Introduction
Although there has been progress in treatment in the
recent decade, patients with glioblastoma (GBM) still only have a
median survival of 9 months and 5-year survival rate of 9.8%
(1). Exploration of
glioma-initiating cells (GICs) impelled us to recognize the
biological behavior of glioma from a new perspective. GICs are
identified to be the root of tumorigenesis, invasion, angiogenesis
and treatment resistance (2). It
is also regarded as the important target to treat malignant glioma
(3). Further investigation of GICs
in vitro and in vivo may promote the effective
strategies and methods to treat glioma.
The correct GICs sources and the suitable
experimental animal models are necessary for research on GICs in
vivo (4). It has been reported
that GICs could be isolated from human primary glioma samples and
glioma cell lines (5–7). A number of implanted models based on
the injection of GICs into immunodeficient or immunocompetent mice
are being used to investigate GICs in vivo (7–9).
However, whether these implanted models can reflect malignant
biological behavior of GICs accurately is unclear.
Although human primary glioma cells preserve the
majority of glioma characteristics, the heterogeneous mesenchymal
microenvironment and the deficiency of immunological elements may
induce the large discrepancy of biological features between human
primary glioma and xenograft in immunodeficient animal. Murine
malignant glioma cell line GL261 was created by intracranial
injection of 3-methylcholantrene into C57/BL6 mice and widely used
in glioma research (10). In order
to confirm a reliable experimental animal model for research on
GICs, we isolated neurosphere-like tumor cells from GL261
(GL261-NS) and primary human glioma specimens (PGBM-NS), injected
GL261-NS into C57/BL6 mice brain to establish a syngeneic
orthotopically transplanted model and injected PGBM-NS into
NOD/SCID mouse brain to establish a xenograft model. We detected
the different tumorigenic characteristics of GICs and analyzed the
tumor inflammatory microenvironment in the two models.
Materials and methods
Patients and specimens
Tumor tissues were obtained from the Neurosurgery
Department of Daping Hospital. Five patients with primary GBM
underwent curative resection between 2008 and 2009 and samples from
these patients were used for primary glioma cell culture (Table I). The protocol was approved by the
institutional Research Ethics Board at Daping Hospital, Chongqing,
China.
 | Table I.Summary of specimens examined. |
Table I.
Summary of specimens examined.
| Patient no. | Code | Age (years)/
gender | Histology/ WHO
grade | Location | CD133-positive
cells in P-NS (%) | Nestin-positive
cells in P-NS (%) | Olig2-positive
cells in P-NS (%) |
|---|
| 1 | G14 | 52/F | GBM/IV | Temporal L | 59.30 | 71.60 | 54.90 |
| 2 | G95 | 50/F | GBM/IV | Frontoparietal
R | 21.60 | 59.10 | 70.60 |
| 3 | G74 | 44/M | GBM/IV | Occipital R | 73.50 | 77.30 | 92.50 |
| 4 | G20 | 72/M | GBM/IV | Temporal L | 63.30 | 53.30 | 46.70 |
| 5 | G103 | 40/F | GBM/IV | Frontal L | 17.70 | 82.70 | 12.30 |
| Total | | | | | 47.1±25.6 | 68.8±12.3 | 55.4±29.8 |
Cell cultures
GL261 cell line was obtained from American Type
Culture Collection (ATCC, Manassas, VA, USA). GL261-NS were
obtained and cultured as we previously described (11). We cultured GL261 cells and
decreased one half of FBS concentration every 3 days. GL261 cells
were seeded into defined stem cell medium after 6 days. GICs from
human glioma tissues and adhesive glioma cells were isolated and
identified as we previously described (12). The primary tumor cells were
detached with trypsin (Gibco) and suspended in defined stem cell
medium described previously at a density of 1,000 cells/ml. After 7
days, the spheres appeared.
Flow cytometry
Glioma cells were stained with
fluorochrome-conjugated antibodies (Abs) for CD133 (Abcam,
Cambridge, MA, USA), Nestin (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), Olig2 (Santa Cruz), glial fibrillary acidic protein
(GFAP) (Zhongshan Jinqiao Biotech, China), microtubule-associated
protein 2 (MAP2) (Millpore, MA, USA) or myelin basic protein (MBP)
(Santa Cruz) or control Ab (B&D, USA). Data were acquired on a
Gallios flow cytometer (Beckman Coulter, Miami, FL, USA) and
analyzed with FlowJo software.
Immunohistochemisty and
immunofluorescence
Glioma cells were incubated with Abs for CD133
(Abcam), Nestin (Santa Cruz), Olig2 (Santa Cruz), GFAP (Zhongshan
Jinqiao Biotech), MAP2 (Millpore) or MBP (Santa Cruz). Negative
controls were performed by replacing the specific primary Abs with
isotype matched control Abs at the same concentration. The primary
Abs bound on tumor cells were reacted with FITC or Cy3-conjugated
goat anti-rabbit or anti-mouse IgG (Sigma, St. Louis, MO, USA). The
cells were then counterstained with 4′,6-diamidino-2-phenylindole
(DAPI, Sigma) to reveal the nuclei. Immunohistochemistry (IHC) was
performed on paraffin-embedded sections. The tumor sections were
incubated with Abs for CD3 (Beijing Biosynthesis Biotech, China),
CD20 (Santa) or Iba1 (Abcam), followed by detection using a
ChemMate Detection kit (Dako, Glostrup, Denmark). Negative controls
were performed as previously mentioned. A positive reaction was
indicated by brown color using DAB and was counterstained with
hematoxylin.
Western blotting
The tumor tissues were resuspended in lysis buffer
(Pierce, Rockford, IL, USA). After centrifugation, the supernatants
were dissolved in Laemmli sample buffer (Pierce) and heated at 95°C
for 5 min. Equal amounts of cellular proteins were separated by 10%
SDS-PAGE, immunoblotted with Abs for IL-2 (Beijing Biosynthesis
Biotech), IL-4 (Santa Cruz), B7-1 and IL-8 (Santa Cruz) and
visualized with a commercial ECL kit (Pierce). To confirm that each
lane received the same amount of proteins, the blots were stripped
and reprobed with rabbit anti-human/mouse GAPDH Ab (Santa
Cruz).
