Introduction
Cancer stem cells (CSCs) possess ability to self
renew, high tumorigenic activity, resistance to anticancer drugs
and radiation and cause cancer recurrence (1–4).
Successful targeting of CSCs is believed to lead to development of
curative cancer therapy. The presence of CSCs has been detected in
colorectal cancers (CRCs) and CD133, CD44, CD166, CD24 and ALDH
expressing cells have been reported as CSC candidates in CRC
(5–11). Though numerous markers have been
identified as candidates for CSCs as the study of CSC has
progressed, few reports have confirmed the presence of these
markers. The development of CSC therapies will require identifying
CSC markers that reliably identify CSCs.
CSCs have been reported to possess high metastatic
ability in various cancers (12–15).
The assessment of markers for cancer cell metastasis might also be
important for the study of CSC in CRC. CD49f, also known as
integrin α6 (ITGA6), is reportedly associated with tumor cell
invasion and metastasis in CRC (16–21).
The chemokine receptor CXCR4 plays a critical role in cancer cell
metastasis via stromal interaction and hypoxia-related pathways
(12,22–26).
To isolate CSCs in CRC, we focused on the changes in markers during
cell differentiation, given that CSCs construct a cancer-cell
society in a hierarchical manner by producing differentiated cancer
cells. Forced cell differentiation leads to depletion of CSCs and
results in the decay of the cancer cell hierarchy in brain tumors
and colon cancer (27–30). Thus, adaptation of cell
differentiation assay is useful for the isolation of immature-cell
phenotypes (31). In this study,
with the aim of identifying CSC markers, we assessed changes in
expression levels of known CSC markers of CRC- and
metastasis-associated markers induced during cell differentiation.
Using serial transplantation of clinical CRC samples, we also
assessed the tumorigenic activity of isolated cell fractions to
determine whether they exhibited self-renewal activity.
Materials and methods
Tumor cell preparation and cell
culture
HT29 was cultured in McCoy’s medium 5A
(Invitrogen)/10% fetal bovine serum (FBS; Equitech-Bio) with 2 mM
L-glutamine (Invitrogen), 100 μg/ml penicillin G and 100
U/ml streptomycin (Invitrogen). Caco2 was cultured in RPMI-1640
(Invitrogen)/10% FBS with 100 μg/ml penicillin G and 100
U/ml streptomycin. HT29 was obtained from the American Type Culture
Collection (ATCC) and Colo201 was obtained from the Japanese
Collection of Research Bioresources Cell Bank (JCRB). The clinical
colorectal cancer samples were obtained from Kyushu University at
Beppu and Osaka University upon patients’ informed consent and
approval by the Research Ethics Board at Kyushu University and
Osaka University in Japan.
Sodium butyrate treatment and alkaline
phosphatase assay
Cell differentiation was induced with sodium
butyrate (NaBT; Wako) as previously reported (31). Briefly, HT29 and Caco2 cells were
dissociated with 0.25% trypsin and 0.02% EDTA and 1×106
cells were subsequently seeded into 10-cm plastic flasks (BD;
Becton-Dickinson). The next day, 5 mM sodium butyrate (NaBT; Wako)
was added and the culture was incubated for 72 h. The expression
level of alkaline phosphatase was determined by ELISA with
SensoLyte™ pNPP Alkaline Phosphatase Assay kit (AnaSpec) according
to the manufacturer’s protocol.
Transplantation of human colon cancer
cells into NOD/ SCID mice
Colon cancer cells obtained from three independent
patients were suspended in a 1:1 mixture of media and basement
membrane matrix high concentration (BD; Becton-Dickinson) and
inoculated subcutaneously into the axillary and inguinal regions of
NOD/SCID mice (5 weeks of age) under anesthesia. After 8–12 weeks,
tumors were removed and analyzed by flow cytometry as described
below. All of the xenograft lines were originally implanted into
NOD/SCID mice subcutaneously and not cultivated or expanded in
vitro. To study the tumorigenic activity of isolated cell
populations, cell doses of 1×104 and 5×103 of
cells were inoculated into the axillary and inguinal regions,
respectively, of NOD/SCID mice. Tumorigenicity was evaluated 6
weeks after NOD/SCID transplantation.
