Introduction
The ability of carcinoma cells to spread to other
sites within the body requires different biological processes
including modulation of adhesion molecules on the cell surface.
ADAMs are known to exert adhesive functions as well as proteolytic
activity (1,2), implying a critical role in carcinoma
metastasis. ADAMs are type-I transmembrane glycoproteins sharing a
common structure (3,4). Starting from the N-terminus ADAM17,
like ADAM10, comprises of a signal peptide and a pro-, a
catalytic-, a disintegrin- and a membrane-proximal domain, followed
by a transmembrane and an intracellular region (3,5). The
prodomain is cleaved during maturation in the Golgi apparatus
(6). The catalytic domain contains
a zinc-binding motif with three conserved histidine residues
(HEXGHXXGXXHD) and is responsible for the ectodomain shedding of
cell-surface receptors or ligands (3,6). The
disintegrin domain is implicated in interactions between ADAMs and
integrins, thereby mediating cell adhesion (7,8)
and/or inhibiting enzymatic activity (9,10).
The membrane-proximal domain has been suggested to be involved in
regulatory affairs, dimerisation as well as in substrate
recognition (5,11–16).
The cytoplasmic region has been shown to interact with proteins
containing SH3 domains and may play a role in regulating protease
activity as well as intracellular trafficking (17–19).
ADAM17 is highly expressed in various cancer cells
(20,21). Indeed, it has been documented that
ADAM17 is upregulated in ovarian cancer (22,23),
colon carcinoma (24), lung cancer
(25) and other types of cancer
(26). Furthermore, it was shown
for several types of human cancer that shedding of growth factors
by ADAM17 is correlated with the pathogenesis of these diseases
(27). Interestingly, all these
studies focused on the proteolytic function of ADAM17. They
considered ADAM17 as the major sheddase of growth factors required
for cancer progression and disregarded its adhesive properties.
Since ADAM17 is known to interact with α5β1 integrin (7), we aimed to clarify the role of ADAM17
as an adhesion molecule. Therefore, the disintegrin domain of
ADAM17 was expressed as a soluble domain and tested for its ability
to bind to fibroblasts/carcinoma cells and to enhance ADAM17
shedding activity. Moreover, the effect of silencing ADAM17 was
investigated with respect to fibroblast-carcinoma cell
interactions. Taken together, these data show that fibroblast and
carcinoma cells adhere to the disintegrin domain of ADAM17 and that
ADAM17 is involved in the interaction between carcinoma and
fibroblasts.
Materials and methods
Antibodies
For western blot analysis anti-integrin α5 antibody
(sc-10729) was purchased from Santa Cruz Biotechnology (Santa Cruz
Biotechnology, Heidelberg, Germany). A300, A300E and A318 are mouse
anti-human ADAM17 monoclonal antibodies generated in our laboratory
(28). While A300 and A318 bind to
disintegrin domain, A300E recognizes the membrane-proximal domain
of ADAM17 (29,30).
Cell culture
Mouse embryonic fibroblasts (MEF) and human
embryonic kidney 293 cells (HEK-293) were cultured in Dulbecco's
modified Eagle's medium (DMEM) high-glucose supplemented with 10%
fetal calf serum (FCS), penicillin (60 mg/l) and streptomycin (100
mg/l) (PAA Laboratories, Cölbe, Germany) in 5% CO2 at
37°C in a humidified atmosphere. The human carcinoma cell lines
(MDA-MB-231, Panc89 and Colo357 from Professor Holger Kalthoff,
UKSH Kiel, Germany; NCI-H292 from Dr Anja Baumgart, TU Munich,
Germany) were cultured in RPMI-1640 medium supplemented with 10%
FCS, penicillin (60 mg/l) and streptomycin (100 mg/l).
Expression and purification of the
soluble disintegrin domain of ADAM17
The coding sequence of the human ADAM17 disintegrin
domain (amino acid residue 475 to 580) was cloned into pcDNA3.1 zeo
(−). Thereby the signal sequence of the human IL-6R, followed by a
His- and a myc-tag was placed in front of the disintegrin domain.
HEK-293 cells were transfected using TurboFect (Fermentas, St.
Leon-Rot, Germany) according to the manufacturer's instruction and
stably transfected cells were selected. The supernatant of these
cells was harvested and the disintegrin domain purified by its
N-terminal His-tag using a HisTrap column (GE Healthcare, Freiburg,
Germany) followed by size-exclusion chromatography (Superdex200
column, GE Healthcare). The identity and purification was confirmed
by SDS-PAGE and western blot analysis.
Cell-adhesion assays
Microtiter tissue culture plates (Sarstedt,
Nümbrecht, Germany) were coated with indicated concentrations of
the disintegrin domain (28) in
coating buffer (0.5 M carbonate-bicarbonate buffer, pH 9.6) at 4°C
overnight. Plates were blocked with heat-denatured BSA (85°C for 10
min, 1% BSA in Ca2+/Mg2+-free PBS) for 1 h at
room temperature. In parallel, MEF and carcinoma cells were
detached with trypsin. The cells were resuspended in complete
medium (DMEM, 10% FCS, 0.1% BSA, 1 mM CaCl2, 1 mM
MnCl2, 1 mM MgCl2). Plates were washed,
3×104 cells/well were added and incubated for 1 h at
37°C. In case of the competition assays, cells were first incubated
for 1 h at 37°C with the soluble disintegrin domain, washed and
then added to the plates coated with the disintegrin domain.
Non-adhering cells were removed by washing with complete medium,
while attached cells were quantified using titer blue reagent
(Promega, Mannheim, Germany). Adhesion was expressed as percentage
relative to FCS control, which was set arbitrarily to 100%
(31).
Cell-cell adhesion assays
The adhesion assay of carcinoma cells to fibroblasts
has been previously described (31). Briefly, carcinoma cells were
labeled with 10 μM Calcein AM (Invitrogen, Darmstadt,
Germany) for 1 h at 37°C. Labeled cells (3×104) were
washed three times with wash solution (HBSS, 1 mM MnCl2,
1 mM MgCl2, 1 mM CaCl2), incubated with or
without disintegrin domain for 1 h at 37°C and seeded onto 80%
confluent monolayers of MEF in 96-well plates for 30 min. In a
parallel experiment, anti-integrin β1 inhibitory antibody (clone
P4C10, Millipore, Schwalbach, Germany) was added to carcinoma cells
instead of disintegrin domain. Not adhered cells were removed by
washing, while attached cells were lysed by adding 20 μl of
10% Triton X-100 and qualified by measurement of their fluorescence
(480 nm/530 nm) using fluorescence reader (BioTek, Bad
Friedrichshall, Germany). Statistical analysis was performed with
Student's t-test (http://www.physics.csbsju.edu/stats/t-test_bulk_form.html).