Colony formation assay and
characterization of resistance to cytotoxic agents
Cultured cells were made into single cell suspension
in defined stem cell medium. Cell density was adjusted into 10
cells/ml. Seeded 100-μl cell suspension in every well of
96-well plate and marked the wells with a single cell. Two weeks
later, neurospheres and small colonies were scored as
colony-forming unit. Cells (5,000/well) were plated in 100 ml per
well in 96-well plates and Nimustine were added. Cell viability was
measured after 72 h with the CellTiter 96 Aqueous Non-radioactive
assay (Promega).
Orthotopic glioma implantation
All procedures involving mice were conducted in
accordance with the Guidelines of Animal Experiments of Third
Military Medical University. Glioma cells were injected
orthotopically into the brains of 6-week-old female C57/BL6 mice or
female NOD/SCID mice (n=5 each group, Center of Experimental
Animals, Third Military Medical University, Chongqing, China). Mice
were maintained for 100 days. The brains of euthanized mice were
collected and fixed in 4% paraformaldehyde for the subsequent
preparation of sections.
Statistical analysis
Data were analyzed with SPSS10.0 statistical
software. When two groups were compared, the unpaired dependent
sample t-test was used. P<0.05 was considered statistically
significant.
Results
Stem cell markers were upregulated in
GL261-NS and PGBM-NS
We seeded GL261 cells and decreased one half of FBS
concentration every 3 days. When the FBS concentration was reduced
to 2.5%, cell clusters at the top of adhesive cells (Fig. 1A) were collected and suspended in
defined stem cell medium. These single cells developed into
neurospheres containing 10–20 cells after 6 days (Fig. 1B). After 2 weeks, the size of these
spheres expanded 10- to 30-fold (Fig.
1C). Immunofluorescence staining showed majority of tumor cells
in neurospheres were positive for CD133, Nestin and Olig2 (Fig. 1D–F). Flow cytometry confirmed that
the percentages of GL261-NS that were positive for GFAP, MAP2 and
