Introduction
Living organisms are exposed daily to microbial
infections and pathogens. In order to defend themselves against
such infectious agents, they have developed potent defensive
mechanism, i.e., innate and adaptive immunity. In innate immunity,
antimicrobial peptides (AMPs) that possess potent antibiotic
activity against bacteria, fungi and even certain viruses play
important roles in the host defense mechanisms of most living
organisms including plants, insects, amphibians and mammals
(1–3).
Insect AMPs are cationic and amphipathic. Although
insect AMPs display variable length, sequences and structures, most
AMPs have relatively small (<5 kDa) molecular masses (4,5). In
case of insect defensins, that was first isolated from the culture
medium of an embryonic cell line of the flesh fly, Sarcophaga
peregrine (6), are members of
a widely distributed family of AMPs. Insect defensin contains six
conserved cysteine residues engaged in three intradisulfide bonds
(4,5) and have antimicrobial activity against
Gram-positive bacteria and fungi (5,7).
Interestingly, several insect AMPs show cytotoxic
effects against a broad range of cancer cell lines such as mouse
myeloma, melanoma, lymphomas, leukemia, breast cancer and lung
cancer (8–12). Coprisin belongs to the defensin
family of insect AMPs, and has been identified from dung beetle,
Copris tripartitus (13)
and its analogue CopA3 showing cytotoxicity against cancer cell
lines as well as strong antibacterial activity against microbes
(14–16).
Previously, we characterized the antibacterial
activity of the synthetic analogue of harmoniasin, HaA4 that was
identified from the ladybug, Harmonia axyridis. Active
region of harmoniasin was defined and selected to be modified as a
homodimeric peptide. HaA4 displayed more potent antibacterial
activity than that of the native peptide (17). HaA4 might also retain cytotoxic
effect on cancer cells similarly to some other AMPs. Therefore, we
investigated the anticancer activity of the HaA4 peptide against
two human leukemia cell types in the present study and report that
the anticancer effect of HaA4 is caused by necrosis and
apoptosis.
Materials and methods
Peptide synthesis
Harmoniasin is a defensin-like peptide consisting of
50 amino acid residues with three intra-disulfide bonds. Because of
the large molecular weight and disulfide bonds, we designed a
variety of analogues based on the harmoniasin sequence in a
previous study (17). The
resulting homodimer peptide, named HaA4, was synthesized and
provided by Anygen Co., Ltd. (Gwangju, Korea).
Cell culture
Raw 264.7, Jurkat and U937 cells were maintained in
DMEM and RPMI-1640 medium containing 10% FBS, penicillin G (100
U/ml) and streptomycin (100 μg/ml) (Invitrogen, Carlsbad,
CA, USA), respectively. Cells were cultured at 37°C in a humidified
incubator with 5% CO2.
Cell viability assay
Cells were plated into 96-well tissue culture plates
(2×104 cells/well) and treated with various
concentrations (50, 100, 150 and 200 μg/ml) of HaA4 or
without HaA4. After incubation for 24 h, viability of the cancer
cells was measured using the CellTiter 96 AQueous One Solution Cell
Proliferation Assay according to the manufacturer's protocol
(Promega, Madison, WI, USA). Optical density was measured at 490 nm
with a microplate reader (Beckman DTX 8800 multi detector).
Reversal of viability reduction by HaA4 was attempted by treatment
with Z-VAD-FMK (Promega), a broad-spectrum caspase inhibitor at
indicated concentration.
LDH release assay
Cell membrane integrity was analyzed by measuring
LDH activity. LDH activity was measured using a Cytotoxicity
Detection kit (Roche Applied Science). In brief, the cells were
seeded at 1×104 cells/well into a 96-well tissue culture
plate in assay medium (RPMI-1640 containing 1% FBS). The cells were
treated with different doses of HaA4. After 24 h of incubation, 5
μl of lysis solution was added to high control samples as a
positive control and the plate incubated for an additional 15 min.
Then, 100 μl reaction mixture was added to each well on the
96-well plate and incubated for 15 min. Finally, 50 μl stop
solution was added to each well on the plate and the absorbance at
490 nm was measured using a microplate reader. The percent
cytotoxicity was calculated by the following equation: Cytotoxicity
(%) = (exp. value − low control)/(high control − low control) ×
100.
Annexin V/propidium iodide (PI)
staining
Jurkat and U937 cells were plated into 6-well tissue
culture plates (1×106 cells/well) and treated with
various concentrations (50, 100, 150 and 200 μg/ml) of HaA4
or without HaA4. After incubation for 4 h, cells were harvested and
washed twice with cold PBS and once with 1X binding buffer (0.01 M
HEPES/NaOH (pH 7.4), 0.14 M NaCl, 2.5 mM CaCl2). Cells
were prepared in 100 μl of the binding buffer
(1×105 cells) and then, added with 5 μl of FITC
Annexin V and PI. The cells were gently mixed by vortex and
incubated for 15 min at room temperature in the dark. After the
incubation, 400 μl of 1X binding buffer was added to each
tube. Stained cells were measured by flow cytometry with a BD
FACSCalibur cytometer (BD Biosciences) and CellQuest software (BD
Biosciences) was used for analysis of the results.
Acridine orange/ethidium bromide
staining
Cells were seeded in 6-well tissue culture plates
(1×106 cells/well), treated without or with HaA4 (50,
100 and 150 μg/ml) for 24 h and the cells were washed with
PBS. Then, the cells were stained with mixture of acridine orange
(3 μg/ml) and ethidium bromide (10 μg/ml) and
observed immediately using AxioImager Z1 fluorescence microscope
(Carl Zeiss, Germany).