Generation of DN-ADAM17 stable transfect
MDA-MB-231 cells
The dominant negative (DN)-ADAM17 is a construct of
human ADAM17 that lacks the catalytic domain (32). The human ADAM17 sequence (starting
from amino acid residue 475) is placed directly behind the signal
peptide of human interleukin-6 in pcDNA3.1 expressing a gentamycin
resistance (32). MDA-MB-231 cells
were transfected with the plasmid using TurboFect transfection
reagent (Fermentas) according to the manufacturer's protocol.
Stable transfected clones were selected and grown in medium
containing G418 (PAA Laboratories). The expression of DN-ADAM17 was
confirmed by flow cytometry and western blot analysis and clone 5
(DN-5) was chosen for further experiments. The DN-ADAM17 construct
contains a C-terminal His- and myc-tag.
Co-immunoprecipitation
Stably transfected MDA-MB-231 cells (DN-5) were
cultured in RPMI-1640 medium, harvested, lysed in 250 μl
lysis buffer [20 mM Tris-HCl pH 7.6, 150 mM NaCl, 2 mM EDTA and
‘complete’ protease inhibitor (Roche Applied Science, Mannheim,
Germany)] and centrifuged for 15 min at 14,000 rpm. Cell lysate
(100 μl) was mixed with 50 μl of protein-G agarose
(Thermo Scientific-Pierce, Bonn, Germany) and 2.5 μg
anti-α5β1 integrin antibodies (NKI-SAM-1, TS2/16; Biozol, Eching,
Germany) or 5 μg anti-ADAM17 A300E (28). As control, 100 μl of cell
lysate was mixed with 50 μl of protein-G agarose without
antibodies. The samples were incubated 2 h at 4°C by gentle
agitation, washed five-times with 1,000 μl of lysis buffer
containing 1 mM CaCl2 and prepared for western blot
analysis. To detect the immunoprecipitated proteins, 20 μl
SDS denatured protein cell lysate was separated on a 12.5%
polyacrylamide gel. After separation by SDS-PAGE, proteins were
transferred onto a polyvinylidene-fluoride (PVDF) membrane (GE
Healthcare). The PVDF membrane was blocked with 5% milk powder in
TBS-T (10 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.1% Tween-20) for 2 h
and then incubated overnight at 4°C with rabbit anti-myc tag
(1/1,000; Cell Signaling, Frankfurt, Germany) or with anti-α5
integrin antibody (sc-10729, Santa Cruz Biotechnology) in 1% milk
powder. After washing, the blots were incubated for 1 h with an
anti-rabbit IgG POD conjugate and visualized by chemiluminescence
using an ECL kit (GE Healthcare).
ELISA
Microtiter plates (Nunc, Denmark) were coated with
50 μl (10 μg/ml) per well of human purified α5β1
integrin (gift from Dr Humphries, University of Manchester,
Manchester, UK) in coating buffer (PBS, 1 mM MnCl2) and
incubated at 4°C overnight. Then the plates were washed with
washing solution (TBS, 1 mM MnCl2, 0.1% BSA) and blocked
with 5% BSA in TBS for 3 h at room temperature. After washing, 50
μl of recombinant disintegrin domain (80 μg/ml) plus
50 μl of mouse anti-disintegrin monoclonal antibodies (A300,
A318; 20 μg/ml) (28) or
anti-His-tag antibody (1/500; Cell Signaling) were added and
incubated for 3 h at room temperature. Blank control was performed
with PBS. The plates were washed with washing solution and the
antibodies were detected by anti-mouse IgG-POD conjugate (Southern
Biotech, Birmingham, AL, USA) diluted 1/3,000 in washing buffer.
After washing, 80 μl of BM blue POD substrate (Roche Applied
Science) was added to each well for 10 min. Then, the reaction was
stopped by adding 80 μl of 0.8 M
H2SO4. Measurement of the optical density
(OD) was performed at 450 nm/540 nm on a plate reader (Tecan,
Männedorf, Switzerland).
Flow cytometry (FACS)
Cells (5×105) were washed twice in FACS
solution (PBS, 0.05% NaN3) and stained for 1 h at 4°C
with 2 μg/ml of anti-ADAM17 (A300E) antibody directed
against the membrane-proximal domain of the enzyme (28). After washing, 2 μl of
allophycocyanin-conjugated goat anti-mouse Ig (Jackson
ImmunoResearch Laboratories, West Grove, PA, USA) was added. The
cells were then incubated for 1 h at 4°C and analyzed with a
FACScan flow cytometer (BD Biosciences, USA). Cell populations of
interest were gated and analyzed using BD FACSDiva software.
Shedding of amphiregulin into the cell
culture medium
MDA-MB-231 cells were seeded on 24-well plate
(70–80% confluence) and incubated overnight at 37°C in RPMI medium
containing 10% FCS. The soluble disintegrin domain of ADAM17 was
added to a final concentration of 20 μg/ml in serum-free
medium and incubated for 8 h. The matrix metal-loprotease
inhibitor, marimastat (MM), was used at final concentration of 3
μM. The supernatant was collected and the levels
amphiregulin were determined using a sandwich ELISA (DuoSet Kit;
R&D Systems, Minneapolis, MN, USA) in accordance with the
manufacturer's instructions.
Results
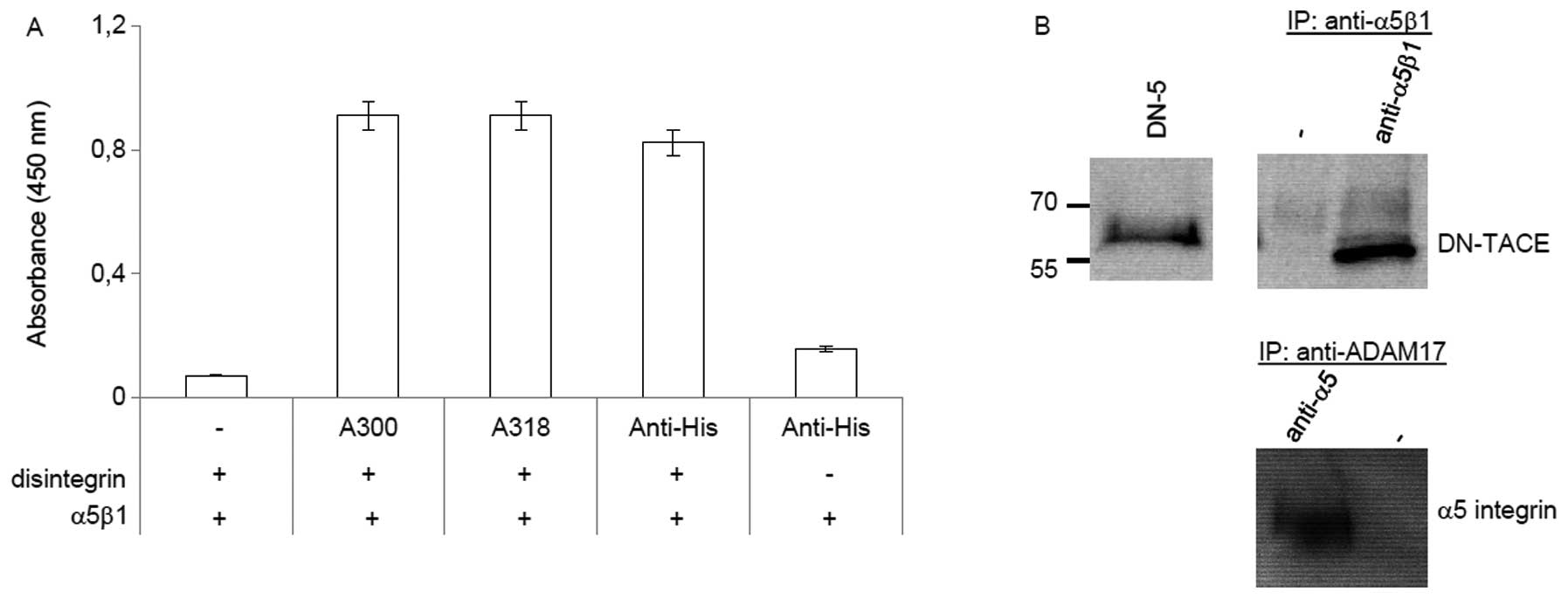
The disintegrin domain of ADAM17
interacts directly with integrin α5β1
A previous study has shown that ADAM17 interacts
with α5β1 integrin (7). To verify
the specificity of this interaction, we performed an ELISA with the
purified disintegrin domain of ADAM17 and the α5β1 integrin
(Fig. 1A). Binding of the
disintegrin domain to the purified α5β1 integrin was detected by
monoclonal mouse anti-disintegrin domain antibodies (A300, A318)
(28) and confirmed by an anti-His
antibody. The ELISA data demonstrate that the disintegrin domain
and the α5β1 integrin interact directly. Co-immunoprecipitation was
used as second approach to confirm the interaction between the
disintegrin domain and the α5β1 integrin. Thereby, DN-ADAM17 was
detectable in cell lysates from MDA-MB-231 cells stably transfected
with the ADAM17 deletion mutant and could be co-immunoprecipitated
using anti-α5β1 antibodies (Fig.