Digestion of cancer tissues
Colon cancer tissues were minced with a sterile
scalpel, washed twice with DMEM/10% FBS with 100 μg/ml
penicillin G and 100 U/ml streptomycin (Invitrogen) and placed in
DMEM/10% FBS with 2 mg/ml collagenase A (Roche) solution. The
mixture was incubated at 37°C for ≤2 h to allow complete digestion.
Every 15 min, the solution was mixed in a 10-ml pipette to
encourage dissociation. Cells were filtered through 40-μm
nylon mesh and washed twice and cell fragments and debris were
subsequently eliminated by Ficoll (GE Healthcare) density gradient
centrifugation. Cells were stained for flow cytometry or subsequent
transplantation into NOD/SCID mice.
Flow cytometric analysis and cell
sorting
To characterize colon cancer-initiating cells, the
following antibodies were used: APC- or PE-conjugated anti-human
CD133/1 (clone AC133, mouse IgG2a, Miltenyi-Biotec), FITC- or
PE-conjugated anti-human CD44 (clone G44–26, mouse IgG2b, BD),
FITC- or PE-conjugated anti-human CD49f (clone GoH3, Rat IgG2a,
BD), FITC-conjugated anti-human CD166 (clone N6B6, mouse IgG2a,
BD), FITC-conjugated anti-human CD24 (clone ML5, mouse IgG2a, BD)
and PE-Cy7-conjugated anti-human CXCR4 (CD184; clone 12G5, mouse
IgG2a, BD).
To isolate human cells from mouse xenografts,
biotinylated anti-mouse H-2Kd (clone SF1-1.1, mouse
IgG2a, BD) and biotinylated anti-mouse CD45 (clone 30-F11, mouse
IgG2b, BD) were used. Streptavidin-conjugated APC-Cy7 (BD) was used
as secondary antibody. Doublet cells were eliminated with
FSC-A/FSC-H and SSC-A/SSC-H. Dead and dying cells were eliminated
with 7-amino-actinomycin D (7-AAD; BD). Samples were analyzed and
sorted with BD FACSAria II flow cytometer (Becton-Dickinson) and
data were analyzed with Diva software (Becton-Dickinson).
Results
Screening of colorectal cancer stem cell
markers by differentiation assay
To assess whether representative CSC markers were in
fact associated with cell differentiation, we applied sodium
butyrate (NaBT) cell differentiation assay to HT29 and Caco2
(31–33). We assessed the expression of CD44
(8), CD133 (5,6,9),
CD166 (8) and CD24 (9) before and after the NaBT treatment. We
also assessed CD49f and CXCR4 expression, because CD49f was
reported as a marker of breast (34), prostate (35) and glioblastoma (36) CSCs and CXCR4 was reported to be a
marker of pancreas CSCs (12).
CD49f and CXCR4 is also known to deeply associate with cancer
metastasis (12,16–26).
The expression of CD44 was drastically reduced by NaBT treatment
(rate of change, −99.2% in HT29 and −97.2% in Caco2; average,
−98.2%). Expression of CD49f was also reduced by NaBT treatment
(−73.7% in HT29 and −75.0% in Caco2; average, −74.4%). The percent
change in CD133 expression was not significantly high (−26.0% in
HT29 and −9.8% in Caco2; average, −17.9%) compared to those of CD44
and CD49f. The expression of CD166 was markedly reduced in HT29
(−82.7%) but not in Caco2 (−16.0%). The expression of CXCR4 was
slightly reduced by NaBT treatment (−8.4% in HT29, −10.0% in Caco2;
average, −9.2%). The expression of CD24 was slightly reduced in
HT29 (−15.2%), but significantly increased in Caco2 (+431.0%)
(Table I). We focused on CD49f in
subsequent studies because its expression was sharply altered by
NaBT treatment in both HT29 and Caco2.
 | Table I.Changes in positive ratios of cell
surface markers following NaBT-induced differentiation. |
Table I.