MBP were, respectively, 2.1±0.7, 1.7±0.6 and 0.3±0.07% (Fig. 1J–L). However, the percentage of
GL261-NS was positive for CD133, Nestin and Olig2 were 25.3±3.9,
72.5±6.8 and 81.3±5.5%, respectively (Fig. 1G–I). When cultured in medium with
FBS, GL261-NS adhered to the bottom of culture flask. After 5 days,
all the cells in the neurosphere migrated out from the spheres and
differentiated into GL261-AC (Fig. 2A
and B). Immunofluorescence staining confirmed GL261-AC
expressed only slightly stems cell markers (data not shown) and
differentiated into cells that were positive for GFAP, MAP2 and MBP
(Fig. 2C–E). Flow cytometry showed
that the percentage of GL261-AC positive for GFAP, MAP2 and MBP was
76.3±6.5, 64.6±7.7 and 8.3±1.7%, respectively (Fig. 2F). However, the percentage of
GL261-AC positive for CD133, Nestin and Olig2 was 1.3±0.4, 27.1±5.6
and 3.9±0.8% respectively (Fig.
2G). We also isolated PGBM-NS from five GBM multiform patients
(Table I). The PGBM-NS expressed
CD133 (47.1±25.6%), Nestin (68.8±12.3%) and Olig2 (55.4±29.8%). It
is noteworthy that the percentage of PGBM-NS positive for stem cell
markers largely varied among individuals (Fig. 2J).
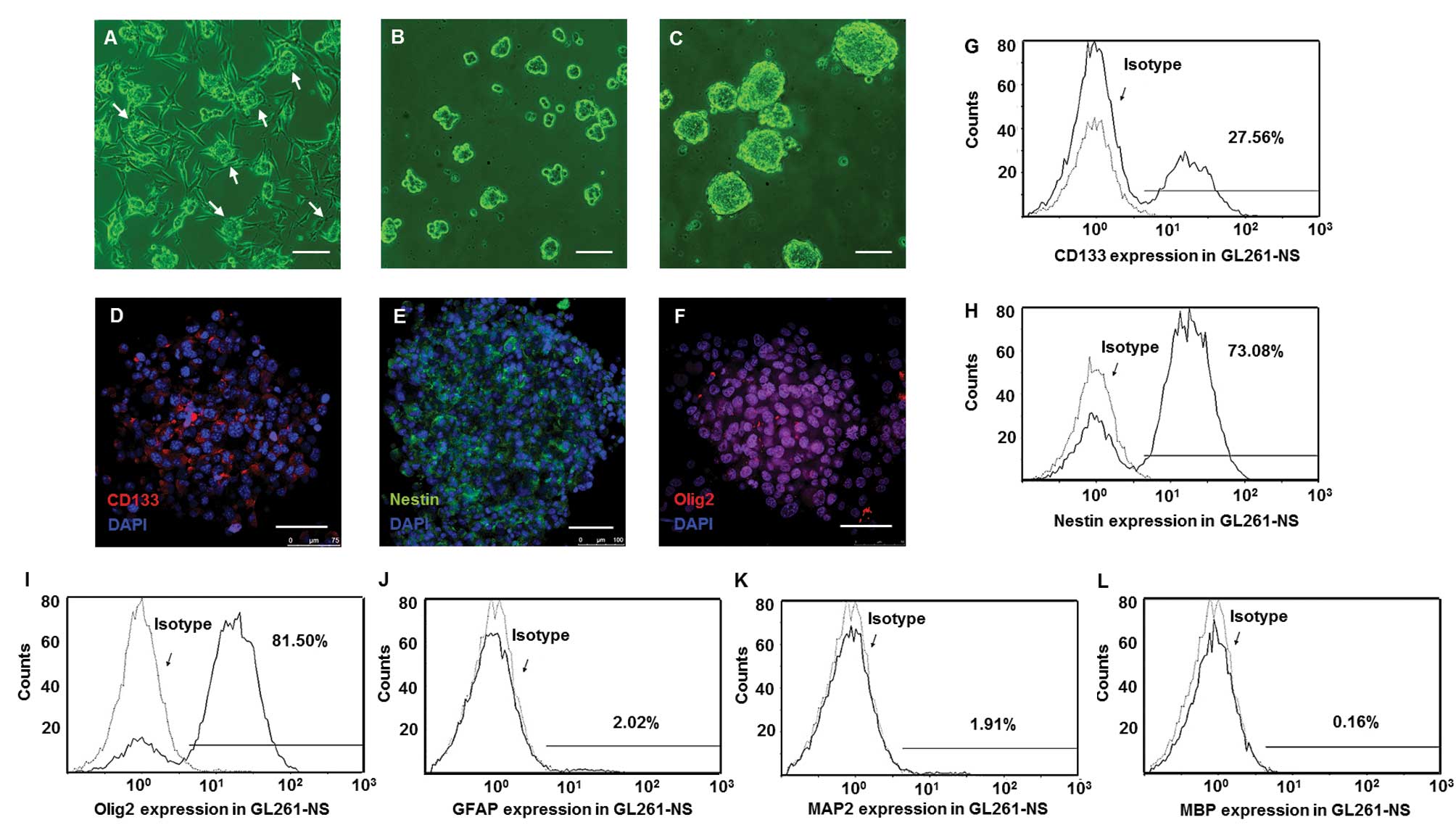 | Figure 1.GICs were enriched in GL261-NS and
expressed stem cell markers. The clusters (arrows) of GL261 cells
appeared in the medium with low concentration of FBS (A),
neurospheres (GL261-NS) derived from the clusters and expanded in
defined stem cell medium (B and C). Immunofluorescence of stem cell
makers CD133 (D, red), Nestin (E, green) and Olig2 (F, red). Flow
cytometry analysis showed the percentage of GL261-NS expressing
CD133, Nestin and Olig2 (G-I), the percentage of GL261-AC
expressing GFAP, MAP2 and MBP (J-L). (A-C) Phase contrast
microscopy; scale bar, 200 μm. (D-F) Merged images,
counterstained with DAPI showing nuclei in blue. Laser confocal
scanning microscopy; scale bar, 100 μm. |
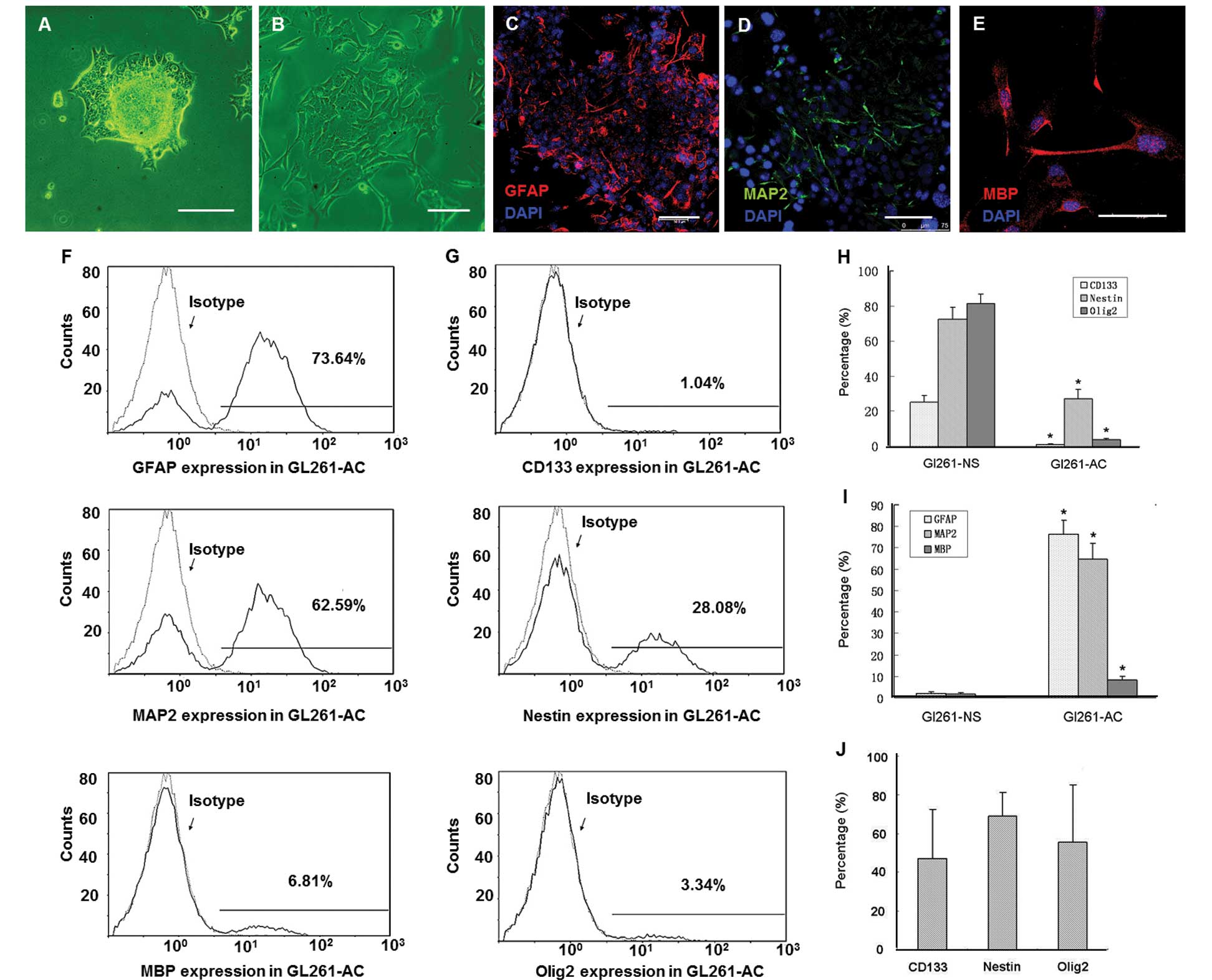 | Figure 2.GL261-NS possesses multi-lineage