1B). In addition, α5 integrin could be detected after
precipitation with anti-ADAM17 antibody (A300E) from cell lysate of
DN-ADAM17 stable transfected MDA-MB-231 cells (Fig. 1B). Thus, the disintegrin domain of
ADAM17 binds to the α5β1 integrin.
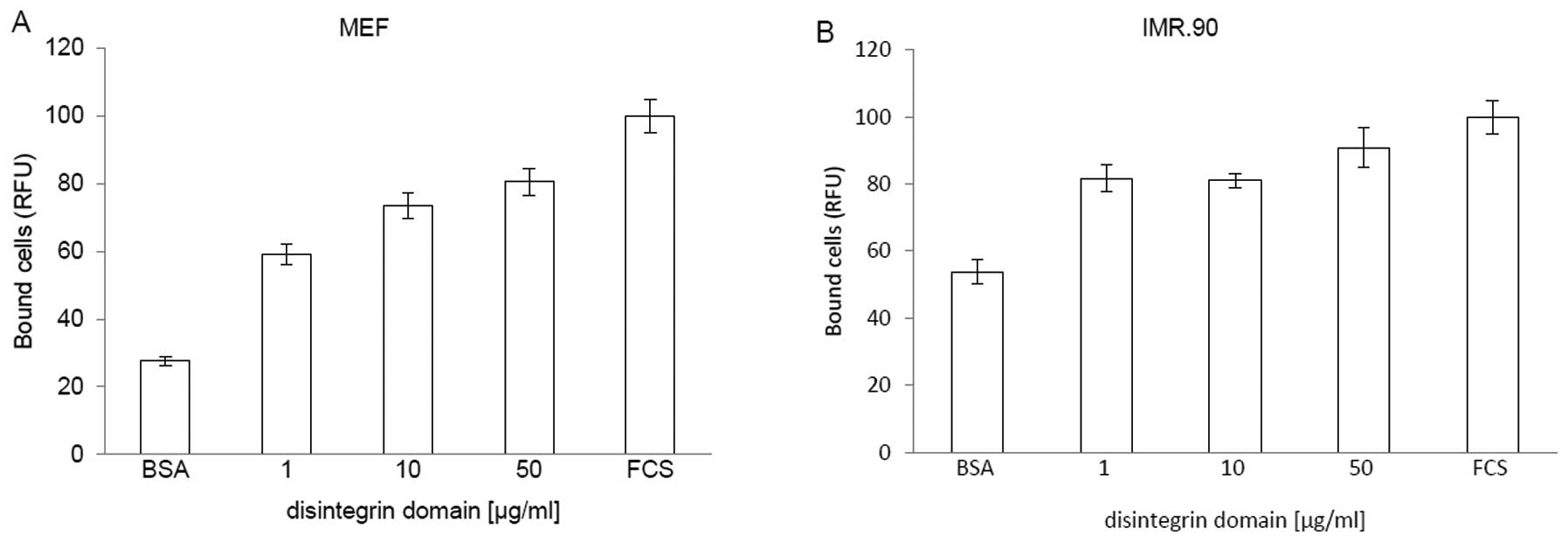
Fibroblast cells adhere to the
disintegrin domain of ADAM17
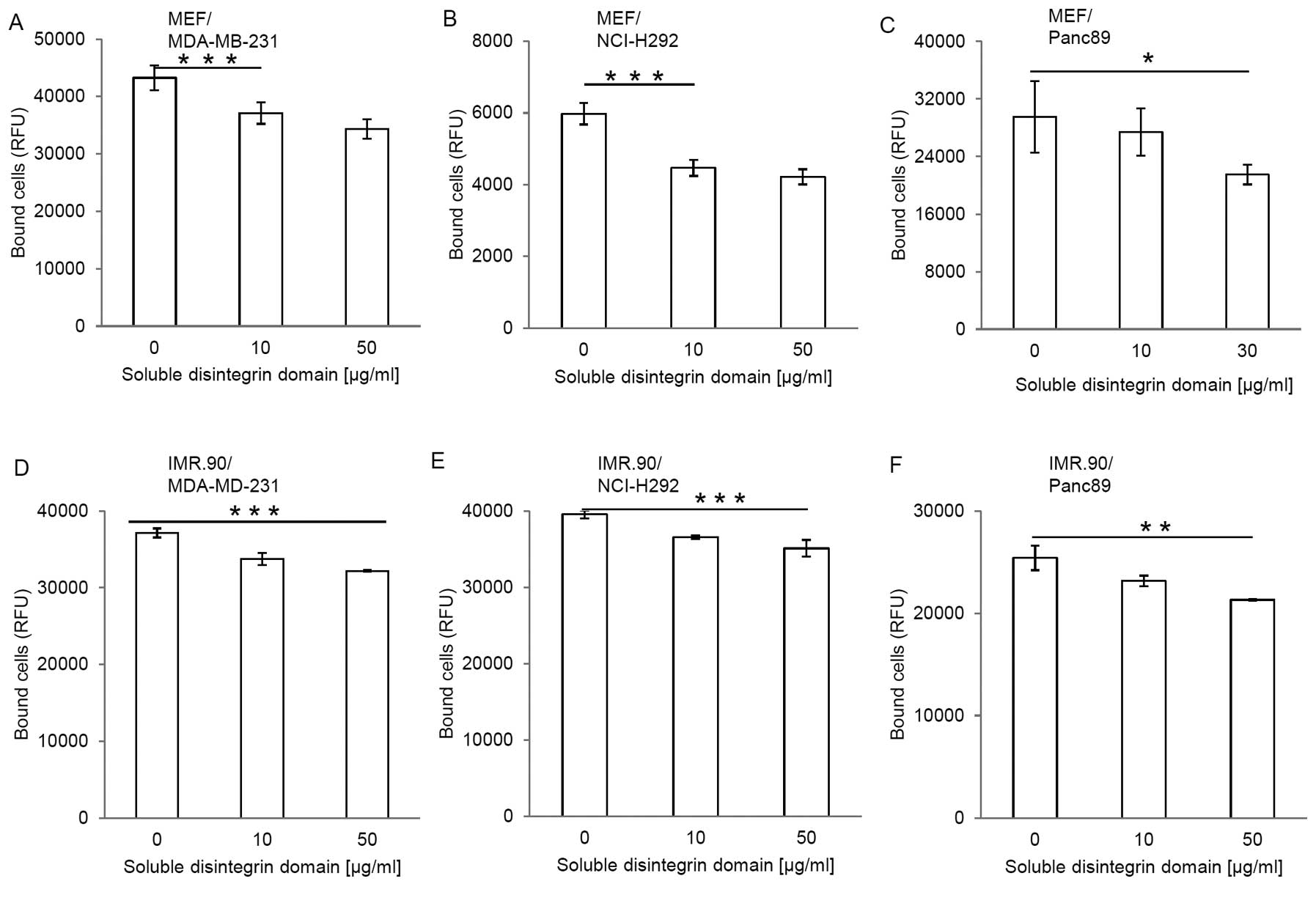
To examine the adhesive properties of ADAM17, we
performed cell adhesion assays using fibroblasts and the
disintegrin domain coated on cell culture plates (Fig. 2). Fibroblasts were chosen because
they express a wide range of different integrins including α5β1
(33). The amount of fibroblasts
bound to the plates coated with FCS was considered as a positive
control and set to 100%. A coating-concentration of 50 μg/ml
disintegrin domain leads to a maximal adhesion of MEF cells
(Fig. 2A). Further increase in the