Changes in positive ratios of cell
surface markers following NaBT-induced differentiation.
| Marker | HT29
| Caco2
| Average of rate of
change (%) |
|---|
| Control (%) | NaBT treat (%) | Rate of change
(%) | Control (%) | NaBT treat (%) | Rate of change
(%) |
|---|
| CD44 | 77.8 | 0.6 | −99.2 | 14.2 | 0.4 | −97.2 | −98.2% |
| CD133 | 90.1 | 67.7 | −26.0 | 93.6 | 84.4 | −9.8 | −17.9% |
| CD49f | 99.9 | 26.3 | −73.7 | 32.8 | 8.3 | −75.0 | −74.4% |
| CD166 | 49.2 | 8.5 | −82.7 | 55.4 | 46.7 | −16.0 | −49.4% |
| CD24 | 97.2 | 82.4 | −15.2 | 6.2 | 32.9 | 431.0 | +207.9% |
| CXCR4 | 40.7 | 37.3 | −8.4 | 1.0 | 0.9 | −10.0 | −9.2% |
Flow cytometric analysis of primary colon
cancer cells
Expression levels of representative colorectal CSC
markers (CD44, CD133 and CD166) and the candidate marker CD49f were
confirmed in four cases of primary colon cancer by flow cytometry.
Tumor cells were obtained independently from mouse xenografted
clinical colorectal cancer cells. The expression of CD44 and CD133
were 30.3±14.0 and 35.5±14.7%, respectively. Double staining of
CD44 and CD133 indicated that tumor cells were constructed by
CD44+CD133+,
CD44+CD133−,
CD44−CD133+ and CD44–CD133− cell
fractions, as we previously reported (31). CD49f-expressing cells (8.0±2.6%)
were localized in the CD44+ and CD133+ cell
fractions. CD166-expressing cells (7.0±3.4%) were also localized in
the CD44+ cell fraction but were present in both
CD133-positive and -negative fractions. Double staining of CD49f
and CD166 indicated that tumor cells could be divided into
CD49f+CD166+ (4.8±2.7%),
CD49f+CD166− (3.2±1.3%),