DNA fragmentation assay
For the DNA fragmentation assay, 2×106
cells were seeded into 6-well plates and treated with 200
μg/ml HaA4 or without HaA4 for 24 h. Cells were collected,
washed once with PBS, lysed in a solution containing 10 mM Tris-HCl
(pH 7.4), 10 mM EDTA (pH 8.0) and 0.5% Triton X-100 on ice for 30
min and then centrifuged at 15,000 rpm for 5 min. The supernatant
was digested with 0.1 mg of RNase A/ml and 1 mg of proteinase K/ml
for 1 h at 55°C in the presence of 1% sodium dodecyl sulfate (SDS).
DNA was extracted from the digested supernatant with phenol and
chloroform, precipitated in cold ethanol and subjected to
electrophoresis on 2% agarose gels containing ethidium bromide. DNA
fragments were visualized by UV light.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL assay)
Jurkat and U937 cells were plated into 6-well plates
(2×106/ml) and treated with or without HaA4 (200
μg/ml) for 24 h. TUNEL assay was performed with DeadEnd™
Fluorometric TUNEL system according to the manufacturer's
instructions (Promega) to determine apoptotic cells.
Immunoblot analysis
Cells were washed with cold PBS and lysed in buffer
[150 mM NaCl, 50 mM Tris-HCl (pH 8.0), 5 mM EDTA and 1% Nonidet
P-40]. Equal amount of protein was separated by SDS-polyacrylamide
gel electrophoresis (12% SDS-PAGE) and transferred onto a
nitrocellulose membrane. The antigen-antibody complexes were
detected using FluorChem (Alpha Innotech, USA). Polyclonal
antibodies against caspase-7, -9, PARP and AIF were obtained from
Cell Signaling Technology (MA, USA). The β-actin antibody was
purchased from Sigma-Aldrich (St. Louis, MO, USA) and the
broad-spectrum caspase inhibitor, Z-VAD-FMK, was obtained from
Promega.
Results and Discussion
The peptide
The primary amino acid sequence of the synthetic
harmoniasin analogues are shown in Table I. HaA4 peptide was used in this
study. Dimerized peptides by disulfide bond such as magainin 2 and
melittin analogues showed stronger antimicrobial activity than the
monomeric forms (18,19). Moreover, halocidin dimer congeners
derived from halocidin, a dimeric α-helical structure peptide that
was purified from the tunicate Halocynthia aurantium showed
more potent antibacterial activity than the its monomer forms
(20). Therefore, dimerization of
the AMPs is suggested to potentiate their biological activity in an
undefined way.
 | Table I.Sequence of harmoniasin
analogues. |
Table I.
Sequence of harmoniasin
analogues.
| Peptide | Amino acid
sequence | Mass (Da)
|
|---|
| Measured | Theoretical |
|---|
| HaNP |  | 2134.8 | 2134.4 |
| HaA4 |  | 2330.2 | 2330.8 |
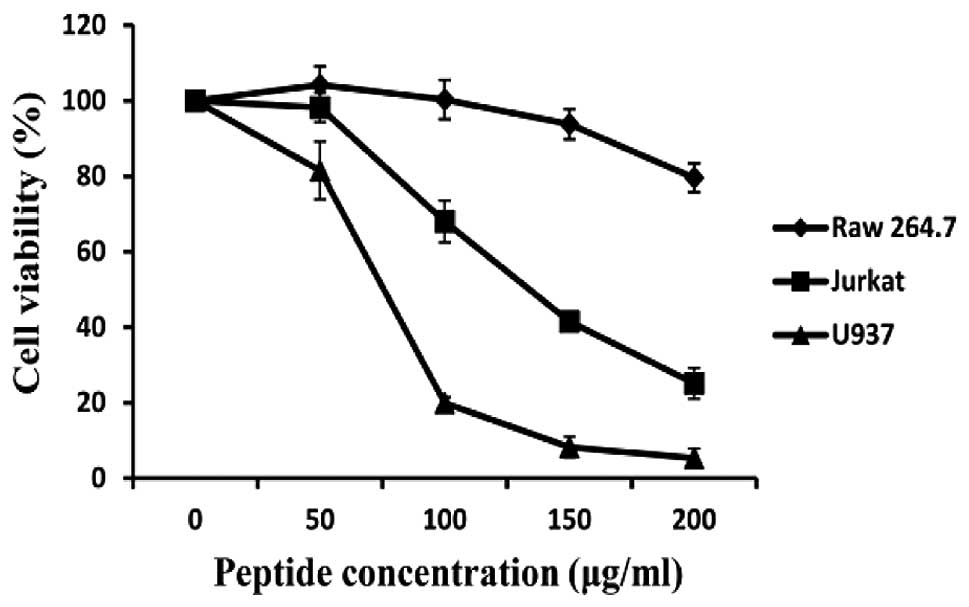
HaA4 markedly decreases cell viability of
leukemia cell lines
Recently, we showed that synthetic HaA4 exerts
antibacterial effect without hemolytic activities (17). We attempted to determine the effect
of the synthetic peptide HaA4 on cell growth and survival of human
leukemia cells (Jurkat and U937) in this study. Cancer cells were
treated with various concentrations (50, 100, 150 and 200
μg/ml) of HaA4 for 24 h and the cell viability was measured
by MTS assay. As shown in Fig. 1,
HaA4 decreased the viability of the leukemia cells in a
dose-dependent manner. In particular, viability of the cells
precipitated by >70% at 200 μg/ml of HaA4, while >70%
of Raw 264.7 cells remained viable. Therefore, our results suggest
that HaA4 should exert a potent anticancer activity against human
leukemia cells.