concentration of the disintegrin domain did not improve binding of
the MEFs (data not show). Also human fibroblasts IRM.90 bind to the
disintegrin domain of ADAM17 in concentration-dependent manner
(Fig. 2B).
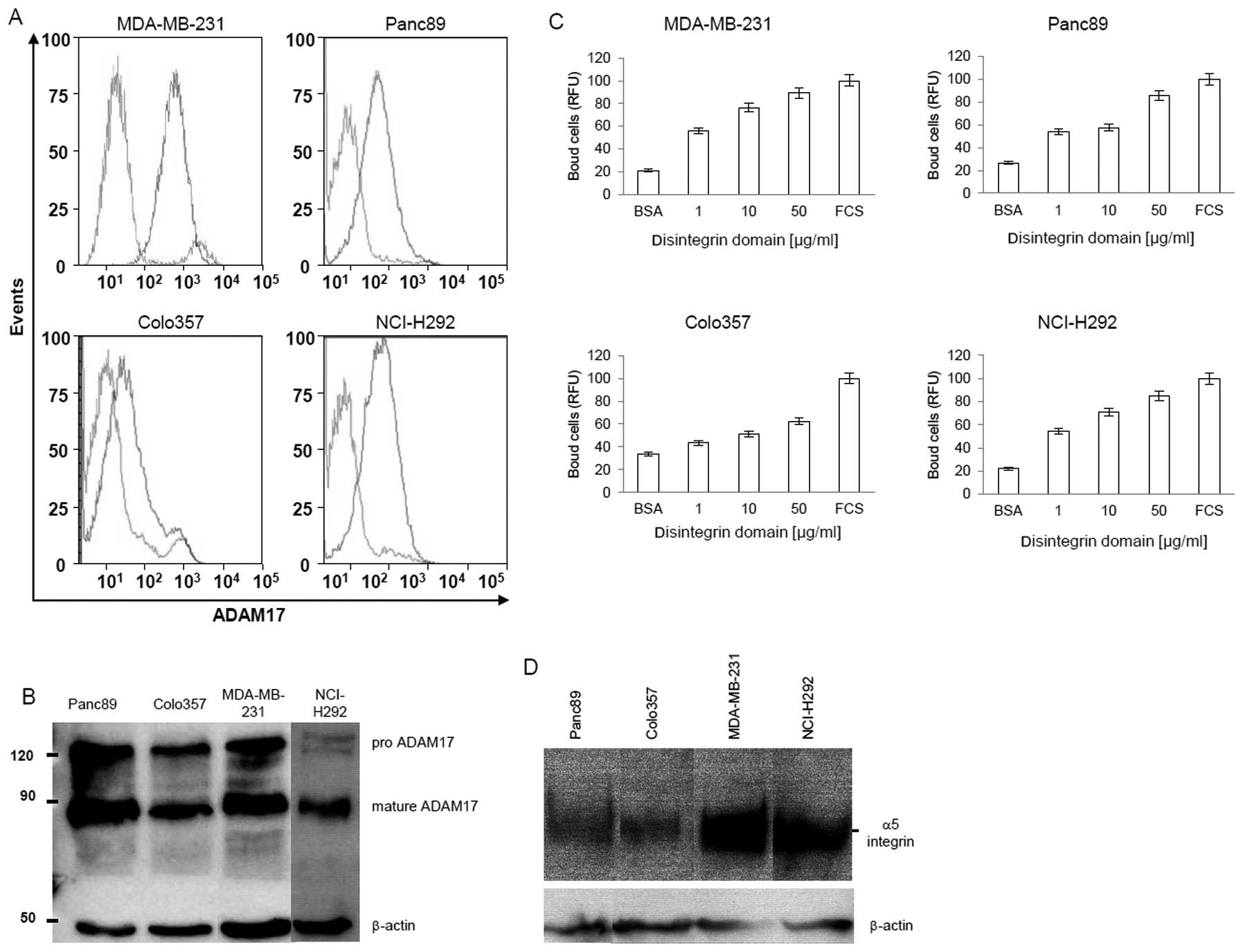
Carcinoma cells express ADAM17 and adhere
to the disintegrin domain
FACS (Fig. 3A) and
western blot (Fig. 3B) analysis
demonstrate that carcinoma cells express high levels of ADAM17.
This led to the assumption that ADAM17 may be involved in the
interactions between fibroblast and carcinoma cells and raised the
question, whether upregulation of ADAM17 in carcinoma cells
increases the cell-cell interactions between fibroblasts and
carcinoma cells. To address this question two assays were
performed. First, we analyzed the binding of carcinoma cells to the
immobilized disintegrin domain. Secondly, the interaction between
fibroblast and carcinoma cells expressing different level of ADAM17
was evaluated.