CD49f−CD166− (90.8±8.2%) and
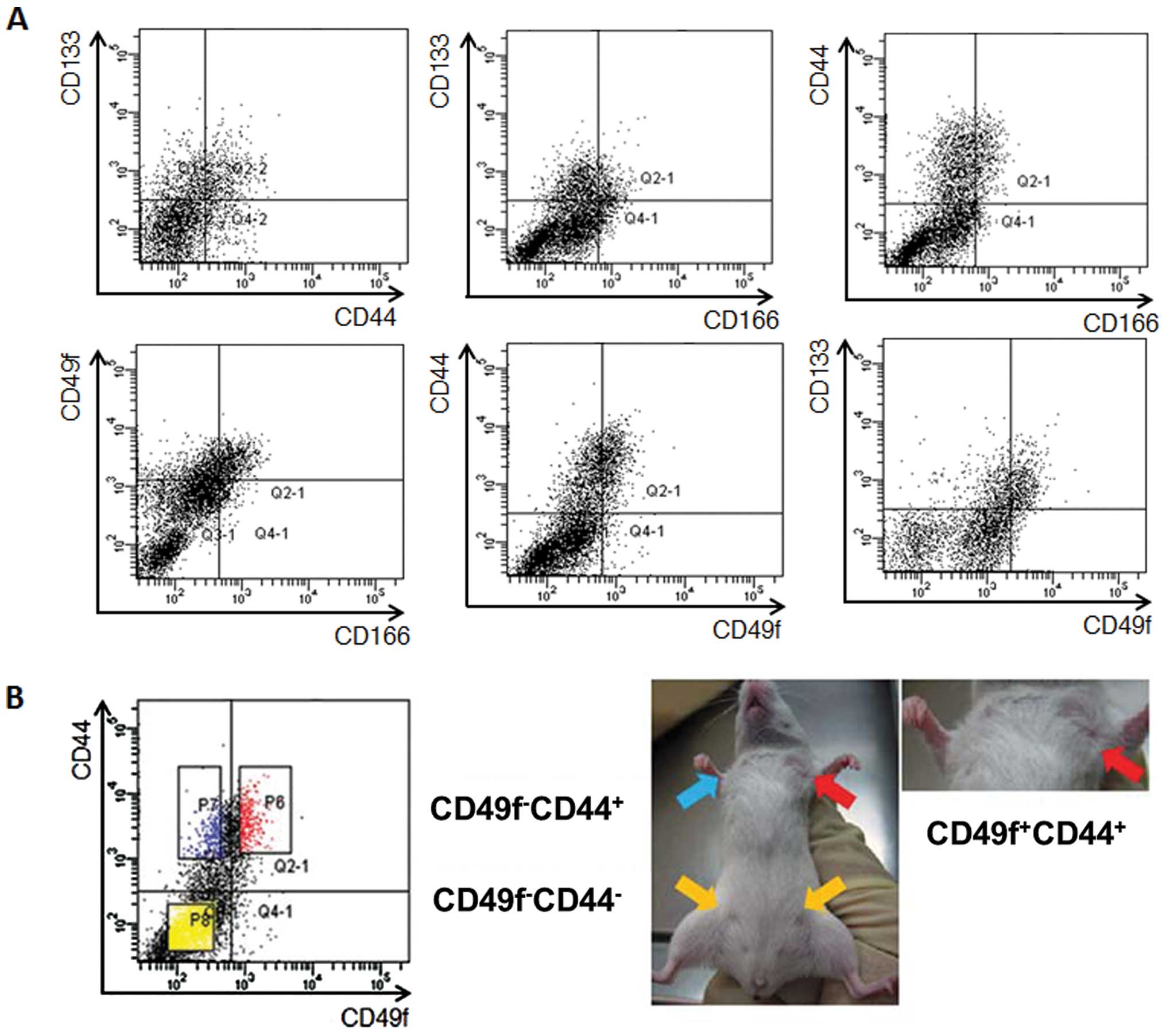
CD49f-CD166+ (2.2±1.7%) cell fractions (Fig. 1A).
Tumorigenic activity in NOD/SCID
mouse
To assess the tumorigenic and self-renewal
activities of isolated tumor cell fractions, we applied a serial
transplantation technique. As reported previously (31), the
CD133−CD44− and
CD133+CD44−fractions formed no tumors,
whereas only the CD133+CD44+ fraction formed
a tumor (3/3 cases in 1×104 cells and 3/4 cases in
5×103 cells), suggesting that CD44 has a more important
role in tumorigenic activity than CD133. Expression of CD166 led to
no difference in tumorigenicity; both CD166-positive and -negative
cells formed tumors when they expressed CD133 or CD44. But the rate
of tumor formation at the cell dose of 5×103 was lower
in the CD133+ fraction to some extent
(CD133+CD166+; 1/3; 33.3%) compared to that
of CD44+ fraction (CD44+CD166+;
2/3; 66.7%), also suggesting that CD44 is more closely associated
with tumorigenicity than CD133. Next, the tumorigenic activity of
CD49f-expressing cells was assessed. Both the
CD133−CD49f− and
CD133+CD49f− cell fractions formed no tumors,
whereas the CD133+CD49f+ cell fraction formed
tumors at cell doses of 1×104 and 5×103.
Combined analysis of CD49f with CD44 revealed that only
CD44+CD49f+ fraction formed tumors at the
cell doses of 1×104 and 5×103.
CD44−CD49f− and
CD44+CD49f− fraction formed no tumors
(Fig. 1B and Table II). Expression of CXCR4 did not
affect the tumorigenicity (3/3 in both 1×104 and
5×103; data not shown).
 | Table II.Tumor-initiating ability of the cell
populations. |
Table II.