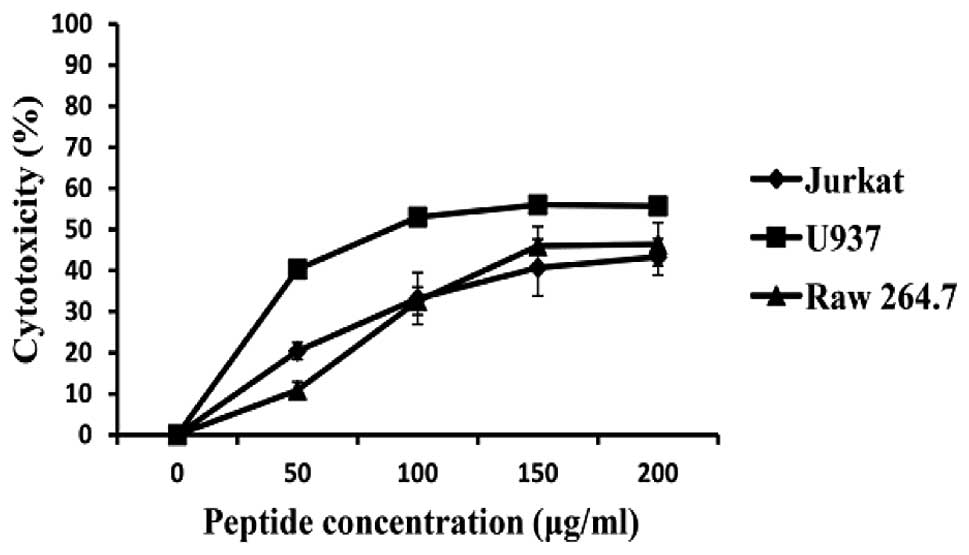
Effect of HaA4 on the integrity of cancer
cell membrane
We attempted to characterize the effects of HaA4 on
the integrity of cancer cell membranes by detecting the LDH
activity. As shown in Fig. 2, the
amount of LDH release increased in a dose-dependent manner in both
cancer cell types and the percentage of cytotoxicity appeared to
reach plateau with the elevation of the peptide concentration.
Although level of LDH release from both was similar, LDH release
from U937 was a little higher than that from Jurkat. Maximal
cytotoxicity at 200 μg/ml HaA4 was 43.3 and 55.7% for Jurkat
and U937 cells, respectively. However, HaA4 showed cytotoxic
activity against Raw 264.7 cells and the LDH release was similar to
Jurkat cells. Base on the results, we surmised that lytic activity
of HaA4 is influenced by the presence of serum. Although HaA4 had
no hemolytic activity in our previous report (17), cell selectivity of HaA4 including
serum stability needs to be examined further. Finally, when
compared to the results from the MTS assay, the reduction in cell
viability was 30–40% higher than expected from cytotoxicity. The
observed discrepancy suggested that additional factors including
apoptosis and growth inhibition as well as necrosis could play a
critical role in the viability reduction by HaA4.
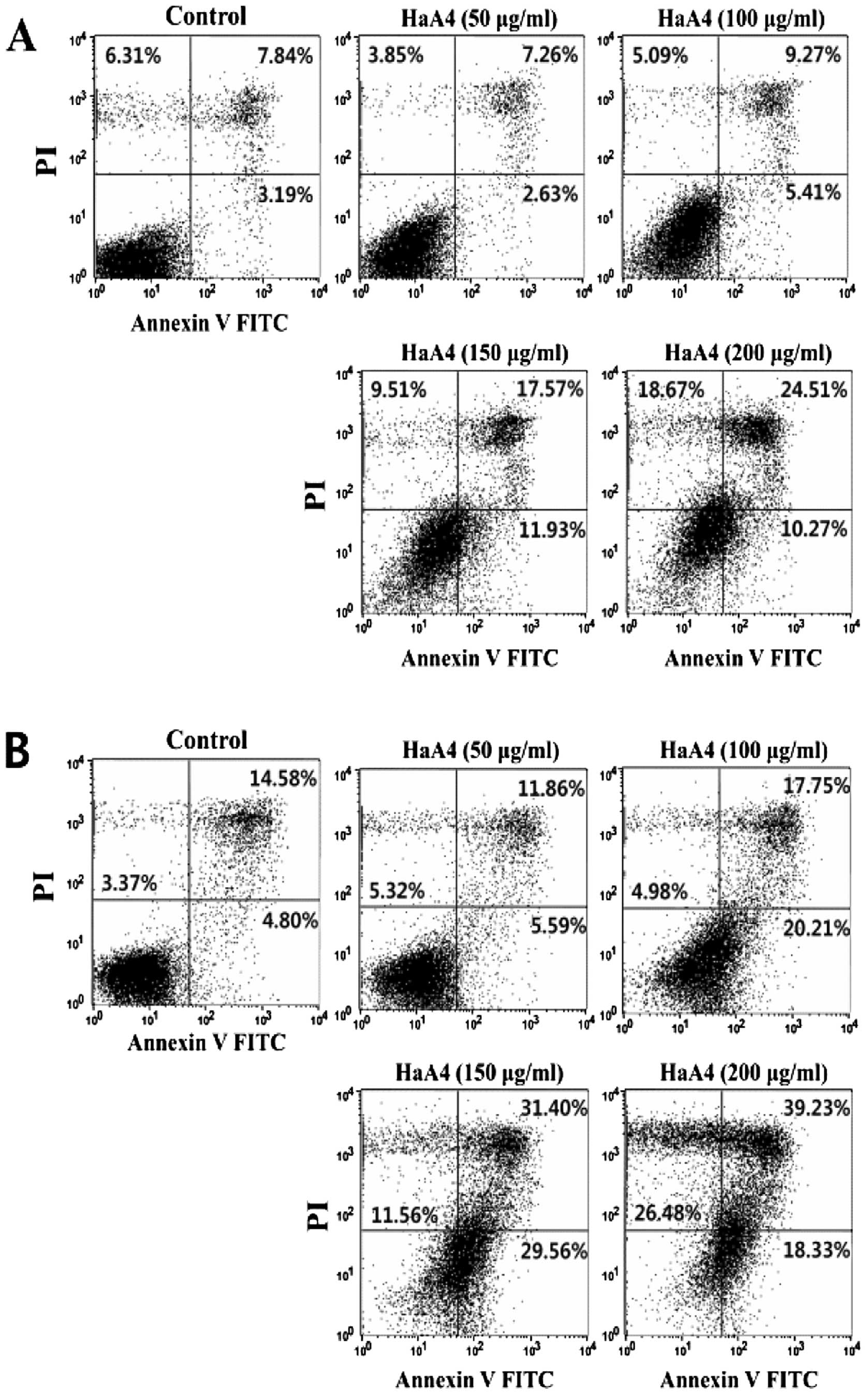