Fig. 3C shows that
various carcinoma cells (MDA-MB-231, Colo357, Panc89, NCl-H292)
bind in a dose-dependent manner to solid phase coupled disintegrin
domain. In case of three cells lines (MDA-MB-231, Panc89, NCl-H292)
the maximal binding reaches 60% at a coating-concentration of 50
μg/ml disintegrin domain, in comparison to the positive
control coated with FCS; while only 20% of Colo357 cells bind to
the disintegrin domain coated surface. This difference correlates
to some extent with the expression level of α5 integrin of these
cells (Fig. 3D).
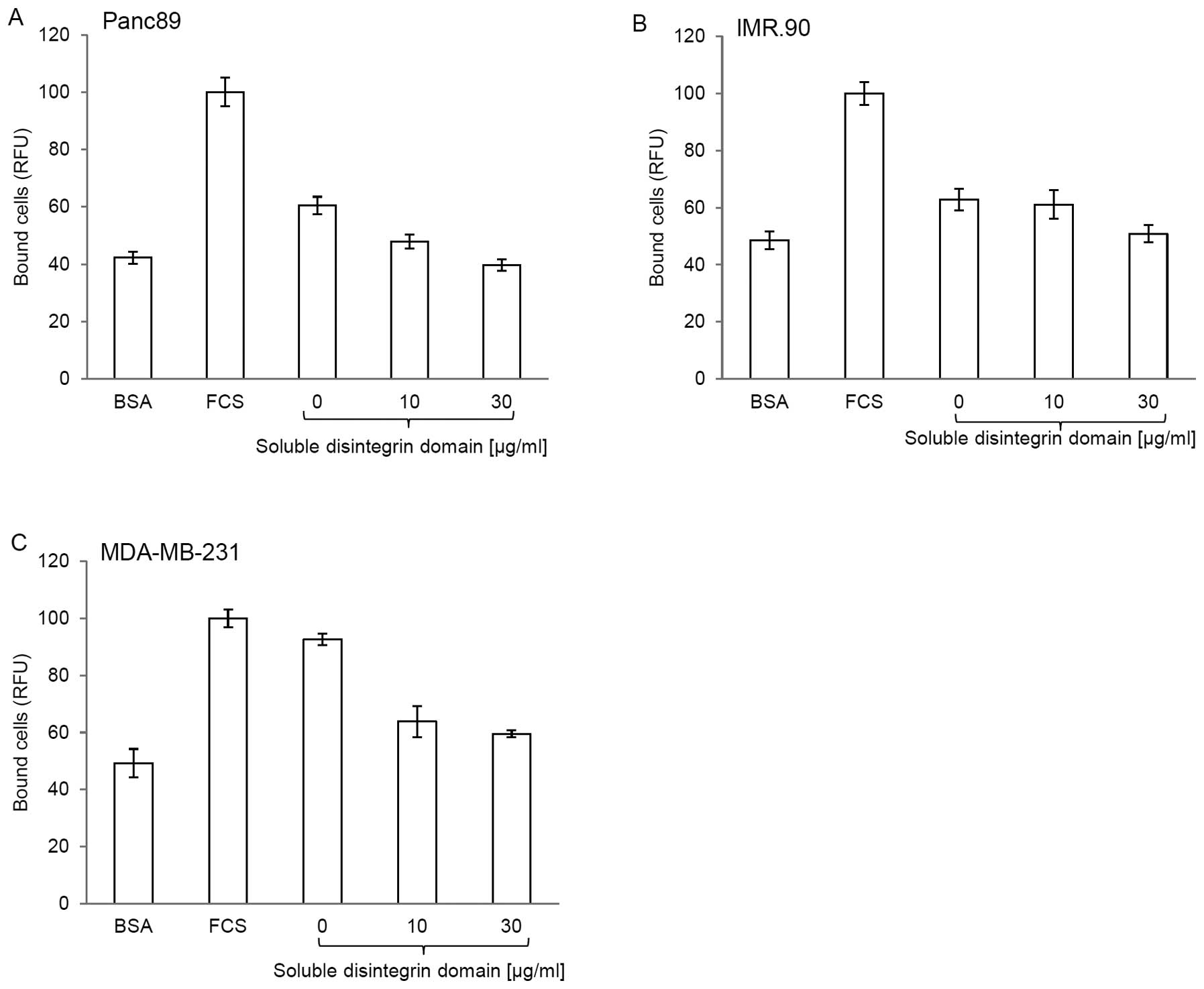
To prove that carcinoma cells and the disintegrin
domain interact specifically and directly, we performed a
competition assay using soluble and immobilized disintegrin domain.
Pretreatment of Panc89 (Fig. 4A),
IMR.90 (Fig. 4B) and MDA-MB-231
(Fig. 4C) cells with the soluble
disintegrin domain significantly reduced the binding of these cells
to the disintegrin domain coated solid phase. A pretreatment with
30 μg/ml of soluble disintegrin domain results in all three
cell lines to a decrease in adhesion down to the level of the
negative control (plates coated with BSA). In summary, these
results indicate that carcinoma cells express α5 integrin and
adhere to the disintegrin domain of ADAM17.
ADAM17 mediates fibroblast-carcinoma cell
interactions
To evaluate the role of the disintegrin domain at a
cellular level, cell-cell interaction assays between fibroblasts
and carcinoma cells in the presence or absence of the disintegrin
domain of ADAM17 were performed. In these experiments, the
MDA-MB-231 (Fig. 5A), NCI-H292
cells (Fig. 5B) and Panc89
(Fig. 5C) were labeled with
Calcein AM, treated with different concentrations of the soluble
disintegrin domain and seeded on top of confluent MEF monolayers.
The MDA-MB-231, NCI-H292 and Panc89 cells attached to MEFs in the
absence of the soluble disintegrin domain. Pretreatment of
MDA-MB-231, NCI-H292 and Panc89 cells with the soluble disintegrin
domain significantly reduced the interactions between carcinoma and
murine fibroblast cells. Similar interactions were observed between
the carcinoma and human fibroblasts IMR.90 (Fig. 5D–F). Thus, ADAM17 mediates
cell-cell interactions, which can be inhibited by the soluble
disintegrin domain.