Tumor-initiating ability of the cell
populations.
| Cell
population | 10,000 cells | 5,000 cells |
|---|
|
CD133−CD44− | (−) 0/3 | (−) 0/4 |
|
CD133+CD44− | (−) 0/3 | (−) 0/4 |
|
CD133+CD44+ | (+) 3/3 | (+) 3/4 |
|
CD133−CD166− | (−) 0/3 | (−) 0/3 |
|
CD133+CD166− | (+) 3/3 | (+) 1/3 |
|
CD133+CD166+ | (+) 3/3 | (+) 1/3 |
|
CD44−CD166− | (−) 0/3 | (−) 0/3 |
|
CD44+CD166− | (+) 3/3 | (+) 2/3 |
|
CD44+CD166+ | (+) 3/3 | (+) 2/3 |
|
CD133−CD49f− | (−) 0/4 | (−) 0/4 |
|
CD133+CD49f− | (−) 0/4 | (−) 0/4 |
|
CD133+CD49f+ | (+) 3/4 | (+) 6/8 |
|
CD44−CD49f− | (−) 0/4 | (−) 0/6 |
|
CD44+CD49f− | (−) 0/4 | (−) 0/6 |
|
CD44+CD49f+ | (+) 4/4 | (+) 6/8 |
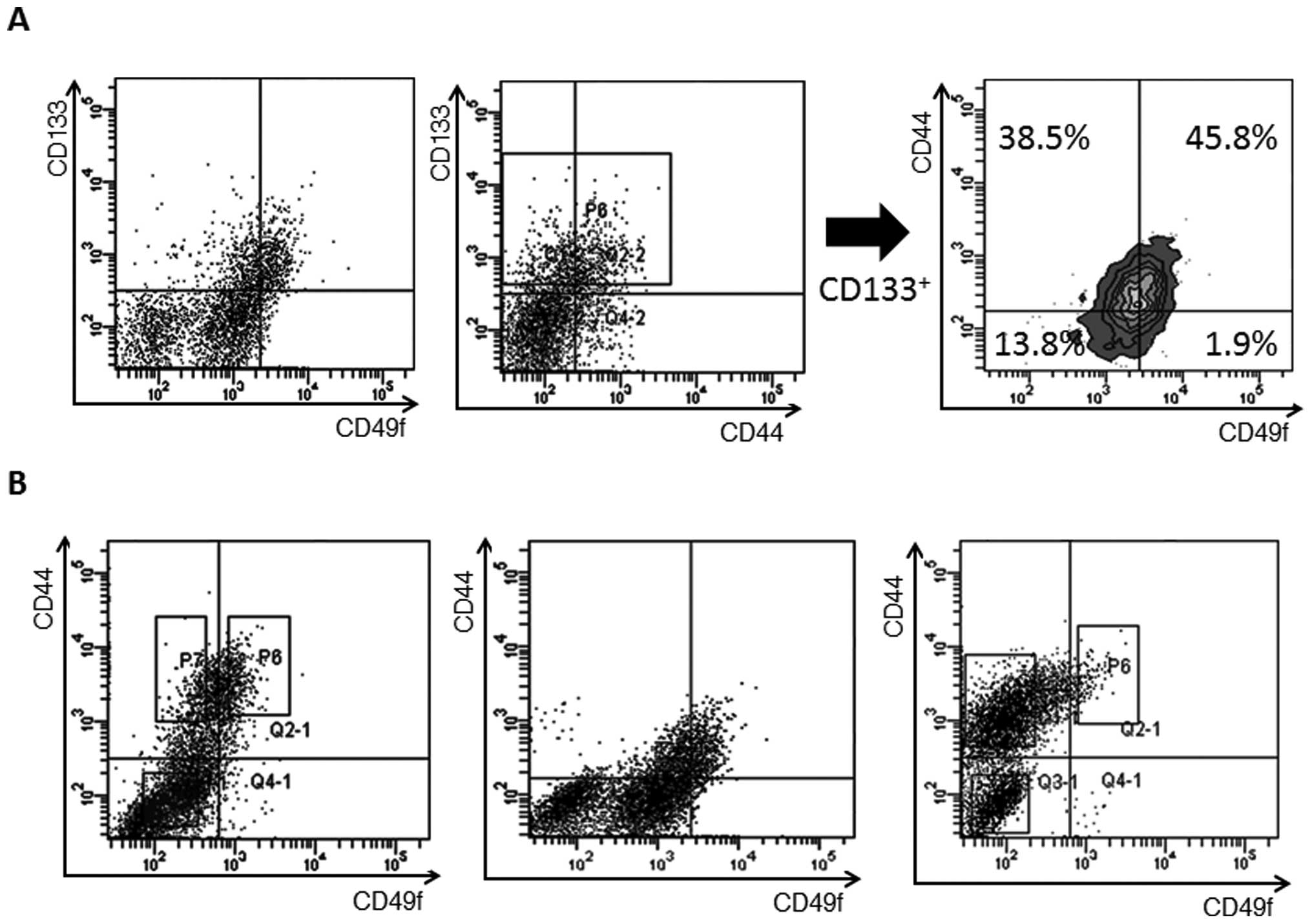
Expression analysis of CD133, CD44 and
CD49f
The associations of expression of CD133 with CD49f
and of CD44 with CD49f were assessed in primary colon cancer cells.