HaA4 induces apoptosis and necrosis in
leukemia cells
In order to further characterize mechanism of the
viability reduction, we assessed the involvement of apoptosis.
Apoptosis (programmed cell death) is a pivotal physiological
process that is required for the normal development and maintenance
of tissue homeostasis in multicellular organisms (21). During apoptosis, certain
morphological characteristics are involved, such as membrane
blebbing, phosphatidyl inositol exposure, nuclear and cytoplasmic
shrinkages, chromatic condensation and DNA fragmentation (22). Apoptosis was examined by Annexin
V/PI staining of the HaA4-treated leukemic cells. Annexin V binding
to the HaA4-treated leukemia cells was gradually increased as the
peptide concentration elevated. Annexin V-positive cell population
reached maximum at 150 μg/ml HaA4, while Annexin
V/PI-positive at 200 μg/ml HaA4 (Fig. 3). These results indicated that HaA4
should induce both apoptosis and necrosis depending on the
concentration of HaA4. Necrosis appeared prevailing over apoptosis
at higher concentration of HaA4.
Previously it has been reported that piscidin-1, a
cationic peptide isolated from the mast cells of hybrid striped
bass (23), also causes apoptosis
and necrosis at a low concentration and necrotic effect at a high
concentration for a short period in HT1080 cells (24). Piscidin-1 has a net charge of +3