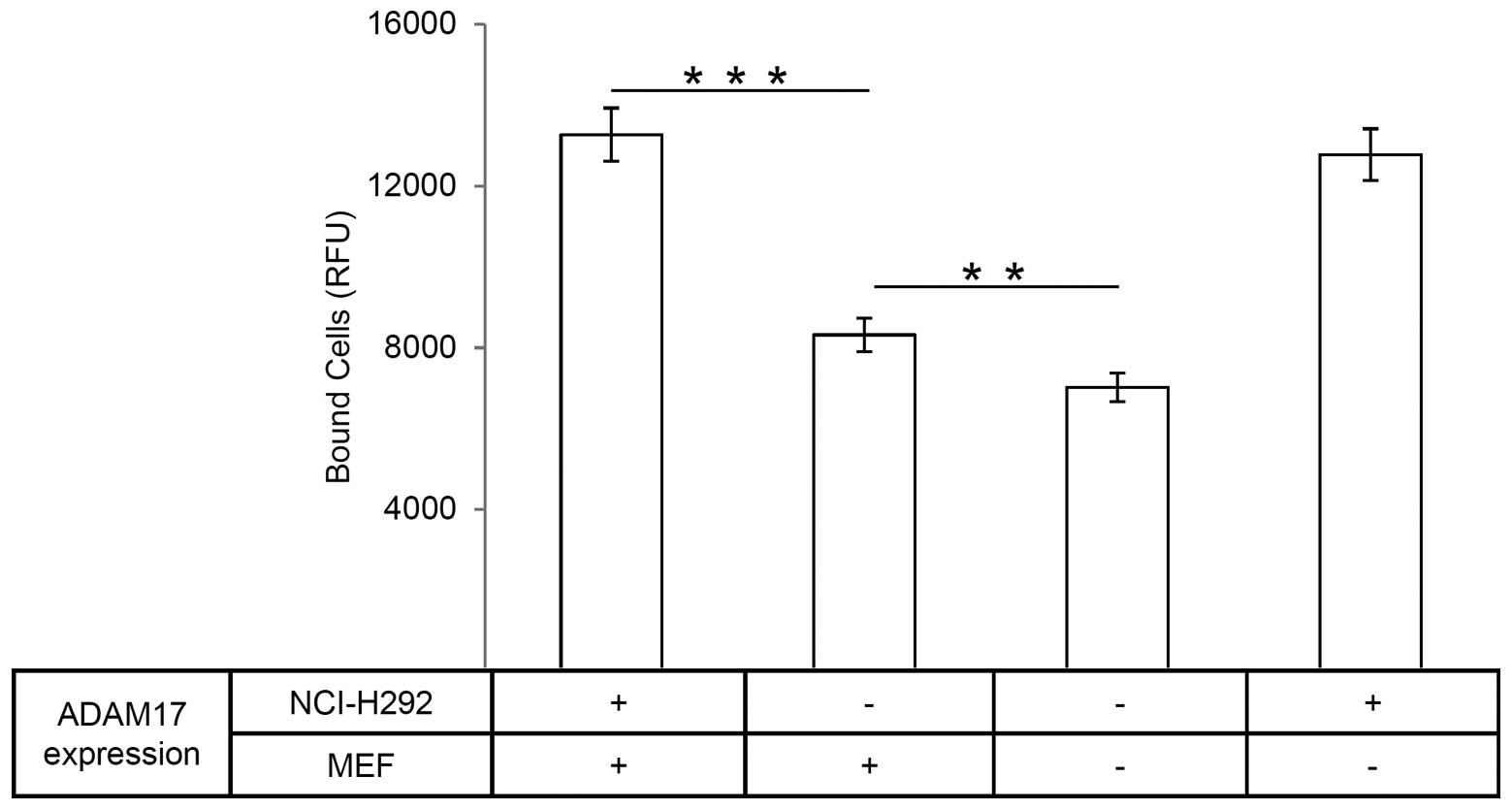
Silencing of ADAM17 reduces
fibroblast-carcinoma cell interaction
In order to evaluate further the adhesive property
of the disintegrin domain, we performed cell-cell interaction
assays using ADAM17 deficient cells. Whereas ADAM17 deficient cells
(ADAM17ex/ex MEFs) were derived from
ADAM17ex/ex mice (34),
ADAM17 silencing in NCI-H292 cells was achieved using an ADAM17
shRNA (35).
ADAM17ex/ex mice were generated by insertion of new exon
within the gene of ADAM17. This new exon starts with an in-frame
translational stop codon. Homozygous ADAM17ex/ex mice
were viable and showed a dramatic loss of ADAM17 protein in all
cell types (34). In line with the
above results, the amount of ADAM17 knockdown NCI-H292 (−) cells
attaching to wt-MEF (+) was significantly reduced as compared to
the wt-NCI-H292 (+) cells (Fig.
6). Furthermore, silencing ADAM17 in MEF, using
ADAM17ex/ex MEFs (−), and in NCI-H292 (−) cells
decreased further the cell-cell interaction by about half in
comparison to the control (Fig.
6). These results indicate that ADAM17 acts as an adhesion
molecule and mediates fibroblast-carcinoma cell interaction.
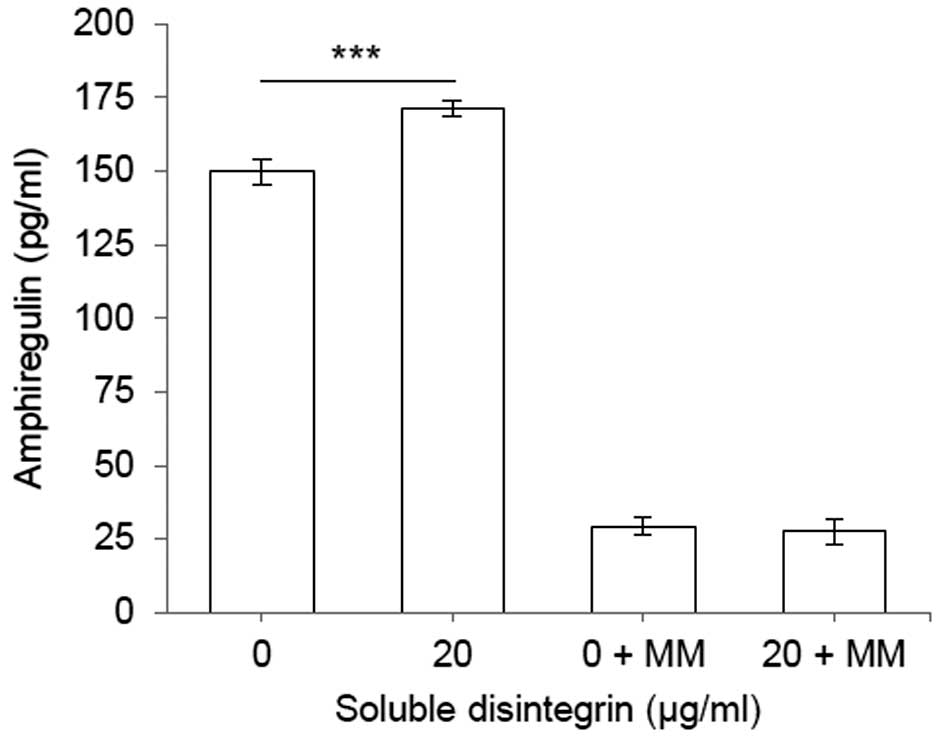
Soluble disintegrin domain of ADAM17
induces shedding of amphiregulin from MDA-MB-231 cells
The interaction between α5β1 integrin and ADAM17
keeps the enzyme in an inactive state (9,10).