Expression analysis of CD49f and CD133 revealed that 52.3% of
CD49f+ cells co-expressed CD133, whereas 47.7% of
CD133+ cells were negative for CD49f (Fig. 2A). A combined analysis of CD49f,
CD44 and CD133 revealed that the CD133+ cells contained
45.8% of CD49f+CD44+ cells, 38.5% of
CD49f-CD44+ cells, 13.8% of
CD49f−CD44− cells and a very small number of
CD49f+CD44− cells (Fig. 2A).
Expression of CD49f was confirmed in each of the
three cases of primary colon cancer and the percentage of
CD49f+ cells in CD44+ cells ranged from 8.5
to 50.2% (30.5±20.9%). The CD44+ cell fraction was
divided into CD44+CD49f+ and
CD44+CD49f− fractions, but CD49f+
cells were localized in the CD44+ cell fraction in each
of the three cases (Fig. 2B). In
view of the finding that both CD133+CD49f+
cells and CD44+CD49f+ cells possess
tumorigenic activity and that CD49f+ cells localized in
the CD133+ and CD44+ cell fractions,
CD49f+ cells may be the best candidate for colorectal
CSCs.
Discussion
This study demonstrated that CD49f is an important
marker for efficient enrichment of colorectal CSCs. In colorectal
cancer, CD44+, CD133+, CD166+,
CD24+ and aldehyde dehydrogenase (ALDH)-positive cells
have been reported as a candidate for colorectal CSCs (5–11).
This study aimed to identify novel and definitive CSCs markers in
CRC by evaluation of the tumor-initiation efficiency of known and
candidate CSCs. To achieve this purpose, we applied cell
differentiation and serial transplantation assays. Similar to
normal stem cells, CSCs possess self-renewal ability and produce
differentiated cells in the cancer cell hierarchy. It is reported
that bone morphogenetic proteins (BMPs) induce cell differentiation
in colorectal CSCs (30) and in
brain tumor-initiating cells (28,29).
Because the content of such immature cell phenotypes will decrease
during cellular differentiation process, it is reasonable to assess
the rate of change of cell surface markers for identification and
evaluation of candidate CSC markers. One method for inducing for
cancer cell differentiation is the use of NaBT (31–33).
In the assessment of two CRC cell lines, the expression levels of
both CD44 and CD49f were sharply decreased after the forcing of
cell differentiation with NaBT. That the expression of CD133 and
CD166 was not much altered by cell differentiation compared to CD44
and CD49f suggested that CD44 and CD49f are enriched in the
immature cell fraction more than CD133 and CD166. This result is
partly supported by a report that CD44 may be more suitable as a
cancer-initiating cell marker than CD133 (8). The expression of CD24 and CXCR4
appeared random in HT29 and Caco2.
Assessment of self-renewal activity is important for
confirmation of the stemness of an isolated cell fraction (1–4). By
the use of cell materials from xenografted primary colon cancers
for tumorigenic assessment, it is relatively easy to assess the
self-renewal ability of targeted cell populations. In our
experiments, tumors formed in immunodeficient mice invariably
re-form tumors in serially transplanted syngenic mice (31). Thus, absence of tumor formation in
a given isolated cell population indicates the loss of self-renewal
activity of this isolated cell fraction. In the tumorigenic
assessment, CD49f displayed promising ability for tumorigenic cell
enrichment; CD44+ or CD133+ cells exhibited
no tumorigenic activity when they were negative for CD49f
expression. In the expression analysis, CD49f+ cells
were localized in the CD44+ and CD133+ cell
fractions. In our previous report (31) and in this study, we have confirmed
that CD44+CD133+ cells are tumor-initiating
cells. The CD44+CD133+ cell fraction
contained 38.5% of CD49f− cells and 45.8% of
CD44+ cells. These results suggest that
CD49f+ cells may be the best candidates for CSCs and
tumor-initiating cells in CRC.