differentiation potential. GL261-AC spilled out from the edges of
tumorspheres at 24 h after plating in DMEM/ F12 containing 10% FBS
(A). The cells in the neurosphere migrated from the spheres and
differentiated into GL261-AC in 5 days (B). Immunofluorescence of
differentiation makers GFAP (C, red), MAP2 (D, green) and MBP (E,
red). Flow cytometry analysis showed the percentage of GL261-AC
expressing GFAP, MAP2 and MBP (F and I), the percentage of GL261-AC
expressing CD133, Nestin and Olig2 (G and H). The percentage of
human primary neurosphere-like glioma cells expressing CD133,
Nestin and Olig2 (J). (A and B) Phase contrast microscopy; scale
bar, 100 μm. (C–E) Merged images, counterstained with DAPI
showing nuclei in blue. Laser confocal scanning microscopy; scale
bar, 75 μm. |
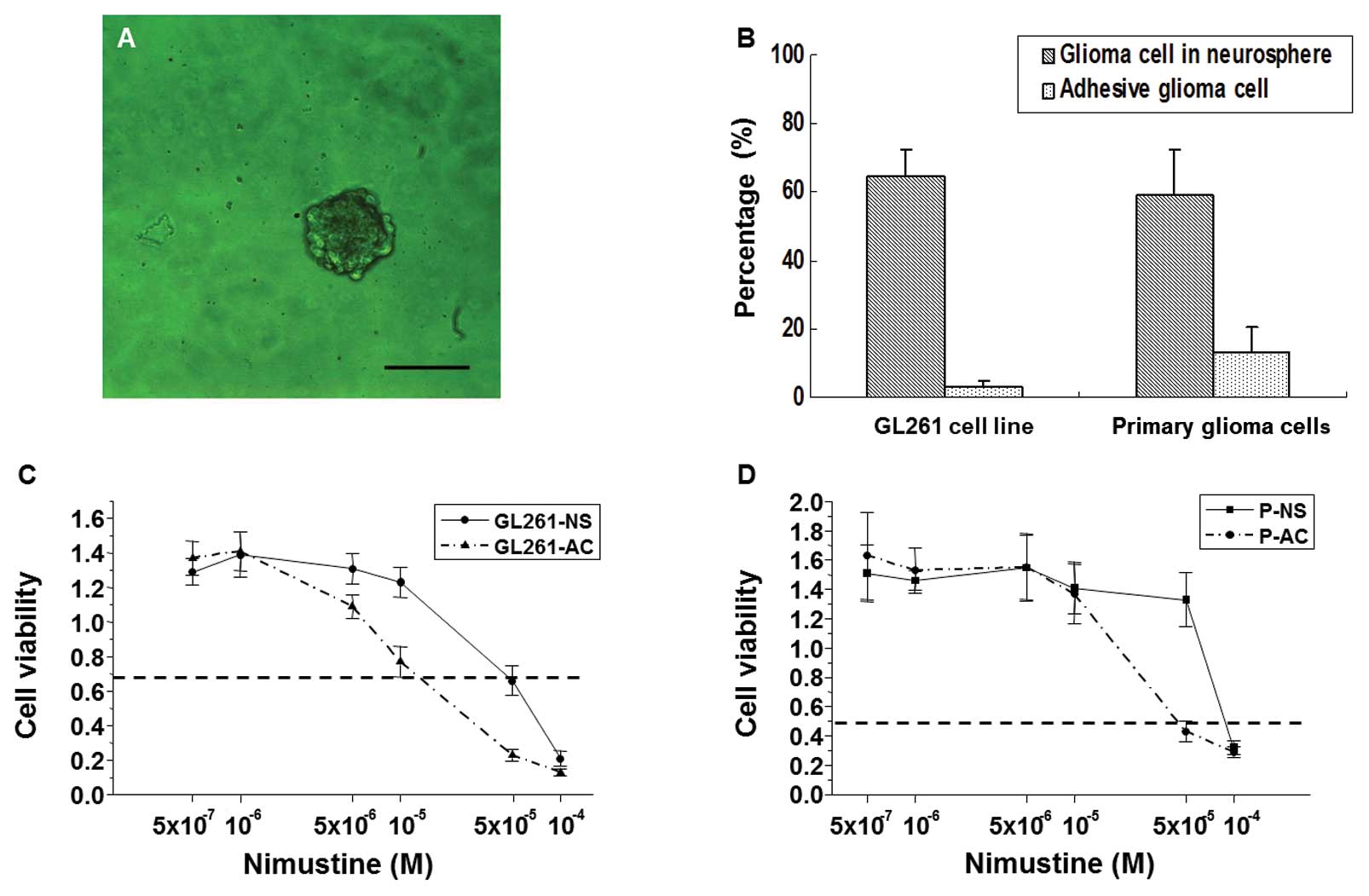
GL261-NS and PGBM-NS exhibit increased
self-renewal potential and chemoresistance
The ability of colony formation in define stem cell
medium represented the self-renewal potential (13), we seeded GL261-NS and GL261-AC in
suspension cultures, GL261-NS showed an ~21-fold increase in
tumorsphere-forming capacity compared to GL261-AC (64.7±7.7 versus
3.3±1.5 spheres per 100 cells; Fig. 3A
and B). Although PGBM-NS also possessed a self-renewal
potential as strong as GL261-NS (59.0±13.0 spheres per 100 cells),
PGBM-AC showed a higher percentage of colony-forming units
(14.6±5.2 spheres per 100 cells) than GL261-AC. So, PGBM-NS showed
only an ~4-fold increase in tumorsphere-forming capacity compared
to PGBM-AC (Fig. 3B). It has been
reported that drug treatment of glioma cell populations induces
enrichment of GICs (8,14). We found that GL261-NS and PGBM-NS
were both more resistant than GL261-AC and PGBM-AC to nimustine,
the commonly used chemotherapeutic drugs. Primary glioma cells
possessed stronger resistance to nimustine than GL261 cells.
GL261-NS showed ~30-fold increase in IC50 of nimustine
compared with GL261-AC, whereas PGBM-NS showed only 3-fold increase
in IC50 of nimustine compared with PGBM-AC (Fig. 3C and D).