and HaA4 has a net charge of +2 at pH 7.0, which might function in
the anticancer activity. Net charge of a peptide is an important
parameter for antitumor activity (25). Thus, it is supposed that the
positively charged cationic peptide could interact with anionic
cancer cell membrane electrostatically and damage the membrane
integrity.
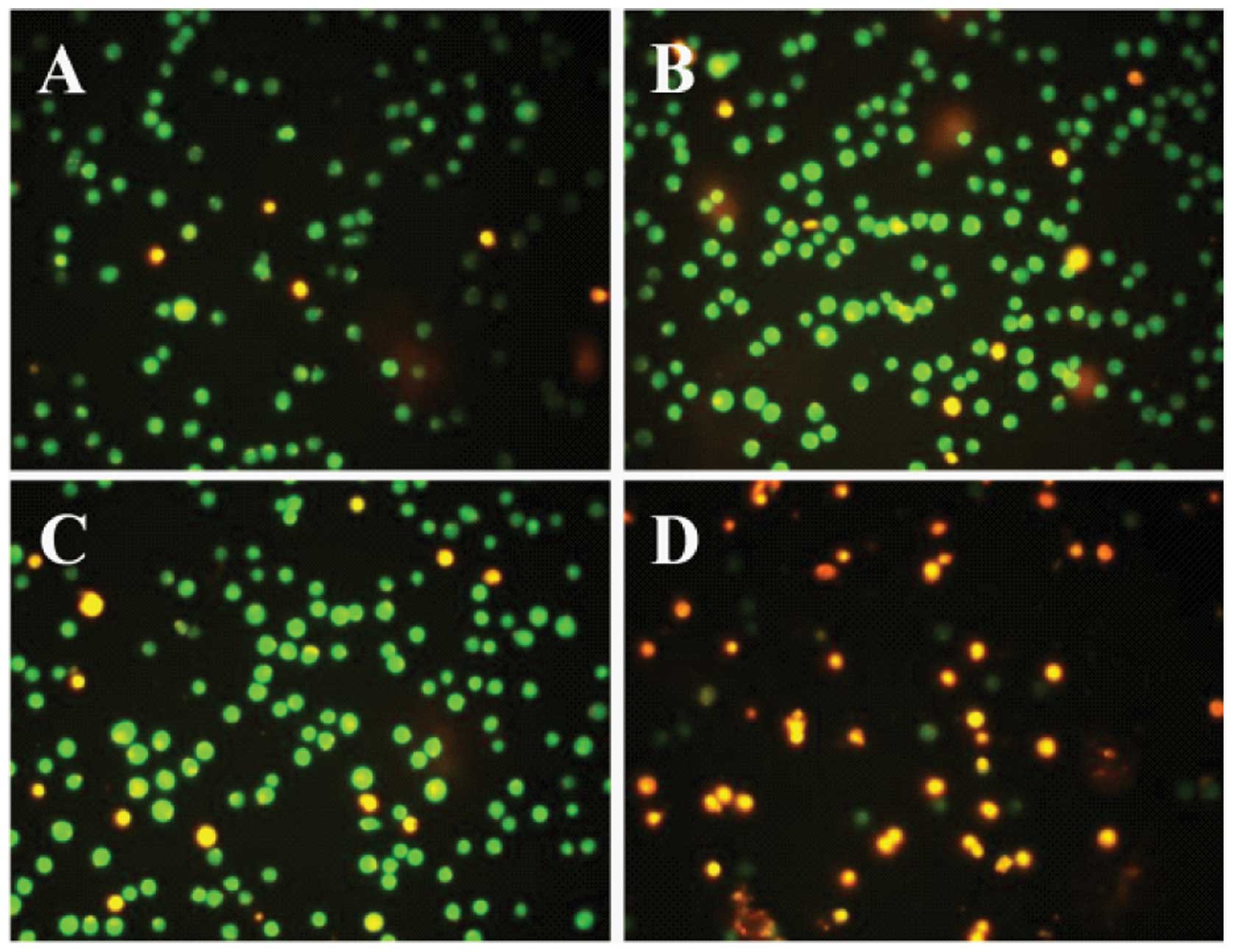
Acridine orange/ethidium bromide
staining
To verify Annexin V/PI assay results, HaA4-treated
Jurkat cells were stained with acridine orange/ethidium bromide.
After HaA4 treatment for 24 h, majority of cells exhibited green
fluorescence in control, while diffused or orange-colored nuclei
were increased in HaA4-treated cells with increase of HaA4
concentration. The cells treated with 150 μg/ml HaA4
developed orange and orange-red fluorescence, indicating membrane
disruption (Fig. 4). U937 cells
presented similar results (data not shown). These results support
that HaA4 could induce both apoptosis and necrosis at high
concentrations.
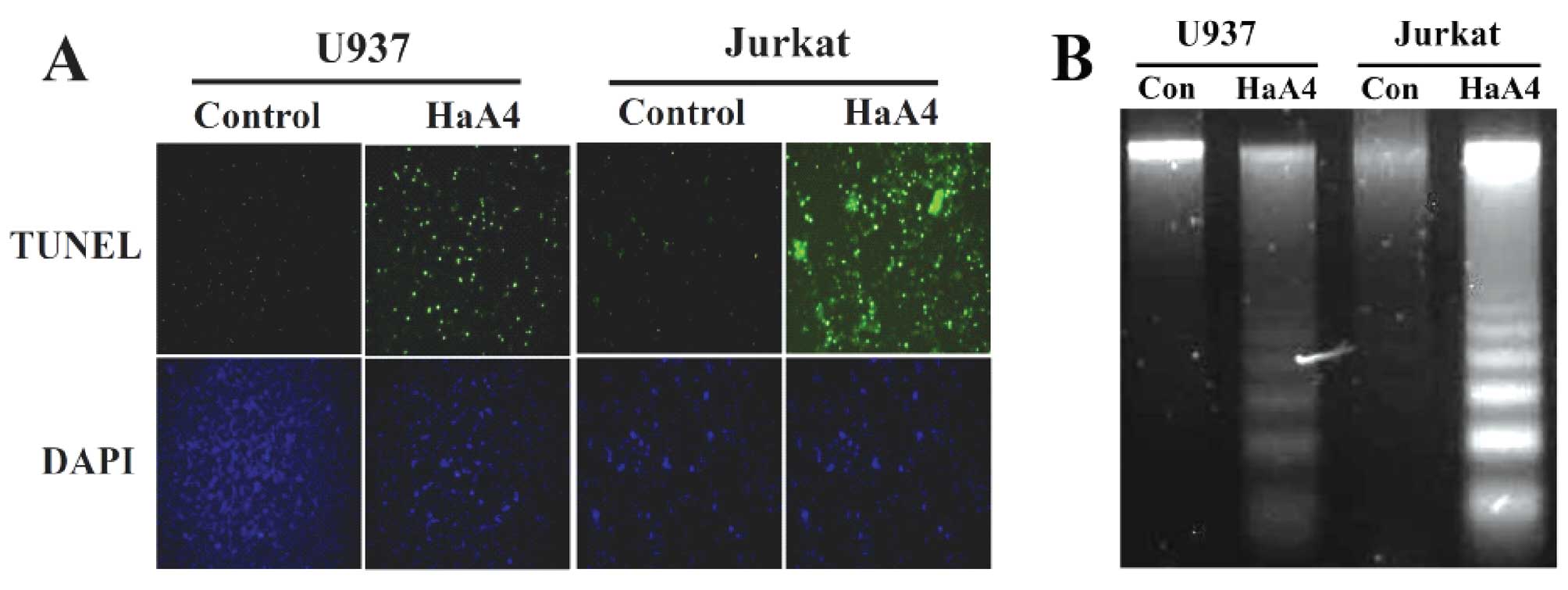
HaA4-induced DNA fragmentation
In order to further determine whether apoptosis is
involved in the viability reduction of the leukemia cells, we
performed TUNEL assay and agarose gel electrophoresis for
chromosomal DNA after treating these cells with 200 μg/ml of
HaA4 for 24 h. As shown in Fig.