CD49f, also known as integrin α6 (ITGA6), is a major
laminin receptor and mediates cell adhesion (16,18,19,21).
In colon cancer, expression of CD49f has been reported to be
associated with tumor cell invasion and metastasis via
integrin-mediated cell signaling and adhesion to the extracellular
matrix (16,18,19,21).
CSCs in various cancers show high metastatic potency (12–15).
In the liver metastasis model of CRC cells, it is reported that
α6-integrin expression on circulating CRC cells mediates cell
adhesion in hepatic microcirculation and extravasation into liver
parenchyma (18). In addition,
inhibition of CD49f by specific antibody resulted in reduction of
cancer cell extravasation and migration (21). Successful targeting of
CD49f-expressing cells may contribute to the development of a novel
radical cancer treatment. Of course, it is necessary to assess if
the inhibition of CD49f+ cells actually disintegrates
the cancer cell hierarchy via suppression of self-renewal activity.
It will also be necessary to assess the metastatic activity of
CD49f expressing cells in a future study, given that we could not
identify meta-static lesions in the term of this study using
subcutaneous transplantation.
Acknowledgements
This study was supported by a
Grant-in-Aid for Young Scientists (Start-up) from the Japan Society
for the Promotion of Science (23800039) and by a Grant-in-Aid for
Young Scientists from the Yasuda Medical Foundation.
References
|
1.
|
Lapidot T, Sirard C, Vormoor J, et al: A
cell initiating human acute myeloid leukaemia after transplantation
into SCID mice. Nature. 367:645–648. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kim CF, Jackson EL, Woolfenden AE, et al:
Identification of bronchioalveolar stem cells in normal lung and
lung cancer. Cell. 121:823–835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Pardal R, Clarke MF and Morrison SJ:
Applying the principles of stem-cell biology to cancer. Nat Rev
Cancer. 3:895–902. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
6.
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ieta K, Tanaka F, Haraguchi N, et al:
Biological and genetic characteristics of tumor-initiating cells in
colon cancer. Ann Surg Oncol. 15:638–648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Dalerba P, Dylla SJ, Park IK, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Vermeulen L, Todaro M, de Sousa Mello F,
et al: Single-cell cloning of colon cancer stem cells reveals a
multi-lineage differentiation capacity. Proc Natl Acad Sci USA.
105:13427–13432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Huang EH, Hynes MJ, Zhang T, et al:
Aldehyde dehydrogenase 1 is a marker for normal and malignant human
colonic stem cells (SC) and tracks SC overpopulation during colon
tumorigenesis. Cancer Res. 69:3382–3389. 2009. View Article : Google Scholar
|
|
11.
|
Todaro M, Francipane MG, Medema JP and
Stassi G: Colon cancer stem cells: promise of targeted therapy.
Gastroenterology. 138:2151–2162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hermann PC, Huber SL, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Liu R, Wang X, Chen GY, et al: The
prognostic role of a gene signature from tumorigenic breast-cancer
cells. N Engl J Med. 356:217–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Pang R, Law WL, Chu AC, et al: A
subpopulation of CD26+ cancer stem cells with metastatic
capacity in human colorectal cancer. Cell Stem Cell. 6:603–615.