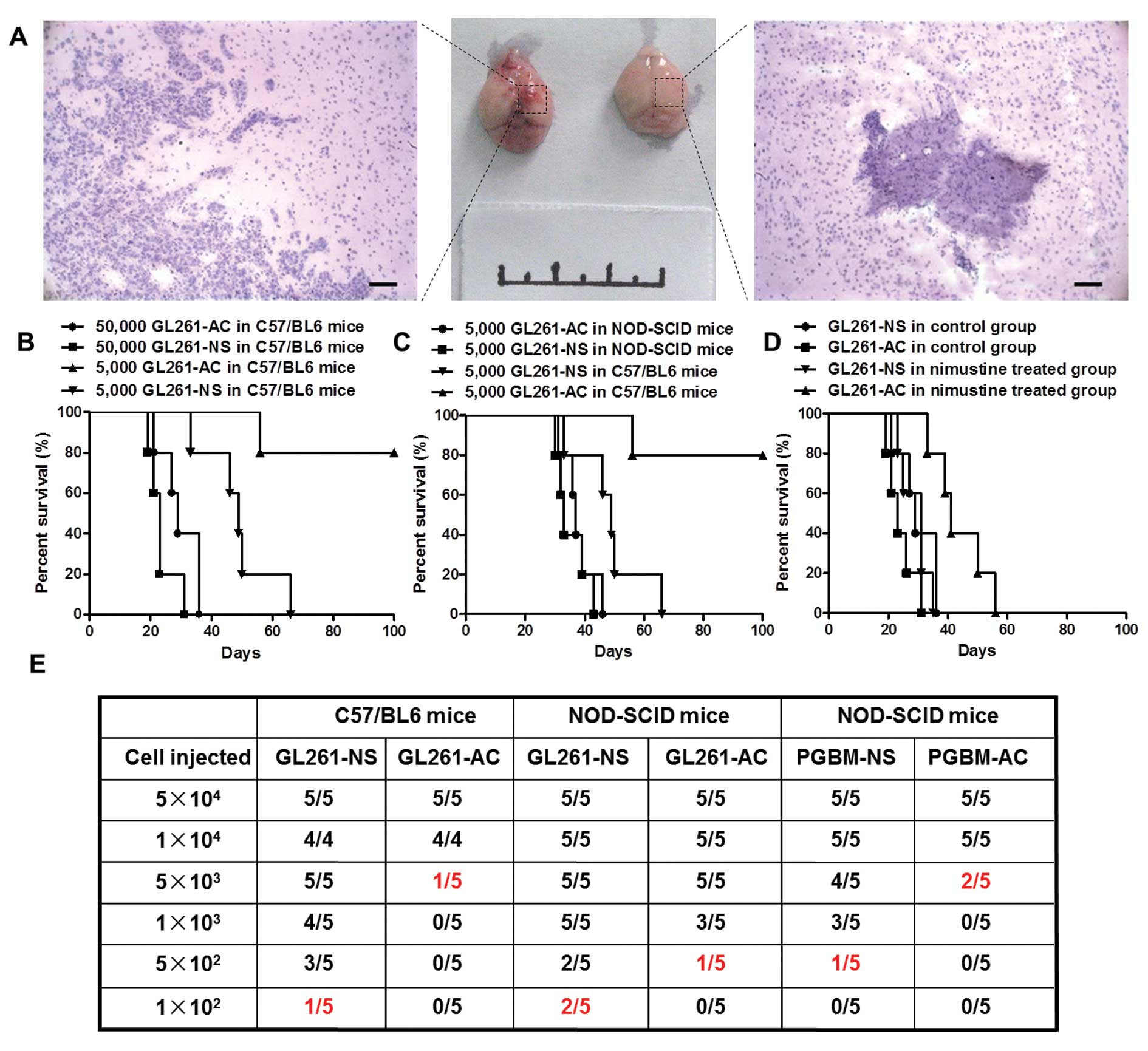
Neurosphere-like glioma cells show more
enhanced tumorigenicity in syngeneic graft model than in
heterogeneous immunodeficiency model
Many studies have reported that PGBM-NS showed
enhanced tumorigenicity in immunodeficient mice (15–18).
We postulated that this model might not be able to reveal the
biological feature of GICs accurately because of immunodeficiency
and individual differences among patients. To test this hypothesis,
we assessed the functional potential of GICs by detecting their
in vivo tumor-seeding ability. We confirmed that GL261-NS
possessed stronger tumorigenicity than GL261-AC in C57/BL6 mice.
Hemorrhage and necrosis appeared at the inoculation sites in the
5×103 GL261-NS injection group. However, there is no
abnormality in the inoculation sites of GL261-AC injection group
(Fig. 4A). The xenografts derived
from GL261-NS showed the typical features of human GBM, obvious
cellular atypia, high density of micro-vessels and numerous
abnormal mitotic figures. The margins between tumor and normal
brain tissue were unclear, showing intraparenchymal invasion
pattern of the tumors. In contrast, tumors formed by GL261-AC
rarely showed histological characteristics of malignant glioma
(Fig. 4A). Kaplan-Meier survival
analysis confirmed that GL261-NS is significantly more aggressive
than GL261-AC in C57/BL6 mice. The median survival of mice injected
with 5×103 GL261-NS was 48.8±11.8 days, whereas only one
mouse injected with 5×103 GL261-AC was dead at 56 days
after inoculation, the other four mice were all alive at 100 days
(P<0.01). However, we did not find significant difference in
survival time between 5×104 GL261-NS group (23.4±4.6
days) and 5×104 GL261-AC group (29.8±6.4 days, P= 0.105)
(Fig. 4B). Unexpectedly, GL261-NS
did not show enhanced tumorigenicity in immunodeficient mice, there
was no significant difference in survival time of NOD/SCID mice
between 5×103 GL261-NS group (35.4±5.4 days) and
5×103 GL261-AC group (37.8±5.5 days, P=0.505), the
survival time of NOD/SCID mice was shorter than C57/BL6 mice when
injected with the equal number of tumor cells (Fig. 4C). We inoculated orthotopically
3×104 GL261-AC and 3×104 GL261-NS
respectively into C57/BL6 mice, and treated these mice with
nimustine (5 mg/kg) or vehicle after 7 days of inoculation with
daily administration. Nimustine-treated animals seeded with
GL261-AC showed an obvious trend toward better prognosis compared
to vehicle-treated animals, the survival time was from 29.8±6.4 to
43.8±9.1 days. But the treatment of nimustine had less impact on
survival in animals injected with GL261-NS, which indicated
GL261-NS possessed increased drug resistance relative to GL261-AC
in vivo (Fig. 4D).