5A, the number of TUNEL-positive apoptotic cells was
significantly increased in Jurkat and U937 cells treated with HaA4
when compared with the untreated cells. In agreement with the
results, the chromosomal DNA of Jurkat and U937 cells was
fragmented in nucleosomal ladder by HaA4 (Fig. 5B). Based on these results, we
assured that such pro-apoptotic effects of HaA4 should contribute
to the viability reduction of the leukemia cells.
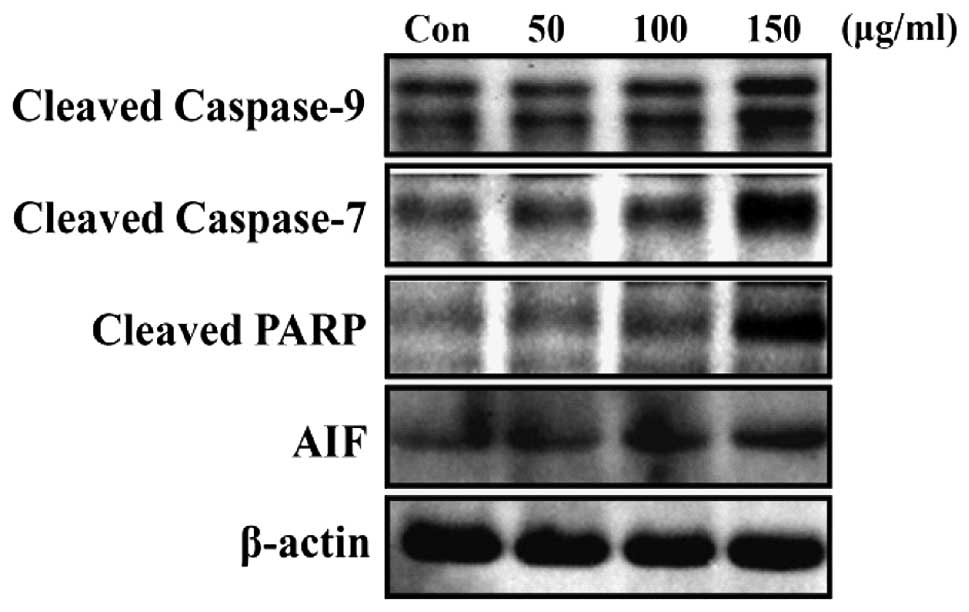
HaA4 induces apoptosis in the leukemia
cells via a caspase-dependent pathway
Since apoptosis can proceed via either
caspase-dependent or -independent signaling pathways (26,27),
the involvement of caspases in HaA4-induced leukemia cell apoptosis
was assessed. As shown in Fig. 6,
a marked increase in the cleavage of caspase-7, -9 and PARP was
observed. Subsequently, the potential role of apoptosis inducing
factor (AIF), a caspase-independent apoptosis regulator on
HaA4-induced apoptosis was investigated. However, we could not
observe converted mature form of AIF (Fig. 6). These results suggest that
HaA4-mediated leukemia cell apoptosis might be associated with the
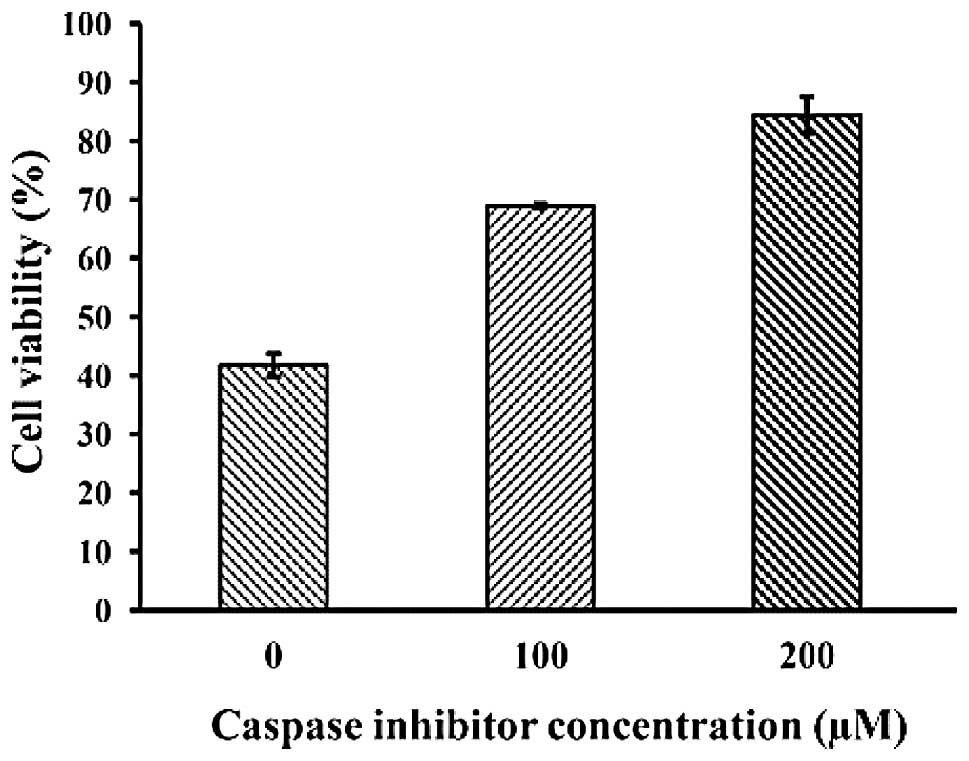
activation of caspase. Moreover, the decreased cell viability by
HaA4 treatment in the MTS assay recovered in the presence of
Z-VAD-FMK, a pan-caspase inhibitor (Fig. 7), demonstrating that HaA4-induced
leukemia cell apoptosis is dependent on the activation of the
caspase family proteins.