This finding raises the assumption that the soluble disintegrin
domain of ADAM17 should be able to induce shedding by competing
with the wild-type molecule for the binding to α5β1 integrin.
Thereby the complex between the integrin and enzyme should be
dissolved, releasing an active ADAM17.
To test this hypothesis, we measured the shedding of
amphiregulin in MDA-MB-231 cells incubated with or without the
soluble disintegrin domain (Fig.
7). Amphiregulin, a substrate of ADAM17 (36), belongs to the epidermal growth
factor family, and acts as mitogenic stimulator through binding to
epidermal growth factor receptors (37). The resulting data showed that
treatment of these cells with the soluble disintegrin domain
increases shedding of amphiregulin in comparison to control cells
treated with PBS. The shedding induced by soluble disintegrin could
be inhibited by metalloprotease inhibitor, marimastat. Thus,
soluble disintegrin domain binds to α5β1 integrin and consequently
activates the wild-type molecule, most likely by releasing ADAM17
out of the inhibitory complex.
Discussion
The disintegrin domain of many ADAM family members
has been shown to act as an adhesion ligand for integrins (8,38).
ADAM15 interacts with various integrins, including αvβ3-, αvβ1-,
α9β- and α5β1-integrin (39),
while ADAM2 binds to α6β1 integrin, ADAM9 to α6β1-, αvβ5- and
α3β1-integrin (31,40) and ADAM28 to α4β1 integrin (8). In line with the work of Bax et
al(7), we demonstrate that the
recombinant soluble disintegrin domain of ADAM17 binds directly to
the purified α5β1 integrin and is able to precipitate the
DN-ADAM17; confirming that the disintegrin domain of ADAM17 is a
specific ligand for the α5β1 integrin and that the domain located
C-terminally of the disintegrin domain of ADAM17 is not needed for
this purpose. Additionally, the soluble disintegrin domain only is
able to release ADAM17 out of the inhibitory complex, allowing the
enzyme to shed its substrate amphiregulin.
In a next step, the adhesive property of ADAM17 at a
cellular level was examined. MEF cells adhere strongly and
dose-dependently to the immobilized disintegrin domain.
Interestingly, carcinoma cells, which express high levels of
ADAM17, are also able to adhere to the disintegrin domain. The
adhesion of carcinoma cells to the immobilized disintegrin domain
was inhibited by the soluble disintegrin domain, indicating a
specific and direct interaction between carcinoma cells and ADAM17
via the disintegrin domain. These results demonstrate that
fibroblasts as well as carcinoma cells adhere to ADAM17 via the
α5β1 integrins, which is in good agreement with the data of Bax
et al(7).
On the other hand, it has been shown that ADAM17 is
upregulated in ovarian cancer (22,23),
colon carcinoma (24), lung cancer
(25,35), head and neck cancer (41) and its expression was associated
with tumour invasion and metastasis (21,42).
In these studies, the authors correlated metastasis and shedding of
different growth factors induced by upregulation of ADAM17, but did
not further analyze the resulting enhancement of adhesive
properties of cancer cells to fibroblasts. For that purpose,
cell-cell interaction assays between fibroblasts and carcinoma
cells in the presence and absence of the soluble disintegrin domain
of ADAM17 were performed. The soluble disintegrin domain reduces
the fibroblast-carcinoma cell interaction, indicating that ADAM17
mediates directly the interaction between fibroblasts and carcinoma
cells.
Similar effects have been shown for ADAM9 (31). The disintegrin domain of ADAM9
mediates a direct interaction between melanoma cells and
fibroblasts and ADAM9 improves the invasive ability of melanoma
cells (31). The same observation
has been made in case of ADAM17 where silencing of ADAM17
expression in NCI-H292 cells leads to a high reduction in their
ability to migrate (27,43). Therefore, ADAM17 mediated shedding
of growth factor and adhesion molecules seem not to be the only
tumour promoting factor of the metalloprotease. In addition, ADAM17
mediates the interaction between fibroblasts and carcinoma cells
via its disintegrin domain, which seems to be an important factor
for carcinoma invasion. The ADAM17/α5β1 integrin complex keeps the
protease in an inactive state. Release of the ADAM17 from this
complex leads to an active ADAM17 (9,10),
which then is able to shed membrane-bound growth factors, like
amphiregulin, resulting in tumour growth. Therefore, ADAM17
promotes migration as well as proliferation of tumour cells. The
combined action of this metalloprotease makes ADAM17 an even more
interesting target for cancer therapy.
Acknowledgments
This study was supported by the Deutsche
Forschungsgemeinschaft (SFB 877, A6, Z3) and the cluster of
excellence ‘Inflammation at Interfaces’.
References
|
1.
|
Brocker CN, Vasiliou V and Nebert DW:
Evolutionary divergence and functions of the ADAM and ADAMTS gene
families. Hum Genomics. 4:43–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
White JM: ADAMs: modulators of cell-cell
and cell-matrix interactions. Curr Opin Cell Biol. 15:598–606.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Takeda S: Three-dimensional domain
architecture of the ADAM family proteinases. Semin Cell Dev Biol.
20:146–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Liu H, Shim AH and He X: Structural
characterization of the ectodomain of a disintegrin and
metalloproteinase-22 (ADAM22), a neural adhesion receptor instead
of metalloproteinase: insights on ADAM function. J Biol Chem.
284:29077–29086. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Janes PW, Saha N, Barton WA, et al: Adam
meets Eph: an ADAM substrate recognition module acts as a molecular
switch for ephrin cleavage in trans. Cell. 123:291–304. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Reiss K and Saftig P: The ‘a disintegrin
and metalloprotease’ (ADAM) family of sheddases: physiological and
cellular functions. Semin Cell Dev Biol. 20:126–137. 2009.
|
|
7.
|
Bax DV, Messent AJ, Tart J, et al:
Integrin alpha5beta1 and ADAM-17 interact in vitro and co-localize
in migrating HeLa cells. J Biol Chem. 279:22377–22386. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
McGinn OJ, English WR, Roberts S, Ager A,
Newham P and Murphy G: Modulation of integrin alpha4beta1 by ADAM28
promotes lymphocyte adhesion and transendothelial migration. Cell
Biol Int. 35:1043–1053. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Saha A, Backert S, Hammond CE, Gooz M and
Smolka AJ: Helicobacter pylori CagL activates ADAM17 to
induce repression of the gastric H, K-ATPase alpha subunit.