Tumor-seeding assay with different cellular amounts indicated that
tumors could be generated with 1×102 GL261-NS in C57/
BL6 mice, which was 50-fold less than the cellular number of
GL261-AC that was required for tumor survival. However, the
discrepancy of tumor-seeding ability between GL261-NS and GL261-AC
did not appear in NOD/SCID mice. The amount of PGBM-NS that was
needed to generate a tumor was ~10-fold less than that of PGBM-AC
in NOD/SCID mice (Fig. 4E).
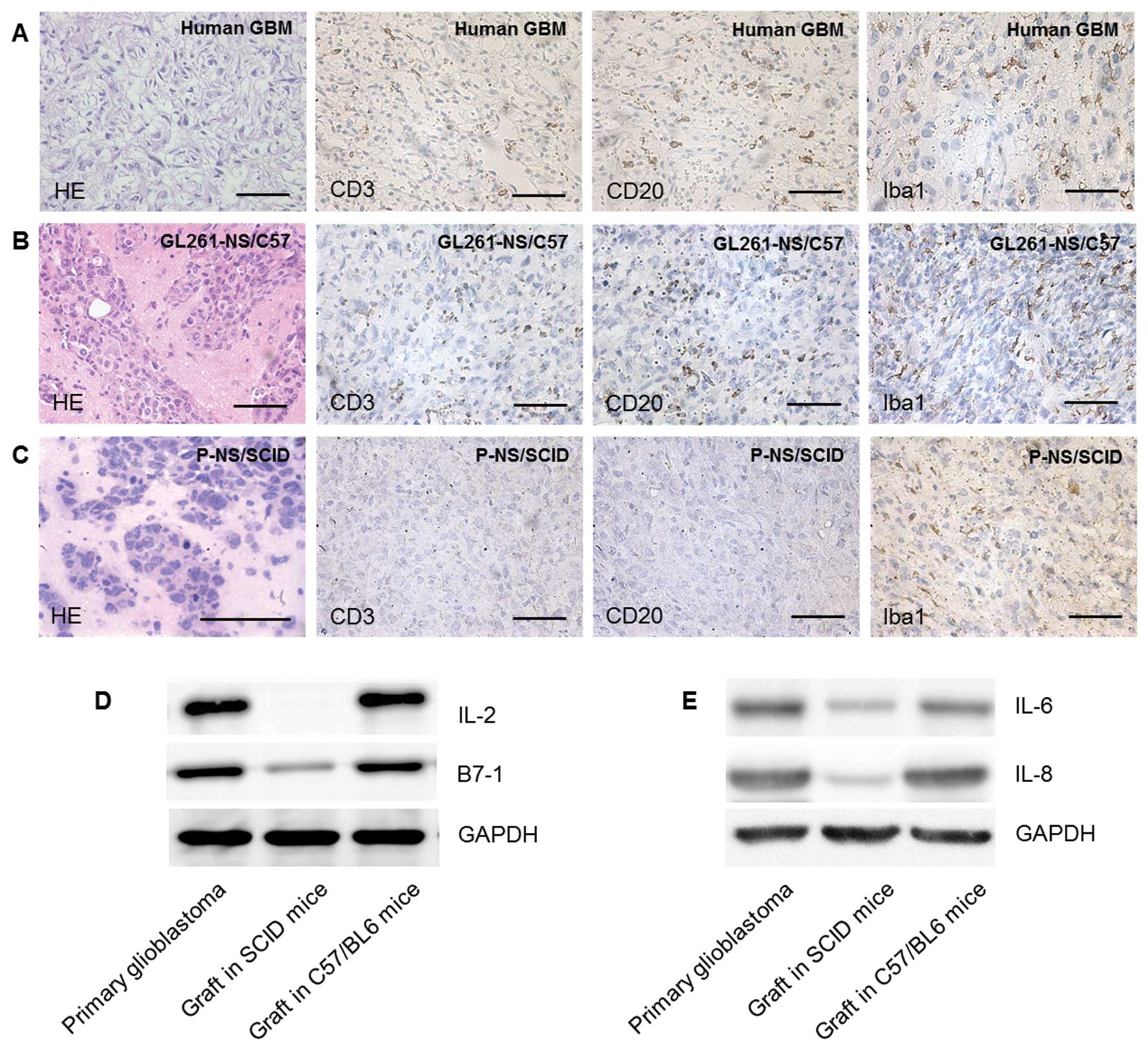
Glioma derived from GL261-NS in C57/BL6
mimicked the primary human glioma histologically better than glioma
derived from PGBM-NS in NOD/SCID mice
Grafts formed by GL261-NS and PGBM-NS both grew
rapidly with pleomorphism and high density of microvessels, which
are typically seen in human GBM multiforme (Fig. 5A–C). Immunocytes and
immunomediators that are indispensable components of the neoplastic
microenvironment can modulate the biological behavior of
malignances (19–21). IHC showed various immunocytes,
including macrophage, DC, B cell and T cell, infiltrated into the
tumor region of primary GBM (22–24).
Similar composition and percentage of infiltrating immunocytes
could be detected in grafts formed by GL261-NS in C57/ BL6 mice,
but not in grafts formed by human PGBM-NS in immunodeficient mice
(Fig. 5A–C). Similarly, many kinds
of immunomediators, such as IL-2, IL-4, B7-1, IL-8, which play
important roles in human primary glioma immunity were absent or
reduced in grafts formed by human PGBM-NS in immunodeficient mice
(Fig. 5D and E). Because of the
lack of impacts from immune system, the orthotopic implantation of
human PGBM-NS in immunodeficient mice may not be a reliable model
to evaluate the biological characteristics of GICs in vivo,
the syngeneic glioma implantation model made by GL261-NS in C57/BL6
mice was more desirable.
Discussion
The high incidence, high mortality and limited
advances in glioma treatment impelled us to inspect the methodology
and technology of glioma research (25). GICs have been recognized as the
insurmountable obstacle for glioma curability (26–28).