Most antibacterial and anticancer peptides employ
cell membrane disruption by lytic activity, or some peptides employ
apoptosis in cancer cells through mitochondrial damage. It is
believed that the mode of action originates from electrostatic
interaction between cationic peptides and anionic cell wall
components of bacterial and cancer cells. To date, there are four
possible different models (toroidal, carpet, barrel-stave and
aggregate channel) of AMP action mechanisms for membrane
permeabilization (28). In
previous studies, α-helical peptides were shown to need more than
20 amino acid residues to span the entire thickness of the
eukaryotic cell membranes for the barrel-stave mechanism (29,30).
Thus, the relatively small size of HaA4 along with aurein 1.2
(31) and citropin 1.1 (32), isolated from frogs suggest that
these AMPs mediate their membranolytic effect through the carpet
mechanism (33). In addition, it
has been reported that bovine lactoferricin binds to the cell
membrane and causes cell membrane disruption followed by entry of
the peptide to the cytoplasm of Jurkat T-leukemia cells and damage
to mitochondrial membrane (34).
Based on the results of our previous study (17), it was postulated that HaA4 acts on
anticancer activity similar to bovine lactoferricin, although the
exact mechanism of HaA4 has to be elucidated.
In this report, we have shown that HaA4 is a good
candidate for a new anticancer therapeutic agent as described
above. As a consequence, we could identify necrotic effects of HaA4
via LDH activity detection (Fig.
2) and Annexin V and PI staining (Fig. 3) and we also observed that HaA4
indicates apoptotic effects. Additionally, apoptosis of the
leukemic cells by HaA4 was dependent on the activation of caspase
(Figs. 6 and 7), a regulator of a caspase-dependent
pathway. Overall, our present study revealed that HaA4 should
retain anticancer activity against human leukemia cells (Jurkat and
U937) and the activity might ascribe to necrosis and apoptosis of
the leukemia cells.
Acknowledgements
This study was supported by a grant
from the Next-Generation BioGreen 21 Program (no. PJ008158) and
partially supported by a grant (no. PJ008706) from the Agenda
Program, Rural Development Administration, Republic of Korea.
References
|
1.
|
Lehrer RI, Lichtenstein AK and Ganz T:
Defensins: antimicrobial and cytotoxic peptides of mammalian cells.
Annu Rev Immunol. 11:105–128. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Zasloff M: Antimicrobial peptides of
multicellular organisms. Nature. 415:389–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Koczulla AR and Bals R: Antimicrobial
peptides: current status and therapeutic potentials. Drugs.
63:389–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bulet P, Hetru C, Dimarcq JL and Hoffmann
D: Antimicrobial peptides in insects; structure and function. Dev
Comp Immunol. 23:329–344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bulet P and Stocklin R: Insect
antimicrobial peptides: structures, properties and gene regulation.
Protein Pept Lett. 12:3–11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Matsuyama K and Natori S: Purification of
3 antibacterial proteins from the culture medium of NIH-Sape-4, an
embryonic cell line of Sarcophaga peregrina. J Biol Chem.
263:17112–17116. 1988.PubMed/NCBI
|
|
7.
|
Bulet P, Cociancich S, Reuland M, Sauber
F, Bischoff R, Hegy G, Van Dorsselaer A, Hetru C and Hoffmann JA: A
novel insect defensin mediates the inducible antibacterial activity
in larvae of the dragonfly Aeschna cyanea (Paleoptera,
Odonata). Eur J Biochem. 209:977–984. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Baker MA, Maloy WL, Zasloff M and Jacob
LS: Anticancer efficacy of Magainin2 and analogue peptides. Cancer
Res. 53:3052–3057. 1993.PubMed/NCBI
|
|
9.
|
Moore AJ, Devine DA and Bibby MC:
Preliminary experimental anticancer activity of cecropins. Pept
Res. 7:265–269. 1994.PubMed/NCBI
|
|
10.
|
Soballe PW, Maloy WL, Myrga ML, Jacob LS
and Herlyn M: Experimental local therapy of human melanoma with
lytic magainin peptides. Int J Cancer. 60:280–284. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Xiao YC, Huang YD, Xu PL, Zhou ZQ and Li
XK: Pro-apoptotic effect of cecropin AD on nasopharyngeal carcinoma
cells. Chin Med J (Engl). 119:1042–1046. 2006.PubMed/NCBI
|
|
12.
|
Iwasaki T, Ishibashi J, Tanaka H, Sato M,
Asaoka A, Taylor D and Yamakawa M: Selective cancer cell
cytotoxicity of enantiomeric 9-mer peptides derived from beetle
defensins depends on negatively charged phosphatidylserine on the
cell surface. Peptides. 30:660–668. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hwang JS, Lee J, Kim YJ, Bang HS, Yun EY,
Kim SR, Suh HJ, Kang BR, Nam SH, Jeon JP, Kim I and Lee DG:
Isolation and characterization of a defensin-like peptide
(Coprisin) from the dung beetle, Copris tripartitus. Int J
Pept. View Article : Google Scholar : 2009.PubMed/NCBI
|
|
14.
|
Kang JK, Hwang JS, Nam HJ, Ahn KJ, Seok H,
Kim SK, Yun EY, Pothoulakis C, Lamont JT and Kim H: The insect
peptide coprisin prevents Clostridium difficile-mediated
acute inflammation and mucosal damage through selective
antimicrobial activity. Antimicrob Agents Chemother. 55:4850–4857.
