhous gastric
cancer cells may act as ‘soil’ for peritoneal dissemination.
Cancer. 77:1668–1675. 1996.PubMed/NCBI
|
|
30
|
Nakazawa K, Yashiro M and Hirakawa K:
Keratinocyte growth factor produced by gastric fibroblasts
specifically stimulates proliferation of cancer cells from
scirrhous gastric carcinoma. Cancer Res. 63:8848–8852. 2003.
|
|
31
|
Elenbaas B and Weinberg RA: Heterotypic
signaling between epithelial tumor cells and fibroblasts in
carcinoma formation. Exp Cell Res. 264:169–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kinugasa S, Abe S, Tachibana M, Hishikawa
Y, Yoshimura H, Monden N, Dhar DK, Nagasue N and Nagaoka S: Over
expression of transforming growth factor-beta1 in scirrhous
carcinoma of the stomach correlates with decreased survival.
Oncology. 55:582–587. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shinto O, Yashiro M, Kawajiri H, Shimizu
K, Shimizu T, Miwa A and Hirakawa K: Inhibitory effect of a TGFbeta
receptor type-I inhibitor, Ki26894, on invasiveness of scirrhous
gastric cancer cells. Br J Cancer. 102:844–851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yashiro M, Chung YS and Sowa M: Role of
orthotopic fibroblasts in the development of scirrhous gastric
carcinoma. Jpn J Cancer Res. 85:883–886. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Inoue T, Chung YS, Yashiro M, Nishimura S,
Hasuma T, Otani S and Sowa M: Transforming growth factor-beta and
hepatocyte growth factor produced by gastric fibroblasts stimulate
the invasiveness of scirrhous gastric cancer cells. Jpn J Cancer
Res. 88:152–159. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koyama T, Yashiro M, Inoue T, Nishimura S,
Hirakawa YS and Chung K: TGF-beta1 secreted by gastric fibroblasts
up-regulates CD44 H expression and stimulates the peritoneal
metastatic ability of scirrhous gastric cancer cells. Int J Oncol.
16:355–362. 2000.PubMed/NCBI
|
|
37
|
Tamura T, Sasaki Y, Nishiwaki Y and Saijo
N: Phase I study of paclitaxel by three-hour infusion: hypotension
just after infusion is one of the major dose-limiting toxicities.
Jpn J Cancer Res. 86:1203–1209. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matsuoka H, Furusawa M, Tomoda H and Seo
Y: Difference in cytotoxicity of paclitaxel against neoplastic and
normal cells. Anticancer Res. 14:163–167. 1994.PubMed/NCBI
|
|
39
|
Gloushankova NA, Lyubimova AV, Tint IS,
Feder HH, Vasiliev JM and Gelfand IM: Role of the microtubular
system in morphological organization of normal and
oncogene-transfected epithelial cells. Proc Natl Acad Sci USA.
91:8597–8601. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schiff PB and Horwitz SB: Taxol stabilizes
microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA.
77:1561–1565. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu S, Goldschmidt-Clermont PJ and Dong C:
Transforming growth factor-β-induced inhibition of myogenesis is
mediated through Smad pathway and is modulated by microtubule
dynamic stability. Circ Res. 94:617–625. 2004.
|
|
42
|
Shi Y, Hata A, Lo RS, Massagué J and
Pavletich NP: A structural basis for mutational inactivation of the
tumour suppressor Smad4. Nature. 388:87–93. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi Y and Massague J: Mechanisms of TGF-
signaling from cell membrane to the nucleus. Cell. 113:685–700.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ten Dijke P, Goumans MJ, Itoh F and Itoh
S: Regulation of cell proliferation by Smad proteins. J Cell
Physiol. 191:1–16. 2002.PubMed/NCBI
|
|
45
|
Liu Q, Mao H, Nie J, Chen W, Yang Q, Dong
X and Yu X: Transforming growth factor β1 induces
epithelial-mesenchymal transition by activating the JNK-Smad3
pathway in rat peritoneal mesothelial cells. Perit Dial Int.
28:S88–S95. 2008.
|
|
46
|
Saika S, Yamanaka O, Ikeda K,
Kim-Mitsuyama S, Flanders KC, Yoo J, Roberts AB, Nishikawa-Ishida
I, Ohnishi Y, Muragaki Y and Ooshima A: Inhibition of p38MAP kinase
suppresses fibrotic reaction of retinal pigment epithelial cells.
Lab Invest. 85:838–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wendling J, Marchand A, Mauviel A and
Verrecchia F: 5-fluorouracil blocks transforming growth
factor-beta-induced alpha 2 type I collagen gene (COL1A2)
expression in human fibroblasts via c-Jun NH2-terminal
kinase/activator protein-1 activation. Mol Pharmacol. 64:707–713.
2003. View Article : Google Scholar
|
|
48
|
Zhang D, Sun L, Xian W, Liu F, Ling G,
Xiao L, Liu Y, Peng Y, Haruna Y and Kanwar YS: Low-dose paclitaxel
ameliorates renal fibrosis in rat UUO model by inhibition of
TGF-beta/Smad activity. Lab Invest. 90:436–447. 2010. View Article : Google Scholar : PubMed/NCBI
|