The experience from the normal stem cell research indicates the
stem cell niche is essential for regulating the biological behavior
of stem cell, the stem cells which break away from the niche can
not maintain stemness and original characteristics (29). Recent studies confirmed
inflammatory cells are the indispensable components of the tumor
stem cell niche, inflammatory cells and inflammatory mediators
regulate the malignant behavior of tumor stem cells (30,31).
However, the conventional model for in vivo assay is
engrafting GICs into immunodeficient animals. It is unclear whether
the research model lacking regulation of immune system can reflect
the malignant behavior of GICs in vivo. We found that GICs
were enriched in GL261-NS which showed potential for self-renewal
and multi-directional differentiation. GL261-NS expressed neural
stem cell markers and possessed stronger chemoresistance than
GL261-AC in vitro and in vivo. The differences of
tumor-seeding ability between GICs and adhesive glioma cells were
shown more adequately in the syngeneic graft model, compared with
the heterogenic immunodeficient model. Inflammatory components of
glioma in C57/BL6 mice mimicked primary GBM much more closely than
the components in NOD/SCID mice.
Animal models of glioma can be divided into three
categories: the induced spontaneous tumor model, the engrafted
tumor model and the genetically engineered tumor model (4). The spontaneous glioma model can be
induced by chemical reagents or oncogenic virus, the course of
progress and histological phenotype of spontaneous glioma model are
similar to primary human glioma. However, the ambiguous genetic
background and prolonged incubation limit its application. The
genetically engineered tumor model is created by transgenic
technology allowing the investigator to activate or turn off
certain specific genes and produce the animal model with specific
genetic pattern of primary human glioma (32,33).
However, there are still great differences in pathogenesis between
the genetically engineered model and human tumor (4). Based on the good repeatability and
the high penetrance, the engrafted model is still the most wildly
used model for evaluating new therapeutic concepts for glioma
(34). The engrafted models are
the xenograft model and the syngraft model. The failure of existing
therapies necessitates investigation of new therapeutic strategies
for glioma. Among these promising strategies are the activation of
the specific immune response to GICs or reversing the
immunosuppressive GIC-niche (35,36).
However, the first challenge is that animal models of primary GICs
faithfully reflect the interaction between GICs and the host’s
immune system. The pan-immune deficiency in the NOD/SCID or athymic
mice obviously restricts the application of the xenograft models. A
more reliable animal model for research on GICs is needed.
The GL261 originates from the brain of C57/BL6 mice,
and is cultured by serial syngeneic transplantation of tumor pieces
(37). The genetic mutation
pattern of GL261 is clear. GL261 is a moderate immunogenic tumor
cell line with low level of MHC class I molecules, absent MHC class
II molecules and moderate level of costimulatory molecules
(38). Approximately 30% of GL261
cells expressed the neural stem cell marker Nestin and could be
cultured into the suspension neurosphere-like cells (GL261-NS) in
stem cell defined medium. Similar to human primary glioma cells,
GL261-NS has a relatively high fraction of CD133+ glioma
cells, which are candidate GICs. This cell population has been
reported to be low immunogenic, thus the tumor derived from GICs
may model the human condition and recapitulate the immune status of
glioma more reliably (39). Tumor
immunoediting plays crucial roles in the process of tumorigenesis
and progression (40). The immune
pressure eliminates nascent tumor cells and results in tumor
progression with reduced immunogenicity. Immune system not only
eradicates tumor cells, but also shapes the malignant disease and
screens out the subclone glioma cell that can gain growth
advantage. The xenografts, which derived by implanting PGBM-NS into
immunodeficient animal, may ignore the immune shaping process and
can not mimic the progression of primary human glioma objectively.
The innate and specific immune responses in the syngeneic model
make this model an improvement over xenograft models to reflect the
interaction between GICs and immune system and to investigate
promising immunotherapy on GICs. Our model confirmed that the
incubation period of GL261-NS glioma in C57/BL6 mice is longer than
that in NOD/SCID mice (data not shown). GL261-AC could survive and
form a tumor in immunodeficient mice, but the same amount of
GL261-AC could not grow into a tumor in immunocompetent mice. The
prognosis of immunodeficient mice bearing glioma derived by
GL261-NS was much worse than that of immunocompetent mice. These
results suggested that the immune system carried out a selective
pressure on GICs in the process of tumorigenesis and
progression.
In conclusion, implantation of GL261-NS into C57/BL6
mice is a reliable syngeneic graft model since it is indeed a
highly reproducible and easy-to-establish model system that can
reflect the interplay of GICs and immune components. However, we
should still pay attention to the immunologic changes induced by
the implantation itself. The discrepancies in neoplastic mechanism
and progression between mouse implantation model and human GBM
always exist. Moreover, human gliomas could have different genetic
mutation patterns that induce various malignant biologic behavior,
we do not imagine modeling the diverse primary gliomas with single
one kind of animal model. The above data urge us to modify our
animal glioma model further and create different kinds of animal
models with specific genetic mutations in order to mimic the human
malignant glioma more accurately.
Acknowledgements
This study was supported by the
National Natural Science Foundations of China (NSFC, nos. 30901538
and 81270039) and the medical research from Chongqing Municipal
Health Bureau (no. 2011-2-427).
References
|
1.
|
Stupp R, Hegi ME, Mason WP, et al: Effects
of radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009.
|
|
2.
|
Stiles CD and Rowitch DH: Glioma stem
cells: a midterm exam. Neuron. 58:832–846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: an emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sughrue ME, Yang I, Kane AJ, et al:
Immunological considerations of modern animal models of malignant
primary brain tumors. J Transl Med. 7:842009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wei J, Barr J, Kong LY, et al:
Glioblastoma cancer-initiating cells inhibit T-cell proliferation
and effector responses by the signal transducers and activators of
transcription 3 pathway. Mol Cancer Ther. 9:67–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Dziurzynski K, Wei J, Qiao W, et al:
Glioma-associated cytomegalovirus mediates subversion of the
monocyte lineage to a tumor propagating phenotype. Clin Cancer Res.