2011.PubMed/NCBI
|
|
15.
|
Kim IW, Kim SJ, Kwon YN, Yun EY, Ahn MY,
Kang DC and Hwang JS: Effects of the synthetic coprisin analog
peptide, CopA3 in pathogenic microorganisms and mammalian cancer
cells. J Microbiol Biotechnol. 22:156–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kang BR, Kim H, Nam SH, Yun EY, Kim SR,
Ahn MY, Chang JS and Hwang JS: CopA3 peptide from Copris
tripartitus induces apoptosis in human leukemia cells via a
caspase-independent pathway. BMB Rep. 45:85–90. 2012.
|
|
17.
|
Kim IW, Lee JH, Park HY, Kwon YN, Yun EY,
Nam SH, Kim SR, Ahn MY and Hwang JS: Characterization and cDNA
cloning of a defensin-like peptide, harmoniasin, from Harmonia
axyridis. J Microbiol Biotechnol. 22:1588–1590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hara T, Kodama H, Kondo M, Wakamatsu K,
Takeda A, Tachi T and Matsuzaki K: Effects of peptide dimerization
on pore formation: antiparallel disulfide-dimerized magainin 2
analogue. Biopolymers. 58:437–446. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Takei J, Remenyi A, Clarke AR and Dempsey
CE: Self-association of disulfide-dimerized melittin analogues.
Biochemistry. 37:5699–5708. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Jang WS, Kim CH, Kim KN, Park SY, Lee JH,
Son SM and Lee IH: Biological activities of synthetic analogs of
halocidin, an antimicrobial peptide from the tunicate
Halocynthia aurantium. Antimicrob Agents Chemother.
47:2481–2486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Wyllie AH: Apoptosis: An overview. Br Med
Bull. 53:451–465. 1997. View Article : Google Scholar
|
|
22.
|
Raff M: Cell suicide for beginners.
Nature. 396:119–122. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Silphaduang U and Noga EJ: Peptide
antibiotics in mast cells of fish. Nature. 414:268–269. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lin HJ, Huang TC, Muthusamy S, Lee JF,
Duann YF and Lin CH: Piscidin-1, an antimicrobial peptide from fish
(hybrid striped bass Morone saxatilis x M. chrysops),
induces apoptotic and necrotic activity in HT1080 cells. Zoolog
Sci. 29:327–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Diao Y, Han W, Zhao H, Zhu S, Liu X, Feng
X, Gu J, Yao C, Liu S, Sun C and Pan F: Designed synthetic analogs
of the α-helical peptide temporin-La with improved antitumor
efficacies via charge modification and incorporation of the
integrin αvβ3 homing domain. J Pept Sci. 18:476–486. 2012.
|
|
26.
|
Zeuner A, Eramo A, Testa U, Felli N,
Pelosi E, Mariani G, Srinivasula SM, Alnemri ES, Condorelli G,
Peschle C and De Maria R: Control of erythroid cell production via
caspase-mediated cleavage of transcription factor SCL/Tal-1. Cell
Death Differ. 10:905–913. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kitanaka C, Kato K and Tanaka Y: Ras
protein expression and autophagic tumor cell death in
neuroblastoma. Am J Surg Pathol. 31:153–155. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Li Y, Xiang Q, Zhang Q, Huang Y and Su Z:
Overview on the recent study of antimicrobial peptides: origins,
functions, relative mechanisms and application. Peptides.
37:207–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Shai YC: Molecular recognition between
membrane-spanning peptides. Trends Biochem Sci. 20:460–464. 1995.
View Article : Google Scholar
|
|
30.
|
Epand RM, Shai YC, Segrest JP and
Anantharamaiah GM: Mechanisms for the modulation of membrane
bilayer properties by amphipathic helical peptides. Biopolymers.
37:319–338. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Rozek T, Wegener KL, Bowie JH, Olver IN,
Carver JA, Wallace JC and Tyler MJ: The antibiotic and anticancer
active aurein peptides from the Australian Bell Frogs Litoria
aurea and Litoria raniformis the solution structure of
aurein 1.2. Eur J Biochem. 267l:5330–5341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Doyle J, Brinkworth CS, Wegener KL, Carver
JA, Llewellyn LE, Olver IN, Bowie JH, Wabnitz PA and Tyler MJ: nNOS
inhibition, antimicrobial and anticancer activity of the amphibian
skin peptide, citropin 1.1 and synthetic modifications. The
solution structure of a modified citropin 11 Eur J Biochem.
270:1141–1153. 2003.PubMed/NCBI
|
|
33.
|
Hoskin DW and Ramamoorthy A: Studies on
anticancer activities of antimicrobial peptides. Biochim Biophys
Acta. 1778:357–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Mader JS, Richardson A, Salsman J, Top D,
de Antueno R, Duncan R and Hoskin DW: Bovine lactoferricin causes
apoptosis in Jurkat T-leukemia cells by sequential permeabilization
of the cell membrane and targeting of mitochondria. Exp Cell Res.
313:2634–2650. 2007. View Article : Google Scholar : PubMed/NCBI
|