Gastroenterology. 139:239–248. 2010. View Article : Google Scholar
|
|
10.
|
Gooz P, Dang Y, Higashiyama S, Twal WO,
Haycraft CJ and Gooz M: A disintegrin and metalloenzyme (ADAM) 17
activation is regulated by alpha5beta1 integrin in kidney mesangial
cells. PLoS One. 7:e333502012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Milla ME, Leesnitzer MA, Moss ML, et al:
Specific sequence elements are required for the expression of
functional tumor necrosis factor-alpha-converting enzyme (TACE). J
Biol Chem. 274:30563–30570. 1999. View Article : Google Scholar
|
|
12.
|
Lorenzen I, Trad A and Grötzinger J:
Multimerisation of A disintegrin and metalloprotease protein-17
(ADAM17) is mediated by its EGF-like domain. Biochem Biophys Res
Commun. 415:330–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Reddy P, Slack JL, Davis R, et al:
Functional analysis of the domain structure of tumor necrosis
factor-alpha converting enzyme. J Biol Chem. 275:14608–14614. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Tape CJ, Willems SH, Dombernowsky SL, et
al: Cross-domain inhibition of TACE ectodomain. Proc Natl Acad Sci
USA. 108:5578–5583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lorenzen I, Lokau J, Düsterhöft S, et al:
The membrane-proximal domain of A Disintegrin and Metalloprotease
17 (ADAM17) is responsible for recognition of the interleukin-6
receptor and interleukin-1 receptor II. FEBS Lett. 586:1093–1100.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Smith KM, Gaultier A, Cousin H, Alfandari
D, White JM and DeSimone DW: The cysteine-rich domain regulates
ADAM protease function in vivo. J Cell Biol. 159:893–902. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Soond SM, Everson B, Riches DW and Murphy
G: ERK-mediated phosphorylation of Thr735 in TNFalpha-converting
enzyme and its potential role in TACE protein trafficking. J Cell
Sci. 118:2371–2380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Edwards DR, Handsley MM and Pennington CJ:
The ADAM metalloproteinases. Mol Aspects Med. 29:258–289. 2008.
View Article : Google Scholar
|
|
19.
|
Kommaddi RP, Thomas R, Ceni C, Daigneault
K and Barker PA: Trk-dependent ADAM17 activation facilitates
neurotrophin survival signaling. FASEB J. 25:2061–2070. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
McGowan PM, McKiernan E, Bolster F, et al:
ADAM-17 predicts adverse outcome in patients with breast cancer.
Ann Oncol. 19:1075–1081. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
McGowan PM, Ryan BM, Hill AD, McDermott E,
O'Higgins N and Duffy MJ: ADAM-17 expression in breast cancer
correlates with variables of tumor progression. Clin Cancer Res.
13:2335–2343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Tanaka Y, Miyamoto S, Suzuki SO, et al:
Clinical significance of heparin-binding epidermal growth
factor-like growth factor and a disintegrin and metalloprotease 17
expression in human ovarian cancer. Clin Cancer Res. 11:4783–4792.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Rosso O, Piazza T, Bongarzone I, et al:
The ALCAM shedding by the metalloprotease ADAM17/TACE is involved
in motility of ovarian carcinoma cells. Mol Cancer Res.
5:1246–1253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Blanchot-Jossic F, Jarry A, Masson D, et
al: Up-regulated expression of ADAM17 in human colon carcinoma:
co-expression with EGFR in neoplastic and endothelial cells. J
Pathol. 207:156–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Dijkstra A, Postma DS, Noordhoek JA, et
al: Expression of ADAMs (‘a disintegrin and metalloprotease’) in
the human lung. Virchows Arch. 454:441–449. 2009.
|
|
26.
|
Badoual C, Bouchaud G, Agueznay Nel H, et
al: The soluble alpha chain of interleukin-15 receptor: a
proinflammatory molecule associated with tumor progression in head
and neck cancer. Cancer Res. 68:3907–3914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zheng X, Jiang F, Katakowski M, Zhang ZG,
Lu QE and Chopp M: ADAM17 promotes breast cancer cell malignant
phenotype through EGFR-PI3K-AKT activation. Cancer Biol Ther.
8:1045–1054. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Trad A, Hedemann N, Shomali M, Pawlak V,
Grotzinger J and Lorenzen I: Development of sandwich ELISA for
detection and quantification of human and murine a disintegrin and
metalloproteinase 17. J Immunol Methods. 371:91–96. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Trad A, Hansen HP, Shomali M, et al:
ADAM17-overexpressing breast cancer cells selectively targeted by
antibody-toxin conjugates. Cancer Immunol Immunother. Sep 1–2012,
(Epub ahead of print).
|
|
30.
|
Yamamoto K, Trad A, Baumgart A, et al: A
novel bispecific single-chain antibody for ADAM17 and CD3 induces
T-cell-mediated lysis of prostate cancer cells. Biochem J.
445:135–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Zigrino P, Nischt R and Mauch C: The
disintegrin-like and cysteine-rich domains of ADAM-9 mediate
interactions between melanoma cells and fibroblasts. J Biol Chem.
286:6801–6807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Chalaris A, Rabe B, Paliga K, et al:
Apoptosis is a natural stimulus of IL6R shedding and contributes to
the proinflammatory trans-signaling function of neutrophils. Blood.
110:1748–1755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Jovic M, Naslavsky N, Rapaport D, Horowitz
M and Caplan S: EHD1 regulates beta1 integrin endosomal transport:
effects on focal adhesions, cell spreading and migration. J Cell
Sci. 120:802–814. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Chalaris A, Adam N, Sina C, et al:
Critical role of the disintegrin metalloprotease ADAM17 for
intestinal inflammation and regeneration in mice. J Exp Med.
207:1617–1624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Baumgart A, Seidl S, Vlachou P, et al:
ADAM17 regulates epidermal growth factor receptor expression
through the activation of Notch1 in non-small cell lung cancer.
Cancer Res. 70:5368–5378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Sternlicht MD and Sunnarborg SW: The
ADAM17-amphiregulin-EGFR axis in mammary development and cancer. J
Mammary Gland Biol Neoplasia. 13:181–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Kuramochi H, Nakajima G, Kaneko Y, et al:
Amphiregulin and Epiregulin mRNA expression in primary colorectal
cancer and corresponding liver metastases. BMC Cancer. 12:882012.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Toquet C, Colson A, Jarry A, et al: ADAM15
to alpha5beta1 integrin switch in colon carcinoma cells: A late
event in cancer progression associated with tumor dedifferentiation
and poor prognosis. Int J Cancer. 130:278–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Lucas N and Day ML: The role of the
disintegrin metalloproteinase ADAM15 in prostate cancer
progression. J Cell Biochem. 106:967–974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Desiderio UV, Zhu X and Evans JP: ADAM2
interactions with mouse eggs and cell lines expressing
alpha4/alpha9 (ITGA4/ITGA9) integrins: implications for
integrin-based adhesion and fertilization. PLoS One. 5:e137442010.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Kornfeld JW, Meder S, Wohlberg M, et al:
Overexpression of TACE and TIMP3 mRNA in head and neck cancer:
association with tumour development and progression. Br J Cancer.
104:138–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Gooz M: ADAM-17: the enzyme that does it
all. Crit Rev Biochem Mol Biol. 45:146–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Zheng X, Jiang F, Katakowski M, Lu Y and
Chopp M: ADAM17 promotes glioma cell malignant phenotype. Mol
Carcinog. 51:150–164. 2012. View Article : Google Scholar : PubMed/NCBI
|