17:4642–4649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Pellegatta S, Poliani PL, Corno D, et al:
Neurospheres enriched in cancer stem-like cells are highly
effective in eliciting a dendritic cell-mediated immune response
against malignant gliomas. Cancer Res. 66:10247–10252. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yu SC, Ping YF, Yi L, et al: Isolation and
characterization of cancer stem cells from a human glioblastoma
cell line U87. Cancer Lett. 265:124–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Bao S, Wu Q, Sathornsumetee S, et al: Stem
cell-like glioma cells promote tumor angiogenesis through vascular
endothelial growth factor. Cancer Res. 66:7843–7848. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Maes W and Van Gool SW: Experimental
immunotherapy for malignant glioma: lessons from two decades of
research in the GL261 model. Cancer Immunol Immunother. 60:153–160.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yi L, Xiao H, Xu M, et al:
Glioma-initiating cells: a predominant role in
microglia/macrophages tropism to glioma. J Neuroimmunol. 232:75–82.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yi L, Zhou ZH, Ping YF, et al: Isolation
and characterization of stem cell-like precursor cells from primary
human anaplastic oligoastrocytoma. Mod Pathol. 20:1061–1068. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yang S, Wang B, Guan C, et al:
Foxp3+IL-17+ T cells promote development of
cancer-initiating cells in colorectal cancer. J Leukoc Biol.
89:85–91. 2011.
|
|
14.
|
Beier D, Schulz JB and Beier CP:
Chemoresistance of glioblastoma cancer stem cells - much more
complex than expected. Mol Cancer. 10:1282011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Galli R, Binda E, Orfanelli U, et al:
Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Calabrese C, Poppleton H, Kocak M, et al:
A perivascular niche for brain tumor stem cells. Cancer Cell.
11:69–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Krishnamurthy S, Dong Z, Vodopyanov D, et
al: Endothelial cell-initiated signaling promotes the survival and
self-renewal of cancer stem cells. Cancer Res. 70:9969–9978. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Li Z, Bao S, Wu Q, et al:
Hypoxia-inducible factors regulate tumorigenic capacity of glioma
stem cells. Cancer Cell. 15:501–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar
|
|
20.
|
Bayne LJ, Beatty GL, Jhala N, et al:
Tumor-derived granulocyte-macrophage colony-stimulating factor
regulates myeloid inflammation and T cell immunity in pancreatic
cancer. Cancer Cell. 21:822–835. 2012. View Article : Google Scholar
|
|
21.
|
Charles NA, Holland EC, Gilbertson R,
Glass R and Kettenmann H: The brain tumor microenvironment. Glia.
59:1169–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hussain SF, Yang D, Suki D, Aldape K,
Grimm E and Heimberger AB: The role of human glioma-infiltrating
microglia/ macrophages in mediating antitumor immune responses.
Neurooncol. 8:261–279. 2006.PubMed/NCBI
|
|
23.
|
Wiencke JK, Accomando WP, Zheng S, et al:
Epigenetic biomarkers of T-cells in human glioma. Epigenetics.
7:1391–1402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Hong TM, Teng LJ, Shun CT, Peng MC and
Tsai JC: Induced interleukin-8 expression in gliomas by
tumor-associated macrophages. J Neurooncol. 93:289–301. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Guryanova OA, Wu QL, Cheng L, et al:
Non-receptor tyrosine kinase BMX maintains self-renewal and
tumorigenic potential of glioblastoma stem cells by activating
STAT3. Cancer Cell. 19:498–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Lamszus K and Gunther HS: Glioma stem
cells as a target for treatment. Target Oncol. 5:211–215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Chen J, McKay RM and Parada LF: Malignant
glioma: lessons from genomics, mouse models, and stem cells. Cell.
149:36–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Li Z, Wang H, Eyler CE, Hjelmeland AB and
Rich JN: Turning cancer stem cells inside out: an exploration of
glioma stem cell signaling pathways. J Biol Chem. 284:16705–16709.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Wagers AJ: The stem cell niche in
regenerative medicine. Cell Stem Cell. 10:362–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Ginestier C, Liu S, Diebel ME, et al:
CXCR1 blockade selectively targets human breast cancer stem cells
in vitro and in xenografts. J Clin Invest. 120:485–497. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Sansone P, Storci G, Tavolari S, et al:
IL-6 triggers malignant features in mammospheres from human ductal
breast carcinoma and normal mammary gland. J Clin Invest.
117:3988–4002. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Weiss WA, Burns MJ, Hackett C, et al:
Genetic determinants of malignancy in a mouse model for
oligodendroglioma. Cancer Res. 63:1589–1595. 2003.PubMed/NCBI
|
|
33.
|
Hu X, Pandolfi PP, Li Y, Koutcher JA,
Rosenblum M and Holland EC: mTOR promotes survival and astrocytic
characteristics induced by Pten/AKT signaling in glioblastoma.
Neoplasia. 7:356–368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Szatmari T, Lumniczky K, Desaknai S, et
al: Detailed characterization of the mouse glioma 261 tumor model
for experimental glioblastoma therapy. Cancer Sci. 97:546–553.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Hatiboglu MA, Wei J, Wu AS and Heimberger
AB: Immune therapeutic targeting of glioma cancer stem cells.
Target Oncol. 5:217–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Wei J, Barr J, Kong LY, et al:
Glioma-associated cancer-initiating cells induce immunosuppression.
Clin Cancer Res. 16:461–473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Ausman JI, Shapiro WR and Rall DP: Studies
on the chemotherapy of experimental brain tumors: development of an
experimental model. Cancer Res. 30:2394–2400. 1970.PubMed/NCBI
|
|
38.
|
Daga A, Orengo AM, Gangemi RM, et al:
Glioma immuno-therapy by IL-21 gene-modified cells or by
recombinant IL-21 involves antibody responses. Int J Cancer.
121:1756–1763. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Abdouh M, Facchino S, Chatoo W, Balasingam
V, Ferreira J and Bernier G: BMI1 sustains human glioblastoma
multiforme stem cell renewal. J Neurosci. 29:8884–8896. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Kim R, Emi M and Tanabe K: Cancer
immunoediting from immune surveillance to immune escape.
Immunology. 121:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|