2010.
|
|
15.
|
Cordenonsi M, Zanconato F, Azzolin L, et
al: The Hippo transducer TAZ confers cancer stem cell-related
traits on breast cancer cells. Cell. 147:759–772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kitayama J, Nagawa H, Tsuno N, et al:
Laminin mediates tethering and spreading of colon cancer cells in
physiological shear flow. Br J Cancer. 80:1927–1934. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Daemi N, Thomasset N, Lissitzky JC, et al:
Anti-beta4 integrin antibodies enhance migratory and invasive
abilities of human colon adenocarcinoma cells and their MMP-2
expression. Int J Cancer. 85:850–856. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Enns A, Gassmann P, Schlüter K, Korb T,
Spiegel HU, Senninger N and Haier J: Integrins can directly mediate
metastatic tumor cell adhesion within the liver sinusoids. J
Gastrointest Surg. 8:1049–1059. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Dydensborg AB, Teller IC, Groulx JF, et
al: Integrin alpha6Bbeta4 inhibits colon cancer cell proliferation
and c-Myc activity. BMC Cancer. 9:2232009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Sakthianandeswaren A, Christie M,
D’Andreti C, et al: PHLDA1 expression marks the putative epithelial
stem cells and contributes to intestinal tumorigenesis. Cancer Res.
71:3709–3719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Robertson JH, Yang SY, Winslet MC and
Seifalian AM: Functional blocking of specific integrins inhibit
colonic cancer migration. Clin Exp Metastasis. 26:769–780. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Vanharanta S, Shu W, Brenet F, et al:
Epigenetic expansion of VHL-HIF signal output drives multiorgan
metastasis in renal cancer. Nat Med. 19:50–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Wu B, Chien EY, Mol CD, et al: Structures
of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide
antagonists. Science. 330:1066–1071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Murphy PM: Chemokines and the molecular
basis of cancer metastasis. N Engl J Med. 345:833–835. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Liotta LA: An attractive force in
metastasis. Nature. 410:24–25. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Müller A, Homey B, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001.PubMed/NCBI
|
|
27.
|
Ikushima H, Todo T, Ino Y, Takahashi M,
Miyazawa K and Miyazono K: Autocrine TGF-beta signaling maintains
tumorigenicity of glioma-initiating cells through Sry-related
HMG-box factors. Cell Stem Cell. 5:504–514. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Piccirillo SG, Reynolds BA, Zanetti N, et
al: Bone morphogenetic proteins inhibit the tumorigenic potential
of human brain tumour-initiating cells. Nature. 444:761–765. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Chirasani SR, Sternjak A, Wend P, et al:
Bone morphogenetic protein-7 release from endogenous neural
precursor cells suppresses the tumourigenicity of stem-like
glioblastoma cells. Brain. 133:1961–1972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Lombardo Y, Scopelliti A, Cammareri P, et
al: Bone morphogenetic protein 4 induces differentiation of
colorectal cancer stem cells and increases their response to
chemotherapy in mice. Gastroenterology. 140:297–309. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Haraguchi N, Ohkuma M, Sakashita H, et al:
133+CD44+ population efficiently enriches
colon cancer initiating cells. Ann Surg Oncol. 15:2927–2933.
2008.
|
|
32.
|
Sussman NL, Eliakim R, Rubin D, Perlmutter
DH, DeSchryver-Kecskemeti K and Alpers DH: Intestinal alkaline
phosphatase is secreted bidirectionally from villous enterocytes.
Am J Physiol. 257:G14–G23. 1989.PubMed/NCBI
|
|
33.
|
Augeron C and Laboisse CL: Emergence of
permanently differentiated cell clones in a human colonic cancer
cell line in culture after treatment with sodium butyrate. Cancer
Res. 44:3961–3969. 1984.PubMed/NCBI
|
|
34.
|
Jo M, Eastman BM, Webb DL, Stoletov K,
Klemke R and Gonias SL: Cell signaling by urokinase-type
plasminogen activator receptor induces stem cell-like properties in
breast cancer cells. Cancer Res. 70:8948–8958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Liao CP, Adisetiyo H, Liang M and
Roy-Burman P: Cancer-associated fibroblasts enhance the
gland-forming capability of prostate cancer stem cells. Cancer Res.
70:7294–7303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Lathia JD, Gallagher J, Heddleston JM, et
al: Integrin alpha 6 regulates glioblastoma stem cells. Stem Cell.
6:421–432. 2010.PubMed/NCBI
|